Published online Nov 15, 2014. doi: 10.4291/wjgp.v5.i4.514
Revised: July 1, 2014
Accepted: September 6, 2014
Published online: November 15, 2014
Alcohol consumption is one of the leading causes of liver diseases and liver-related death worldwide. The gut is a habitat for billions of microorganisms which promotes metabolism and digestion in their symbiotic relationship with the host. Alterations of gut microbiome by alcohol consumption are referred to bacterial overgrowth, release of bacteria-derived products, and/or changed microbiota equilibrium. Alcohol consumption also perturbs the function of gastrointestinal mucosa and elicits a pathophysiological condition. These adverse effects caused by alcohol may ultimately result in a broad change of gastrointestinal luminal metabolites such as bile acids, short chain fatty acids, and branched chain amino acids. Gut microbiota alterations, metabolic changes produced in a dysbiotic intestinal environment, and the host factors are all critical contributors to the development and progression of alcoholic liver disease. This review summarizes recent findings of how alcohol-induced alterations of gut microbiota and metabolome, and discusses the mechanistic link between gastrointestinal dyshomeostasis and alcoholic liver injury.
Core tip: Excessive alcohol consumption causes alcoholic liver disease (ALD) with the mechanisms of pathogenesis largely unknown. Alterations of gut microbiota and metabolites are critical contributors to the development of ALD, which may lead to identification of therapeutic targets for ALD. This review summarizes recent findings of how alcohol-induced alterations of gut microbiota and metabolome, and discusses the mechanistic link between gastrointestinal dyshomeostasis and alcoholic liver injury.
- Citation: Zhong W, Zhou Z. Alterations of the gut microbiome and metabolome in alcoholic liver disease. World J Gastrointest Pathophysiol 2014; 5(4): 514-522
- URL: https://www.wjgnet.com/2150-5330/full/v5/i4/514.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v5.i4.514
Alcohol abuse is one of the leading causes of liver disease-related morbidity and mortality worldwide. Alcoholic liver disease (ALD) may progress from steatosis (fatty liver) to steatohepatitis, liver cirrhosis, and eventually hepatic carcinoma[1,2]. According to the National Institute on Alcoholic Abuse and Alcoholism, liver cirrhosis is the 12th leading cause of death in the United States, about 50% of which are alcohol related[3]. Even though enormous efforts have been made, the pathogenesis of ALD is still poorly understood, which makes the progress in finding proper treatments slow. In the last decade, the role of the gastrointestinal tract (GI) in the pathogenesis and progression of ALD has drawn more and more attention. It is estimated that there are multiple times more microbial cells in the gut than the total number of cells in the human body[4]. The microbes contribute to a complex of biological processes such as digestion[5], synthesis of vitamins[6], and regulation of immunity[7]. Disruption of intestinal homeostasis and alterations in the gut microbiome and metabolome contribute to the pathogenesis of many disorders including ALD[4,8,9]. This review summarizes recent findings on how alcohol affects the composition of the gut microbiota and metabolites, and discusses the mechanistic link between GI dyshomeostasis and the pathogenesis of alcohol consumption-induced liver injury.
Quantitative (bacterial overgrowth) and qualitative (dysbiosis) changes of the GI microbiome have long been associated with liver diseases including ALD[10]. Disturbed gut microbiota homeostasis results in dysfunction of the intestinal barrier and translocation of bacteria and/or bacterial products, which eventually contribute to the progression of ALD. Interventions focusing on gut bacteria and/or bacterial products in preventing ALD have drawn increasing attention in the last decade.
Alcohol consumption is well known to elicit bacterial overgrowth along the GI tract[11]. The number of both aerobic and anaerobic bacteria cultures of jejunal aspirates from alcoholic patients was distinctly higher than that from the control patients[12]. Similar trends were observed in patients with alcoholic cirrhosis[13]. Bacterial overgrowth has also been documented in experimental models of ALD[14,15]. Overgrowth of bacteria affects ethanol metabolism. Experimental induction of bacteria overgrowth resulted in enhanced endogenous and/or exogenous ethanol metabolism and high concentrations of acetaldehyde in both the intestinal lumen and the portal blood[16-18]. Oral administration of metronidazole, an antibiotic drug, led to a higher level of intracolonic acetaldehyde by increasing aerobic bacteria and reducing anaerobic bacteria in the intestine[19]. On the other hand, intracolonic acetaldehyde accumulation was prevented by antibiotic ciprofloxacin, which decreased colonic microbiota and fecal alcohol dehydrogenase activity[20].
Bacterial translocation is defined as the passage of viable bacteria from the GI tract to extraintestinal sites, such as the mesenteric lymph node, liver, kidney, and bloodstream. Experimental induction of small bowel bacterial overgrowth caused bacterial translocation in association with hepatic inflammation in rats[21]. The translocation of bacteria has been reported as early as 14 d after alcohol consumption in rats[22], while some studies did not show significant bacterial translocation after alcohol administration for 2 wk[23,24]. Moreover, Yan et al[14] reported that the bacterial translocation occurred prior to changes observed in the microbiome in a mouse model of continuous intragastric alcohol feeding for up to 3 wk. On the contrary, in a rat model of ALD combined with bacterial inoculation, rats chronically fed with alcohol presented markedly less bacterial translocation to the mesenteric lymph nodes and to the other organs examined compared to rats fed with an isocaloric liquid diet[25].
Bacteria, particularly the Gram-negative bacteria, produce endotoxins in the GI tract. Under physiological condition, endotoxin is excluded out of the body along with feces, and only trace amount of endotoxin can penetrate through the GI epithelium to the systemic circulation due to the gut barrier[26]. Alcohol consumption increases the serum endotoxin level, namely endotoxemia. The development of endotoxemia mainly results from bacterial overgrowth and/or increased gut permeability. Endotoxemia has been well documented in patients with ALD[26], and the blood endotoxin levels correlate well with tumor necrosis factor α (TNF-α) levels and the severity of ALD[27-29]. Elevated endotoxin in systemic circulation activates hepatic Kupffer cells via Toll-like receptor 4 to produce inflammatory cytokines and chemokines which, in turn, attract neutrophils and monocytes into the liver[30]. In addition to endotoxin, other bacterial products, such as bacterial DNA, peptidoglycan, and flagellin, could also translocate from the intestinal lumen to extraintestinal space and organs, and play a critical role in ALD progression. It was reported that bacterial DNA was elevated in the plasma of patients with alcoholic cirrhosis[31]. Bacterial DNA is recognized by TLR9 and sensitizes the liver to endotoxin-induced injury[32]. Alcohol exposure increased peptidoglycan levels and injected peptidoglycan deteriorated liver injury and inflammation in alcohol-fed mice[33,34].
Intestinal barrier dysfunction has been repeatedly reported in alcohol-induced endotoxemia and liver damage. Alcoholic patients showed increased gut permeability to a variety of macromolecules, such as polyethyleneglycol, lactulose/mannitol, or 51CrEDTA[35-38]. In animal studies, gut permeability to macromolecules such horse radish peroxidase was also increased in association with alcohol-induced endotoxemia and liver damage[39-43]. Orally administrated lipopolysaccharide could be detected in the plasma of acute alcohol-intoxicated mice but not in the control mice[44]. Chronic alcohol exposure reduced the distribution of tight junction proteins, but did not significantly affect the intestinal histopathology[45]; and the gut leakiness only occurred in the ileum instead of in the duodenum or jejunum[45]. Taken together, intestinal barrier dysfunction enables bacteria and bacterial products to translocate from the intestinal lumen to the liver which, as a result, facilitates the development of ALD.
Alcohol consumption not only results in quantitative changes of the intestinal microbiota, but also leads to enteric dysbiosis. Enteric dysbiosis refers to an imbalance in the intestinal bacterial composition that participates in the normal activities of the GI tract. Clinical studies have shown that patients with alcoholic cirrhosis had a lower proportion of Bacteroidetes and higher ones of Proteobacteria in the colon as compared to alcoholic patients without liver cirrhosis[46]. In another study, patients with alcoholic liver cirrhosis showed higher amounts of Prevotellaceae in the feces compared to cirrhotic patients with hepatitis B or healthy controls[47]. Animal studies also demonstrated that alcohol consumption for 10 wk altered colonic mucosa-associated microbiota composition in rats[48]. The abundance of Bacteroidetes and Verrucomicrobia were elevated in the cecum of mice intragastrically fed alcohol for 3 wk, while Firmicutes bacteria (including Lactobacillus, Pediococcus, Leuconostoc, and Lactococcus) were predominant in the control mice[14]. A recent animal study showed that chronic alcohol feeding for 8 wk caused a decline in the abundance of both Bacteroidetes and Firmicutes phyla, with a proportional increase in the Gram-negative Proteobacteria and Gram-positive Actinobacteria phyla in mice feces[15].
The interactions between alcohol-induced liver injury and alterations in the amount/proportion of certain bacteria phylum remain largely unknown. The Proteobacteria phylum includes Gram-negative bacteria, most of which are regarded as pathogenic species. Alcohol exposure-induced Proteobacteria expansion in the GI tract strongly indicates a link between alcohol-induced alterations of gut microbiota and the elevated plasma endotoxin level as well as hepatic inflammation. Studies have described opportunistic infections of Corynebacterium, a member of the Actinobacteria phylum, in individuals with ALD[49,50]. As mentioned above, intestinal bacteria like Escherichia coli metabolize alcohol and increase luminal acetaldehyde levels through alcohol dehydrogenase-dependent[51] or catalase-dependent pathway[52]. Acetaldehyde is known to disrupt the intestinal barrier through disassembling tight junction proteins[53-56], which implicates another mechanism of how microbiota participate in the development of ALD. The relevance of the gut microbiota changes for ALD progression still requires further investigation.
Efforts on exploring therapeutic strategies for treating ALD have been made for decades, and one of the major attempts was to ameliorate alcohol-induced endotoxemia. Indeed, animal studies demonstrated that abrogating endotoxin signal cascade in the liver by administration of antibiotics[57] or neutralization of circulating endotoxin[58], led to attenuation of alcohol-induced cytokine production and liver damage. Dietary supplementation of milk osteopontin prevented alcohol-induced liver injury through blocking enteric Gram-negative bacterial translocation and the endotoxin-mediated effects in the liver[59].
The effects of probiotics and prebiotics in modulating alcohol-induced liver injury in both patients with ALD and experimental models have been widely studied and the related references are summarized in Table 1. The first report was the study by Nanji et al[60], which showed that Lactobacillus GG treatment reduced endotoxemia and the severity of ALD. Treatment with Lactobacillus GG attenuated alcohol-induced intestinal barrier stress, gut leakiness, and liver injury in rats[40,61-64] and mice[65,66]. A short-term therapy with Bifidobacterium bifidum and Lactobacillus plantarum 8PA3 to alcoholic patients lowered plasma alanine aminotransferase and aspartate aminotransferase levels, restored the gut microbiota, and improved ALD compared to patients treated with standard therapy (abstinence plus vitamins) alone[67]. Another human study showed that Lactobacillus casei Shirota administration for 4 wk restored neutrophil phagocytic capacity in alcoholic cirrhotic patients[68]. Notably, the beneficial effects of probiotics were achieved not only by live bacteria, but also by heat-inactivated bacteria[63,69] or bacteria culture supernatant[70].
| Probiotic/prebiotic | Subjects | Duration of treatment | Outcome | Ref. | Year |
| Probiotics | |||||
| Lactobacillus rhamnosus GG | Male Wistar rats | 1 mo | Probiotic feeding reduced alcohol-induced endotoxemia and liver injury | Nanji et al[60] | 1994 |
| A mixture containing 450 billion bacteria (VSL #3) | Alcoholic cirrhosis patients | 3 mo | Treatment of probiotic lowered plasma levels of cytokines and oxidative stress parameters | Loguercio et al[103] | 2005 |
| L. casei Shirota | Alcoholic cirrhosis patients | 4 wk | Probiotic supplementation restored neutrophil phagocytic capacity | Stadlbauer et al[68] | 2008 |
| Heat-killed L. brevis SBC8803 | C57BL/6N mice | 35 d | L. brevis SBC8803 ameliorated alcohol-induced liver injury and fatty liver | Segawa et al[69] | 2008 |
| Bifidobacterium bifidum and L. plantarum 8PA3 | Male Russian adults | 5 d | Patients treated with probiotics had significantly lower ALT and AST activity, and restored gut microbiota compared to patients treated with standard therapy alone | Kirpich et al[67] | 2008 |
| GG | Male Sprague-Dawley rats | 10 wk | L. GG reduced alcohol-induced gut leakiness and blunted alcohol-induced oxidative stress and inflammation both in the intestine and liver | Forsyth et al[40] | 2009 |
| L. rhamnosus GG | Male C57BL/6N mice | Last 2 wk of the 8-wk feeding | L. GG supplementation reduced alcohol-induced endotoxemia and hepatic steatosis | Wang et al[65,66] | 2011, 2013 |
| L.paracasei | Male Fischer 344 rats | 10 wk | L. paracasei altered the fatty acid composition of the plasma and liver | Komatsuzaki et al[61] | 2012 |
| L. rhamnosus GG culture supernatant | Male C57BL/6N mice | 5 d | Bacteria-free L. GG culture supernatant ameliorated acute alcohol-induced gut leakiness and liver injury | Wang et al[70] | 2012 |
| Combined Bifidobacterium, Lactobacillus, Enterococcus and Bacillus cereus tablets | Male Sprague-Dawley rats | Up to 8 wk | Probiotic administration reduced plasma elevated-endotoxin levels caused by alcohol and altered gut microbiota | Zhang et al[62] | 2012 |
| Live or heat-killed VSL #3 | Male rats | Up to 12 h | VSL #3 administration reduced plasma endotoxin level and cytokine production caused by acute alcohol exposure | Chang et al[63] | 2013 |
| Heat-killed L. casei MYL01 | HepG2 cells | 20 h | L. casei MYL01 modulated proinflammatory cytokine production | Chiu et al[104] | 2014 |
| Escherichia coli Nissle 1917 secreting pyrroloquinoline quinone | Male Foster rats | 10 wk | Probiotic treatment ameliorated alcohol-induced oxidative damage and hyperlipidemia in rats | Singh et al[64] | 2014 |
| Prebiotics | |||||
| L. rhamnosus GG or oats | Male Sprague-Dawley rats | 10 wk | Supplementation of L. rhamnosus GG or oats prevented alcohol-altered colonic musoca-associated microbiota composition in rats | Mutlu et al[48] | 2009 |
| Fructooligosaccharides | Male C57BL/6J mice | 3 wk | Administration of fructooligosaccharides to alcohol-fed mice reduced bacterial overgrowth and ameliorated alcoholic steatohepatitis through partially restoring the host antimicrobial protein Reg3g | Yan et al[14] | 2011 |
Short-chain fructooligosaccharides and other prebiotics are used to stimulate the growth and activity of probiotics such as Lactobacillii and Bifidobacteria. Dietary supplementation of oats prevented alcohol-altered colonic musoca-associated microbiota composition in rats[48]. It was shown that administration of prebiotic (fructooligosaccharides) to alcohol-fed mice reduced bacterial overgrowth and ameliorated alcoholic steatohepatitis through partially restoring the host antimicrobioal protein Reg3g[14].
There are few reports addressing the impact of probiotic and/or prebiotic supplementation on gut microbiome during the development of ALD. To date the most comprehensive study employed 16S ribosome RNA sequencing to characterize gut microbiome changes in mice feces after chronic alcohol exposure and Lactobacillus GG supplementation[15]. Lactobacillus GG not only reduced bacterial overgrowth in alcohol-fed mice, but also prevented the alcohol-induced expansion of the Proteobacteria and Actinobacteria phyla.
Research into alterations in gut metabolome in ALD is unfortunately not as advanced as that for alterations in gut microbiota. To the best of our knowledge, our group, for the first time, applied mass spectrometry-based high throughput technology for characterization of the metabolic alterations of the GI tract contents in a rat model of chronic alcohol consumption. First of all, we conducted a comprehensive metabolite profiling using a high performance liquid chromatography time-of-flight mass spectrometry (HPLC-TOF MS). Secondly, since the HPLC-TOF MS-based profiling approach may not be able to detect or generate accurate data of short chain amino acids (SCFAs) and branched chain fatty acids (BCAAs) due to their volatile properties, a gas chromatography mass spectrometry (GC-MS) was used to quantitatively measure specific metabolic panels of SCFAs and BCAAs. The methods were described in more detail elsewhere[71,72]. Thirdly, a targeted quantitative metabolomics approach for a panel of 20-30 bile acids using ultraperformance liquid chromatography-triple-quadrupole mass spectrometry was utilized[73]. Alcohol consumption markedly altered bile acids[73], increased fatty acids and steroids, decreased carnitines, amino acids, branched chain amino acids, and all short chain fatty acids except for acetic acid[71] in the GI luminal contents of rats after 8-wk of alcohol exposure. Bile acids, SCFAs, and BCAAs were the top three categories among the significantly changed metabolites by alcohol consumption. Therefore, they were quantitatively measured in our study and the results will be discussed in more detail below.
Chronic alcohol consumption resulted in a global metabolite alteration including amino acids, fatty acids, steroids, lipids, carnitine, SCFAs, BCAAs[71], and bile acids[73] along the GI tract of rats. Almost all amino acids detected were decreased in GI contents of alcohol-fed rats compared to the control. Notably, high abundances of alanine, arginine, glutamic acid, proline, and threoine were observed in all the intestinal segments (from duodenum to rectum) and they were dramatically decreased after alcohol exposure. Amino acids derived from dietary protein may serve as substrates for luminal conversion by the gut microbiota which, in turn, regulate the host homeostasis. For example, one constituent of the gut microbiome, Lactobacillus reuteri, is able to convert L-histidine into histamine, which is an immune-regulatory signal suppressing TNF-α production[74]. Intestinal bacteria also involve in converting glutamate to γ-amino butyric acid via glutamate decarboxylase[75]. Taken together, it is possible that the reduced abundance of amino acids in alcohol-fed rats was resulted from a perturbed gut microbial-host co-metabolism under the enteric dysbiosis condition.
The levels of steroids and steroid derivatives were significantly increased after alcohol consumption in the stomach, duodenum, jejunum, and ileum. Carnitines and metabolites involved in lipid metabolism were decreased in alcohol-fed rats. Most of the fatty acids detected were at higher levels including 17-HDoHE and 19,20-DiHDPA, the two metabolic products from docosahexaenoic acid (DHA), and DHA itself. The elevation of DHA and DHA metabolites in the intestinal lumen, especially the large intestine, indicates a disrupted absorption of this nutrient induced by alcohol exposure.
Alcohol consumption significantly perturbed all 21 bile acids detected along the GI tract with the ileum showed the most significant alteration[73]. The concentration of unconjugated bile acids in control rats was low in duodenum (0.04 nmol/mg wet weight), whereas it was increased in the alcohol group (1.30 nmol/mg wet weight). Taurine-conjugated bile acids are the most abundant bile acids in the small intestine and the liver of control rats[73,76,77]. Alcohol consumption led to lower levels of taurine-conjugated bile acids in the duodenum and ileum (0.15 and 0.02 nmol/mg wet weight) compared to control rats (2.39 and 5.66 nmol/mg wet weight, respectively), which made unconjugated bile acids accounted for the largest proportion of the total bile acids in the entire GI tract. Meanwhile, the amount and proportion of taurine-conjugated bile acids were decreased both in the liver and blood[73].
Bile acid metabolism is dependent on the biological activities of the gut microbiota and the host, and both bacterial and hepatic enzymes further modify bile acids during enterohepatic circulation[78,79]. Perturbed gut microbiome may result in a disturbance of bile acid metabolism and reabsorption, leading to altered bile acid profiles in the blood, liver, kidney, and heart[80]. Indeed, inhibiting intestinal microbiota with ampicillin increased the expression of the apical sodium-dependent bile acid transporter (ASBT/Slc10a2) in the brush-border membrane of the ileum, which in turn increased bile acid transport into portal blood[81]. Germ-free mice and rats have a higher proportion of taurine-conjugated bile acids in their livers and intestines[79,82], demonstrating a close association between gut microbiota and bile acid composition. It has been reported that the ratio of glycine-conjugated to taurine-conjugated bile acids is dependent on the hepatic taurine concentration[83]. In our study, we found that the hepatic bile salt taurine to glycine ratio was 30:1 in control rats, while the ratio was 1:1 in alcohol-treated rats. The majority of taurine is usually degraded by the gut microbiota to inorganic sulfate[84]. For this reason, an overgrowth of gut microbiota caused by alcohol exposure would be expected to decrease taurine bioavailability, which provides an explanation for alcohol-induced decrease in taurine-conjugated bile acids in our study. In addition, another investigation suggests that the reduction of taurine in the liver in alcohol-fed mice may be due to the formation and excretion of N-acetyltaurine, a novel metabolite synthesized from taurine and acetate[85].
Acetic acid, propionic acid, and butyric acid are the most predominant SCFAs within the intestine[86]. Our study revealed that the distal intestine (ileum to rectum, especially cecum) processed the majority of SCFAs, within which acetic acid, propionic acid, and butyric acid were predominant (85% in ileum, 94% in cecum, 97% in colon, and 93% in rectum)[71]. Alcohol consumption dramatically reduced all 9 SCFAs detected in the distal intestine except for acetic acid. SCFAs are mainly produced by microbial fermentation of indigestible dietary fibers in the gut[87]. The alteration of SCFAs in alcohol-fed rats may be a result from alcohol-perturbed gut microbiota. The elevated acetic acid levels after alcohol consumption may presumably be due to the oxidation of ethanol to acetaldehyde and subsequent oxidation to acetic acid[88]. Since bacterial aldehyde dehydrogenase activity is limited[18], gut microbiota may not be the major player for the elevated luminal acetic acid level. On the other hand, SCFAs may influence the gut microbiota through stimulating Bifidibacteria growth and inhibiting Gram-negative facultative and anaerobic bacteria[89]. SCFAs are known as energy sources to regulate the homeostasis of the intestine and other organs[86]. In a recent study, SCFAs were approved to be beneficial against alcohol-induced intestinal barrier dysfunction through activating AMP-activated protein kinase in Caco-2 cells[90].
BCAAs are essential nutrients obtained from food, as they cannot be synthesized de novo by mammals[91]. Gut microbiota, however, are capable to produce BCAAs efficiently[92]. BCAA supplementation has been widely used to improve energy metabolism[93,94], insulin resistance[95-97], and severity of liver disease[98]. Our study reported that all three BCAAs, valine, leucine, and isoleucine, in the GI lumen were predominant in the small intestine (duodenum, jejunum, and ileum) and to a lesser extent in the cecum in rats[71]. Alcohol consumption led to significantly lower levels of all three BCAAs in the GI contents[71]. Previous findings have shown that chronic alcohol consumption increased incorporation of leucine into hepatic proteins[99] and accelerated the absorption of leucine from the small intestine[100], which may explain the dramatic reduction of BCAAs in the gut lumen observed in our study. Notably, a low ratio of plasma BCAAs to aromatic amino acids is a hallmark of liver cirrhosis. Indeed, elevated leucine and isoleucine levels were reported in the plasma of non-alcholic steatotic and non-alcoholic steatohepatitic patients compared to healthy controls[101], which indicate the homeostasis of BCAAs may be involved in the pathogenesis of liver diseases. Moreover, branched chain SCFAs, 2-methylpropanoic acid, 2-methylbutyric acid, and 3-methylbutyric acid are derived from the catabolism of BCAAs[102]. The decreased enteric BCAA levels may further contribute to the decreased levels of branched chain SCFAs after alcohol consumption.
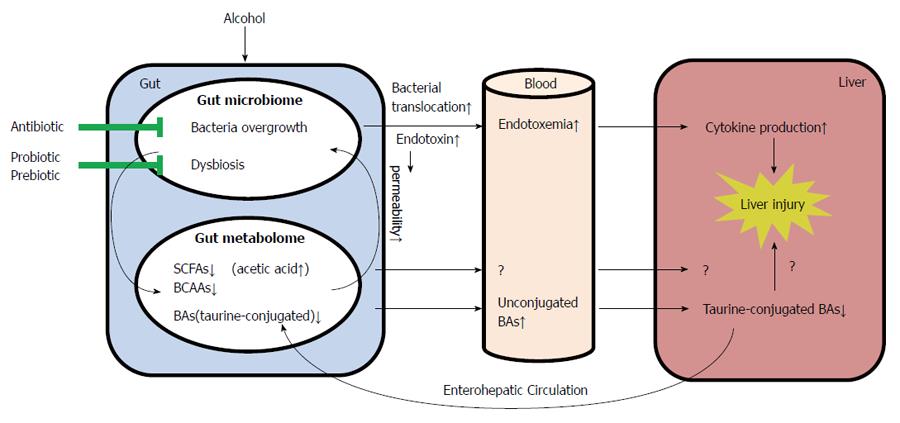
Alcoholic consumption is one of the leading causes of liver diseases and liver-related death worldwide. Of the major factors that contribute to the pathogenesis of ALD, the gut microbiota and metabolites have recently drawn more and more attention. Altered intestinal microbiota and gut-associated endotoxemia are recognized as pathophysiological factors in the development of ALD. Prebiotics and probiotics have been applied to prevent alcohol-induced disease development and progression. Taking the advantages of metabonomics approaches, detailed metabolic profiling provides novel information on alcohol-induced alterations in microbiota-host co-metabolism. The impact of alcohol consumption on the gut microbiome and metabolome during the development of ALD is summarized in Figure 1. Despite the recent progression in understanding the importance of the GI tract in the development of ALD, questions of how alcohol consumption results in gut microbiome and metabolome alterations and what are the consequences of such changes to the host have not been fully addressed. Future investigations on the cause-effect relationship between alterations of gut microbiome/metabolome and the liver pathophysiology will not only provide novel insights into the pathogenesis of ALD but also pave the way to the development of therapeutic interventions to cure ALD.
P- Reviewer: Bashashati M, Lee YY S- Editor: Song XX L- Editor: A E- Editor: Wang CH
| 1. | Miller AM, Horiguchi N, Jeong WI, Radaeva S, Gao B. Molecular mechanisms of alcoholic liver disease: innate immunity and cytokines. Alcohol Clin Exp Res. 2011;35:787-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 136] [Article Influence: 10.5] [Reference Citation Analysis (2)] |
| 2. | Mandrekar P. Epigenetic regulation in alcoholic liver disease. World J Gastroenterol. 2011;17:2456-2464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 60] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1244] [Cited by in F6Publishing: 1342] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 4. | Shoaie S, Nielsen J. Elucidating the interactions between the human gut microbiota and its host through metabolic modeling. Front Genet. 2014;5:86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Breen DM, Rasmussen BA, Côté CD, Jackson VM, Lam TK. Nutrient-sensing mechanisms in the gut as therapeutic targets for diabetes. Diabetes. 2013;62:3005-3013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24:160-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 790] [Cited by in F6Publishing: 825] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 7. | Chen VL, Kasper DL. Interactions between the intestinal microbiota and innate lymphoid cells. Gut Microbes. 2014;5:129-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Parekh PJ, Arusi E, Vinik AI, Johnson DA. The role and influence of gut microbiota in pathogenesis and management of obesity and metabolic syndrome. Front Endocrinol (Lausanne). 2014;5:47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Kanauchi O, Andoh A, Mitsuyama K. Effects of the modulation of microbiota on the gastrointestinal immune system and bowel function. J Agric Food Chem. 2013;61:9977-9983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Chen P, Schnabl B. Host-microbiome interactions in alcoholic liver disease. Gut Liver. 2014;8:237-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Gabbard SL, Lacy BE, Levine GM, Crowell MD. The impact of alcohol consumption and cholecystectomy on small intestinal bacterial overgrowth. Dig Dis Sci. 2014;59:638-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 12. | Bode JC, Bode C, Heidelbach R, Dürr HK, Martini GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30-34. [PubMed] [Cited in This Article: ] |
| 13. | Casafont Morencos F, de las Heras Castaño G, Martín Ramos L, López Arias MJ, Ledesma F, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1996;41:552-556. [PubMed] [Cited in This Article: ] |
| 14. | Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 529] [Cited by in F6Publishing: 563] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 15. | Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, Gobejishvili L, Joshi-Barve S, Ayvaz T, Petrosino J. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8:e53028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 382] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 16. | Baraona E, Julkunen R, Tannenbaum L, Lieber CS. Role of intestinal bacterial overgrowth in ethanol production and metabolism in rats. Gastroenterology. 1986;90:103-110. [PubMed] [Cited in This Article: ] |
| 17. | Väkeväinen S, Tillonen J, Salaspuro M, Jousimies-Somer H, Nuutinen H, Färkkilä M. Hypochlorhydria induced by a proton pump inhibitor leads to intragastric microbial production of acetaldehyde from ethanol. Aliment Pharmacol Ther. 2000;14:1511-1518. [PubMed] [Cited in This Article: ] |
| 18. | Visapää JP, Tillonen J, Salaspuro M. Microbes and mucosa in the regulation of intracolonic acetaldehyde concentration during ethanol challenge. Alcohol Alcohol. 2002;37:322-326. [PubMed] [Cited in This Article: ] |
| 19. | Tillonen J, Väkeväinen S, Salaspuro V, Zhang Y, Rautio M, Jousimies-Somer H, Lindros K, Salaspuro M. Metronidazole increases intracolonic but not peripheral blood acetaldehyde in chronic ethanol-treated rats. Alcohol Clin Exp Res. 2000;24:570-575. [PubMed] [Cited in This Article: ] |
| 20. | Visapää JP, Jokelainen K, Nosova T, Salaspuro M. Inhibition of intracolonic acetaldehyde production and alcoholic fermentation in rats by ciprofloxacin. Alcohol Clin Exp Res. 1998;22:1161-1164. [PubMed] [Cited in This Article: ] |
| 21. | Lichtman SN, Sartor RB, Keku J, Schwab JH. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology. 1990;98:414-423. [PubMed] [Cited in This Article: ] |
| 22. | Napolitano LM, Koruda MJ, Zimmerman K, McCowan K, Chang J, Meyer AA. Chronic ethanol intake and burn injury: evidence for synergistic alteration in gut and immune integrity. J Trauma. 1995;38:198-207. [PubMed] [Cited in This Article: ] |
| 23. | Mason CM, Dobard E, Kolls J, Nelson S. Effect of alcohol on bacterial translocation in rats. Alcohol Clin Exp Res. 1998;22:1640-1645. [PubMed] [Cited in This Article: ] |
| 24. | Fleming S, Toratani S, Shea-Donohue T, Kashiwabara Y, Vogel SN, Metcalf ES. Pro- and anti-inflammatory gene expression in the murine small intestine and liver after chronic exposure to alcohol. Alcohol Clin Exp Res. 2001;25:579-589. [PubMed] [Cited in This Article: ] |
| 25. | Braulio VB, De Queiroz Côrtes M, Borges-Neto AA, Bastos MA, Cruz MS, Fracalanza SE. Inhibition of bacterial translocation by chronic ethanol consumption in the rat. APMIS. 2001;109:809-815. [PubMed] [Cited in This Article: ] |
| 26. | Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881-G884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 27. | Fujimoto M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, Tamagawa T, Kitano H, Kikukawa M, Ann T, Ishii Y. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res. 2000;24:48S-54S. [PubMed] [Cited in This Article: ] |
| 28. | Hanck C, Rossol S, Böcker U, Tokus M, Singer MV. Presence of plasma endotoxin is correlated with tumour necrosis factor receptor levels and disease activity in alcoholic cirrhosis. Alcohol Alcohol. 1998;33:606-608. [PubMed] [Cited in This Article: ] |
| 29. | Urbaschek R, McCuskey RS, Rudi V, Becker KP, Stickel F, Urbaschek B, Seitz HK. Endotoxin, endotoxin-neutralizing-capacity, sCD14, sICAM-1, and cytokines in patients with various degrees of alcoholic liver disease. Alcohol Clin Exp Res. 2001;25:261-268. [PubMed] [Cited in This Article: ] |
| 30. | Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321-1329. [PubMed] [Cited in This Article: ] |
| 31. | Francés R, Benlloch S, Zapater P, González JM, Lozano B, Muñoz C, Pascual S, Casellas JA, Uceda F, Palazón JM. A sequential study of serum bacterial DNA in patients with advanced cirrhosis and ascites. Hepatology. 2004;39:484-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Romics L, Dolganiuc A, Kodys K, Drechsler Y, Oak S, Velayudham A, Mandrekar P, Szabo G. Selective priming to Toll-like receptor 4 (TLR4), not TLR2, ligands by P. acnes involves up-regulation of MD-2 in mice. Hepatology. 2004;40:555-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, Franchimont D, Louis H, Devière J, Le Moine O. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 34. | Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 35. | Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1:179-182. [PubMed] [Cited in This Article: ] |
| 36. | Keshavarzian A, Fields JZ, Vaeth J, Holmes EW. The differing effects of acute and chronic alcohol on gastric and intestinal permeability. Am J Gastroenterol. 1994;89:2205-2211. [PubMed] [Cited in This Article: ] |
| 37. | Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 287] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 38. | Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742-747. [PubMed] [Cited in This Article: ] |
| 39. | Enomoto N, Takei Y, Hirose M, Ikejima K, Miwa H, Kitamura T, Sato N. Thalidomide prevents alcoholic liver injury in rats through suppression of Kupffer cell sensitization and TNF-alpha production. Gastroenterology. 2002;123:291-300. [PubMed] [Cited in This Article: ] |
| 40. | Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43:163-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 292] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 41. | Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 232] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 42. | Keshavarzian A, Choudhary S, Holmes EW, Yong S, Banan A, Jakate S, Fields JZ. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J Pharmacol Exp Ther. 2001;299:442-448. [PubMed] [Cited in This Article: ] |
| 43. | Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 44. | Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. J Pharmacol Exp Ther. 2003;305:880-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. 2010;298:G625-G633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 46. | Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966-G978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 493] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 47. | Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 674] [Cited by in F6Publishing: 701] [Article Influence: 53.9] [Reference Citation Analysis (1)] |
| 48. | Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33:1836-1846. [PubMed] [Cited in This Article: ] |
| 49. | Harnisch JP, Tronca E, Nolan CM, Turck M, Holmes KK. Diphtheria among alcoholic urban adults. A decade of experience in Seattle. Ann Intern Med. 1989;111:71-82. [PubMed] [Cited in This Article: ] |
| 50. | Cericco M, Iglicki F, Guillaumont MP, Schmitt JL, Dupas JL, Capron JP. [Corynebacterium xerosis endocarditis associated with alcoholic cirrhosis]. Gastroenterol Clin Biol. 1996;20:514. [PubMed] [Cited in This Article: ] |
| 51. | Salaspuro V, Nyfors S, Heine R, Siitonen A, Salaspuro M, Jousimies-Somer H. Ethanol oxidation and acetaldehyde production in vitro by human intestinal strains of Escherichia coli under aerobic, microaerobic, and anaerobic conditions. Scand J Gastroenterol. 1999;34:967-973. [PubMed] [Cited in This Article: ] |
| 52. | Tillonen J, Kaihovaara P, Jousimies-Somer H, Heine R, Salaspuro M. Role of catalase in in vitro acetaldehyde formation by human colonic contents. Alcohol Clin Exp Res. 1998;22:1113-1119. [PubMed] [Cited in This Article: ] |
| 53. | Rao RK. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res. 1998;22:1724-1730. [PubMed] [Cited in This Article: ] |
| 54. | Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1280-G1288. [PubMed] [Cited in This Article: ] |
| 55. | Basuroy S, Sheth P, Mansbach CM, Rao RK. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and L-glutamine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G367-G375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Dunagan M, Chaudhry K, Samak G, Rao RK. Acetaldehyde disrupts tight junctions in Caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1356-G1364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 57. | Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218-224. [PubMed] [Cited in This Article: ] |
| 58. | Zhou Z, Wang L, Song Z, Lambert JC, McClain CJ, Kang YJ. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-alpha production. Am J Pathol. 2003;163:1137-1146. [PubMed] [Cited in This Article: ] |
| 59. | Ge X, Lu Y, Leung TM, Sørensen ES, Nieto N. Milk osteopontin, a nutritional approach to prevent alcohol-induced liver injury. Am J Physiol Gastrointest Liver Physiol. 2013;304:G929-G939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 60. | Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc Soc Exp Biol Med. 1994;205:243-247. [PubMed] [Cited in This Article: ] |
| 61. | Komatsuzaki N, Shima J. Effects of live Lactobacillus paracasei on plasma lipid concentration in rats fed an ethanol-containing diet. Biosci Biotechnol Biochem. 2012;76:232-237. [PubMed] [Cited in This Article: ] |
| 62. | Zhang B, Lu XL, Song YH, Shi HT, Li J, Geng Y. [Changes in the intestinal microenvironment during development of alcoholic fatty liver disease and related effects of probiotic therapy]. Zhonghua Ganzangbing Zazhi. 2012;20:848-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 63. | Chang B, Sang L, Wang Y, Tong J, Zhang D, Wang B. The protective effect of VSL#3 on intestinal permeability in a rat model of alcoholic intestinal injury. BMC Gastroenterol. 2013;13:151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 64. | Singh AK, Pandey SK, Naresh Kumar G. Pyrroloquinoline quinone-secreting probiotic Escherichia coli Nissle 1917 ameliorates ethanol-induced oxidative damage and hyperlipidemia in rats. Alcohol Clin Exp Res. 2014;38:2127-2137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Wang Y, Kirpich I, Liu Y, Ma Z, Barve S, McClain CJ, Feng W. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am J Pathol. 2011;179:2866-2875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 66. | Wang Y, Liu Y, Kirpich I, Ma Z, Wang C, Zhang M, Suttles J, McClain C, Feng W. Lactobacillus rhamnosus GG reduces hepatic TNFα production and inflammation in chronic alcohol-induced liver injury. J Nutr Biochem. 2013;24:1609-1615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 67. | Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 340] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 68. | Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48:945-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 69. | Segawa S, Wakita Y, Hirata H, Watari J. Oral administration of heat-killed Lactobacillus brevis SBC8803 ameliorates alcoholic liver disease in ethanol-containing diet-fed C57BL/6N mice. Int J Food Microbiol. 2008;128:371-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 70. | Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol. 2012;303:G32-G41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 71. | Xie G, Zhong W, Zheng X, Li Q, Qiu Y, Li H, Chen H, Zhou Z, Jia W. Chronic ethanol consumption alters mammalian gastrointestinal content metabolites. J Proteome Res. 2013;12:3297-3306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 72. | Zheng X, Qiu Y, Zhong W, Baxter S, Su M, Li Q, Xie G, Ore BM, Qiao S, Spencer MD. A targeted metabolomic protocol for short-chain fatty acids and branched-chain amino acids. Metabolomics. 2013;9:818-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 73. | Xie G, Zhong W, Li H, Li Q, Qiu Y, Zheng X, Chen H, Zhao X, Zhang S, Zhou Z. Alteration of bile acid metabolism in the rat induced by chronic ethanol consumption. FASEB J. 2013;27:3583-3593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 74. | Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, Britton RA, Kalkum M, Versalovic J. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One. 2012;7:e31951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 75. | Kim JY, Chung EJ, Kim JH, Jung KY, Lee WY. Response to steroid treatment in anti-glutamic acid decarboxylase antibody-associated cerebellar ataxia, stiff person syndrome and polyendocrinopathy. Mov Disord. 2006;21:2263-2264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Dupont J, Oh SY, O’Deen LA, Geller S. Cholanoic (bile) acids in hepatic and nonhepatic tissues of miniature swine. Lipids. 1974;9:294-297. [PubMed] [Cited in This Article: ] |
| 77. | García-Cañaveras JC, Donato MT, Castell JV, Lahoz A. Targeted profiling of circulating and hepatic bile acids in human, mouse, and rat using a UPLC-MRM-MS-validated method. J Lipid Res. 2012;53:2231-2241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 198] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 78. | Martin FP, Dumas ME, Wang Y, Legido-Quigley C, Yap IK, Tang H, Zirah S, Murphy GM, Cloarec O, Lindon JC. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model. Mol Syst Biol. 2007;3:112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 326] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 79. | Claus SP, Tsang TM, Wang Y, Cloarec O, Skordi E, Martin FP, Rezzi S, Ross A, Kochhar S, Holmes E. Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol Syst Biol. 2008;4:219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 251] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 80. | Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, Nicholson JK, Holmes E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4523-4530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 474] [Cited by in F6Publishing: 502] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 81. | Miyata M, Yamakawa H, Hamatsu M, Kuribayashi H, Takamatsu Y, Yamazoe Y. Enterobacteria modulate intestinal bile acid transport and homeostasis through apical sodium-dependent bile acid transporter (SLC10A2) expression. J Pharmacol Exp Ther. 2011;336:188-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Wostmann BS. Intestinal bile acids and cholesterol absorption in the germfree rat. J Nutr. 1973;103:982-990. [PubMed] [Cited in This Article: ] |
| 83. | Hardison WG, Proffitt JH. Influence of hepatic taurine concentration on bile acid conjugation with taurine. Am J Physiol. 1977;232:E75-E79. [PubMed] [Cited in This Article: ] |
| 84. | Hepner GW, Sturman JA, Hofmann AF, Thomas PJ. Metabolism of steroid and amino acid moieties of conjugated bile acids in man. 3. Cholyltaurine (taurocholic acid). J Clin Invest. 1973;52:433-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 63] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 85. | Shi X, Yao D, Chen C. Identification of N-acetyltaurine as a novel metabolite of ethanol through metabolomics-guided biochemical analysis. J Biol Chem. 2012;287:6336-6349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 86. | Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1076] [Cited by in F6Publishing: 1351] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 87. | Cummings JH. Short chain fatty acids in the human colon. Gut. 1981;22:763-779. [PubMed] [Cited in This Article: ] |
| 88. | Israel Y, Orrego H, Carmichael FJ. Acetate-mediated effects of ethanol. Alcohol Clin Exp Res. 1994;18:144-148. [PubMed] [Cited in This Article: ] |
| 89. | Andoh A, Tsujikawa T, Fujiyama Y. Role of dietary fiber and short-chain fatty acids in the colon. Curr Pharm Des. 2003;9:347-358. [PubMed] [Cited in This Article: ] |
| 90. | Elamin EE, Masclee AA, Dekker J, Pieters HJ, Jonkers DM. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J Nutr. 2013;143:1872-1881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 91. | Tom A, Nair KS. Assessment of branched-chain amino Acid status and potential for biomarkers. J Nutr. 2006;136:324S-330S. [PubMed] [Cited in This Article: ] |
| 92. | Park JH, Lee SY. Fermentative production of branched chain amino acids: a focus on metabolic engineering. Appl Microbiol Biotechnol. 2010;85:491-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 93. | Higuchi N, Kato M, Miyazaki M, Tanaka M, Kohjima M, Ito T, Nakamuta M, Enjoji M, Kotoh K, Takayanagi R. Potential role of branched-chain amino acids in glucose metabolism through the accelerated induction of the glucose-sensing apparatus in the liver. J Cell Biochem. 2011;112:30-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 94. | Kawaguchi T, Izumi N, Charlton MR, Sata M. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology. 2011;54:1063-1070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 95. | She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 96. | Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56:1647-1654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 400] [Cited by in F6Publishing: 392] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 97. | Ikehara O, Kawasaki N, Maezono K, Komatsu M, Konishi A. Acute and chronic treatment of L-isoleucine ameliorates glucose metabolism in glucose-intolerant and diabetic mice. Biol Pharm Bull. 2008;31:469-472. [PubMed] [Cited in This Article: ] |
| 98. | Shirabe K, Yoshimatsu M, Motomura T, Takeishi K, Toshima T, Muto J, Matono R, Taketomi A, Uchiyama H, Maehara Y. Beneficial effects of supplementation with branched-chain amino acids on postoperative bacteremia in living donor liver transplant recipients. Liver Transpl. 2011;17:1073-1080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 99. | Baraona E, Leo MA, Borowsky SA, Lieber CS. Pathogenesis of alcohol-induced accumulation of protein in the liver. J Clin Invest. 1977;60:546-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 245] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 100. | Martines D, Morris AI, Billington D. The effect of chronic ethanol intake on leucine absorption from the rat small intestine. Alcohol Alcohol. 1989;24:525-531. [PubMed] [Cited in This Article: ] |
| 101. | Kalhan SC, Guo L, Edmison J, Dasarathy S, McCullough AJ, Hanson RW, Milburn M. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism. 2011;60:404-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 368] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 102. | Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1150] [Cited by in F6Publishing: 1148] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 103. | Loguercio C, Federico A, Tuccillo C, Terracciano F, D’Auria MV, De Simone C, Del Vecchio Blanco C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540-543. [PubMed] [Cited in This Article: ] |
| 104. | Chiu YH, Tsai JJ, Lin SL, Lin MY. Lactobacillus casei MYL01 modulates the proinflammatory state induced by ethanol in an in vitro model. J Dairy Sci. 2014;97:2009-2016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |