Published online Jul 24, 2019. doi: 10.5306/wjco.v10.i7.256
Peer-review started: February 12, 2019
First decision: June 7, 2019
Revised: June 22, 2019
Accepted: July 22, 2019
Article in press: July 22, 2019
Published online: July 24, 2019
Locoregional recurrence of breast cancer is challenging for clinicians, due to the various former treatments patients have undergone. However, treatment of the recurrence with systemic therapy and subsequent reirradiation of chest wall is accompanied by increased toxicities, particularly radiation-induced cardiovascular disease. Reirradiation by proton beam therapy (PBT) enables superior preservation of adjacent organs at risk as well as concurrent dose escalation for delivery to the gross tumor. This technology is expected to improve the overall outcome of recurrent breast cancer.
A 47-year-old female presented with an extensive locoregional recurrence at 10 yr after primary treatment of a luminal A breast cancer. Because of tumor progression despite having undergone bilateral ovarectomy and systemic therapy, the patient was treated with PBT BE total dose of 64.40 Gy to each gross tumor and 56.00 Gy to the upper mediastinal and retrosternal lymphatics including the entire sternum in 28 fractions. Follow-up computed tomography showed a partial remission, without evidence of newly emerging metastasis. At 19 mo after the PBT, the patient developed a radiation-induced pericardial disease and pleural effusions with clinical burden of dyspnea, which were successfully treated by drainage and corticosteroid. Cytological analysis of the puncture fluid showed no malignancy, and the subsequent computed tomography scan indicated stable disease as well as significantly decreased pericardial and pleural effusions. The patient remains free of progression to date.
PBT was a safe and effective method of reirradiation for locoregionally recurrent breast cancer in our patient.
Core tip: The treatment of recurrent breast cancer is very complex and not standardized. Patients with locoregional recurrence and gross residual tumor despite systemic therapy and surgery demand further options with potentially curative intention. Presented here is a patient with a late locoregionally recurrent breast cancer, showing significant reduction of gross tumor disease after reirradiation by proton beam therapy. In the 2 years since, the patient has remained free of tumor progression.
- Citation: Lin YL. Reirradiation of recurrent breast cancer with proton beam therapy: A case report and literature review. World J Clin Oncol 2019; 10(7): 256-268
- URL: https://www.wjgnet.com/2218-4333/full/v10/i7/256.htm
- DOI: https://dx.doi.org/10.5306/wjco.v10.i7.256
Breast cancer (BC) is the second most common cancer disease worldwide. The global cancer research project GLOBOCAN 2012 reported an approximate 1.67 million new cases diagnosed in 2012, accounting for 25% of all cancers in women and ranking 5th among the total cancer-related deaths. At the initial diagnosis of BC, the likelihood of locoregional and distant recurrence and overall prognosis can be assessed according to clinicopathological features, such as tumor size, grading, lymph node (LN) involvement, expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2), and proliferation index Ki-67. The incidence rate of local recurrence at 10 years after breast conserving therapy ranges from 10% to 22% and that for mastectomy from 5% to 15%, occurring on average within the first 5 years after the primary treatment. Furthermore, local recurrence is associated with increased appearance of regional LN and distant metastases, namely in 10%-30% of patients after breast conserving therapy and in 30%-50% of those after mastectomy[1].
BC recurrence is a real challenge for clinicians owing to the variety of previous treatments a patient can undergo [i.e., breast conserving therapy, mastectomy, neoadjuvant or adjuvant systemic therapy, and radiotherapy (RT)] as well as the co-existence of LN and distant metastases. Patients with recurrent BC, even in the absence of distant metastasis, suffer from severe mental and corporal stress related to the local tumor progression and which, particularly the latter, can manifest ulceration, hemorrhage, stench, pain, brachial plexus palsy, and lymphedema. Thus, multidisciplinary treatment approaches are required, consisting of breast and plastic surgery, medical and radiation oncology, pathology, radiology, psycho-oncology, and specific wound management for ulcerating tumors.
Based on the favorable physical property of the Bragg peak[2], proton beam therapy (PBT) enables an optimal planning of reirradiation for locoregional recurrence of BC. This technology not only spares adjacent uninvolved breast tissue and other organs at risk (OAR), such as heart, esophagus, lungs, spinal cord and brachial plexus, but also allows for dose escalation of the irradiation to partial breast and chest wall (CW).
A 47-year-old female presented with a progressive locoregional recurrence at 10 years after the initial diagnosis of a luminal A BC. As to her clinical symptoms, she complained only of intermittent pain in the parasternal area on both sides.
The breast magnet resonance imaging (MRI) performed in May 2014 primarily showed suspicious sternal metastasis, but the sternum biopsy did not reveal any malignancy. The subsequent mammography of both breasts also excluded local recurrence. Over a year later, in July 2015, a computed tomography (CT) scan revealed a tumor mass in the right para- and presternal area (between the 2nd and 5th rib) with invasion of cartilage and ventral pleura, and extending to the subcutis and contralateral parasternal region. Moreover, several enlarged mediastinal LN were detected, along with a pleural lesion in the anterior segment of right upper lobe and bony metastasis in the sternum body. Biopsy of the CW recurrence confirmed a moderately differentiated invasive carcinoma with ER 8, PR 8 (according to Allred score), HER2 negativity, and Ki-67 10%.
After a laparoscopic ovarectomy (both sides) was performed, the patient chose to participate in the MONALEESA-3 study investigating efficacy of fulvestrant in combination with ribociclib (vs placebo). In addition, she commenced biphosphonate therapy with zoledronic acid. In February 2016, she dropped out of the study due to progressive disease with newly-occurring parasternal LN metastases and slowly growing pleural metastasis; her endocrine therapy was switched to letrozole. However, the parasternal CW recurrence on the right side continued to grow, reaching roughly 30 mm × 50 mm × 20 mm in size. The patient participated in consults for both cyber knife therapy in another clinic and PBT at the Rinecker Proton Therapy Center, and eventually decided to undergo the latter.
At the age of 37, the patient had been diagnosed with a multifocal invasive ductal carcinoma, presenting as four cancerous lesions measuring up to 1.6 cm, as well as an extended ductal carcinoma in situ of the right breast. She underwent, first, a segment resection with axillary LN dissection. In the second session, she underwent an ablation of the right breast. The postoperative tumor stage was pT1c(m) pTis(4cm) G2 pN1a(2/23) cM0 L0 V0 R0. The receptor status (according to Allred score) was ER 8, PR 8, HER2-negative.
After completing 6 cycles of postoperative chemotherapy with docetaxel, doxorubicin and cyclophosphamide, the patient received adjuvant RT with photon beams to the right CW and supraclavicular, axillary and retrosternal lymphatic drainage pathways (at a total dose of 50 Gy in 25 fractions). A sequential electron boost treatment (providing a further 10 Gy in 5 fractions) was given to the tumor bed in the lower and inner quadrants over the period of June to July 2006. Under endocrine therapy with zoladex (until June 2009) and tamoxifen (until July 2012), there was no sign of tumor recurrence in the follow-ups. Consequently, the patient underwent a breast reconstruction with deep inferior epigastric perforator flap in January 2011.
Beside lymphedema of her right arm, a tumor conglomerate was noted on the right parasternal CW; although, the skin surface was still intact and without ulceration. The common clinical examination of cardiopulmonary and neurological status did not reveal any pathological findings.
No special laboratory test was arranged.
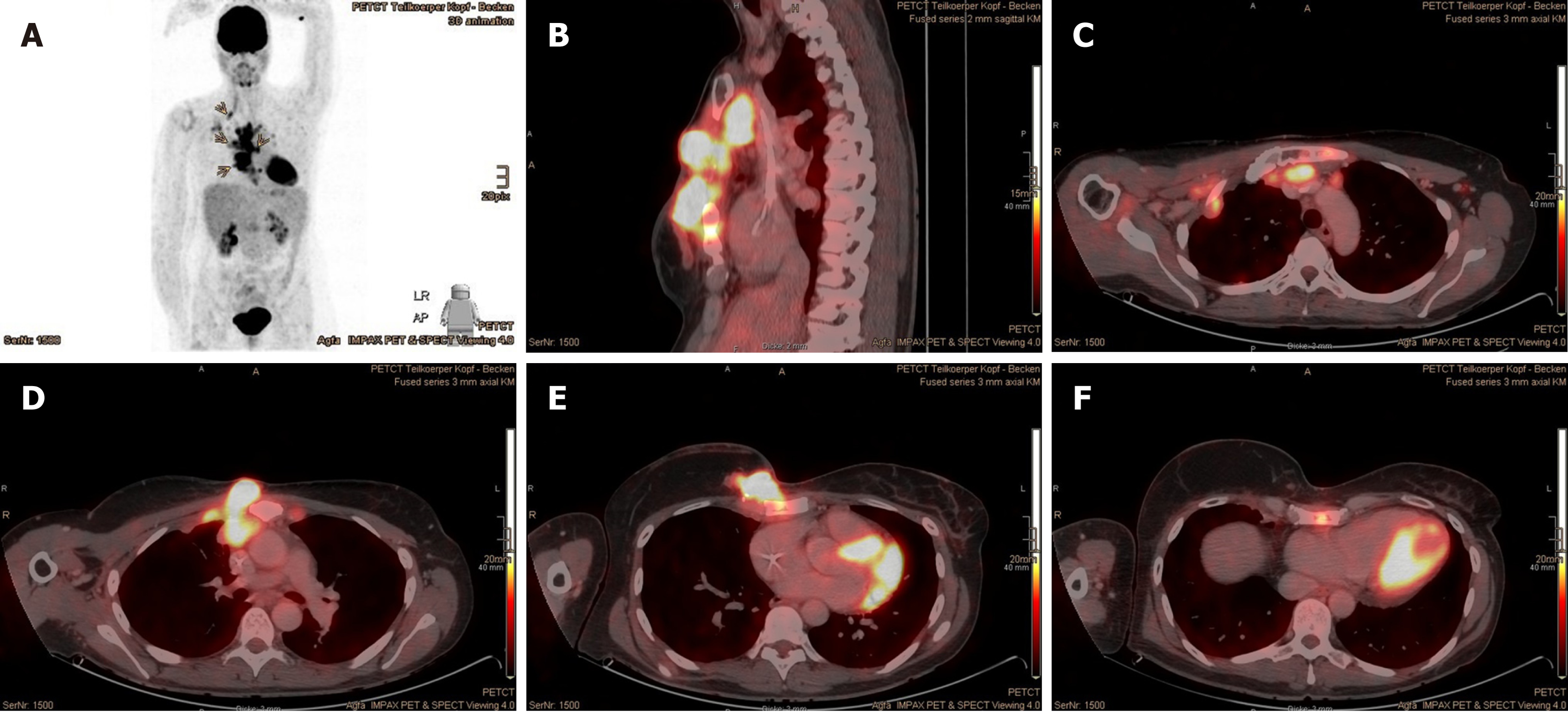
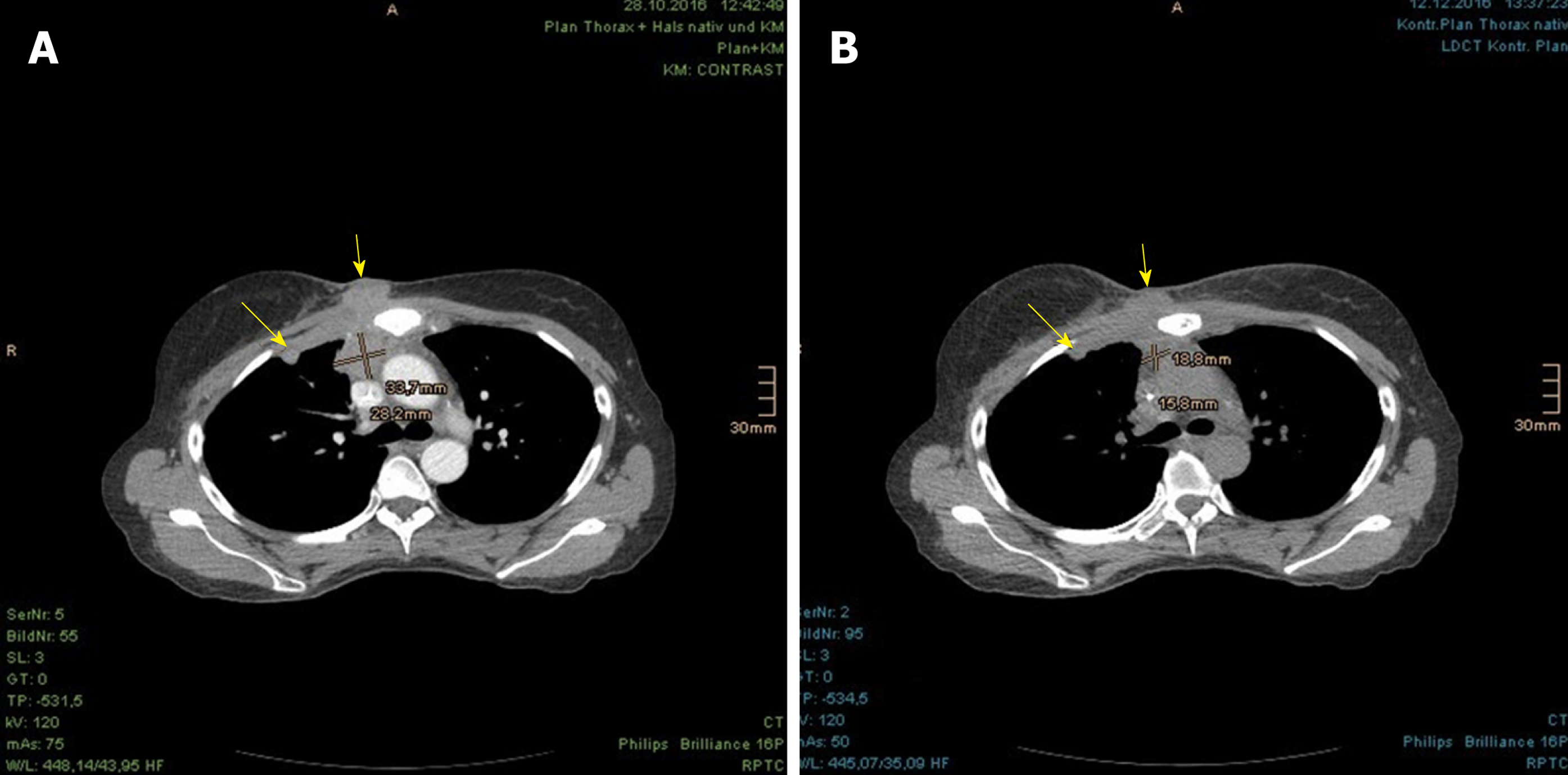
Prior to the PBT, the patient underwent positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro-D-glucose (18F-FDG) PET/CT at our center, displaying intensively increased uptake in the LN stations of the right armpit, upper mediastinum, aortic arch on the left, and retrosternal in front of the right heart ventricle, as well as in the corpus sterni and on the CW in the right parasternal area with invasion of right breast, subcutis and mediastinum (Figure 1). Over and above that, pleural metastases with aspect of lymphangiosis carcinomatosa were detected in the right upper lobe, with adhesion on the interlobe of the right lower lobe apex (Figure 2).
This approach was not specified according to the unequivocal histopathological and 18F-FDG PET/CT findings.
Recurrent BC.
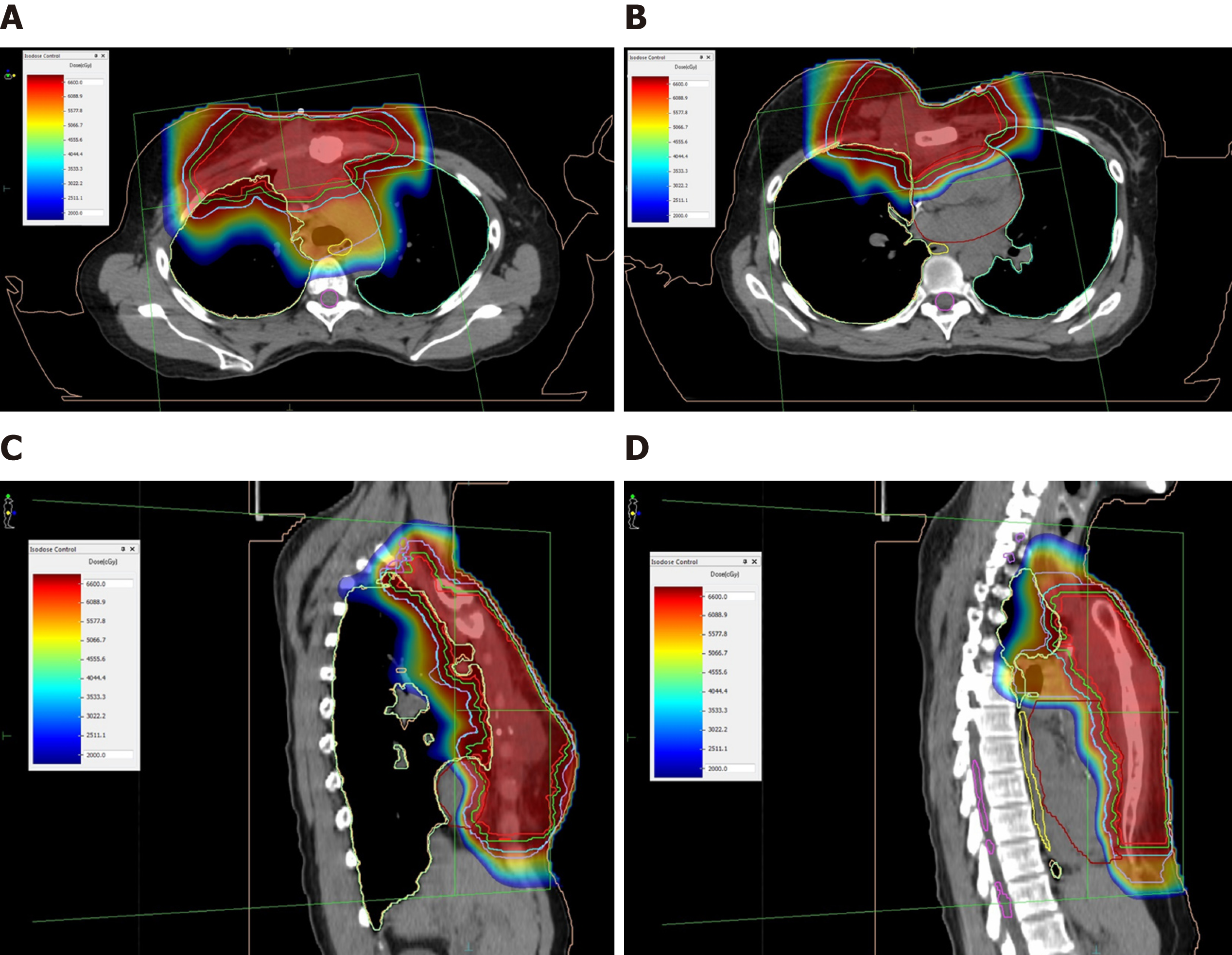
The patient was treated at the Rinecker Proton Therapy Center from November to December 2016. The PBT was delivered in 28 fractions (at a total dose of 64.40 Gy; relative biological effectiveness (referred to as RBE)) to the tumor lesions on the right CW, including the adjacent pleura, LN metastases in the right axillary, parasternal and mediastinal area and sternum metastasis. The superior mediastinal and retrosternal lymphatic drainage pathways and the entire sternum also received 56 Gy (RBE) in 28 fractions, concurrently (Figure 3). For the purpose of accurate reproduction of the target, the patient was positioned with custom immobilization devices, consisting of vacuum cushion, breastboard and Beekley Spots as fiducial markers. To estimate the deviation of the target by respiratory motion, the patient underwent CT simulation in flat and free breathing states during the planning stage, as well as weekly CT scans (performed as controls) during the treatment and including fusion with the planning CT. We used only one irradiation field, from the 352 degree gantry angle with a field size of 22.8 cm in width and 28.8 cm in length. The irradiation direction was chosen in the best way to compensate the difference of chest wall in anterior-posterior direction due to respiratory motion. The pencil beam scanning technique, depriving energy of 75-250 MeV from a superconducting cyclotron at our center, enables an intensity modulated proton therapy, which was employed with the anticipation of homogeneous target volume coverage, sparing of uninvolved surrounding tissue and OAR, as well as certain dose escalation.
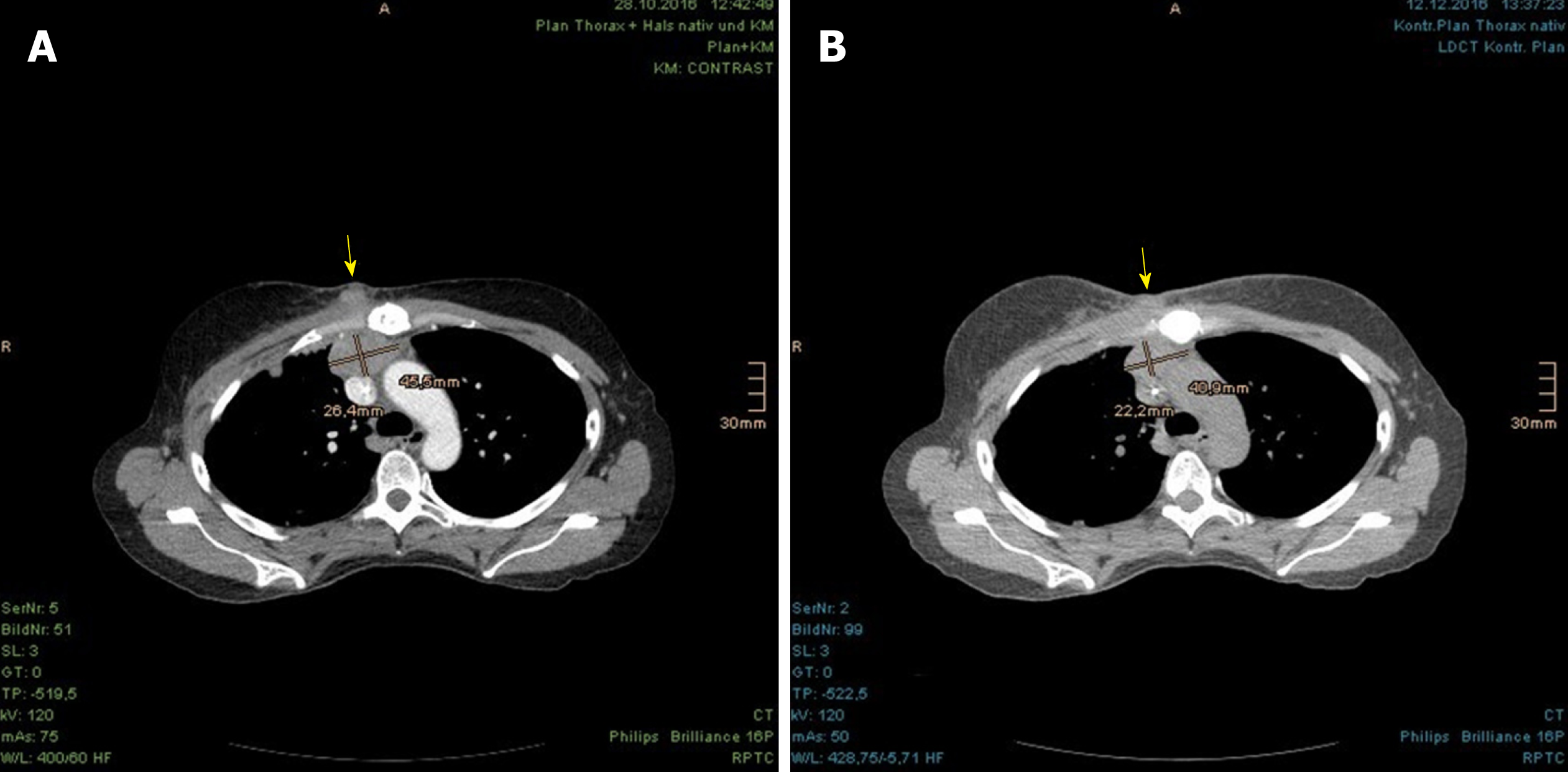
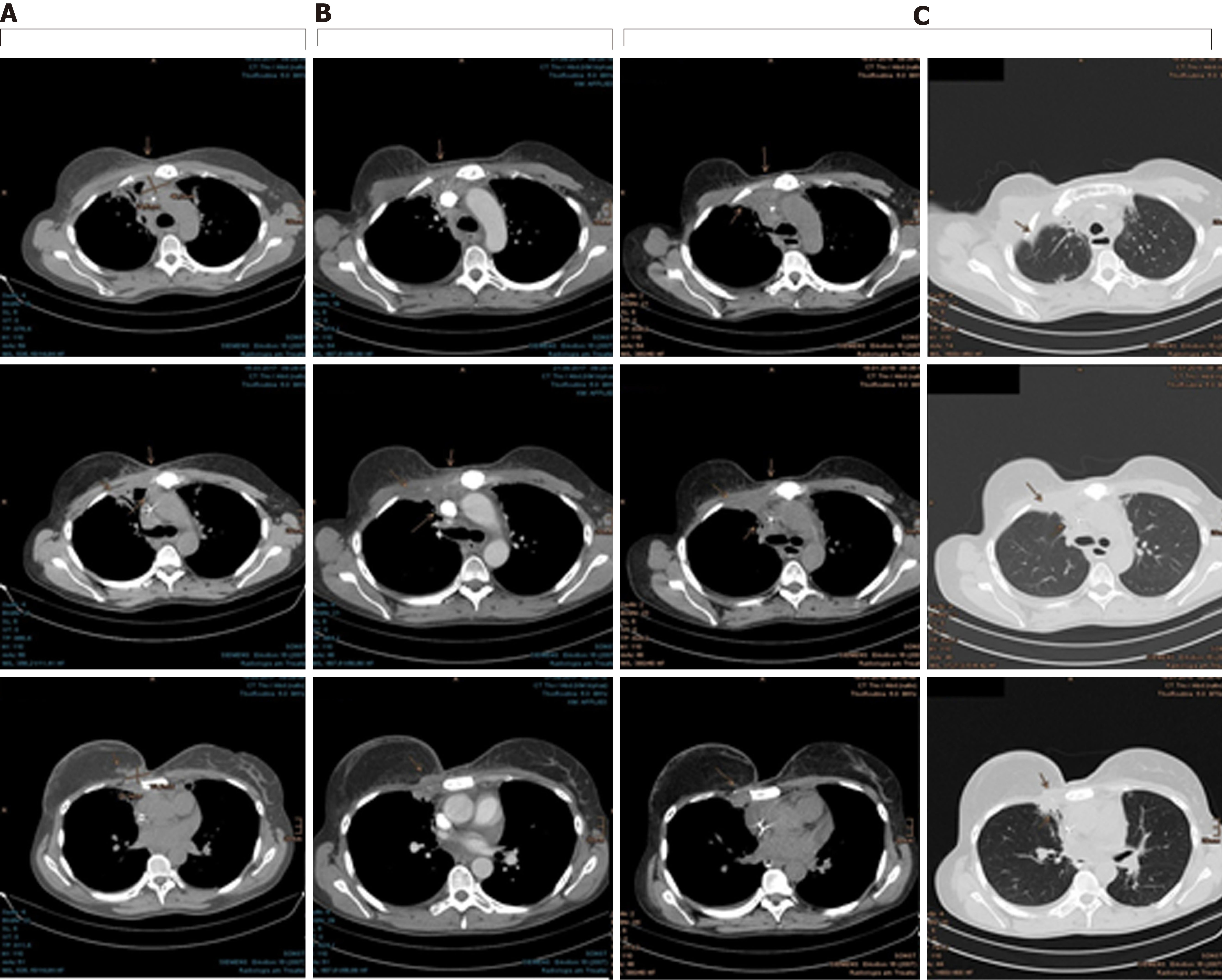
The patient tolerated the PBT well and reported only dysphagia with reflux and cough now and then, defined as grade 2 by the Common Terminology Criteria for Adverse Events (commonly known as CTCAE). The skin showed radiation dermatitis of grade 2 CTCAE, with dry desquamation in the parasternal and submammary locations. Because of the remarkable radiation dosage to the lungs, a regimen of ciprofloxacin (10 d), prednisolone and omeprazole (6 wk) was recommended as prevention against radiation pneumonitis. Already in the course of the treatment, a diminution of the parasternal tumor nodules was observed clinically and radiographically by the weekly-performed controls with low-dose CT scan (Figures 4-6).
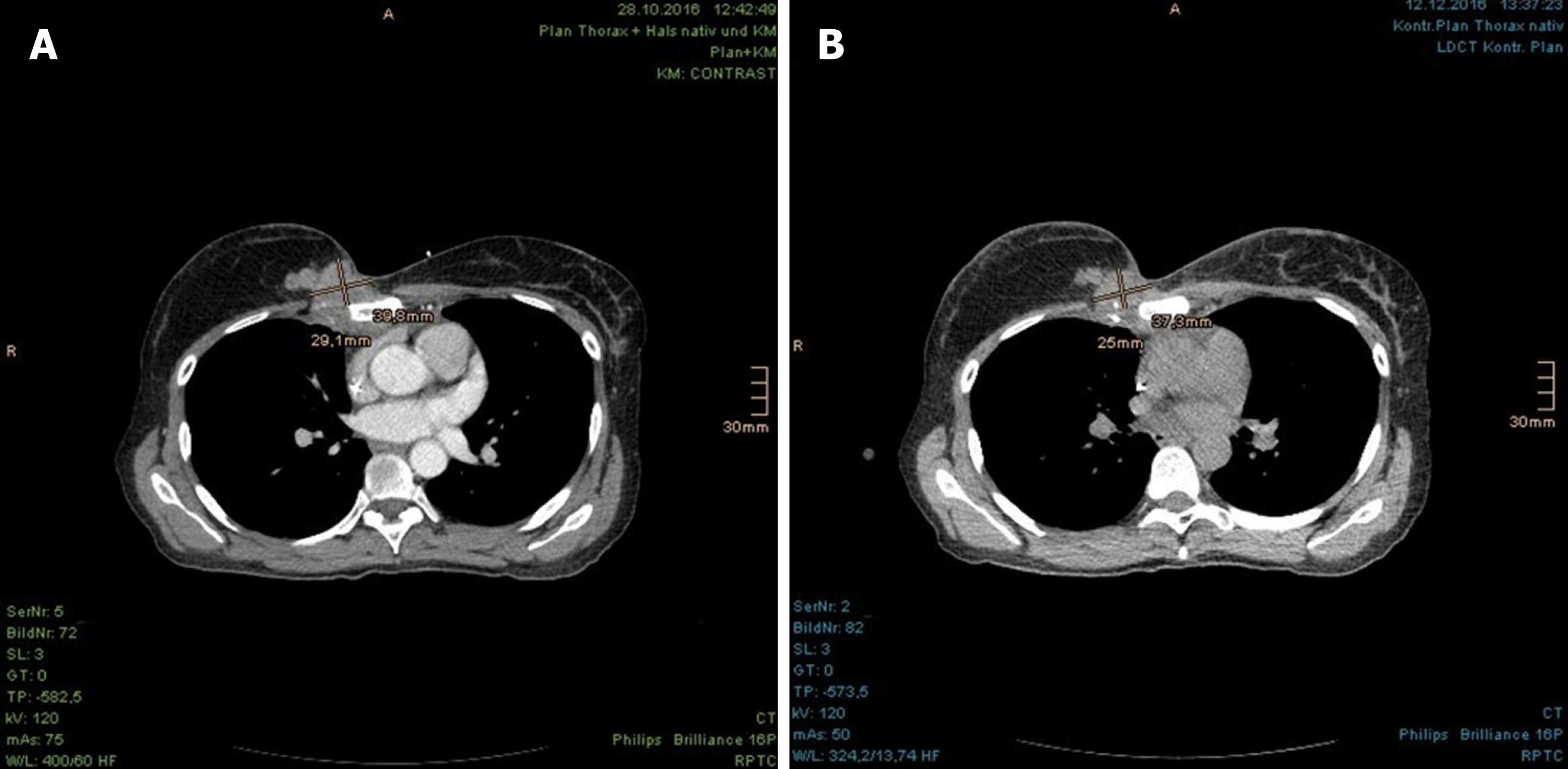
In the first follow-up, at 3 mo after the PBT, the CT scan revealed significant regression of the CW recurrence and the LN metastases (Figure 7A). Subjectively, the patient complained of consistent skin hyperpigmentation with itching, in spite of frequent skin care. Furthermore, she reported coughing with clear sputum, but felt generally sound and undisturbed. The difficulties in swallowing had completely diminished. She was able to move her right arm better and had less pain. The lymphedema, which had existed since the axillary LN dissection, did not deteriorate after the PBT but was reportedly improved by movements like swimming. Regarding the most concerned brachial plexus palsy due to reirradiation of the extended locoregional recurrence, a mere tingling paresthesia of the right arm had occurred at 8 mo after the end of the PBT treatment and ameliorated spontaneously within 1 mo. In this time period, the arm strength was not impaired and the lymphedema of right arm remained unchanged. Subsequently, the patient only reported subtle paresthesia on the fingertips and heaviness of the right arm, and she underwent regular lymphatic drainage. Apart from the chronic cough with difficulties in expectorating due to stiffness of the neck, there was no other clinical complaint.
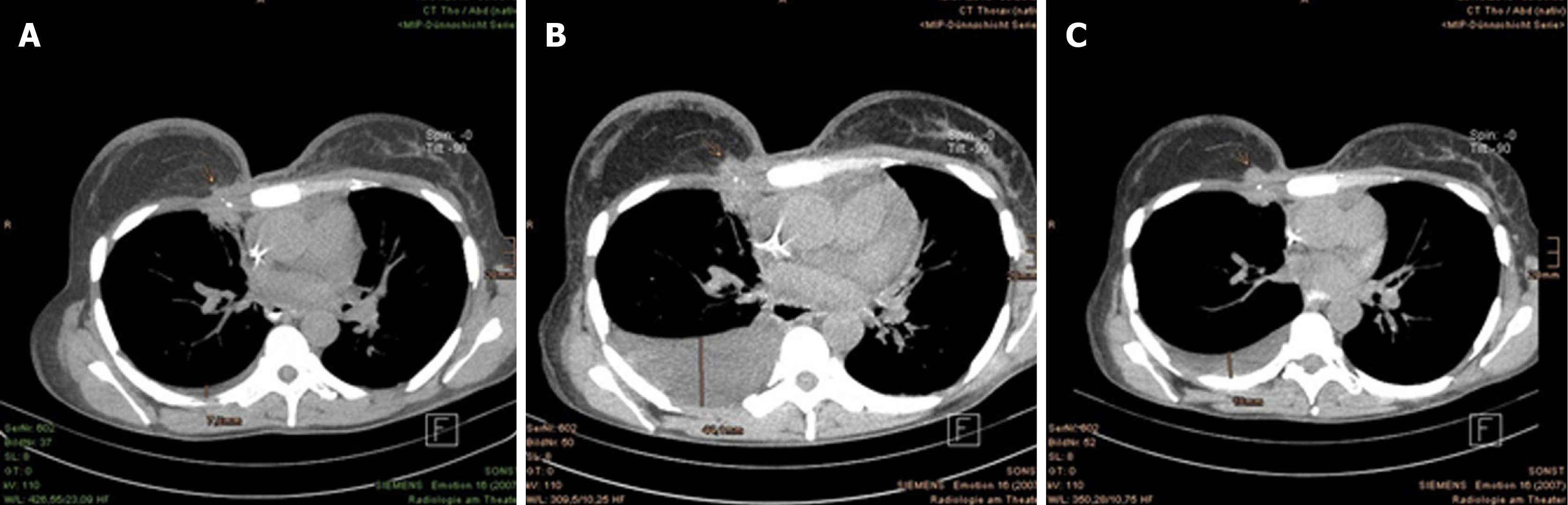
The CT scans of thorax and abdomen, taken at the 9th and 13th mo after PBT, demonstrated further reduction of the tumor mass at right of the sternum, as well as constant postradiogenic changes in both paramediastinal regions and in the right lung apex (Figure 7B and C), excluding any new metastasis. Early 2018, the patient indicated dysphonia with occasional dyspnea and cough stimulated by cold in winter. The examination by a staff otolaryngologist exhibited laryngeal edema with soreness, excluding vocal cord paralysis. The patient attended and completed speech therapy, and no further treatment was recommended for this condition. Aside from persisting lymphedema of the right arm, the patient reported tension of the right shoulder muscles, which the fibrosis contributed to as well. The skin continued to appear discreetly flushed, consistent with teleangiectasia. There was no tumorous proturbance that was visible, but the right breast remained in its slightly swollen state since the PBT.
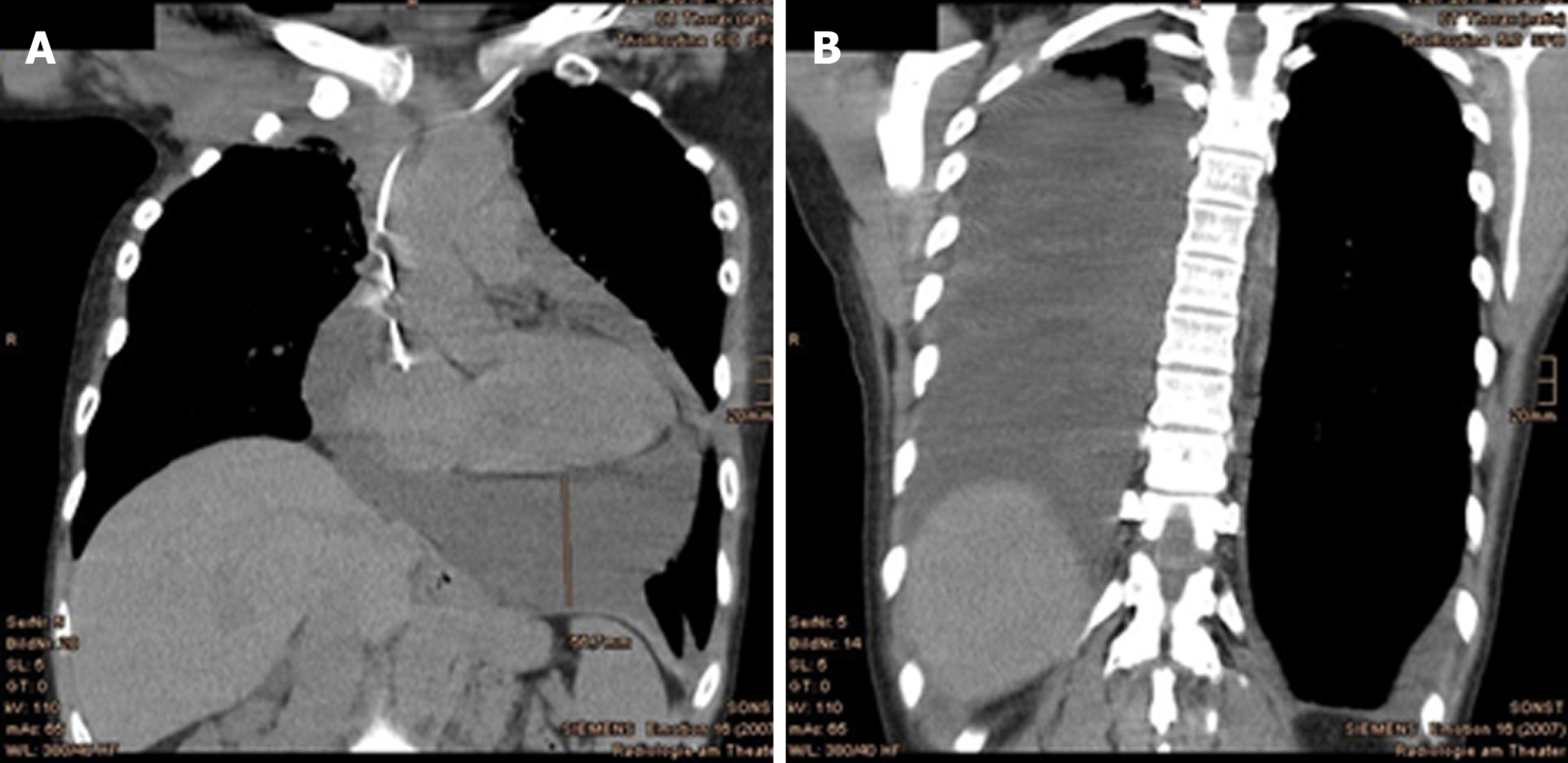
In July 2018, the follow-up CT scan showed an increase in pericardial and pleural effusion on the right (Figure 8). Assessment of the pericardial effusion (about 4 cm wide) by a staff cardiologist found no evidence of hemodynamic compromise. Instinctively, the patient reported overall stable general condition, except for burden of dyspnea, corresponding to class II in the New York Heart Association (commonly known as NYHA) classification system. Otherwise, the dysphonia, coughing and shoulder tension were bettered by physiotherapy and speech therapy. In addition, a newly-appeared soft tissue augmentation in the right parasternal area (Figure 9A and B) prompted referral to the Breast Cancer Institute by her oncologist. Nevertheless, the pericardial and pleural effusions became gradually significant thereafter. A cardiac MRI performed after the first puncture revealed acute pericarditis without myocardial involvement or cardiac wall motion abnormalities.
In the following months, a total amount of 1650 mL of pericardial effusions and 3000 mL of pleural effusions were drained, all sans evidence of malignancy. Starting with the first drainage, the patient had been treated with colchicine, ibuprofen and torasemide. According to the reproduction of effusions in the interval of 2 wk and ruling-out of other differential diagnoses, such as rheumatic diseases, a probationary treatment with corticosteroid was initiated. From this time forward, the pericardial and pleural effusions distinctly declined (Figure 10).
In the most recent CT scan, taken in October 2018, there was no definite sign of tumor progression. Considering the stable size of the right parasternal soft tissue density (Figure 9C), no further investigations (i.e., biopsy and 18F-FDG PET/CT) were arranged by the Breast Cancer Institute. The patient continued the present systemic therapy with letrozole and denosumab. She experienced incremental advances in physical ability and appetite, and even reported a noticeable reduction in the lymphedema in her right arm, which allowed her to begin swimming anew.
Recognition and appreciation of the various biological subtypes of BC can help clinicians predict the recurrence pattern and determine the most appropriate and effective treatment concept[3]. In the literature, several approaches to analyze hormone receptor status as a predictor of outcome in BC patients have been reported. According to the National Cancer Institute’s Surveillance, Epidemiology, and End Results program (commonly known as SEER; 1992-2002), ER-negative and ER-positive cases rendered very different consequences in hazard rates of cancer-specific death. Specifically, at 17 mo the ER-negative hazard rates peaked at 7.5% per year and thereafter declined, while the ER-positive hazard rates had no distinct early peak but showed a consistent rate of 1.5%-2% per year. The falling ER-negative and constant ER-positive hazard rates finally traversed at 7 years. Thereupon, the prognosis seemed to be better for the ER-negative cases[4].
Intriguingly, the recurrence rate is significantly higher in ER-negative cases for the first 2 years of follow-up and is associated with rising emergence of visceral and soft tissue metastases. ER-positive tumors, in contrast, metastasize more frequently to bone[5]. With regard to this, Campbell et al[6] proposed a new combined endocrine receptor immunohistochemistry scoring system as a more powerful predictor of patient outcome, being especially important for early BC with positivity for ER.
A retrospective study of 300 patients with recurrent disease concluded that hormone receptor-positive and HER2-negative BC had higher risk of recurrence later than 5 years from the initial treatment or after diagnosis, particularly concomitant with high ER titer (> 50%) and low nuclear grade, and predominantly spread to the bone; larger (> 2 cm), node-positive and HER2-positive tumors were predicted for early recurrence[7]. A recent Dutch study demonstrated that the risk of first recurrence was highest in the second year after BC diagnosis, including a second peak around years 8-9[8]. In that study, young age (< 40 years), tumor size, positive LN metastases, tumor grade 2-3, multifocality, and lack of systemic therapy were identified as prognostic predictors for first recurrence. Interestingly, another study by Lynch et al[9] could not define multifocal or multicentric BC as an independent risk factor for locoregional recurrence. Those patients with multifocal or multicentric disease showed a comparable locoregional control rate as those with unifocal tumor, regardless of treatment type (i.e., breast conserving therapy or mastectomy, or mastectomy with postmastectomy RT)[9].
Correlated to this knowledge, our case presented herein yielded several adverse prognostic factors, including young age, multifocality, large tumor size and axillary LN metastases at the initial diagnosis of BC. The ER and PR status of our patient was highly positive, while the HER2 status was negative. She had received the adjuvant endocrine therapy for a total of 6 years, but developed unresectable tumor recurrence at 3 years after ending the tamoxifen therapy. The review published by Wimmer et al[10] validated the benefit of prolonged endocrine therapy beyond 5 years on recurrence-free and disease-free survival for patients with hormone receptor-positive BC, particularly when tamoxifen was followed by an aromatase inhibitor. The updated version of the American Society of Clinical Oncology clinical practice guideline in 2014 recommended a prolongation of adjuvant endocrine therapy with tamoxifen, from 5 years to 10 years, in pre- and perimenopausal patients; for those with postmenopausal status, after 5 years of adjuvant therapy, the tamoxifen should be switched to an aromatase inhibitor or the patient should continue on tamoxifen for another 5 years[11]. Consequently, our patient should be a candidate benefiting from extended adjuvant endocrine therapy of 10 years.
Unfortunately, the elongated treatment is associated with aggravated adverse effects, namely thromboembolic events, bone density loss and additional risk for endometrial cancer. Apart from the well-known risk factors for tumor recurrence like nodal-positive and large tumors, feasible predictive markers are required to evaluate patients who are most likely to benefit from protracted endocrine therapy. Sestak et al[12] pointed out the importance of identifying BC patients with high risk of late (distant) recurrence precisely by use of prognostic and predictive biomarkers or multi-gene signatures or liquid biopsies. The improved expertise of molecular markers will enable a planning of individualized therapies for patients[12].
Beyond that, the early and accurate detection of recurrent disease is of essential importance. In comparison to conventional imaging, such as sonography, mammography, CT, MRI and bone scintigraphy, 18F-FDG PET/CT is more comprehensive and less time consuming. It has shown distinctly higher predictive values for determination of locoregional and systemic BC recurrence, and consequently has a higher impact on therapeutic management as well as prognostication of survival[13,14]. The maximum standardized uptake value (commonly referred to as the SUVmax) of 18F-FDG PET/CT has been shown to serve incidentally as a useful predictor of postoperative relapse-free survival and overall survival in patients with luminal-type BC[15].
The patient in our case report had not received 18F-FDG PET/CT at the beginning of clinical suspicion. As the diagnosis was confirmed at more than 1 year later, she already presented a locally advanced, unresectable locoregional recurrence with distant metastases. Leastways prior to PBT, she was referred to undergo 18F-FDG PET/CT to obtain a more precise localization of all cancerous manifestations. However, although the 18F-FDG PET/CT was recommended by us (owing to the later noted right parasternal soft tissue augmentation), her supervising oncologist and referring BC institute did not regard it as necessary because of the observed stability in size from July to October 2018.
Concerning the management of locoregional BC recurrence, the treatment is not standardized and demands a multidisciplinary approach[1,16,17]. The German Society of Radiation Oncology updated the radiotherapeutic guidelines and considered RT (e.g., external beam RT, brachytherapy, or intraoperative RT) as an essential part of multimodality treatment in addition to systemic therapy, surgery and hyperthermia[18]. However, it remarked that the largest experience on reirradiation was based on multi-catheter brachytherapy and that prospective clinical trials were required to define the selection criteria, long-term local control, and toxicity.
A multi-institutional review of repeat irradiation of breast and CW for locally recurrent BC (median dose of the first and second course of RT being 60 Gy and 48 Gy) found the overall complete response rate to be 57%; strictly speaking, the rates were 67% and 39% with and without concurrent hyperthermia, respectively. The 1-yr local disease-free survival rate for patients with gross disease was 53%, while the rate for those short of gross disease reached 100%[19]. In another study of radiation-naive patients with isolated locoregionally recurrent BC after mastectomy, the presence of residual gross disease at the time of RT was recognized as the most crucial prognostic factor for any outcome, as well. Even a 10% dose escalation (54 Gy to complete CW and regional lymphatics, and 12 Gy boost to CW flap and any other recurrent sites) did not exhibit any remarkable improvements in locoregional control and survival[20].
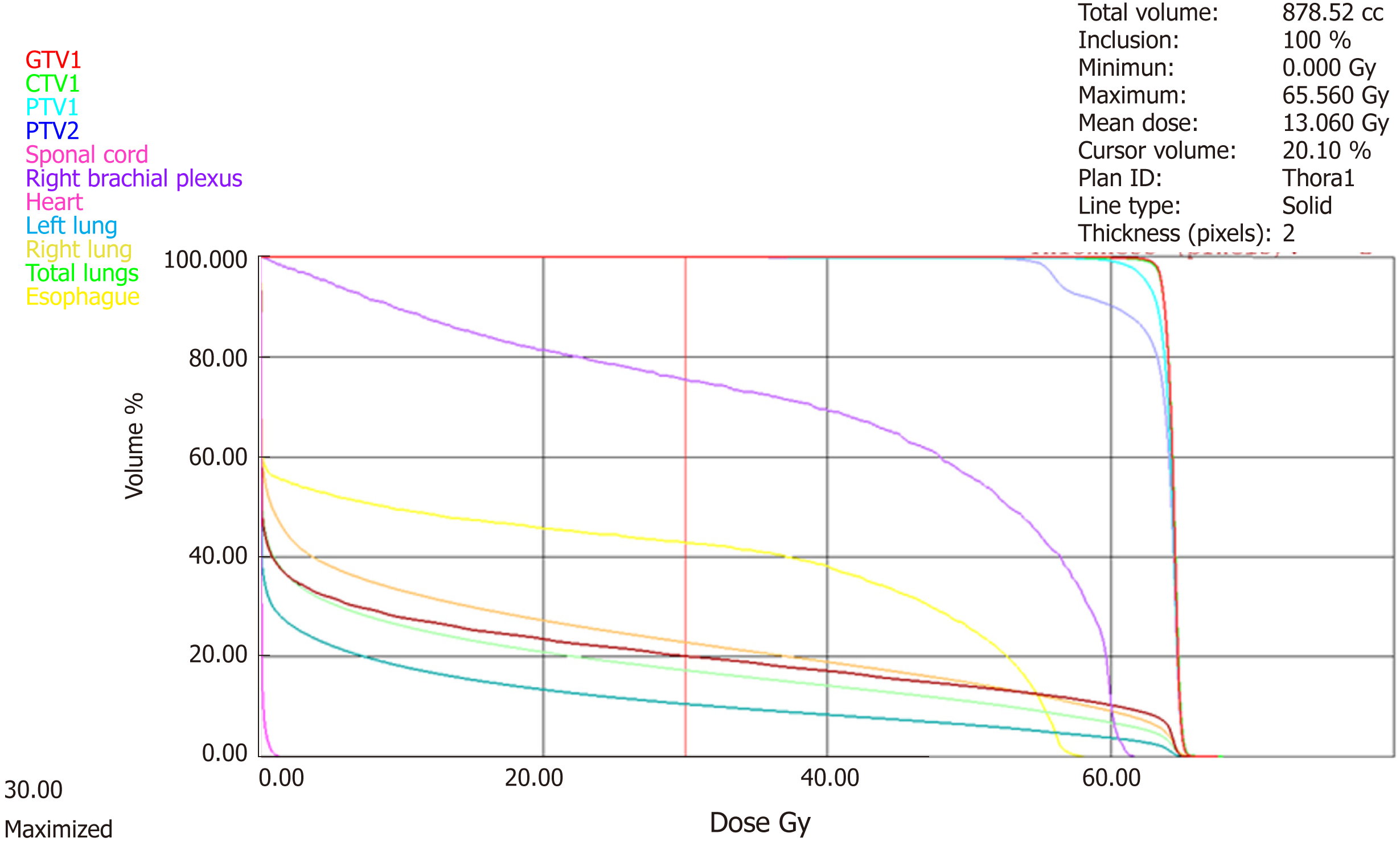
Siglin et al[21] reviewed the literature on reirradiation for locally recurrent BC, which had been demonstrated as feasible for its toxicity and response rates. Nonetheless, the increased risk of toxicity in repeat CW radiation with cumulative dose of ≥ 100 Gynamely, skin ulceration, lymphedema, brachial plexopathy, soft-tissue and bone necrosis, rib fracture, pneumonitis and cardiomyopathynecessitates this procedure to be undertaken with caution. In our case report, the patient received a cumulative dose from partial CW treatments ranging from 106 Gy to 124.40 Gy. The dose burden of OAR in the second course of our treatment with PBT is listed in Table 1. Even though there was an interval of 10 years between both courses of RT, the recovery and tolerance of our patient’s OAR remained varied and unreliable.
| Organ at risk | Minimum dose, Gy | Maximum dose, Gy | Mean dose, Gy |
| Spinal cord | 0 | 1.37 | 0.06 |
| Right brachial plexus | 0.20 | 61.57 | 43.70 |
| Heart | 0 | 65.56 | 13.06 |
| Left lung | 0 | 65.60 | 7.38 |
| Right lung | 0 | 67.55 | 14.79 |
| Esophagus | 0 | 58.04 | 22.89 |
In general, the acutely reacting tissues (skin, mucosa, lung) are deemed to repair radiation damage within a few months and can tolerate a repeat RT. On the other hand, late responding organs (brain, heart and kidney) do show none or limited long-term recovery[22,23]. Applying to the patient herein, the acute and late toxicities of skin, esophagus, brachial plexus and lungs were largely moderate but a radiation-induced pericardial disease became relevant at 19 mo after the PBT. This is known as one of the most common and earliest variants of radiation-induced cardiovascular disease, beside coronary heart disease, cardiomyopathy, valvular dysfunction and conduction abnormalities, and appears if a significant portion of heart volume sustains a critical radiation dose.
According to the data from the Quantitative Analysis of Normal Tissue Effects in the Clinic (referred to as QUANTEC) effort, the likelihood of pericarditis is less than 15% in compliance with the following dose constraints: mean heart dose < 26 Gy and V30 < 46 %. The mean heart dose of our patient treated with PBT was 13.06 Gy, and the heart volume receiving ≥ 30 Gy was 20% (Figure 11). Even if both the QUANTEC recommended heart dose restrictions were not exceeded, a limited recovery of the heart from the former RT would be expected. Furthermore, the consequence of previous and present systemic therapies, particularly those with established cardiotoxic side effects such as anthracyclines and trastuzumab, should also be taken into account. Indeed, a retrospective cohort study found comparable risk of cardiac ischemia and stroke among the tamoxifen-only and aromatase inhibitor-only users but detected, unexpectedly, an association between the sole and sequential use of an aromatase inhibitor and increased risk of other cardiac events (i.e., heart failure, cardiomyopathy, dysrhythmia, valvular disease and pericarditis)[24].
Since publication of the supposed proportionally growing rate of ischemic heart disease at the mean dose to the heart by 7.4% per gray[25], clinicians have been appealed to contemplate cardiac dose and risk factors in choosing the appropriate RT technique for BC patients. PBT evidently offers a refinement of cardiopulmonary events in comparison to conventional RT with photon and electron beams. The treatment planning comparison studies have verified the dosimetric advantage in use of PBT for locally advanced and left-sided BC, with respect to homogeneous target volume coverage and reduction of radiation dose exposure to surrounding uninvolved tissue, in favor of decreasing the cardiopulmonary toxicities and radiation-induced second malignancies[26,27].
Although most of the clinical experience has been based on passive scattering and uniform scanning technique, pencil beam scanningused on our patientfacilitates a faster treatment delivery with a single irradiation field and certain degree of skin sparing[28]. However, both PBT and conventional RT are confronted with inter- and intrafraction uncertainty due to breath and heart motions and set-up inconstancy. Techniques like deep inspiration breath-hold and respiratory gating were developed to reduce the position variability of target and OAR[29]. In a recent comparative treatment planning study on the use of deep inspiration breath-hold technique for Hodgkin’s lymphoma patients, plans with intensity modulated proton therapy showed superior results in decrease of all dose/volume parameters of the OAR compared to those with intensity modulated RT in the form of volumetric modulated arc therapy[30].
Still, in our experience of practical implementation, these techniques postulate training and compliance of patients beforehand, as well as compatibility between respiratory control devices and PBT facilities and consistent beam delivery to abbreviate the breath-hold time and daily treatment duration. Mostly, the latter remains challenging for larger centers, which need to share the beam among several gantries.
This case report demonstrates that even patients with locally advanced recurrent BC can benefit from a local treatment with PBT. Apart from later arising pericardial and pleural effusions that were successfully treated by drainage and corticosteroid, the acute toxicities as well as the locoregional control, progression-free survival, cosmetic result and quality of life at 2 years after PBT are satisfactory and encouraging. Since gross residual disease despite prior surgery and systemic therapy represents an essential factor for locoregional control and survival outcome, further comprehensive investigations into the simultaneous use of radiosensitizers, such as hyperthermia, with PBT become compelling above all.
The patient demonstrates acceptable acute and late side effects after a dose-escalated reirradiation of a vast tumor recurrence, followed by a progression-free survival of 2 years since the treatment. Although a radiation-induced pericardial disease occurred at 19 mo, it was successfully treated by drainage and corticosteroid. PBT serves as a safe and effective therapy for locoregionally recurrent BC and improves the outcome of gross tumor disease significantly.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chang Z, Schietroma M S-Editor: Ma YJ L-Editor: A E-Editor:Ma YJ
| 1. | Voinea SC, Sandru A, Blidaru A. Management of Breast Cancer Locoregional Recurrence. Chirurgia (Bucur). 2017;112:429-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Tommasino F, Durante M. Proton radiobiology. Cancers (Basel). 2015;7:353-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 173] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 3. | Cadoo KA, Fornier MN, Morris PG. Biological subtypes of breast cancer: current concepts and implications for recurrence patterns. Q J Nucl Med Mol Imaging. 2013;57:312-321. [PubMed] [Cited in This Article: ] |
| 4. | Anderson WF, Chen BE, Jatoi I, Rosenberg PS. Effects of estrogen receptor expression and histopathology on annual hazard rates of death from breast cancer. Breast Cancer Res Treat. 2006;100:121-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Hess KR, Pusztai L, Buzdar AU, Hortobagyi GN. Estrogen receptors and distinct patterns of breast cancer relapse. Breast Cancer Res Treat. 2003;78:105-118. [PubMed] [Cited in This Article: ] |
| 6. | Campbell EJ, Tesson M, Doogan F, Mohammed ZMA, Mallon E, Edwards J. The combined endocrine receptor in breast cancer, a novel approach to traditional hormone receptor interpretation and a better discriminator of outcome than ER and PR alone. Br J Cancer. 2016;115:967-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Wangchinda P, Ithimakin S. Factors that predict recurrence later than 5 years after initial treatment in operable breast cancer. World J Surg Oncol. 2016;14:223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Geurts YM, Witteveen A, Bretveld R, Poortmans PM, Sonke GS, Strobbe LJA, Siesling S. Patterns and predictors of first and subsequent recurrence in women with early breast cancer. Breast Cancer Res Treat. 2017;165:709-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Lynch SP, Lei X, Hsu L, Meric-Bernstam F, Buchholz TA, Zhang H, Hortobágyi GN, Gonzalez-Angulo AM, Valero V. Breast cancer multifocality and multicentricity and locoregional recurrence. Oncologist. 2013;18:1167-1173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Wimmer K, Strobl S, Bolliger M, Devyatko Y, Korkmaz B, Exner R, Fitzal F, Gnant M. Optimal duration of adjuvant endocrine therapy: how to apply the newest data. Ther Adv Med Oncol. 2017;9:679-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Rowden D, Solky AJ, Stearns V, Winer EP, Griggs JJ. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255-2269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 523] [Cited by in F6Publishing: 533] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 12. | Sestak I, Cuzick J. Markers for the identification of late breast cancer recurrence. Breast Cancer Res. 2015;17:10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Cochet A, David S, Moodie K, Drummond E, Dutu G, MacManus M, Chua B, Hicks RJ. The utility of 18 F-FDG PET/CT for suspected recurrent breast cancer: impact and prognostic stratification. Cancer Imaging. 2014;14:13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Chang HT, Hu C, Chiu YL, Peng NJ, Liu RS. Role of 2-[18F] fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in the post-therapy surveillance of breast cancer. PLoS One. 2014;9:e115127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Aogi K, Kadoya T, Sugawara Y, Kiyoto S, Shigematsu H, Masumoto N, Okada M. Utility of (18)F FDG-PET/CT for predicting prognosis of luminal-type breast cancer. Breast Cancer Res Treat. 2015;150:209-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Harms W, Geretschläger A, Cescato C, Buess M, Köberle D, Asadpour B. Current Treatment of Isolated Locoregional Breast Cancer Recurrences. Breast Care (Basel). 2015;10:265-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Belkacemi Y, Hanna NE, Besnard C, Majdoul S, Gligorov J. Local and Regional Breast Cancer Recurrences: Salvage Therapy Options in the New Era of Molecular Subtypes. Front Oncol. 2018;8:112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Harms W, Budach W, Dunst J, Feyer P, Fietkau R, Haase W, Krug D, Piroth MD, Sautter-Bihl ML, Sedlmayer F, Souchon R, Wenz F, Sauer R; Breast Cancer Expert Panel of the German Society of Radiation Oncology (DEGRO). DEGRO practical guidelines for radiotherapy of breast cancer VI: therapy of locoregional breast cancer recurrences. Strahlenther Onkol. 2016;192:199-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Wahl AO, Rademaker A, Kiel KD, Jones EL, Marks LB, Croog V, McCormick BM, Hirsch A, Karkar A, Motwani SB, Tereffe W, Yu TK, Sher D, Silverstein J, Kachnic LA, Kesslering C, Freedman GM, Small W. Multi-institutional review of repeat irradiation of chest wall and breast for recurrent breast cancer. Int J Radiat Oncol Biol Phys. 2008;70:477-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Skinner HD, Strom EA, Motwani SB, Woodward WA, Green MC, Babiera G, Booser DJ, Meric-Bernstam F, Buchholz TA. Radiation dose escalation for loco-regional recurrence of breast cancer after mastectomy. Radiat Oncol. 2013;8:13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Siglin J, Champ CE, Vakhnenko Y, Anne PR, Simone NL. Radiation therapy for locally recurrent breast cancer. Int J Breast Cancer. 2012;2012:571946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Nieder C, Milas L, Ang KK. Tissue tolerance to reirradiation. Semin Radiat Oncol. 2000;10:200-209. [PubMed] [Cited in This Article: ] |
| 23. | Das S, Patro KC, Mukherji A. Recovery and tolerance of the organs at risk during re-irradiation. J Curr Oncol. 2018;1:23-28. [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Haque R, Shi J, Schottinger JE, Chung J, Avila C, Amundsen B, Xu X, Barac A, Chlebowski RT. Cardiovascular Disease After Aromatase Inhibitor Use. JAMA Oncol. 2016;2:1590-1597. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987-998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2429] [Cited by in F6Publishing: 2505] [Article Influence: 227.7] [Reference Citation Analysis (0)] |
| 26. | MacDonald SM, Jimenez R, Paetzold P, Adams J, Beatty J, DeLaney TF, Kooy H, Taghian AG, Lu HM. Proton radiotherapy for chest wall and regional lymphatic radiation; dose comparisons and treatment delivery. Radiat Oncol. 2013;8:71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Hernandez M, Zhang R, Sanders M, Newhauser W. A treatment planning comparison of volumetric modulated arc therapy and proton therapy for a sample of breast cancer patients treated with post-mastectomy radiotherapy. J Proton Ther. 2015;1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Cuaron JJ, MacDonald SM, Cahlon O. Novel applications of proton therapy in breast carcinoma. Chin Clin Oncol. 2016;5:52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Shah C, Badiyan S, Berry S, Khan AJ, Goyal S, Schulte K, Nanavati A, Lynch M, Vicini FA. Cardiac dose sparing and avoidance techniques in breast cancer radiotherapy. Radiother Oncol. 2014;112:9-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 30. | Baues C, Marnitz S, Engert A, Baus W, Jablonska K, Fogliata A, Vásquez-Torres A, Scorsetti M, Cozzi L. Proton versus photon deep inspiration breath hold technique in patients with hodgkin lymphoma and mediastinal radiation: A Planning Comparison of Deep Inspiration Breath Hold Intensity Modulation Radiotherapy and Intensity Modulated Proton Therapy. Radiat Oncol. 2018;13:122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |