INTRODUCTION
Pulmonary arteriovenous malformations (PAVM) are congenital low pressure right-to-left shunts in the pulmonary vasculature bypassing the capillaries and thus causing decreased oxygenation of the arterial blood and reduced filtering of the blood. Most PAVM are high-flow shunts but a few are not and may consequently go undetected for a long period. Furthermore, PAVM patients can be asymptomatic because they have had their malformations for several years but will typically present with hypoxaemia and polycythaemia. Thus, they often have exercise intolerance, cyanosis and drumstick fingers. In addition, they have an increased risk of paradoxical emboli to the systemic circulation and subsequent infarctions and abscesses in the organs, especially the brain. Severe neurological events such as transitory cerebral ischaemia, stroke, and cerebral abscess occur in 30%-40% of patients with untreated PAVM[1-3] and 40%-50% of patients with PAVM have a history of migraine, headache or transient ischemic attacks[4]. Thus, there is an increased prevalence of neurologic symptoms among patients with hereditary haemorrhagic telangiectasia (HHT) (Mb. Osler-Weber-Rendu)[2]. There is also a risk of PAVM rupture, especially during pregnancy, with resulting severe bleeding which may be lethal[2].
Therefore, there is evidence-based indication to treat these malformations[2].
In more than 75% of cases, PAVM appear in patients with HHT[5]. About 25%-30% of all patients with HHT have PAVM[1,4]. The prevalence of HHT is estimated to be 1 per 5000-10 000[6,7] and the prevalence of PAVM in the population is about 1 per 20 000. PAVM and shunts are rarely seen in relation to other diseases like hepatic cirrhosis, mitral valve stenosis, post trauma, actinomycosis, tuberculosis, and schistosomiasis[8].
HEREDITARY HAEMORRHAGIC TELANGIECTASIA
HHT is an autosomal dominant inherited vascular disease with high penetrans but variable symptomatology. Two subtypes have been defined with mutations in chromosome 9q34 in type 1 and mutations in chromosome 12q31 in type 2. Furthermore, the SMAD4 gene has been shown to be involved in a very rare HHT type combined with juvenile polyposis[9]. The manifestations of HHT are localized to the capillaries and are caused by a defect in normal growth and repair of endothelial cells[10]. Thus, there is a tendency for capillary vascular telangiectasias to form in the skin and mucosa membranes and arteriovenous malformations in the internal organs, most commonly the lungs, but almost all organs can be affected. Epistaxis is the most frequent symptom and is seen in about 95% of patients with HHT. PAVM is seen in about 45% of patients with HHT type 1, and in 12% of patients with HHT type 2[1,11]. Evidence-based international guidelines for the diagnosis and management of HHT have been published recently[12].
PULMONARY ARTERIOVENOUS MALFORMATIONS
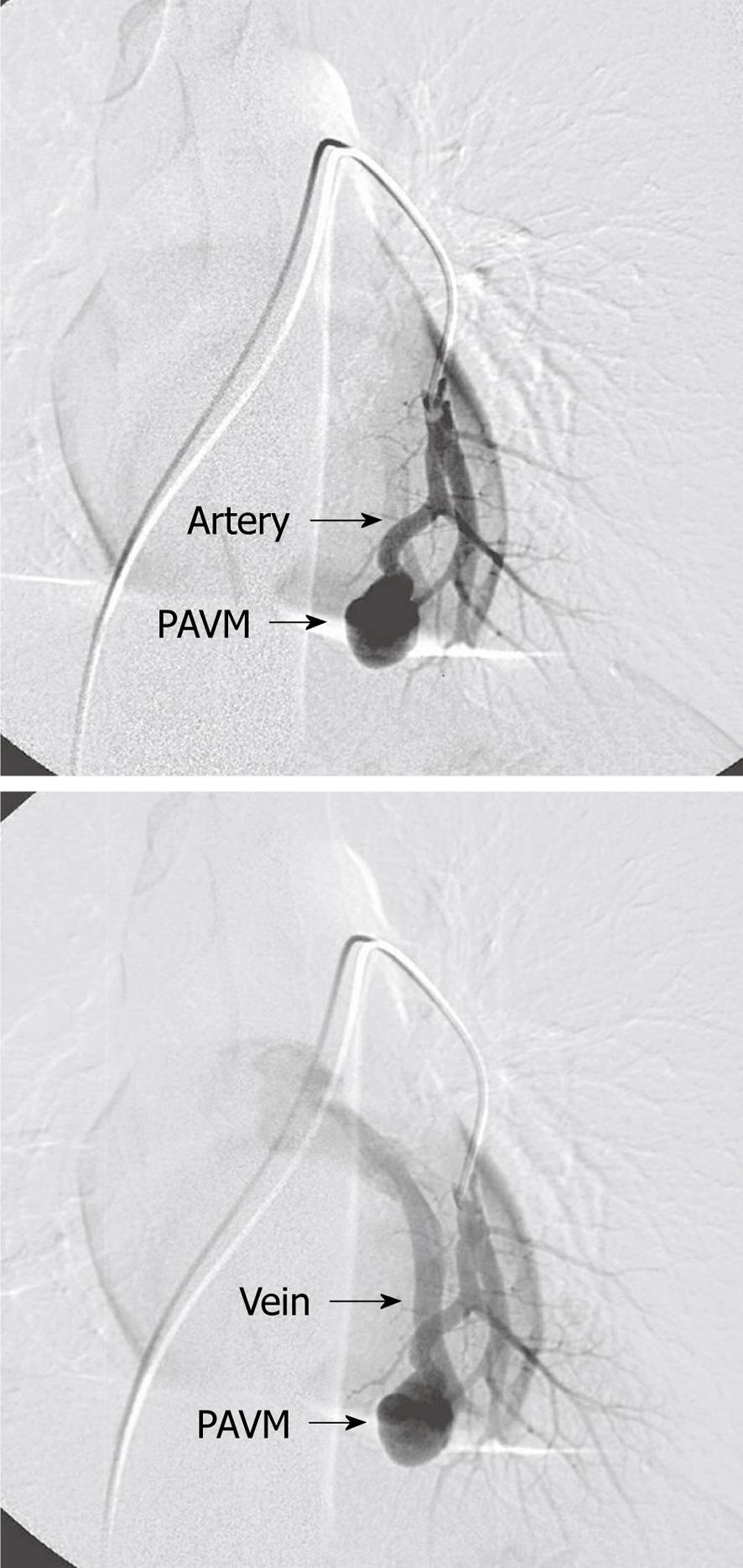
Most patients with PAVM are discovered by screening patients with HHT[7]. PAVM are congenital but are usually not discovered until adult age because they grow with age. The morphology ranges from complex structures supplying and draining an aneurismal sac to small telangiectatic vessels. PAVM can be simple with only one afferent feeding segmental artery and draining vein (Figure 1) or complex with more than one supplying arteries to the PAVM (Figure 2A). Most PAVM are localized to the lower lobes but they can be seen all over the lungs (Figure 3A). It is generally accepted that there is indication for embolization when the feeding artery is 3 mm or more in diameter[2]. Patients with PAVM and without known HHT should be examined for HHT.
Figure 1 Simple pulmonary arteriovenous malformations with one afferent (feeding) artery and one efferent (draining) vein.
PAVM: Pulmonary arteriovenous malformations.
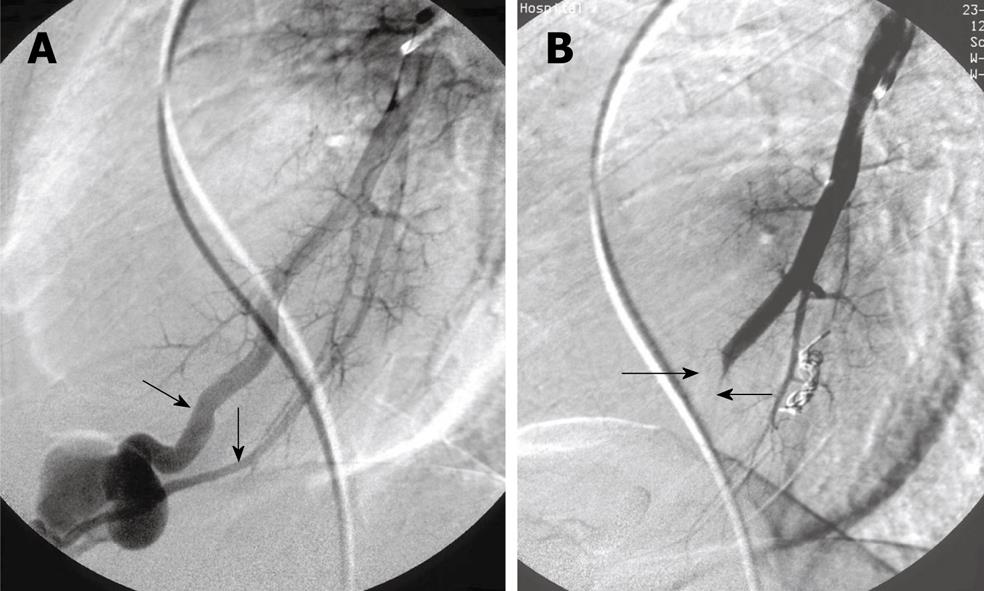
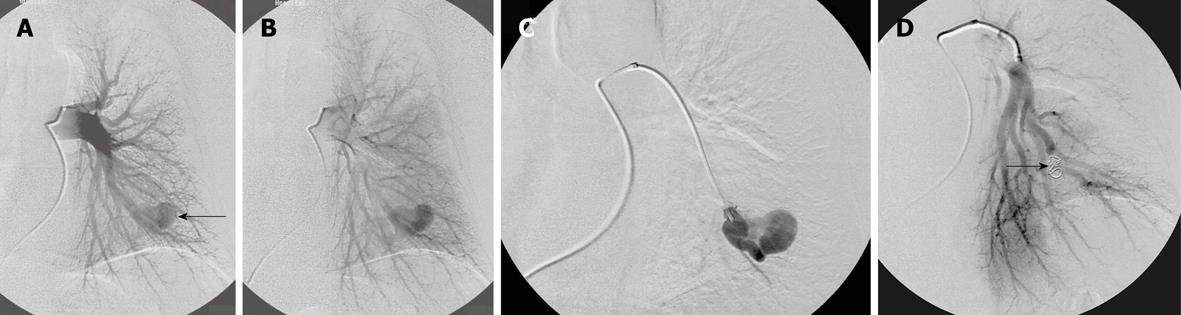
Figure 2 Complex pulmonary arteriovenous malformations.
A: Two afferent (feeding) arteries (arrows); B: After embolization with balloon in one feeding artery (arrows) and coils in the other.
Figure 3 Multiple pulmonary arteriovenous malformations.
A: In both lungs; B: Multiple pulmonary arteriovenous malformations (PAVM) after embolization. One small PAVM on the right side has been left untreated because of small sized feeding artery (arrow).
DIAGNOSIS
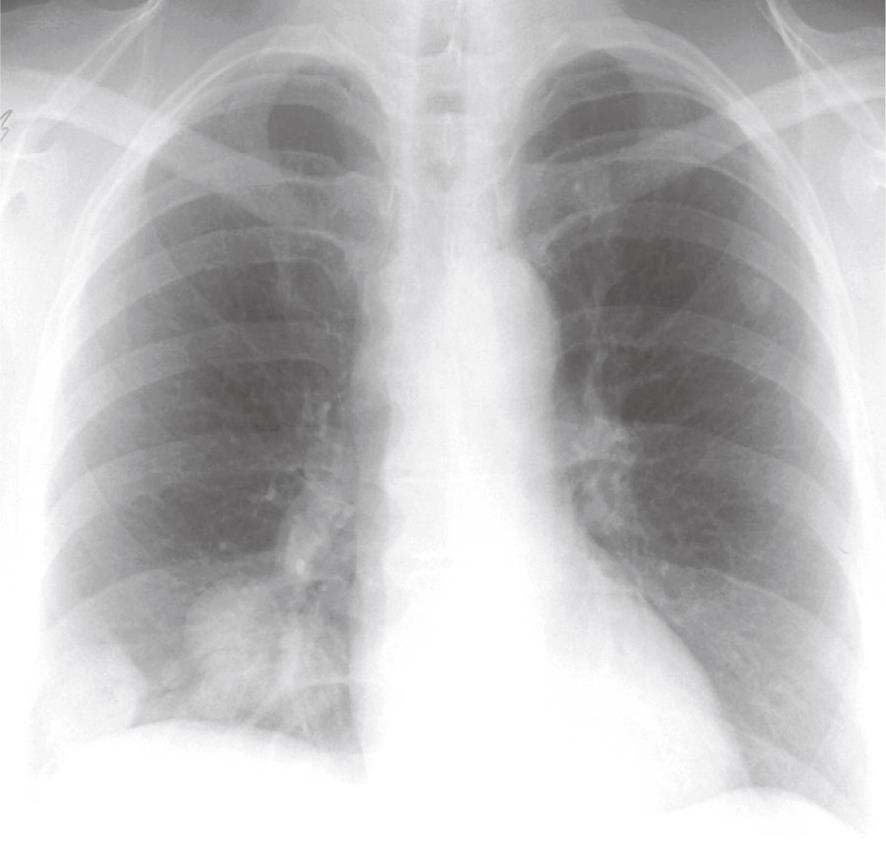
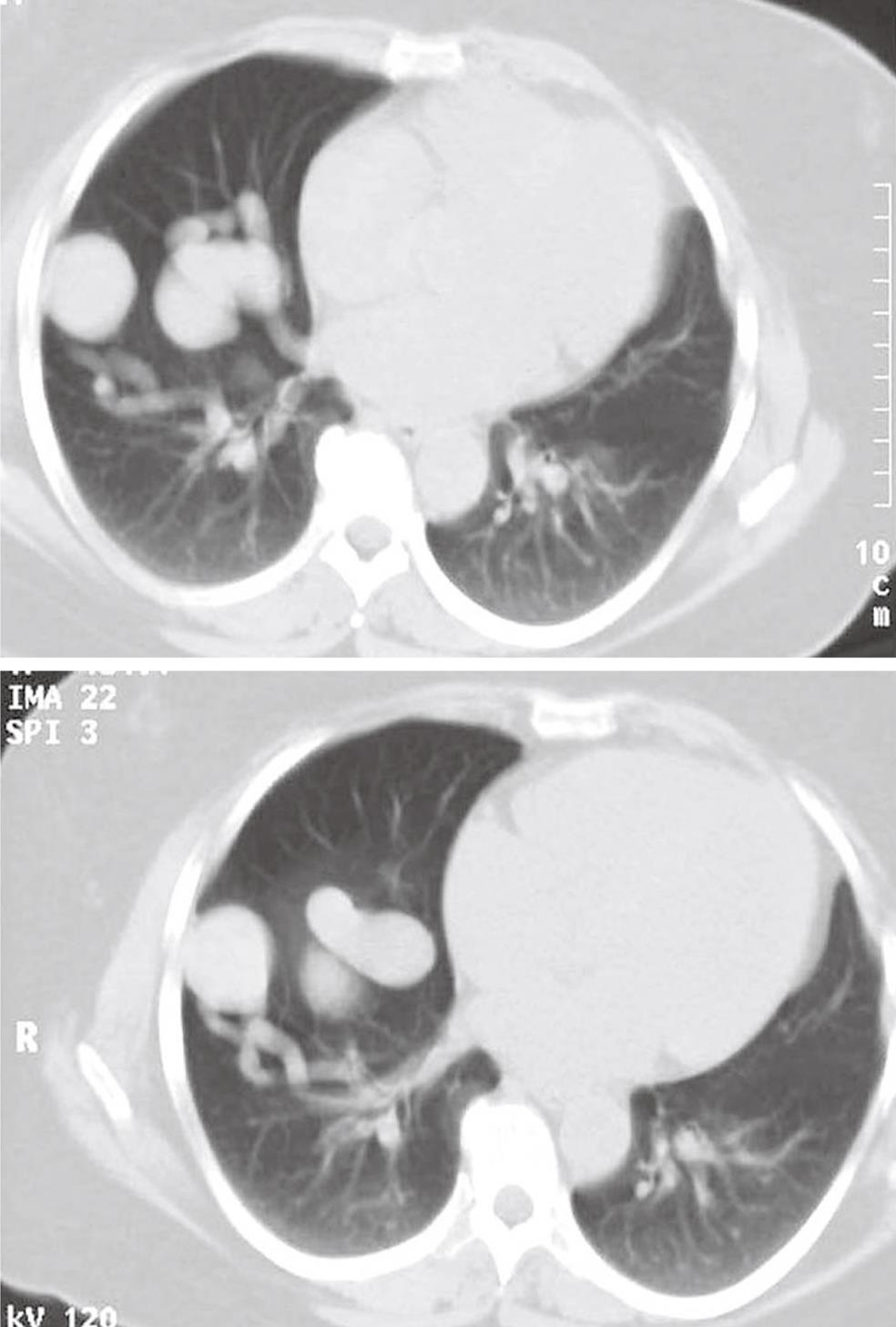
Most patients are found by screening of patients and their closest relatives with HHT[1,7], but some patients without HHT are occasionally found based on patient history (dyspnoea on exertion, cerebral insult/abscess, hemoptysis), clinical findings or pathological findings of rounded tumors on chest X-rays (Figure 4). Thus, some have been suspected of pulmonary malignancy in the initial phase. Lung biopsy should, however, be avoided as it might induce severe bleeding. Only about 66% of PAVM are visible on chest film depending on the size and location. Other patients will primarily be admitted to hospital because of neurological complications and with undiagnosed PAVM. Suspected patients will have a clinical examination, chest X-ray and contrast echocardiography performed initially. If echo contrast bubbles after injection into a peripheral vein appear in left-sided heart chambers a couple of heart cycles after being visible in right-sided chambers, this is indicative of a right-to-left shunt in the lungs. If these examinations are suggestive of PAVM, further examinations will include multi-slice computed tomography (CT) without contrast media, arterial blood gas analysis (partial pressure of oxygen in arterial blood), and pulmonary angiography[13,14]. On multi-slice CT, the characteristic afferent and efferent vessels to the PAVM sac will often be visible (Figure 5)[15,16]. Finally, pulmonary angiography is performed with catheterization through the femoral vein and selective contrast injection in both pulmonary arteries in at least two projections, and if needed additional projections in the anatomic position, that best profiles the artery and its entrance into the malformation.
Figure 4 Pulmonary arteriovenous malformations in both lungs, visible on the chest X-ray.
Figure 5 Computed tomography of the chest showing pulmonary arteriovenous malformations with afferent and efferent vessels.
All patients with HHT and their closest relatives, patients with hepatorenal syndrome, with unexplained dyspnoea on exertion, cerebral abscess or stroke should be screened for PAVM.
EMBOLIZATION METHODS
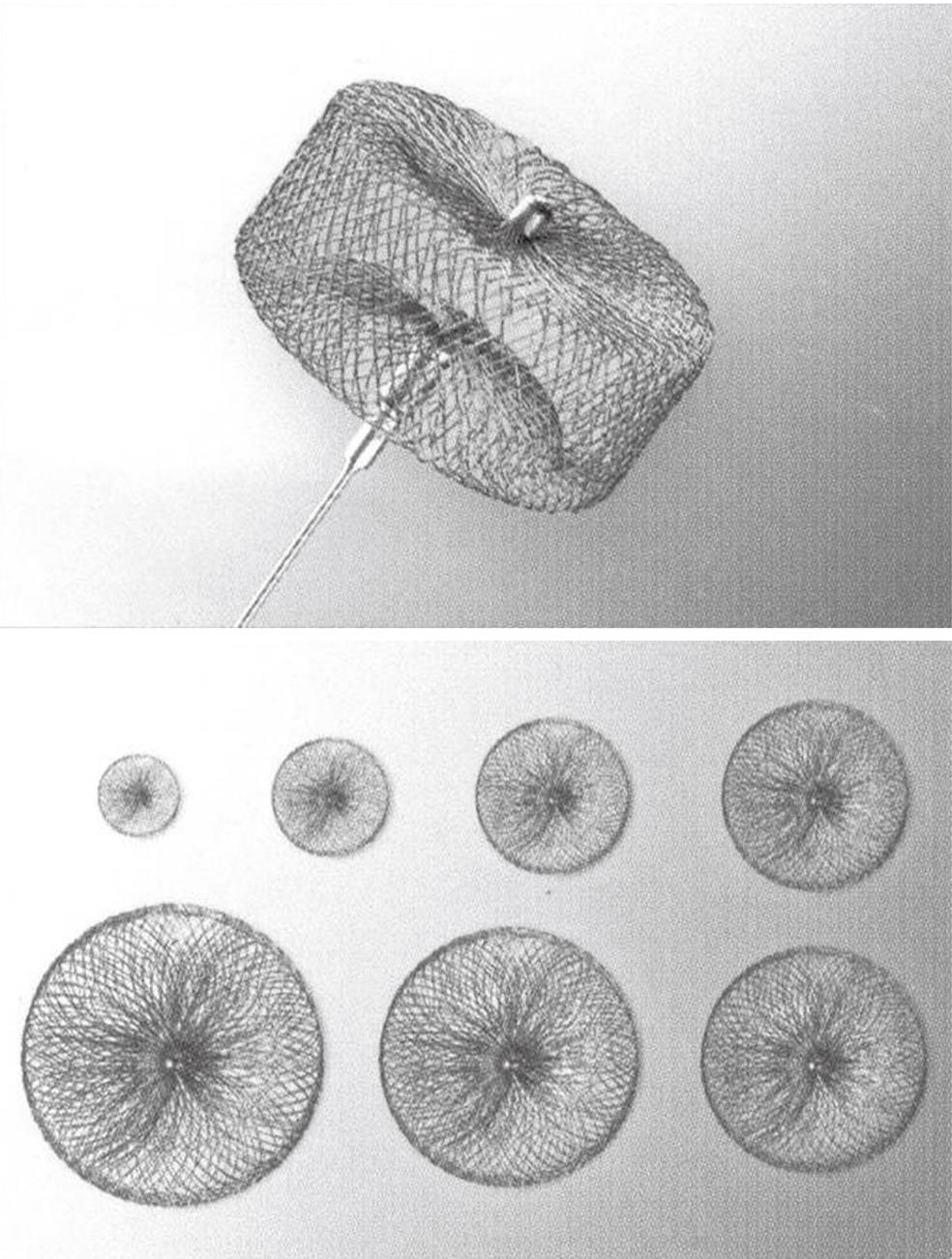
In 1977, percutaneous transluminal embolization of PAVM was introduced as a treatment option[17,18]. This treatment has since shown to be effective with a high success rate and few complications[2,3,19-22], and has therefore replaced surgery as the first-line treatment. The advantages of embolization are that the afferent feeding artery to the PAVM is occluded as close as possible to the PAVM sac and at the same time spares the adjacent normal pulmonary arteries (Figures 2, 3B, 6 and 7). Furthermore, selective, percutaneous, transluminal embolization is a minimally invasive procedure which is performed under local analgesia and without need of convalescence. Embolization is performed using transfemoral venous access to the pulmonary arteries. A 7F J-shaped guiding catheter with a 5F diagnostic vertebral J-shape or another appropriate shape according to the angulation of the target vessels inside is then inserted. A curved hydrophilic guidewire is used inside the catheters for crossing the right-sided heart chambers and for selective catheterization of the pulmonary artery branches. Coils can be delivered through the diagnostic catheter and microcoils through a microcatheter inside the diagnostic catheter. Use of coaxial or triaxial catheters allow for precise placement of the coils. After embolization, the PAVM usually shrinks and leaves a fibrous scar. Transcatheter embolization is established as the preferred treatment for PAVM which have one (simple) (Figures 6 and 7) or more (complex) (Figure 2) feeding arteries of more than 3 mm or smaller if amenable to embolization[2]. Embolization is usually performed with coils, but years ago detachable silicone balloons were also used[13,14,23]. Pushable coils come in all sizes and a huge variety of shapes. They are easy to deliver through a diagnostic or microcatheter and are relatively cheap. Careful “packing” and cross-sectional occlusion by the coils is essential for embolization. It can be achieved using the “anchor” or “scaffold” technique[4] and may reduce the recanalization rate. Recanalization or primary insufficient embolization after use of coils has, however, been described in about 8%-15% of cases[19,20,24-26]. Detachable coils are even more precise and safe to deliver and can be retracted and replaced if necessary before final delivery, but they are more expensive and are usually not routinely used. Vascular plugs are increasingly used[27] (Figures 7 and 8). The vascular plug is a self-expandable, cylindrical nitinol wire-mesh occlusion device. A microscrew is welded to the plug and attached to a delivery wire. They are available in diameters of 4-22 mm and the newest versions pass through 5-8F catheters or long sheaths. They are also detachable and can be retracted, removed, exchanged or repositioned very precisely and safely before final delivery by a precise, controlled detachment. In tortuous and small calibrated vessels, coils are preferred because they are more flexible during delivery through the catheter and available in small sizes. In most cases, multiple coils are necessary to achieve complete occlusion while one plug often will do the job, and it is more time consuming to deliver multiple coils than one plug. The potential for recanalization or incomplete occlusion by the plug is probably lower than that of the coils, especially if it is not possible to pack the coils properly. Personal experience with and preference for a certain device will often be decisive for the choice made.
Figure 6 Simple pulmonary arteriovenous malformations.
A, B: Before embolization; C: After selective catheterization of the feeding artery; D: After embolization with coils (arrow).
Figure 7 Embolization of simple pulmonary arteriovenous malformations with vascular plug.
Figure 8 One version of a vascular plug.
EMBOLIZATION RESULTS
Technical success with occlusion of the feeding artery is achieved in about 95%-100% of cases in experienced hands[13,14,28]. A significant reduction of the pulmonary shunt after embolotherapy has been demonstrated by contrast echocardiography and by raised arterial blood partial pressure of oxygen[13,14,19]. Furthermore, the majority of patients experience a higher functional level and better performance after treatment[14,19]. Embolization is a safe treatment and complications and adverse effects during and after PAVM embolization are not severe. Side effects of this therapy are seen in about 10%-20% of cases[2,13]. Self-limited pleurisy with or without a small pulmonary infiltration and respiratory chest pain usually starting 2-4 d after treatment is seen in 10%-15%[2,14], precordial pain in 2%-5%, primary coil dislocation or malpositioning during delivery in 4%. Catheter-induced bradycardia and ectopic heart beats are common during catheterization but are self-limiting and will disappear after removal or replacement of the catheter from the heart. A transient rise in temperature for 1 or 2 d is common following embolization (post embolization syndrome). Secondary dislocation of coils after deployment and mortality have never been reported in relation to this treatment[2,20]. Clinical and anatomical evaluation after embolization of PAVM is important to detect persistent or reperfused lesions because of recanalization, presence of accessory feeding arteries, pulmonary collateral vessels, and bronchial collateral vessels and growth of non-embolized lesions. These patients will often have symptoms but a significant minority are asymptomatic[29].
CONCLUSION
Embolization of PAVM is a definitive treatment and is a well-established method with a significant effect on oxygenation of the blood and prophylactic effect on paradoxical emboli to the brain. It is a minimally invasive, safe and lung-preserving treatment with high effectiveness and low morbidity and mortality. Patients with HHT and their first degree relatives should be screened for PAVM.
Peer reviewer: Edwin JR van Beek, MD, PhD, Med, FRCR, SINAPSE, Chair of Medical Imaging, Clinical Imaging Research Centre, CO.19, Queen’s Medical Research Institute, 47 Little France Crescent, Edinburgh, EH16 4TJ, United Kingdom
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM