Published online May 15, 2016. doi: 10.4291/wjgp.v7.i2.211
Peer-review started: September 5, 2015
First decision: November 30, 2015
Revised: January 3, 2016
Accepted: March 7, 2016
Article in press: March 9, 2016
Published online: May 15, 2016
Although insulin resistance (IR) is strongly associated with nonalcoholic fatty liver disease (NAFLD), the association of IR and NAFLD is not universal and correlation between IR and severity of NAFLD is still controversial. In this review, we summarize recent evidence that partially dissociates insulin resistance from NAFLD. It has also been reported that single-nucleotide polymorphisms in the diacylglycerol acyltransferase gene, rather than IR, account for the variability in liver fat content. Polymorphisms of the patatin-like phospholipase 3 gene have also been reported to be associated with NAFLD without metabolic syndrome, which suggests that genetic conditions that promote the development of fatty changes in the liver may occur independently of IR. Moreover, environmental factors such as nutrition and physical activity as well as small intestinal bacterial overgrowth have been linked to the pathogenesis of NAFLD, although some of the data are conflicting. Therefore, findings from both genetically engineered animal models and humans with genetic conditions, as well as recent studies that have explored the role of environmental factors, have confirmed the view that NAFLD is a polygenic disease process caused by both genetic and environmental factors. Therefore, IR is not the sole predictor of the pathogenesis of NAFLD.
Core tip: Insulin resistance is considered as the major contributor for the development and progression of nonalcoholic fatty liver disease (NAFLD). However, recent evidence that has shown that non-obese individuals from developing countries are also affected by NAFLD, thus the conventional paradigm of NAFLD as the “hepatic manifestation of metabolic syndrome” has become outdated. Recent studies have highlighted novel pathophysiological mechanisms for the development and progression of NAFLD. Insulin resistance contributes to the disease process, but it is evident that environmental and genetic factors also contribute for development of necroinflammation and subsequent progression to fibrosis. This review provides a summary of current knowledge of the pathogenesis of NAFLD and discusses factors that dissociate insulin resistance from NAFLD.
- Citation: Alam S, Mustafa G, Alam M, Ahmad N. Insulin resistance in development and progression of nonalcoholic fatty liver disease. World J Gastrointest Pathophysiol 2016; 7(2): 211-217
- URL: https://www.wjgnet.com/2150-5330/full/v7/i2/211.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v7.i2.211
Nonalcoholic fatty liver disease (NAFLD) is an emerging public health problem[1] due to increasing prevalence in developed and developing countries. NAFLD is the second leading cause of chronic liver diseases after hepatitis C in Western countries and affects individuals of all age groups[2]. NAFLD includes a wide spectrum of conditions that range from a simple steatosis to nonalcoholic steatohepatitis (NASH) which may further progress to cirrhosis and its complications, in absence of alcohol consumption; or a low daily consumption of alcohol (< 30 g/d for men, < 20 g/d for women)[3-5]. NAFLD has been linked to insulin resistance (IR) and other components of metabolic syndrome such as diabetes mellitus, central abdominal obesity and dyslipidemia[6]. Patients with NAFLD are at an increased risk for all-cause mortality, including liver-related deaths and non-liver-related deaths such as death due to cardiovascular disease and diabetes[7].
Recent evidence that has shown that non-obese individuals from developing countries are also affected by NAFLD; thus, the conventional paradigm of NAFLD as the “hepatic manifestation of metabolic syndrome” has become outdated[8]. Recent studies have highlighted novel pathophysiological mechanisms in the development and progression of NAFLD. IR contributes to the disease process, but it is evident that environmental and genetic factors also have the contribution in the development of necroinflammation and subsequent fibrosis. The dogma of a sequential progression of simple steatosis to NASH to cirrhosis in NAFLD is currently under scrutiny.
The pathogenesis of NAFLD is now conceptualized as a complex and multifaceted process that requires further understanding. This review provides a summary of our current understanding of these processes, particularly the evidence that IR is not the lone predictor for NAFLD, but rather, the disease is multifactorial and may be caused by the involvement of genetic and environmental factors.
We searched MEDLINE, EMBASE, and PubMed using the MeSH terms “insulin resistance”, “nonalcoholic fatty liver disease”, and “nonalcoholic steatohepatitis”. The reference lists of the articles selected for inclusion were also reviewed for additional relevant papers. The search was limited to studies that were reported in the English language and that were published between 1995 and March 2015. Articles that are specifically related to the epidemiology, diagnosis and current treatment strategies for NAFLD and NASH are summarized.
The reported prevalence of NAFLD from Western countries is 20%-30%, from Asian countries is approximately 15%[9-11]. In normal-weight individuals without any known metabolic risk factors, the prevalence of NAFLD is reported to be approximately 16%. However, the prevalence is much higher among high-risk groups such as diabetics (60%), patients with hyperlipidemia (90%) and obese patients undergoing bariatric surgery (91%)[9-13]. Only 20% of patients under the age of 20 have NAFLD, but among patients aged 60 and above, the prevalence is more than 40%[14]. This findings further strengthened in another study where older age is identified as an independent risk factor for disease progression from simple steatosis to NASH and for the development of fibrosis and cirrhosis[15]. Hamabe et al[16] showed that smoking is an independent risk factor for NAFLD. A few studies have also reported ethnic variation in the prevalence of NAFLD, but these reports present contrasting data[17,18]. The risk of mortality is higher in NASH and advanced fibrosis compared with simple steatosis[19]. The progression to advanced fibrosis has been shown to be associated with the patient’s age and the degree of inflammation[20]. In a long-term longitudanal study of 129 patients with NAFLD, Ekstedt et al[19] explored that mortality was not increased in patients with simple steatosis but was increased in NASH patient. Although the mortality was primarily due to cardiovascular disease, liver-related deaths were more common in patients with NASH-related cirrhosis[21].
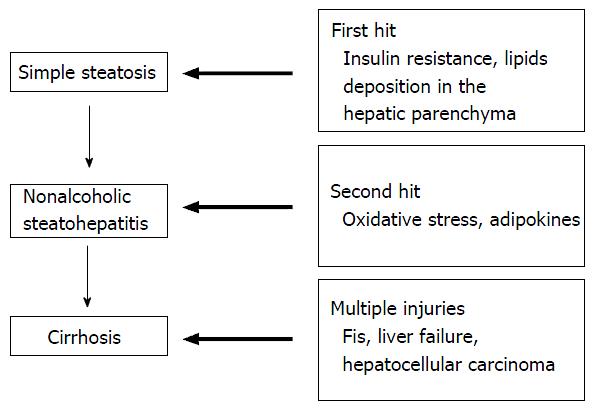
Traditional concept: The two-hit hypothesis: Day et al[22] first proposed the current concept of the “two-hit hypothesis in NAFLD” in 1998 (Figure 1). The first hit is primarily as a result of IR, increased dietary intake and enhanced hepatic lipogenesis there is accumulation of free fatty acids (FFAs) and triglycerides (TGs) in hepatocytes[22]. The second hits is a combination of oxidative stress, lipid peroxidation, mitochondrial dysfunction and the release of inflammatory mediators, which leads to progressive liver injury which constitute steatohepatitis and fibrosis[22]. The activation of proinflammatory pathways and toll-like receptors merge at the junction of two main intracellular signaling pathways known as nuclear factor-κB (NF-κB) and c-Jun N-terminal kinase (JNK)[23,24]. NF-κB activation has been reported in NASH and can lead to increased transcription of many proinflammatory genes, whereas JNK activation causes IR via the direct phosphorylation and degradation of insulin receptor substrate 1 (IRS1); this in turn reduces the intracellular signaling pathway activity downstream of the insulin receptor[23]. Lipid peroxidation can promote the proliferation of stellate cells, which contributes to fibrogenesis[25]. Reactive oxygen species induce the release of cytokines from hepatocytes, which leads to the initiation of various immune-mediated mechanisms that contribute to further liver cell injury. The combination of hyperinsulinemia, hepatic iron and lipid peroxidation induces oxidative stress[17], which can cause mitochondrial dysfunction in NASH and can contribute to TG accumulation and eventually to cell necrosis[11].
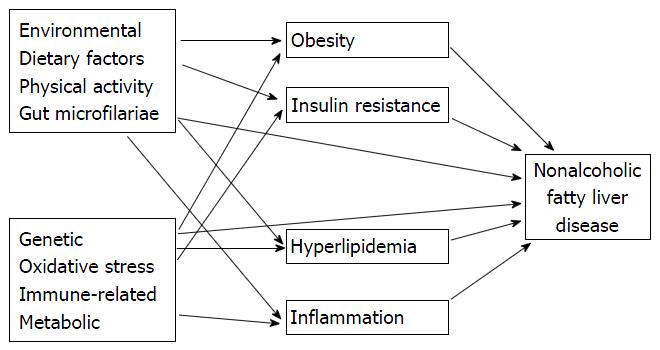
Accumulations of knowledge in recent years have challenged the traditional “two-hit” pathogenesis. Knowledge of interaction between insulin resistance, adipokines, adipose tissue inflammation and other less recognized pathogenic factors has been argued that multiple hits from adipose tissue and the gut occur at the same time and promote liver inflammation (Figure 2). This process suggests that cellular inflammation and insulin resistance occur concurrently[26,27]. Progression of NAFLD to NASH is explained by subsequent “two-hit” theory. In the “multiple-hit” model[28,29] hepatic steatosis may represent an epiphenomenon of several distinct injurious mechanisms including IR rather than a true “first hit”[30]. Hyperinsulinemia, results in increased hepatic de novo lipogenesis and increased adipose tissue lipolysis; leads to an increased efflux of free fatty acids to the liver[31,32]. After the initial development of steatosis, the liver becomes extremely vulnerable. Multipe series of pathogenic and injurious factors including oxidative damage, activation of transforming growth factor-beta pathway, dysregulation of multiple adipokines and apoptpsis and activation of hepatic stellate cell may lead to hepatocyte injury and finally to the progression from simple steatosis to NASH and fibrosis[33]. So multiple factors ineract in the complicated ways for development and progression of steatosis, NASH and fibrosis[14,34,35].
A more recent model has proposed that the development of simple steatosis and NASH follows distinct pathways. The activation of these pathways is a complex process and is not only the result of a simple hepatic insult. Many other factors promote the activation of the pathways that lead to the development of steatosis and NASH[34]. The most important factors include genetic factors, the activation the hedgehog pathway and hepatic progenitor cells[36].
Studies have demonstrated that NAFLD is associated with higher IR compared with controls, even after the exclusion of overweight and obese subjects, and that IR increases with increasing degrees of steatosis[37-40]. IR in NAFLD is predominantly peripheral and occurs in the skeletal muscle and adipose tissue. Peripheral IR in the skeletal muscle causes reduced glucose uptake, which leads to hyperglycemia. In adipose tissue, IR impairs the anti-lipolytic action of insulin, which leads to an increased release of FFA. Elevated plasma concentrations of insulin, glucose, and fatty acids then impair the β-oxidation of fatty acids by negative feedback and promote the uptake of hepatic fatty acids and triglycerides, de novo lipid synthesis (via SREBP) (sterol-regulatory element-binding protein) and the expression of C/enhancer-binding protein (CCAAT/EBP). Insulin resistance also increases the amount of intra-hepatocytic fatty acids via an increase in glycolysis and a decrease in apolipoprotein B-100, which blocks the export of VLDL. The development of IR in NAFLD is most likely related to the imbalance between pro-insulin (adiponectin) and anti-insulin (TNFα) cytokines, specially, those secreted by adipose tissue. Alterations in several molecules, including FFAs, TNFα, membrane glycoprotein PC-1, and leptin, interfere with the insulin signaling pathway. FFAs are both the result and cause of IR. Excess FFAs cause hepatic IR via the down regulation of IRS1 signaling and by the activation of the inhibitor kappa B kinase (IKK-B)/NF-κB pathway. Patients with NAFLD have increased insulin resistance not only in muscle but also in liver and adipose tissue[41], and this reduced insulin sensitivity plays a major role in the pathogenesis of NAFLD. This IR, increases peripheral lipolysis in adipose tissue that leads to increase in the delivery of FFAs to the liver and de novo lipogenesis[17,35]. In addition, lipid overload in pancreatic-B cells leads to dysregulated insulin secretion and changes in the expression of peroxisome proliferator-activated receptor(PPAR)-α, glucokinase, the glucose transporter-2, pre-pro-insulin and pancreatic duodenal homeobox-1, which can lead to IR as a result of FFA-induced B-cell apoptosis[12]. It has been suggested that IR in the liver is sufficient to produce dyslipidemia and increase the risk of atherosclerosis[42]. However, current evidences are not sufficient to demonstrate a consistent association between any particular type of adipokine and the histological severity of NAFLD[43].
Although the development and progression of NAFLD is strongly associted with metabolic syndrome and IR, several studies have evidenced that all obese and diabetic dividuals dont have NAFLD. There are also evideces that NAFLD can occur in nonobese, as well as persons without metabolic syndrome[44]. Therefore, it could be hypothesized that factors other than IR could be the determinant of the development and severity of NAFLD. Familial lustering[45,46] and in the ethnic variation in the prevalence of NAFLD strengthen the initial concept[17]. Single-nucleotide polymorphisms in the adiponectin, interleukin-6, TNFα and apoE genes has been studied[47-49]. Multiethnic genome-wide association study with NAFLD revealed that the patatin-like phospholipase domain containing protein 3 (also known as adiponutrin) gene is strongly associated with hepatic TG content[50]. Allelic variants of the patatin-like phospholipase domain containing protein 3 (PNPLA3) genes have been found to be correlated with amounts of hepatic fat in Hispanics and African-Americans, and to be associted with prevalence of NAFLD. PNPLA3 has also been independently identified in a separate population-based genome-wide study that influences the alanine aminotransferase (ALT) livel[51]. Environmental factors like; sedentary life styles, excess food intake, constituents of food and intestinal bacterial overgrowth have evidences to contribute in the pathogenesis of NAFLD. Obesity resulting from excess food intake and lack of exercise has been proven to contribute to the progression of fibrosis in patients with NAFLD[19]. An increased consumption of meat, soft drinks, saturated fat and cholesterol and a low consumption of fish and polyunsaturated fat (PUFA) were found to be associated with NAFLD[52-55]. Dietary supplementation with PUFA has been demonstrated in randomized control trial to be benifical in regression of fatty liver and reduction of ALT compared to dietery advice alone[56,57]. On the other hand highcarbohydrate and lowfat diets are associated with more progressive disease[58,59]. Conversely, studies in mice[60] and non-human primates[61], exposure to a maternal high-fat diet associated with development and progression of NAFLD in the offspring. Small intestinal bacterial overgrowth increases gut permeability, which leads to portal endotoxemia and increased numbers of circulating inflammatory cytokines, both of which have crucial role in the progression of NAFLD to NASH[62]. Several studies have reported an association between small intestinal bacterial overgrowth and the progression of NAFLD[63-65]. Dietary supplimentatation of probiotics and treatment with antibiotics resulted in benificial effects in NAFLD, which has furter sterngthen the concept[65].
Although simple steatosis and NASH are currently classified as two histological subtypes of NAFLD, the two conditions are likely distinct from both a histological and a pathophysiological standpoint[34]. The American Association for the Study of Liver Diseases has recently suggested the classification of patients within the NAFLD spectrum into two main categories: NASH and “not steatohepatitis, with steatosis” (“simple steatosis”)[66]. Differentiation is on histological variation where NASH is defined by the findings of lobular inflammation, portal inflammation, cellular ballooning, and fibrosis. In contrast, “not steatohepatitis, with steatosis” is characterized by simple fat infiltration with minimal/no inflammation[66]. NASH is a progressive disease, may progress to cirrhosis upto 9%-20% over a period of 5-10 years[67-69]. Vernon et al[9] explored that, only NASH is progressive and associated with the development of cirrhosis and hepatocellular carcinoma. In contrast, “simple steatosis” tends to be stable over time[69]. Though there is recent study of progression of steatosis to NASH and also there is progression to fibrosis[70], In agreement with these findings, Musso et al[71] in a meta-analysis concluded that a minority of patients with pure fatty liver will progress to NASH and only NASH seems to be associated with an increased risk of progressive liver disease[71]. Along these lines, a community based study of NAFLD outcomes has shown that no patients with simple steatosis died during a 7.6-year follow-up, whereas 35% of patients with NASH died during[69]. All these results established that NASH and “not steatohepatitis, with steatosis” are two distinct entities rather than a real progression of histological changes that can progress over time. For this reason, simple steatosis and NASH should be considered as a separate disease entity that develops along a distinct pathogenic pathway with multiple hits. The conceptualisation of these pathophysiological mechanisms would not only improve our biological understanding of NAFLD but may also allow clinicians to interven the patogenesis more accurately in future.
Considering that IR is a primary factor in the pathogenesis of NAFLD, several insulin sensitizers have been used in different settings. Table 1 summarizes a few of these trials. According to these trials, none of these drugs was effective, and thus further studies are warranted to identify their role. Notably, metformin was shown to improve liver injury, but this medication, which is typically used in the treatment of type 2 diabetes, could not prevent fibrosis in patients with steatosis[72]. Additionally, glitazones, which are PPARγ agonists, were found to be efficient in the management of NAFLD via a notable decrease in liver fibrosis[73]. In contrast, another study revealed that pioglitazone does not promote beneficial effects with respect to liver fibrosis, but it diminished inflammation and steatosis[74]. Therefore, further studies are required to elucidate these contradictory results. Additionally, salsalate, a potential anti-diabetic drug that is currently under development, has been shown to improve glycemia in diabetic patients through a downregulation of the proinflammatory IKKβ/NFκB pathway[75]. Additionally, this agent likely improves NAFLD through an induction of adiponectin[76].
| Insulin-sensitizing agent | Results of the study | Relevance to NAFLD | Ref. |
| Metformin | Improvements in liver histology and ALT levels in 30% of patients with NASH | Appears to be beneficial for NAFLD patients but not for non-obese patients with early-stage NAFLD | Loomba et al[72] |
| Pioglitazone | Improvement in the biochemical and histological features of NASH | Could be used as a treatment for NAFLD | Promrat et al[73] |
| Pioglitazone | Improvement in insulin resistance but not in hepatic fibrosis and ALT levels | Not adapted to treat NAFLD | Sanyal et al[74] |
Hepatic steatosis is recognized to be the consequence of a complex interplay among diet, environment and liver and adipose tissues, although a comprehensive understanding of pathogenesis of NAFLD has not yet been complete. Therefore, NAFLD is currently perceived as multifactorial pathogenic disease with both genetic and environmental factors. Genome-wide association studies have identified specific genetic associations that are involved in NAFLD. From a therapeutic point of view, pathogenic-based interventions aimed at the reversal of NAFLD are likely to be a rational approach to the prevention and treatment of hepatic IR, metabolic syndrome and related complications. Further studies are required to explore the relationship among adiponutrin mutations, steatosis and IR. A better understanding of the different factors involved in the pathophysiology of NAFLD will open the opportunity to intervene its progression in future.
P- Reviewer: Hwu CM, Ikura Y S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Zafrani ES. Non-alcoholic fatty liver disease: an emerging pathological spectrum. Virchows Arch. 2004;444:3-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 607] [Cited by in F6Publishing: 625] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, Goodman Z. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874-1882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 434] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 4. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2357] [Cited by in F6Publishing: 2246] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 5. | Scaglioni F, Ciccia S, Marino M, Bedogni G, Bellentani S. ASH and NASH. Dig Dis. 2011;29:202-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Chang E, Park CY, Park SW. Role of thiazolidinediones, insulin sensitizers, in non-alcoholic fatty liver disease. J Diabetes Investig. 2013;4:517-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59:1174-1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 396] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 8. | Das K, Das K, Mukherjee PS, Ghosh A, Ghosh S, Mridha AR, Dhibar T, Bhattacharya B, Bhattacharya D, Manna B. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51:1593-1602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 316] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 9. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2065] [Cited by in F6Publishing: 2143] [Article Influence: 164.8] [Reference Citation Analysis (0)] |
| 10. | Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 814] [Cited by in F6Publishing: 742] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 11. | Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, Frier BM, Van Look LA, Johnston GI, Reynolds RM. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2011;34:1139-1144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 282] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 12. | Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45:600-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 367] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 13. | Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5:1544-1560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 547] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 14. | Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia. 2008;51:1947-1953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 278] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 15. | Attar BM, Van Thiel DH. Current concepts and management approaches in nonalcoholic fatty liver disease. ScientificWorldJournal. 2013;2013:481893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Hamabe A, Uto H, Imamura Y, Kusano K, Mawatari S, Kumagai K, Kure T, Tamai T, Moriuchi A, Sakiyama T. Impact of cigarette smoking on onset of nonalcoholic fatty liver disease over a 10-year period. J Gastroenterol. 2011;46:769-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2633] [Cited by in F6Publishing: 2593] [Article Influence: 129.7] [Reference Citation Analysis (3)] |
| 18. | Bambha K, Belt P, Abraham M, Wilson LA, Pabst M, Ferrell L, Unalp-Arida A, Bass N. Ethnicity and nonalcoholic fatty liver disease. Hepatology. 2012;55:769-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1647] [Cited by in F6Publishing: 1624] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 20. | Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 383] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 21. | Perazzo H, Munteanu M, Ngo Y, Lebray P, Seurat N, Rutka F, Couteau M, Jacqueminet S, Giral P, Monneret D. Prognostic value of liver fibrosis and steatosis biomarkers in type-2 diabetes and dyslipidaemia. Aliment Pharmacol Ther. 2014;40:1081-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2953] [Cited by in F6Publishing: 2955] [Article Influence: 113.7] [Reference Citation Analysis (1)] |
| 23. | Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2445] [Cited by in F6Publishing: 2381] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 24. | Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1612] [Cited by in F6Publishing: 1671] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 25. | Ikura Y, Ohsawa M, Suekane T, Fukushima H, Itabe H, Jomura H, Nishiguchi S, Inoue T, Naruko T, Ehara S. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology. 2006;43:506-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Kim CH, Younossi ZM. Nonalcoholic fatty liver disease: a manifestation of the metabolic syndrome. Cleve Clin J Med. 2008;75:721-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 27. | Asrih M, Jornayvaz FR. Metabolic syndrome and nonalcoholic fatty liver disease: Is insulin resistance the link? Mol Cell Endocrinol. 2015;418 Pt 1:55-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 215] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 28. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1543] [Cited by in F6Publishing: 1629] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 29. | Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 437] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 30. | Cortez-Pinto H, de Moura MC, Day CP. Non-alcoholic steatohepatitis: from cell biology to clinical practice. J Hepatol. 2006;44:197-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Mehta K, Van Thiel DH, Shah N, Mobarhan S. Nonalcoholic fatty liver disease: pathogenesis and the role of antioxidants. Nutr Rev. 2002;60:289-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 184] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 32. | Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, Conti M, Huet S, Ba N, Buffet C. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738-744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 377] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 33. | Harrison SA, Kadakia S, Lang KA, Schenker S. Nonalcoholic steatohepatitis: what we know in the new millennium. Am J Gastroenterol. 2002;97:2714-2724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Yilmaz Y. Review article: is non-alcoholic fatty liver disease a spectrum, or are steatosis and non-alcoholic steatohepatitis distinct conditions? Aliment Pharmacol Ther. 2012;36:815-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 35. | Brea A, Puzo J. Non-alcoholic fatty liver disease and cardiovascular risk. Int J Cardiol. 2013;167:1109-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Day CP. Non-alcoholic steatohepatitis (NASH): where are we now and where are we going? Gut. 2002;50:585-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 37. | Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1458] [Cited by in F6Publishing: 1436] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 38. | Yki-Järvinen H. Liver fat in the pathogenesis of insulin resistance and type 2 diabetes. Dig Dis. 2010;28:203-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 39. | Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430-15435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 678] [Cited by in F6Publishing: 708] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 40. | Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in F6Publishing: 498] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 41. | Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1074] [Cited by in F6Publishing: 1051] [Article Influence: 42.0] [Reference Citation Analysis (1)] |
| 42. | Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 694] [Cited by in F6Publishing: 694] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 43. | Farrell GC, George J, Hall PdlM, McCullough AJ. Fatty liver disease: NASH and related disorders. Malden: John Wiley & Sons; 2008; . [Cited in This Article: ] |
| 44. | Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103-1109. [PubMed] [Cited in This Article: ] |
| 45. | Struben VM, Hespenheide EE, Caldwell SH. Nonalcoholic steatohepatitis and cryptogenic cirrhosis within kindreds. Am J Med. 2000;108:9-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 184] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 46. | Willner IR, Waters B, Patil SR, Reuben A, Morelli J, Riely CA. Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial tendency, and severity of disease. Am J Gastroenterol. 2001;96:2957-2961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 264] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 47. | Musso G, Gambino R, De Michieli F, Durazzo M, Pagano G, Cassader M. Adiponectin gene polymorphisms modulate acute adiponectin response to dietary fat: Possible pathogenetic role in NASH. Hepatology. 2008;47:1167-1177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 48. | Tokushige K, Hashimoto E, Noto H, Yatsuji S, Taniai M, Torii N, Shiratori K. Influence of adiponectin gene polymorphisms in Japanese patients with non-alcoholic fatty liver disease. J Gastroenterol. 2009;44:976-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | Sazci A, Akpinar G, Aygun C, Ergul E, Senturk O, Hulagu S. Association of apolipoprotein E polymorphisms in patients with non-alcoholic steatohepatitis. Dig Dis Sci. 2008;53:3218-3224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 50. | Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461-1465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2233] [Cited by in F6Publishing: 2311] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 51. | Yuan X, Waterworth D, Perry JR, Lim N, Song K, Chambers JC, Zhang W, Vollenweider P, Stirnadel H, Johnson T. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am J Hum Genet. 2008;83:520-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 350] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 52. | Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, Fagà E, Silli B, Pagano G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909-916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 526] [Cited by in F6Publishing: 508] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 53. | Toshimitsu K, Matsuura B, Ohkubo I, Niiya T, Furukawa S, Hiasa Y, Kawamura M, Ebihara K, Onji M. Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition. 2007;23:46-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 54. | Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Blendis L, Halpern Z, Oren R. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol. 2007;47:711-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 383] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 55. | Kim CH, Kallman JB, Bai C, Pawloski L, Gewa C, Arsalla A, Sabatella ME, Younossi ZM. Nutritional assessments of patients with non-alcoholic fatty liver disease. Obes Surg. 2010;20:154-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Spadaro L, Magliocco O, Spampinato D, Piro S, Oliveri C, Alagona C, Papa G, Rabuazzo AM, Purrello F. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liver Dis. 2008;40:194-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 57. | Zhu FS, Liu S, Chen XM, Huang ZG, Zhang DW. Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J Gastroenterol. 2008;14:6395-6400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 137] [Cited by in F6Publishing: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 58. | Solga S, Alkhuraishe AR, Clark JM, Torbenson M, Greenwald A, Diehl AM, Magnuson T. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci. 2004;49:1578-1583. [PubMed] [Cited in This Article: ] |
| 59. | Kang H, Greenson JK, Omo JT, Chao C, Peterman D, Anderson L, Foess-Wood L, Sherbondy MA, Conjeevaram HS. Metabolic syndrome is associated with greater histologic severity, higher carbohydrate, and lower fat diet in patients with NAFLD. Am J Gastroenterol. 2006;101:2247-2253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 60. | Bruce KD, Cagampang FR, Argenton M, Zhang J, Ethirajan PL, Burdge GC, Bateman AC, Clough GF, Poston L, Hanson MA. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50:1796-1808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 326] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 61. | McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 308] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 62. | Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palù G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518-G525. [PubMed] [Cited in This Article: ] |
| 63. | Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 610] [Cited by in F6Publishing: 598] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 64. | Sabaté JM, Jouët P, Harnois F, Mechler C, Msika S, Grossin M, Coffin B. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: a contributor to severe hepatic steatosis. Obes Surg. 2008;18:371-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 65. | Sajjad A, Mottershead M, Syn WK, Jones R, Smith S, Nwokolo CU. Ciprofloxacin suppresses bacterial overgrowth, increases fasting insulin but does not correct low acylated ghrelin concentration in non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;22:291-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 537] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 67. | Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042-2047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 322] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 68. | Ong JP, Younossi ZM. Nonalcoholic fatty liver disease (NAFLD)--two decades later: are we smarter about its natural history? Am J Gastroenterol. 2003;98:1915-1917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2092] [Cited by in F6Publishing: 2023] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 70. | McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148-1155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 687] [Cited by in F6Publishing: 693] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 71. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 886] [Cited by in F6Publishing: 854] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 72. | Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, Modi A, Nagabhyru P, Sumner AE, Liang TJ. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29:172-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 73. | Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 575] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 74. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2215] [Cited by in F6Publishing: 2196] [Article Influence: 156.9] [Reference Citation Analysis (1)] |
| 75. | Goldfine AB, Silver R, Aldhahi W, Cai D, Tatro E, Lee J, Shoelson SE. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1:36-43. [PubMed] [Cited in This Article: ] |
| 76. | Jung TW, Choi HY, Lee SY, Hong HC, Yang SJ, Yoo HJ, Youn B-S, Baik SH, Choi KM. Salsalate and Adiponectin Improve Palmitate-Induced Insulin Resistance via Inhibition of Selenoprotein P through the AMPK-FOXO1α Pathway. PLoS One. 2013;8:e66529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |