Published online Jul 28, 2014. doi: 10.4329/wjr.v6.i7.459
Revised: April 25, 2014
Accepted: May 16, 2014
Published online: July 28, 2014
Since the recognition of disease molecular basis, it has become clear that the keystone moments of medical practice, namely early diagnosis, appropriate therapeutic treatment and patient follow-up, must be approached at a molecular level. These objectives will be in the near future more effectively achievable thanks to the impressive developments in nanotechnologies and their applications to the biomedical field, starting-up the nanomedicine era. The continuous advances in the development of biocompatible smart nanomaterials, in particular, will be crucial in several aspects of medicine. In fact, the possibility of manufacturing nanoparticle contrast agents that can be selectively targeted to specific pathological cells has extended molecular imaging applications to non-ionizing techniques and, at the same time, has made reachable the perspective of combining highly accurate diagnoses and personalized therapies in a single theranostic intervention. Main developing applications of nanosized theranostic agents include targeted molecular imaging, controlled drug release, therapeutic monitoring, guidance of radiation-based treatments and surgical interventions. Here we will review the most recent findings in nanoparticles contrast agents and their applications in the field of cancer molecular imaging employing non-ionizing techniques and disease-specific contrast agents, with special focus on recent findings on those nanomaterials particularly promising for ultrasound molecular imaging and simultaneous treatment of cancer.
Core tip: The development of novel nanomaterials specifically targeting diseased cells has made possible their employment as nanosized contrast agents also for non-ionizing molecular imaging techniques namely, magnetic resonance, ultrasound and optical imaging. Among them, ultrasound imaging might represent the best choice because of its low cost, ease of use and wide availability in clinical practice. Unfortunately, their actual employment in molecular imaging is limited due to their low tissue contrast discrimination. Hence, the described development of novel ultrasound targeted contrast agent may play a crucial role for their use in clinical molecular imaging.
- Citation: Di Paola M, Chiriacò F, Soloperto G, Conversano F, Casciaro S. Echographic imaging of tumoral cells through novel nanosystems for image diagnosis. World J Radiol 2014; 6(7): 459-470
- URL: https://www.wjgnet.com/1949-8470/full/v6/i7/459.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i7.459
One of the hottest research topic of the last decade in the medical field is related to nanomedicine, a new open field of modern medicine relying on advanced nanotechnology applied to medicine. In fact, the latest advances in nanotechnology and their application to the biomedical environment are dramatically changing the overall disease management process, starting from first diagnosis to the evaluation of treatment effects, leading to the concept of personalized medicine, characterized by very early, even pre-symptomatic, diagnosis accompanied by highly-effective targeted therapies[1-4]. At this regard, the introduction of novel nanotechnology-based techniques in medical imaging and drug delivery allows to define personalized diagnoses and therapies, employing minimally invasive approaches based on non-ionizing imaging techniques for early detection of diseases[5]. From these recent advances arises the concept of molecular imaging, which is gaining an increasingly important role in both pathology understanding and specific choice of treatment[6]. Rather than morphological or functional characteristics, molecular imaging techniques are specifically aimed at identifying the molecular causes of disease[7], with consequent ability to detect molecular and cellular processes in living organisms and to allow an early and careful identification and differentiation between healthy and pathological tissues. The basic aspect of molecular imaging is the use of smart contrast agents able to selectively identify specific molecular targets or cellular processes, highlighting them on the corresponding images. The rationale for the development of these new methods is that many diseases have a molecular basis, whose visualization may result in a number of advantages like early diagnosis, precise staging, real-time monitoring of therapeutic treatment, and better prognostic evaluation. The quality of the final result depends on two key-factors: (1) actual ability of contrast agents to reach their specific biological target and binding to it (targeting); and (2) performance of the detection system in terms of sensitivity and contrast enhancement.
Chemical manipulation of drugs and other nanomaterials may allow a controlled modification of some of their properties and bioactivity such as solubility, blood pool retention times, controlled release, highly specific site-targeted delivery. Concerning this particular aspect, surface functionalization with synthetic polymers and/or specific ligands can target nanosized carriers to specific cells and organs within the body after intravenous or subcutaneous injection[8-16]. These approaches may thus be used to enhance detection sensitivity in medical imaging and to improve therapeutic effectiveness with concomitant decrease of side effects. In addition, some of the carriers can be engineered in such a way to be activated by changes in the environmental pH, chemical stimuli, by the application of a rapidly oscillating magnetic field or by the application of an external heat source[9,17-19]. Furthermore, nanoparticles for specific diagnostic purposes can be designed to act as multifunctional agents capable, for example, to simultaneously produce signals that are detectable by more than one imaging techniques, like ultrasound (US) and magnetic resonance imaging (MRI)[20,21].
Although different pathological conditions like atherosclerotic plaques, inflammation, angiogenesis and thrombus formation have been identified as possible targets of these innovative methodologies, the most promising applications of nanomedicine are those related to the new approaches to cancer diagnosis and therapy at cellular and molecular level[5,22-24]. Cancer is widely considered to be one the main cause of death in modern society, characterized by a high mortality rate often due to a late diagnosis available with conventional techniques. Current therapeutic strategies for cancer treatment, which include surgery, chemotherapy and radiotherapy, are largely invasive and exhibit significant toxicities together with a variety of side effects that worsen the quality of life of patients. It is then conceivable that the specific targeting of therapeutic agents (drugs or genes) to tumor tissues may result in a great improvement of treatment effectiveness and decrease of systemic toxicity. For these reasons nanoparticle-mediated drug targeting has been widely explored in recent years, by incorporating anticancer agents into suitable nanocapsules or by attaching therapeutic molecules to nanoparticle surface, and it actually exhibits several advantages like reduced drug dosage, increased pharmaceutical effectiveness, minimal side effects, drug protection against degradation and enhanced drug stability[10,25,26]. Anyway, one of the aspects of absolute novelty introduced by nanovector drug delivery is represented by the possibility of assessing therapy response, by directly monitoring the localization of targeted nanoparticles through non ionizing imaging techniques. Apart from these advantages, however, the possible toxicity related to nanoparticles themselves is an aspect that requires attention. The assessment of the biocompatibility of nanomaterials and their safety profile is in fact of crucial importance not only for patients treated, which can retain these materials for long period of time, but also for the production, management and disposal processes, which should be strictly regulated.
Imaging is a tool of fundamental importance in medical practice in general, and in cancer research in particular. Despite the impressive amount of imaging technologies and their applications available today, early and detailed cancer diagnosis is made possible only by using molecular imaging systems[27]. Among these, positron emission tomography (PET) is currently the only diagnostic technique in clinical use that provides imaging of tumours at molecular level. PET systems can in fact detect abnormal cellular activity well before any anatomical change is visible and structural anomalies detectable by other macroscopic imaging techniques like ultrasound, magnetic resonance (MRI), X-rays or computed tomography (CT). Nevertheless, since the high cost and the involvement of highly ionizing radiation, with consequent risks for patients, operators and environment, PET examinations cannot be routinely used for patient follow-up or for population screening purposes.
However, the recent advances in the development of smart nanoparticle contrast agents (NPCAs) opened new perspectives for diagnostic imaging techniques, allowing on one hand the extension of molecular imaging applications to non-ionizing techniques[28], like MRI[29], ultrasound[23,30] and optical imaging[31,32], and, on the other hand, introducing the possibility of combining highly detailed diagnoses and personalized therapies in single theranostic interventions[5].
A short overview of the most interesting properties of novel NPCAs and a summary of the most significant approaches to early molecular cancer diagnosis by employing non-ionizing techniques in combination with NPCAs will be illustrated in the next subparagraphs.
In recent years, many efforts have been made to synthesize new NPCAs suitable for cellular and molecular imaging through non-ionizing diagnostic techniques. To obtain an effective diagnostic imaging, NPCA must be designed to have the following basic characteristics: long circulating half-life, high vascular endothelium permeability, selective binding to the cellular/molecular target of interest, significant contrast-to-noise ratio enhancement, absence of toxicity, ease of clinical use, and compatibility with standard commercially available imaging systems[22,33].
The very crucial point is the effective interaction of NPCAs with their molecular targets, which is strongly dependent on nanoparticle size. In normal conditions, 50 nm can be considered as the upper size threshold to cross the vascular endothelium and directly target extravascular cells, larger diameters allowing only the recognition of intravascular targets. However, since the consistent difference between normal and tumor vessels, effective targeting of cancer cells beyond the capillary endothelium can occur also with bigger NPCAs. In fact, due to the aberrant angiogenesis, tumor vasculature is more leaky than normal one and exhibits the so-called EPR (enhanced permeability and retention) effect, which results in enhanced permeability and retention of particles that are smaller than the pore diameter of tumor endothelium (typically between 380 and 780 nm)[34-36].
One of the most common strategies to selectively target specific cellular receptors is functionalization, which is the conjugation of NPCA surface with specific ligands. Sometimes, a polymeric coating of particles may be necessary not only to improve particle stability and to modulate their intravascular half-life, but also to increase biocompatibility and to avoid immediate sequestration by the reticulo-endothelial system (RES).
Hitherto, the variety of nanomaterials synthesized that can be used as contrast agents for molecular imaging is very wide. Table 1 provides a list of different nanosized materials, with their chemical-physical properties, applications and the main literature-reported studies, their detailed description being beyond the goal of this review.
| Nanomaterials | Properties | Applications | Ref. |
| Liposomes | Lipid spherical membranes | In vivo ultrasound and MRI molecular imaging | [37,38] |
| Emulsions | Oil-in-water-type mixtures | Ultrasound and MRI | [39-41] |
| Polymers | Single or multiple molecular components | Molecular imaging, drug delivery | [33] |
| Iron particles | Paramagnetism, superparamagnetism | MRI | [42] |
| Gold nanoshells | Infrared absorption | MRI, photonics imaging, in vivo photo-thermal therapy | [43-45] |
| Carbon nanotubes | Fluorescence | In vitro optical imaging | [46-49] |
| Quantum dots | Fluorescence | Optical imaging | [50-53] |
Owing to its high resolution and elevated anatomical contrast, MRI is widely and successfully adopted in clinical routine. However, while standard MRI protocols are effective in detecting global properties of a tissue (e.g., relaxation times T1, T2, etc.), the low sensitivity of these techniques in normal conditions hampers their direct employment for molecular imaging purposes[6].
Nevertheless, the relatively low MRI contrast might be enhanced by using novel nanotechnologies[22]. Indeed, paramagnetic nanoparticles functionalized with several copies of Gd chelates were successfully exploited in both MRI molecular imaging and targeted therapy of atherosclerotic plaques[22,41].
Other clinical applications of MRI molecular imaging, ranging from liver disease to several type of cancers[27], have also been reported by using FeO nanoparticles coated with PEG (polyethylene glycol) or other polymers[54,55].
To further improve MRI sensitivity and image contrast, alternative strategies are currently under evaluation, based mainly on the synthesis of superparamagnetic nanoparticles made of metal alloys with specific chemical and physical properties (e.g., 2CoFe4O, 2MnFe4O, 2NiFe4O, FePt-FeO)[56,57].
Other methodological approaches are aimed at synthesizing multifunctional nanoparticles, detectable by high resolution MRI as well as by less expensive techniques like ultrasound or fluorescence imaging, so taking advantages of different diagnostic techniques with a single contrast agent. At this regard, “in vitro” experiments with dual mode silica nanospheres covered by an outer shell of superparamagnetic nanoparticles (in order to combine MRI and ultrasonography)[21] and with core-shell iron oxide/fluorescent silica nanoparticles (for MRI/fluorescence imaging applications)[58] have been successfully carried out.
Ultrasound imaging is a cheap and widely available technique offering all the previously mentioned exciting perspectives even if some limitations do apply, which are mostly related to the physical needs for wave transmission pathway: some anatomical sites remain not easily reachable because of boundary bone structures like brain, bone marrow, pelvic organs, etc. Furthermore, some technological limitations for 3D and multi-planar imaging acquisitions still remain, which make echographic examinations the first level diagnostic approach and not the ideal candidate for in depth more accurate investigations.
Some of the above described limitations, however, can be overcome by employing ultrasound contrast agents, commercially available for clinical use like microbubbles, and other novel nanosized targeted contrast agents under research development.
All contrast agents approved for routinely use in clinical ultrasound imaging are in the form of aqueous solutions of shell-stabilized gas-filled microbubbles[59]. Under an ultrasonic beam, microbubbles undergo volumetric oscillations with consequent emission of detectable ultrasound signals that can be exploited to enhance image contrast.
Upon controlled structural modifications, microbubbles can acquire targeting specificity, becoming then suitable also for molecular imaging purposes[23]. Based on the strategy adopted[60], microbubble targeting can be passive, in which the intrinsic properties of the shell promote cell adhesion[61,62], or active, in which the shell is functionalized with specific ligands toward target cells or tissues[63-66].
However, since microbubbles diameter ranges in the micrometer scale, they cannot cross endothelium wall, with consequent important limitations in their use to target extravascular cells. As a further limitation, half-lives of circulating microbubbles are in the order of just a few minutes, because of both sequestrations by reticulo-endothelial system (RES) and gas diffusion phenomena[6].
As discussed before, mainly due to their lower size NPCAs show significant intrinsic advantages with respect to microbubbles. In fact, nanoparticles can easily reach extravascular targets through endothelium crossing, and elude RES capturing. Moreover, the variety of specific surface modifications available for nanosized particles is particularly wide, with consequent effective targeting of a wide range of selected pathologies. In the last years most of the experimental work aimed at developing novel NPCAs for ultrasound molecular imaging has focused mainly on testing few type of nanoparticles, namely liposomes, perfluorocarbon nanoemulsions and nanobubbles[67-69].
Recent studies have demonstrated, however, that the use of solid nanoparticles as NPCAs may be even more effective[21,70,71]. With respect to liquid nanoparticles, solid nanomaterials exhibit in fact higher contrast enhancements, since of their higher acoustic impedance with respect to surrounding tissues, and, at the same time, are much more stable than nanobubbles, whose circulating half-life is quite limited by the aforementioned gas diffusion phenomena.
First experiments performed on solid nanoparticles as contrast agents for ultrasound imaging were carried out by using echographic probes working at very high frequencies (30-40 MHz)[72,73], whose clinical usefulness is closely restricted to intravascular or dermatological applications. More recent studies, instead, have demonstrated that silica nanospheres can be effectively detected on conventional echographic images acquired at diagnostic frequencies (7.5-10 MHz). In addition, the coating of silica nanospheres with a shell of smaller superparamagnetic nanoparticles has made possible to obtain dual-mode NCPAs, detectable by both ultrasound and MRI[21].
On the basis of these and other literature findings, the development of silica nanoparticles-based NPCAs for ultrasound molecular imaging seems to be particularly promising since of their well-documented biocompatibility[74-76], ease of functionalization[75] as well as synthesis procedures[76], potential employment as nanovectors for controlled release of drugs[77] or genes[78].
Since of their high sensitivity and non-invasiveness, optical imaging techniques have recently attracted the interest of researchers working on the development of novel molecular imaging protocols[6]. Optical imaging is actually mainly limited to cell biology and other non-clinical applications, due to the very low penetration of visible wavelengths into anatomical tissues. Interestingly, the use of NPCAs also in optical imaging may enhance its potential suitability in clinical applications like molecular detection of tumours. In fact, optically detectable quantum dots targeting cancer cells have been effectively visualized in both “in vitro” and “in vivo” studies[79,80]. Gold nanoshells have been used for optical coherence tomography imaging in a mouse model of colon cancer[81]. Detectable fluorescence has been observed in carbon nanotubes excited at visible wavelengths after uptake by breast cancer cells[82].
Optoacoustic imaging is an emerging technique that combines high sensitivity and elevated contrast of optical imaging with spatial resolutions and penetration depths typical of ultrasound-based techniques[83]. Essentially, when irradiated with near-infrared short laser pulses, tissues emit acoustic waves (photoacoustic effect) that can be detected by ultrasound probes and used for imaging purposes[84]. As an example, the optical absorption of hemoglobin has allowed the optoacoustic visualization of breast tumor microvasculature[85].
Many efforts are in progress to extend the optoacoustic techniques to molecular imaging applications. Particularly promising seem to be, at this regard, noble metal nanoparticles which, as a consequence of surface Plasmon resonance, strongly absorb laser energy with subsequent generation of ultrasound signals. Although several plasmonic nanoparticles have been recently tested as potential NPCAs for optoacoustic imaging[86,87], the metal of choice seems to be gold[86,88-90] because of its high stability, facile chemistry, easy bioconjugation and very low toxicity[87,91-96]. Among the various type of gold nanoparticles, the most studied for molecular optoacoustic imaging applications are nanorods[87,97-103], which are of particular interest since of their high tendency to accumulate in tumors[24] and their potential for simultaneous photothermal therapy[104].
As mentioned before, solid nanoparticles exhibit both higher ultrasound signal enhancement and longer stability as compared to liquid and gaseous particles of the same size. Nevertheless, ultrasound experiments carried out so far on solid nanoparticles at very high frequencies (30-40 MHz)[72,73] have limited clinical usefulness.
We have recently demonstrated that silica nanospheres are effective ultrasound contrast agents already at common diagnostic frequencies, and quantified the contrast enhancement observed as a function of particle concentration and diameter, in a range of clinical usefulness for tumor targeting purposes[70].
Diagnostic power of silica nanospheres of three different diameters (160 nm, 330 nm and 660 nm) was evaluated by measuring ultrasound backscatter in agarose phantoms containing nanoparticles at concentrations ranging from 1010 to 1013 part/mL. Imaging was performed with a digital echograph equipped with a linear transducer operating at 7.5 MHz and linked to a prototype platform for acquisition of unprocessed radiofrequency (RF) data.
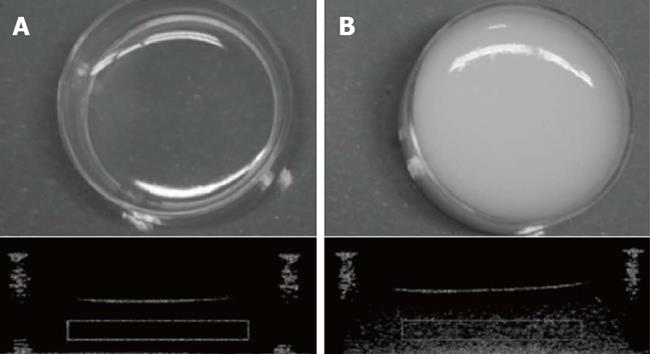
Quantitative off-line analyses showed that while amplitude of nanoparticle-backscattered signals did increase as a linear function of particle concentration, image brightness did not because of saturation effects. However, when nanoparticle diameter, instead of concentration, was increased both backscatter amplitude and image brightness showed significant increments. Taking into account the previously discussed particle size characteristics for effective endothelial crossing and tumor targeting, the best combination was found to be the sample containing 330 nm silica nanospheres at a concentration of about 1 to 2 × 1011 part/mL[70]. Figure 1 shows a typical picture, with the corresponding echographic image, of agarose sample containing 330 nm silica nanoparticles at 2 × 1011 part/mL concentration.
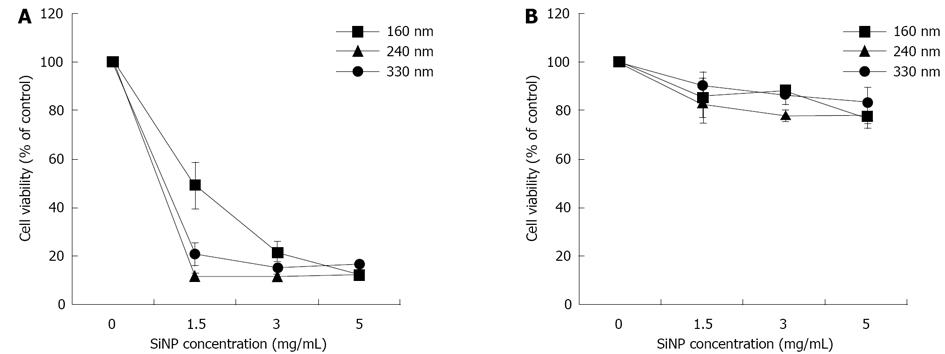
Among the characteristics considered basic for any NPCA to be suitable for clinical molecular imaging, their biocompatibility and effective target recognition are without doubt of major importance. In a recent paper[105] we have evaluated the cytotoxicity of silica nanospheres of different diameters (160 nm, 240 nm and 330 nm) on two different tumor cell lines, namely MCF-7 cells (breast cancer) and HeLa cells (cervical cancer). Moreover, since sometimes polymeric coating of nanoparticle surface may affect significantly their biocompatibility as well as other parameters, we have synthesized and tested both uncoated and Methoxy (polyethyleneoxy) propyltrimethoxysilane (PEG)-coated silica nanospheres. Acoustic behavior of coated and uncoated particles was also investigated. The results obtained, summarized in Figure 2, showed that the incubation of MCF-7 cells with increasing concentration (up to 5 mg/mL) of uncoated silica nanospheres over 72 h caused a remarkable cytotoxicity, which was dependent on nanoparticle diameter, concentration and incubation time, reaching percentages of cell mortality close to 80%. Conversely, in the experiments carried out using PEG-coated silica nanospheres cell viability was only slightly affected, with percentage of cell mortality lower than 30% (considered as threshold value of cytotoxicity by ISO 10993-5 international guide) at any time and at any particle concentration and diameter. Comparable results were obtained when HeLa, instead of MCF-7, cells were assayed.
Acoustic behavior of these nanoparticles was characterized exactly as described above and gave results in good agreement with those already obtained. Interestingly, at the same concentrations, 240 nm nanospheres exhibited ultrasound backscattered signals even slightly stronger than 330 nm nanoparticles, this ensuring a good contrast enhancement together with a more effective targeting potential since of their lower diameter.
Work is in progress in our laboratory aimed at functionalizing 240 nm silica nanoparticles incorporating a fluorescent probe for “in vitro” molecular imaging of hepatocellular carcinoma (HCC), with both ultrasound and laser-scanning confocal microscopy. HCC is the most common among all liver cancer cases (around 75%)[106], and is characterized by the particular feature to express on its cell surface Glypican-3 protein (GPC-3) which, therefore, is a good candidate for specific targeting of HCC cells[107]. On the basis of recent findings by Lee et al[108] demonstrating that a seven amino acid peptide exhibit high affinity in GPC-3 recognizing and binding, we have synthesized GPC-3 peptide-functionalized 240 nm fluorescent silica nanoparticles and tested them on HepG2 cells, a GPC-3 positive human hepatocarcinoma cell line. Interestingly, preliminary results show that, at concentration useful for ultrasound detection, GPC-3-targeted silica nanoparticles exhibit only negligible cytotoxic effects and seem to effectively bind to HepG2 cell plasmamembrane, as revealed by confocal microscopy and transmission electron microscopy. These results, which however require be further substantiating by parallel experiments on GPC-3 negative cells and, more importantly, confirming also “in vivo”, indicate that 240 nm silica nanoparticles might be a very promising theranostic agents since of their high biocompatibility, targeting effectiveness and acoustic behavior.
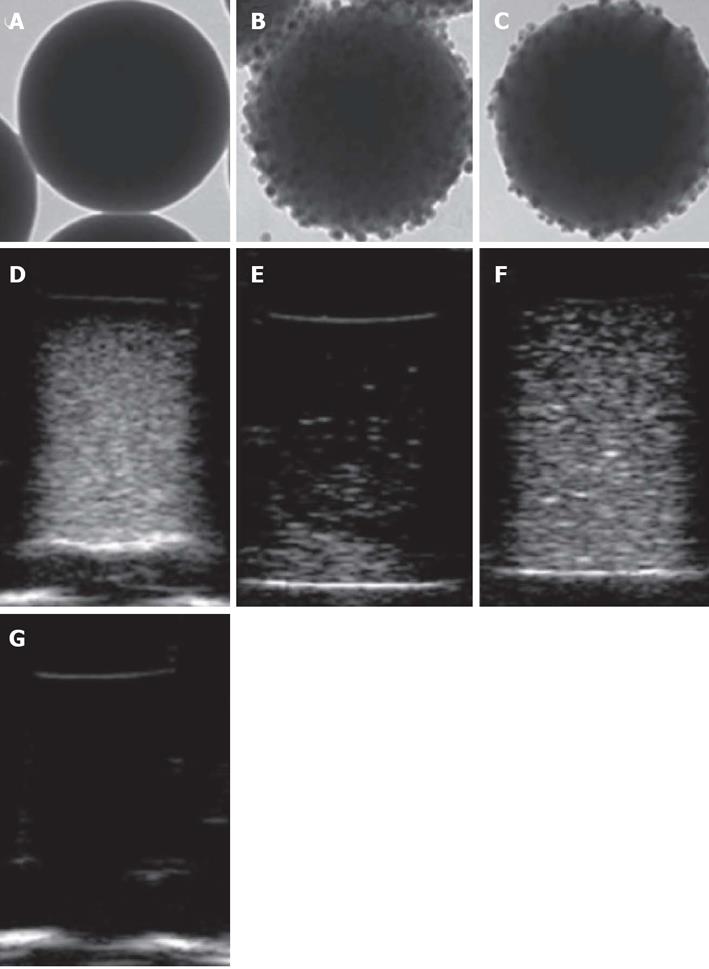
As mentioned in previous paragraphs, our interest in exploring the employability of silica nanoparticles as effective NPCAs was extended to the possibility of designing novel silica-based hybrid nanocomposites for dual-mode molecular imaging, combining MRI and ultrasounds. At this regard, we have developed a simple and efficient synthesis protocol for multi-component nanoparticles having a spherical silica core (160 nm, 330 nm or 660 nm in diameter) coated with an outer shell of smaller superparamagnetic nanoparticles, represented by either 15-nm FeO or 17-nm FePt-FeO nanocrystals[21,109].
To evaluate the potential of these nanocomposites as MRI contrast agents, proton relaxivity measurements were performed at three radio frequency (RF) frequencies: 12.5, 23 and 60 MHz. Both the transversal relaxivity r2 and the longitudinal relaxivity r1 values were calculated for the different silica host nanospheres covered by IO or FePt-IO nanoparticles. As the ratio r2/r1 was greater than 2, all the synthesized systems were classified as good T2-relaxing systems. In particular, for each employed RF frequency and SiNP-core diameter, the r2/r1 ratios of FePt-IO coated SiNPs were higher than those of IO coated SiNPs, indicating that FePt-IO-coated SiNPs are more efficient as MRI negative contrast agents with respect to IO coated SiNPs[110].
Ultrasound measurements were carried out on silica nanospheres dispersed in agarose gel samples, with the employment of a 10-MHz incident ultrasound frequency. As shown in Figure 3, all the nanoparticle-containing phantoms exhibited a clear image enhancement with respect to the pure agarose gel, that was almost completely transparent to ultrasound. Among the three nanoparticle type tested, uncoated silica nanospheres provided the highest image brightness for each considered size, as compared to IO-coated silica nanospheres, whereas FePt-IO nanocrystals showed image enhancements qualitatively analogous to those of pure silica but with a slightly less uniform brightness.
Therefore, the acoustic and magnetic characterization of coated SiNSs shows that FePt-IO, rather than IO, seems to be the best magnetic coating for realizing NPCAs suitable for dual mode molecular imaging through US and MRI techniques.
Nanostructured aluminosilicates are other new materials of particular interest for their potential medical applications. In particular, halloysite clay is a double-layered aluminosilicate spontaneously forming empty tubular structures in the submicrometer range. They size 1 ± 0.5 µm in length, 50 to 70 nm in external diameter and around 15 nm diameter lumen, and are capable of entrapping a wide variety of active agents in the inner lumen, followed by their retention and slow release[111-119]. Moreover, owing to their easy surface functionalization[120] as well as high level of biocompatibility[121], halloysite clay nanotubes (HNTs) present an ideal profile for cell targeting and drug delivery purposes. In fact, HNTs have been recently demonstrated to be successful in intracellular delivery of antisense oligonucleotides[122]. Furthermore, Resveratrol-loaded HNTs have been shown to effectively promote apoptotic cell death in MCF-7 breast cancer cell line[112]. It is then conceivable that therapeutic protocols involving HNTs may take enormous advantage from the possibility of monitoring them through non-invasive imaging techniques. On the basis of these considerations, we have recently explored the feasibility of using HNTs as ultrasound contrast agents for clinical echographic imaging.
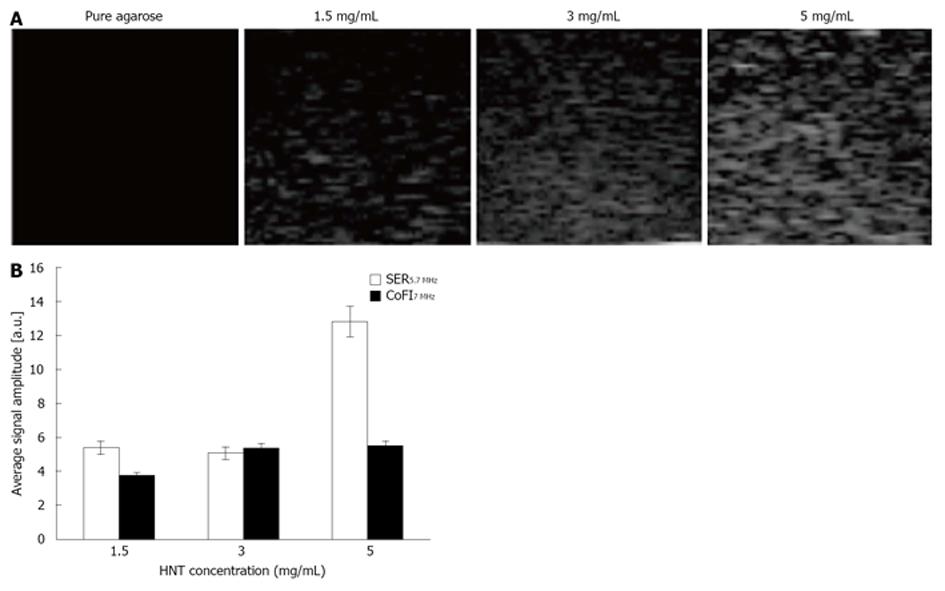
HNT at different concentrations (1.5, 3 and 5 mg/mL) were dispersed in agarose gel and imaged through a commercially available echographic system, employing conventional ultrasonic frequencies (5.7-7 MHz) at an intermediate level of power (50%) of the signal emitted by clinical equipment (Figure 4A).
Acquired data were processed through a dedicated prototypal platform for ultrasonic signal amplitude extraction. The signal enhancement ratio (SER) was calculated between different values of HNT concentration at the considered echographic frequency; additionally, the contribution of frequency increment (CoFI) to the image backscatter was also quantified (Figure 4B). The average contribution of frequency increment from 5.7 to 7 MHz was found to be 4.86 ± 0.80 (corresponding to about 20%), indicating that the increasing HNT concentration determined a nonlinear increment of absolute SER. Hence, it might be useful to study a wider range of HNT concentration in order to achieve safe and effective dose optimization in future clinical application.
Recent progresses in the field of nanotechnology applied to medical diagnostic imaging are overcoming most of the constraints offered by classical clinical approaches: molecular imaging without using ionizing techniques, early diagnosis of major social diseases, targeted tissue local therapies instead of systemic approaches, etc. Echography and ultrasonography provided so far, in the research arena, one of the most promising result by supporting very interesting future clinical perspectives for both diagnosis and therapies still presenting the above mentioned limitations.
Several research applications unveiled many classes of novel nanosystems as effective “theranostic” agents based on both organic and inorganic components. For ultrasound cellular applications the latter certainly offer a wide range of advantages in terms of contrast enhancement, drug loading capabilities, highly effective cell targeting even making possible gene therapy approaches at very low costs.
Nevertheless, many challenges need to be faced in order to translate in clinics those research findings, mainly related to classical difficulties faced by all new drug development steps prior to reach the human clinical trials with additional incognita for the new physical features of novel nano-materials and their eventual toxicity.
Nowadays, our society is experiencing a rapid evolution in terms of population aging, social dynamic modifications accompanied by significant cost reductions in government spending. The real challenge for modern medicine is offering higher medical standards at reduced costs: this ideal objective is not reachable relying on actual classical approaches, but that can be done only pushing medical research toward the new frontiers made feasible by nanotheranostics and nanomedicines, whose main potentialities and challenges still remain unexpressed and unexplored.
P- Reviewer: Chen K, Mani V S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Liu Y, Miyoshi H, Nakamura M. Nanomedicine for drug delivery and imaging: a promising avenue for cancer therapy and diagnosis using targeted functional nanoparticles. Int J Cancer. 2007;120:2527-2537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 426] [Cited by in F6Publishing: 357] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 2. | Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19:311-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1369] [Cited by in F6Publishing: 1078] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 3. | Gewin V. Big opportunities in a small world. Nature. 2009;460:540-541. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Caruthers SD, Wickline SA, Lanza GM. Nanotechnological applications in medicine. Curr Opin Biotechnol. 2007;18:26-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 5. | Casciaro S. Theranostic applications: Non-ionizing cellular and molecular imaging through innovative nanosystems for early diagnosis and therapy. World J Radiol. 2011;3:249-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Pascali G, Conversano F, Casciaro S, Salvadori PA. [Translational perspectives in molecular imaging: methodological evolution and nanostructured materials]. Recenti Prog Med. 2012;103:142-153. [PubMed] [Cited in This Article: ] |
| 7. | Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219:316-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1152] [Cited by in F6Publishing: 954] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 8. | Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818-1822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3286] [Cited by in F6Publishing: 2890] [Article Influence: 144.5] [Reference Citation Analysis (0)] |
| 9. | Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283-318. [PubMed] [Cited in This Article: ] |
| 10. | Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discovery Today. 2003;8:1112-1120. [DOI] [Cited in This Article: ] [Cited by in Crossref: 742] [Cited by in F6Publishing: 546] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 11. | Krämer M, Stumbé JF, Grimm G, Kaufmann B, Krüger U, Weber M, Haag R. Dendritic polyamines: simple access to new materials with defined treelike structures for application in nonviral gene delivery. Chembiochem. 2004;5:1081-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Raja KS, Wang Q, Gonzalez MJ, Manchester M, Johnson JE, Finn MG. Hybrid virus-polymer materials. 1. Synthesis and properties of PEG-decorated cowpea mosaic virus. Biomacromolecules. 2003;4:472-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Fenske DB, MacLachlan I, Cullis PR. Long-circulating vectors for the systemic delivery of genes. Curr Opin Mol Ther. 2001;3:153-158. [PubMed] [Cited in This Article: ] |
| 14. | Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2:750-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1224] [Cited by in F6Publishing: 1080] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 15. | Sudimack J, Lee RJ. Targeted drug delivery via the folate receptor. Advanced Drug Delivery Reviews. 2000;41:147-162. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1104] [Cited by in F6Publishing: 955] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 16. | Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proc Natl Acad Sci USA. 2003;100:6039-6044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 433] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 17. | Drummond DC, Zignani M, Leroux JC. Current status of pH-sensitive liposomes in drug delivery. Progress in Lipid Research. 2000;39:409-460. [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 383] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 18. | Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 2002;16:1217-1226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 777] [Cited by in F6Publishing: 724] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 19. | Clark HA, Hoyer M, Philbert MA, Kopelman R. Optical nanosensors for chemical analysis inside single living cells. 1. Fabrication, characterization, and methods for intracellular delivery of PEBBLE sensors. Anal Chem. 1999;71:4831-4836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 299] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Wickline SA, Neubauer AM, Winter P, Caruthers S, Lanza G. Applications of nanotechnology to atherosclerosis, thrombosis, and vascular biology. Arterioscler Thromb Vasc Biol. 2006;26:435-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Malvindi MA, Greco A, Conversano F, Figuerola A, Corti M, Bonora M, Lascialfari A, Doumari HA, Moscardini M, Cingolani R. Magnetic/silica nanocomposites as dual-mode contrast agents for combined magnetic resonance imaging and ultrasonography. Advanced Functional Materials. 2011;21:2548-2555. [DOI] [Cited in This Article: ] |
| 22. | Wickline SA, Neubauer AM, Winter PM, Caruthers SD, Lanza GM. Molecular imaging and therapy of atherosclerosis with targeted nanoparticles. J Magn Reson Imaging. 2007;25:667-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Conversano F, Casciaro S. Last advances in ultrasound molecular imaging. New technology frontiers in minimally invasive therapies. Lecce (Italy): Lupiensis Biomedical Publications 2007; 161-171. [Cited in This Article: ] |
| 24. | Puvanakrishnan P, Park J, Chatterjee D, Krishnan S, Tunnell JW. In vivo tumor targeting of gold nanoparticles: effect of particle type and dosing strategy. Int J Nanomedicine. 2012;7:1251-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Fahmy TM, Samstein RM, Harness CC, Mark Saltzman W. Surface modification of biodegradable polyesters with fatty acid conjugates for improved drug targeting. Biomaterials. 2005;26:5727-5736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 26. | Couvreur P, Barratt G, Fattal E, Legrand P, Vauthier C. Nanocapsule technology: a review. Crit Rev Ther Drug Carrier Syst. 2002;19:99-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 282] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 27. | Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1876] [Cited by in F6Publishing: 1590] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 28. | Hahn MA, Singh AK, Sharma P, Brown SC, Moudgil BM. Nanoparticles as contrast agents for in-vivo bioimaging: current status and future perspectives. Anal Bioanal Chem. 2011;399:3-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 276] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 29. | Wickline SA, Lanza GM. Nanotechnology for molecular imaging and targeted therapy. Circulation. 2003;107:1092-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Lanza GM, Wickline SA. Targeted ultrasonic contrast agents for molecular imaging and therapy. Curr Probl Cardiol. 2003;28:625-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Tsien RY. Imagining imaging’s future. Nat Rev Mol Cell Biol. 2003;Suppl:SS16-SS21. [PubMed] [Cited in This Article: ] |
| 32. | Herschman HR. Molecular imaging: looking at problems, seeing solutions. Science. 2003;302:605-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 306] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 33. | Hawker CJ, Wooley KL. The convergence of synthetic organic and polymer chemistries. Science. 2005;309:1200-1205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1170] [Cited by in F6Publishing: 1022] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 34. | Pasqualini R, Arap W, McDonald DM. Probing the structural and molecular diversity of tumor vasculature. Trends in Molecular Medicine. 2002;8:563-571. [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95:4607-4612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1791] [Cited by in F6Publishing: 1601] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 36. | Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1332] [Cited by in F6Publishing: 1241] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 37. | Demos SM, Alkan-Onyuksel H, Kane BJ, Ramani K, Nagaraj A, Greene R, Klegerman M, McPherson DD. In vivo targeting of acoustically reflective liposomes for intravascular and transvascular ultrasonic enhancement. JACC. 1999;33:867-875. [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 191] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 38. | Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat Med. 1998;4:623-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 695] [Cited by in F6Publishing: 725] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 39. | Marsh JN, Partlow KC, Abendschein DR, Scott MJ, Lanza GM, Wickline SA. Molecular imaging with targeted perfluorocarbon nanoparticles: quantification of the concentration dependence of contrast enhancement for binding to sparse cellular epitopes. Ultrasound Med Biol. 2007;33:950-958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Lanza GM, Winter P, Caruthers S, Schmeider A, Crowder K, Morawski A, Zhang H, Scott MJ, Wickline SA. Novel paramagnetic contrast agents for molecular imaging and targeted drug delivery. Curr Pharm Biotechnol. 2004;5:495-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Flacke S, Fischer S, Scott MJ, Fuhrhop RJ, Allen JS, McLean M, Winter P, Sicard GA, Gaffney PJ, Wickline SA. Novel MRI contrast agent for molecular imaging of fibrin: implications for detecting vulnerable plaques. Circulation. 2001;104:1280-1285. [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 428] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 42. | Schmitz SA, Coupland SE, Gust R, Winterhalter S, Wagner S, Kresse M, Semmler W, Wolf KJ. Superparamagnetic iron oxide-enhanced MRI of atherosclerotic plaques in Watanabe hereditable hyperlipidemic rabbits. Invest Radiol. 2000;35:460-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 197] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 43. | Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA. 2003;100:13549-13554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3043] [Cited by in F6Publishing: 2245] [Article Influence: 106.9] [Reference Citation Analysis (0)] |
| 44. | Loo C, Lin A, Hirsch L, Lee MH, Barton J, Halas N, West J, Drezek R. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol Cancer Res Treat. 2004;3:33-40. [PubMed] [Cited in This Article: ] |
| 45. | O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209:171-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1410] [Cited by in F6Publishing: 1470] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 46. | Cherukuri P, Bachilo SM, Litovsky SH, Weisman RB. Near-infrared fluorescence microscopy of single-walled carbon nanotubes in phagocytic cells. J Am Chem Soc. 2004;126:15638-15639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 679] [Cited by in F6Publishing: 467] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 47. | Tsyboulski DA, Bachilo SM, Weisman RB. Versatile visualization of individual single-walled carbon nanotubes with near-infrared fluorescence microscopy. Nano Lett. 2005;5:975-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Barone PW, Baik S, Heller DA, Strano MS. Near-infrared optical sensors based on single-walled carbon nanotubes. Nat Mater. 2005;4:86-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 816] [Cited by in F6Publishing: 497] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 49. | Hertel T, Hagen A, Talalaev V, Arnold K, Hennrich F, Kappes M, Rosenthal S, McBride J, Ulbricht H, Flahaut E. Spectroscopy of single- and double-wall carbon nanotubes in different environments. Nano Lett. 2005;5:511-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Akerman ME, Chan WC, Laakkonen P, Bhatia SN, Ruoslahti E. Nanocrystal targeting in vivo. Proc Natl Acad Sci USA. 2002;99:12617-12621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1117] [Cited by in F6Publishing: 1151] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 51. | Chen L, Zurita AJ, Ardelt PU, Giordano RJ, Arap W, Pasqualini R. Design and validation of a bifunctional ligand display system for receptor targeting. Chem Biol. 2004;11:1081-1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Gao X, Nie S. Quantum dot-encoded beads. Meth Mol Biol. 2005;303:61-71. [Cited in This Article: ] |
| 53. | Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6698] [Cited by in F6Publishing: 4751] [Article Influence: 250.1] [Reference Citation Analysis (0)] |
| 54. | Corot C, Robert P, Idée JM, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev. 2006;58:1471-1504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1143] [Cited by in F6Publishing: 919] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 55. | Semelka RC, Helmberger TK. Contrast agents for MR imaging of the liver. Radiology. 2001;218:27-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 268] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 56. | Alric C, Taleb J, Le Duc G, Mandon C, Billotey C, Le Meur-Herland A, Brochard T, Vocanson F, Janier M, Perriat P. Gadolinium chelate coated gold nanoparticles as contrast agents for both X-ray computed tomography and magnetic resonance imaging. J Am Chem Soc. 2008;130:5908-5915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 352] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 57. | Figuerola A, Fiore A, Di Corato R, Falqui A, Giannini C, Micotti E, Lascialfari A, Corti M, Cingolani R, Pellegrino T. One-pot synthesis and characterization of size-controlled bimagnetic FePt-iron oxide heterodimer nanocrystals. J Am Chem Soc. 2008;130:1477-1487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 58. | Yang H, Zhao F, Li Y, Xu M, Li L, Wu C, Miyoshi H, Liu Y. VCAM-1-targeted core/shell nanoparticles for selective adhesion and delivery to endothelial cells with lipopolysaccharide-induced inflammation under shear flow and cellular magnetic resonance imaging in vitro. Int J Nanomedicine. 2013;8:1897-1906. [PubMed] [Cited in This Article: ] |
| 59. | Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2012. Available from: http: //www.ncbi.nlm.nih.gov/books/NBK5330/. [Cited in This Article: ] |
| 60. | Kaufmann BA, Lindner JR. Molecular imaging with targeted contrast ultrasound. Curr Opin Biotechnol. 2007;18:11-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 61. | Kindberg GM, Tolleshaug H, Roos N, Skotland T. Hepatic clearance of Sonazoid perfluorobutane microbubbles by Kupffer cells does not reduce the ability of liver to phagocytose or degrade albumin microspheres. Cell Tissue Res. 2003;312:49-54. [PubMed] [Cited in This Article: ] |
| 62. | Bryant TH, Blomley MJ, Albrecht T, Sidhu PS, Leen EL, Basilico R, Pilcher JM, Bushby LH, Hoffmann CW, Harvey CJ. Improved characterization of liver lesions with liver-phase uptake of liver-specific microbubbles: prospective multicenter study. Radiology. 2004;232:799-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 63. | Willmann JK, Lutz AM, Paulmurugan R, Patel MR, Chu P, Rosenberg J, Gambhir SS. Dual-targeted contrast agent for US assessment of tumor angiogenesis in vivo. Radiology. 2008;248:936-944. [PubMed] [Cited in This Article: ] |
| 64. | Palmowski M, Huppert J, Ladewig G, Hauff P, Reinhardt M, Mueller MM, Woenne EC, Jenne JW, Maurer M, Kauffmann GW. Molecular profiling of angiogenesis with targeted ultrasound imaging: early assessment of antiangiogenic therapy effects. Mol Cancer Ther. 2008;7:101-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 65. | Palmowski M, Peschke P, Huppert J, Hauff P, Reinhardt M, Maurer M, Karger CP, Scholz M, Semmler W, Huber PE. Molecular ultrasound imaging of early vascular response in prostate tumors irradiated with carbon ions. Neoplasia. 2009;11:856-863. [PubMed] [Cited in This Article: ] |
| 66. | Lutz AM, Bachawal SV, Drescher CW, Pysz MA, Willmann JK, Gambhir SS. Ultrasound molecular imaging in a human CD276 expression-modulated murine ovarian cancer model. Clin Cancer Res. 2014;20:1313-1322. [PubMed] [Cited in This Article: ] |
| 67. | Negishi Y, Hamano N, Tsunoda Y, Oda Y, Choijamts B, Endo-Takahashi Y, Omata D, Suzuki R, Maruyama K, Nomizu M. AG73-modified Bubble liposomes for targeted ultrasound imaging of tumor neovasculature. Biomaterials. 2013;34:501-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | Díaz-López R, Tsapis N, Fattal E. Liquid perfluorocarbons as contrast agents for ultrasonography and (19)F-MRI. Pharm Res. 2010;27:1-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 69. | Yin T, Wang P, Zheng R, Zheng B, Cheng D, Zhang X, Shuai X. Nanobubbles for enhanced ultrasound imaging of tumors. Int J Nanomedicine. 2012;7:895-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 70. | Casciaro S, Conversano F, Ragusa A, Malvindi MA, Franchini R, Greco A, Pellegrino T, Gigli G. Optimal enhancement configuration of silica nanoparticles for ultrasound imaging and automatic detection at conventional diagnostic frequencies. Invest Radiol. 2010;45:715-724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 71. | Conversano F, Greco A, Casciaro E, Ragusa A, Lay-Ekuakille A, Casciaro S. Harmonic ultrasound imaging of nanosized contrast agents for multimodal molecular diagnoses. IEEE Trans Instrum Meas. 2012;61:1848-1856. [DOI] [Cited in This Article: ] |
| 72. | Liu J, Levine AL, Mattoon JS, Yamaguchi M, Lee RJ, Pan X, Rosol TJ. Nanoparticles as image enhancing agents for ultrasonography. Phys Med Biol. 2006;51:2179-2189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 73. | Liu J, Li J, Rosol TJ, Pan X, Voorhees JL. Biodegradable nanoparticles for targeted ultrasound imaging of breast cancer cells in vitro. Phys Med Biol. 2007;52:4739-4747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | Jin Y, Kannan S, Wu M, Zhao JX. Toxicity of luminescent silica nanoparticles to living cells. Chem Res Toxicol. 2007;20:1126-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 226] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 75. | BarbIJ C, Bartlett J, Kong L, Finnie K, Lin HQ, Larkin M, Calleja S, Bush A, Calleja G. Silica particles: a novel drug-delivery system. Adv Funct Mater. 2004;16:1959-1966. [DOI] [Cited in This Article: ] |
| 76. | Piao Y, Burns A, Kim J, Wiesner U, Hyeon , T . Designed fabrication of silica-based nanostructured particle systems for nanomedicine applications. Adv Funct Mater. 2008;18:3745-3758. [DOI] [Cited in This Article: ] |
| 77. | Moulari B, Pertuit D, Pellequer Y, Lamprecht A. The targeting of surface modified silica nanoparticles to inflamed tissue in experimental colitis. Biomaterials. 2008;29:4554-4560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 78. | Roy I, Ohulchanskyy TY, Bharali DJ, Pudavar HE, Mistretta RA, Kaur N, Prasad PN. Optical tracking of organically modified silica nanoparticles as DNA carriers: a nonviral, nanomedicine approach for gene delivery. Proc Natl Acad Sci USA. 2005;102:279-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 289] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 79. | Liu L, Yong KT, Roy I, Law WC, Ye L, Liu J, Liu J, Kumar R, Zhang X, Prasad PN. Bioconjugated pluronic triblock-copolymer micelle-encapsulated quantum dots for targeted imaging of cancer: in vitro and in vivo studies. Theranostics. 2012;2:705-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 80. | Poulose AC, Veeranarayanan S, Mohamed MS, Raveendran S, Nagaoka Y, Yoshida Y, Maekawa T, Kumar DS. PEG coated biocompatible cadmium chalcogenide quantum dots for targeted imaging of cancer cells. J Fluoresc. 2012;22:931-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Winkler AM, Rice PF, Drezek RA, Barton JK. Quantitative tool for rapid disease mapping using optical coherence tomography images of azoxymethane-treated mouse colon. J Biomed Opt. 2010;15:041512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Avti PK, Sitharaman B. Luminescent single-walled carbon nanotube-sensitized europium nanoprobes for cellular imaging. Int J Nanomedicine. 2012;7:1953-1964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Wang LV. Multiscale photoacoustic microscopy and computed tomography. Nat Photonics. 2009;3:503-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1063] [Cited by in F6Publishing: 728] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 84. | Oraevsky AA, Karabutov AA. Optoacoustic tomography. Biomedical Photonics Handbook. Boca Raton: CRC Press/Francis and Taylor Group 2003; . [Cited in This Article: ] |
| 85. | Ermilov SA, Khamapirad T, Conjusteau A, Leonard MH, Lacewell R, Mehta K, Miller T, Oraevsky AA. Laser optoacoustic imaging system for detection of breast cancer. J Biomed Opt. 2009;14:024007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 276] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 86. | Lu W, Huang Q, Ku G, Wen X, Zhou M, Guzatov D, Brecht P, Su R, Oraevsky A, Wang LV. Photoacoustic imaging of living mouse brain vasculature using hollow gold nanospheres. Biomaterials. 2010;31:2617-2626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 87. | Song KH, Kim C, Maslov K, Wang LV. Noninvasive in vivo spectroscopic nanorod-contrast photoacoustic mapping of sentinel lymph nodes. Eur J Radiol. 2009;70:227-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 88. | Mallidi S, Larson T, Tam J, Joshi PP, Karpiouk A, Sokolov K, Emelianov S. Multiwavelength photoacoustic imaging and plasmon resonance coupling of gold nanoparticles for selective detection of cancer. Nano Lett. 2009;9:2825-2831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 274] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 89. | Li ML, Wang JC, Schwartz JA, Gill-Sharp KL, Stoica G, Wang LV. In-vivo photoacoustic microscopy of nanoshell extravasation from solid tumor vasculature. J Biomed Opt. 2009;14:010507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 90. | Kim C, Cho EC, Chen J, Song KH, Au L, Favazza C, Zhang Q, Cobley CM, Gao F, Xia Y. In vivo molecular photoacoustic tomography of melanomas targeted by bioconjugated gold nanocages. ACS Nano. 2010;4:4559-4564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 366] [Cited by in F6Publishing: 295] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 91. | Jain PK, El-Sayed IH, El-Sayed MA. Au nanoparticles target cancer. Nano Today. 2007;2:18-29. [DOI] [Cited in This Article: ] |
| 92. | Pan D, Pramanik M, Senpan A, Wickline SA, Wang LV, Lanza GM. A facile synthesis of novel self-assembled gold nanorods designed for near-infrared imaging. JNN. 2010;10:8118-8123. [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 93. | Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38:1759-1782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2191] [Cited by in F6Publishing: 1713] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 94. | Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1855] [Cited by in F6Publishing: 1534] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 95. | Cai QY, Kim SH, Choi KS, Kim SY, Byun SJ, Kim KW, Park SH, Juhng SK, Yoon KH. Colloidal gold nanoparticles as a blood-pool contrast agent for X-ray computed tomography in mice. Invest Radiol. 2007;42:797-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 96. | Chen YS, Hung YC, Liau I, Huang GS. Assessment of the In Vivo Toxicity of Gold Nanoparticles. Nanoscale Res Lett. 2009;4:858-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 470] [Cited by in F6Publishing: 392] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 97. | Eghtedari M, Oraevsky A, Copland JA, Kotov NA, Conjusteau A, Motamedi M. High sensitivity of in vivo detection of gold nanorods using a laser optoacoustic imaging system. Nano Lett. 2007;7:1914-1918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 98. | Ha S, Carson A, Agarwal A, Kotov NA, Kim K. Detection and monitoring of the multiple inflammatory responses by photoacoustic molecular imaging using selectively targeted gold nanorods. Biomed Opt Express. 2011;2:645-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Agarwal A, Huang SW, O’Donnel M, Day KC, Day M, Kotov N, Ashkenazi S. Targeted gold nanorod contrast agent for prostate cancer detection by photoacoustic imaging. J Appl Phys. 2007;102:064701. [DOI] [Cited in This Article: ] |
| 100. | Li PC, Wei CW, Liao CK, Chen CD, Pao KC, Wang CRC, Wu TN, Shieh DB. Photoacoustic imaging of multiple targets using gold nanorods. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54:1642-1647. [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 101. | Kim K, Huang SW, Ashkenazi S, O’Donnell M, Agarwal A, Kotov NA, Denny MF, Kaplan MJ. Photoacoustic imaging of early inflammatory response using gold nanorods. Applied Physics Letters. 2007;90:223901-223903. [DOI] [Cited in This Article: ] |
| 102. | Li PC, Wang CR, Shieh DB, Wei CW, Liao CK, Poe C, Jhan S, Ding AA, Wu YN. In vivo photoacoustic molecular imaging with simultaneous multiple selective targeting using antibody-conjugated gold nanorods. Opt Express. 2008;16:18605-18615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 103. | Conversano F, Soloperto G, Greco A, Ragusa A, Casciaro E, Chiriacò F, Demitri C, Gigli G, Maffezzoli A, Casciaro S. Echographic detectability of optoacoustic signals from low-concentration PEG-coated gold nanorods. Int J Nanomedicine. 2012;7:4373-4389. [PubMed] [Cited in This Article: ] |
| 104. | Samim M, Prashant CK, Dinda AK, Maitra AN, Arora I. Synthesis and characterization of gold nanorods and their application for photothermal cell damage. Int J Nanomedicine. 2011;6:1825-1831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 105. | Chiriac? F, Conversano F, Soloperto G, Casciaro E, Ragusa A, Sbenaglia EA, Dipaola L, Casciaro S. Epithelial cells biocompatibility of silica nanospheres for contrast-enhanced ultrasound molecular imaging. J Nanopart Research. 2013;15:1779-1792. [DOI] [Cited in This Article: ] |
| 106. | Ahn BC, Ronald JA, Kim YI, Katzenberg R, Singh A, Paulmurugan R, Ray S, Hofmann LV, Gambhir SS. Potent, tumor-specific gene expression in an orthotopic hepatoma rat model using a Survivin-targeted, amplifiable adenoviral vector. Gene Ther. 2011;18:606-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 107. | Ho M, Kim H. Glypican-3: a new target for cancer immunotherapy. Eur J Cancer. 2011;47:333-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 108. | Lee YL, Ahn BC, Lee Y, Lee SW, Cho JY, Lee J. Targeting of hepatocellular carcinoma with glypican-3-targeting peptide ligand. J Pept Sci. 2011;17:763-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 109. | Casciaro S, Soloperto G, Greco A, Casciaro E, Franchini R, Conversano F. Effectiveness of functionalized nanosystems for multimodal molecular sensing and imaging in medicine. IEEE Sens J. 2013;13:2305-2312. [DOI] [Cited in This Article: ] |
| 110. | Chiriacò F, Soloperto G, Greco A, Conversano F, Ragusa A, Menichetti L, Casciaro S. Magnetically-coated silica nanospheres for dual-mode imaging at low ultrasound frequency. World J Radiol. 2013;5:411-420. [PubMed] [Cited in This Article: ] |
| 111. | Price RR, Gaber BP, Lvov Y. In-vitro release characteristics of tetracycline HCl, khellin and nicotinamide adenine dineculeotide from halloysite; a cylindrical mineral. J Microencapsul. 2001;18:713-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 240] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 112. | Vergaro V, Lvov YM, Leporatti S. Halloysite clay nanotubes for resveratrol delivery to cancer cells. Macromol Biosci. 2012;12:1265-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 113. | Kelly HM, Deasy PB, Ziaka E, Claffey N. Formulation and preliminary in vivo dog studies of a novel drug delivery system for the treatment of periodontitis. Int J Pharm. 2004;274:167-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 114. | Veerabadran NG, Price RR, Lvov YM. Clay nanotubes for encapsulation and sustained release of drugs. Nano. 2007;2:115-120. [DOI] [Cited in This Article: ] |
| 115. | Lvov YM, Shchukin DG, Möhwald H, Price RR. Halloysite clay nanotubes for controlled release of protective agents. ACS Nano. 2008;2:814-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 769] [Cited by in F6Publishing: 335] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 116. | Veerabadran NG, Mongayt D, Torchilin V, Price RR, Lvov YM. Organized shells on clay nanotubes for controlled release of macromolecules. Macromol Rapid Commun. 2009;30:99-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 117. | Kommireddy D, Ichinose I, Lvov Y, Mills D. Nanoparticle multilayer: surface modification for cell attachment and growth. J Biomed Nanotechnol. 2005;1:286-290. [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 118. | Viseras MT, Aguzzi C, Cerezo P, Cultrone G, Viseras C. Supramolecular structure of 5-aminosalycilic acid/halloysite composites. J Microencapsul. 2009;26:279-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 119. | Soloperto G, Conversano F, Greco A, Casciaro E, Ragusa A, Leporatti S, Lay-Ekuakille A, Casciaro S; Multiparametric evaluation of the acoustic behaviour of halloysite nanotubes for medical echographic image enhancement. IEEE Trans Instrum Meas. 2014;In press. [Cited in This Article: ] |
| 120. | Duartel AH, Lourenco MP, Heine T, Guimares L. Clay mineral nanotubes: stability, structure and properties. Vukovar, Croatia: InTech 2012; . [Cited in This Article: ] |
| 121. | Vergaro V, Abdullayev E, Lvov YM, Zeitoun A, Cingolani R, Rinaldi R, Leporatti S. Cytocompatibility and uptake of halloysite clay nanotubes. Biomacromolecules. 2010;11:820-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 497] [Cited by in F6Publishing: 326] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 122. | Shi YF, Tian Z, Zhang Y, Shen HB, Jia NQ. Functionalized halloysite nanotube-based carrier for intracellular delivery of antisense oligonucleotides. Nanoscale Res Lett. 2011;6:608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |