Published online May 26, 2017. doi: 10.4330/wjc.v9.i5.457
Peer-review started: October 23, 2016
First decision: December 1, 2016
Revised: March 13, 2017
Accepted: April 6, 2017
Article in press: April 10, 2017
Published online: May 26, 2017
To investigate feasibility of combined assessment of biochemical and electrophysiological myocardial impairment markers risk-stratifying patients with chronic heart failure (CHF).
Serum levels of heart-type fatty acid binding protein (H-FABP) as a marker of ongoing myocardial damage and QRS duration on electrocardiogram were measured at admission in 322 consecutive patients with CHF. A prolonged QRS duration was defined as 120 ms or longer. The cut-off value for H-FABP level (4.5 ng/mL) was determined from a previous study. Patients were prospectively followed during a median follow up period of 534 d. The primary endpoint was cardiac deaths and rehospitalization for worsening CHF.
There were 117 primary events, including 27 cardiac deaths and 90 rehospitalizations. Patients were stratified into four groups according to H-FABP level and QRS duration (≥ 120 ms). Multivariate analysis demonstrated that high H-FABP levels [hazard ratio (HR) = 1.745, P = 0.021] and QRS prolongation (HR 1.612, P = 0.0258) were independent predictors of cardiac events. Kaplan-Meier analysis demonstrated that the combination of high H-FABP levels and QRS prolongation could be used to reliably stratify patients at high risk for cardiac events (log rank test P < 0.0001).
Combined assessment of myocardial damage and electrical disturbance can be used to risk-stratify patients with CHF.
Core tip: This was a prospective single center study with 322 consecutive patients with chronic heart failure (CHF) seeking to evaluate the feasibility of combined assessment of biochemical and electrophysiological markers of myocardial impairment for risk-stratifying patients with CHF. QRS prolongation and high heart-type fatty acid binding protein levels are independently associated with cardiac events in patients with CHF.
- Citation: Kadowaki S, Watanabe T, Otaki Y, Narumi T, Honda Y, Takahashi H, Arimoto T, Shishido T, Miyamoto T, Kubota I. Combined assessment of myocardial damage and electrical disturbance in chronic heart failure. World J Cardiol 2017; 9(5): 457-465
- URL: https://www.wjgnet.com/1949-8462/full/v9/i5/457.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i5.457
Chronic heart failure (CHF) is a major health problem with high mortality despite advance in medical therapy[1-3]. Various pathophysiological changes are reportedly associated with initiation and progression in CHF[4]. The role of biomarkers continues to increase in importance to evaluate and risk-stratify CHF patients[5].
Heart-type fatty acid binding protein (H-FABP) is a small molecule protein (14-15 kDa), abundant in cytoplasm of cardiomyocytes and easily leaks to the circulation from damaged myocardium[6-8]. H-FABP is a potential myocardial damage marker. We and others reported that elevated serum H-FABP levels can predict poor outcomes in patients with CHF[9,10]. Progression of CHF is associated with persistent loss of cardiomyocytes, which can be clinically detected as a continuous increase in serum H-FABP levels[11].
Electrocardiography (ECG) is routinely performed and is useful for evaluating the etiology of heart failure. Several electrocardiographic parameters were reported to predict poor outcome in HF patients[12-14]. QRS prolongation indicated electrical disturbance and is associated with left ventricular dyssynchrony and poor cardiac prognosis in patients with CHF[15-17]. Not surprisingly, due to the complex pathogenesis of CHF, a single biomarker cannot be used to predict the absolute risk of future cardiac events. Therefore, the purpose of the present study was to investigate whether a combined measurement of a myocardial damage marker and electrical disturbance can be used to risk-stratify CHF patients.
We prospectively studied 322 patients with CHF, who were admitted to our hospital for the diagnosis or treatment of CHF. The diagnosis of CHF was made by two cardiologists who used the generally accepted Framingham criteria, including a history of dyspnea and symptomatic exercise intolerance, signs of pulmonary congestion, peripheral edema, and radiologic or echocardiographic evidence of left ventricular enlargement or dysfunction. Demographic and clinical data including age, gender, New York Heart Association (NYHA) functional class, and medications at discharge were obtained from hospital medical records and interviews with patients. The diagnoses of hypertension, diabetes mellitus and hyperlipidemia were ascertained from the medical records or current or previous medical therapy. Glomerular filtration rate (GFR) was estimated using the modification of diet in renal disease equation with the Japanese coefficient, as previously reported[18]. The exclusion criteria for the present study were acute coronary syndrome, bundle branch block, pace maker implantation, a serum creatinine concentration > 2.0 mg/dL, and implantation of a heart valve prosthesis.
Standard 12-lead ECG was performed at admission. QRS duration was measured by averaging of all heartbeats all leads. A normal QRS duration was defined as less than 120 ms and a prolonged QRS as 120 ms or longer. Transthoracic echocardiography was performed by physicians who were blinded to the biochemical data.
Venous blood samples were obtained at admission for measurements of serum H-FABP levels. These samples were immediately centrifuged at 2500 G for 15 min at 4 °C. The clarified serum samples were frozen, stored at -70 °C, and thawed just before assay. H-FABP concentration was measured using a two-step sandwich enzyme-linked immunosorbent assay kit (MARKIT-M HFABP, Dainippon Pharmaceutical Co Ltd, Tokyo, Japan) as previously reported[19,20]. The cut-off value for H-FABP concentration (4.5 ng/mL) was determined from a previous study[21]. The same blood samples were used for measurement of plasma brain natriuretic peptide (BNP) concentrations. The samples were transferred to chilled tubes containing of ethylene diamine tetraacetic acid disodium salt (4.5 mg) and aprotinin (500 U/mL), and immediately centrifuged at 1000 G for 15 min at 4 °C. The clarified plasma samples were frozen, stored at -70 °C and thawed just before assay. BNP concentrations were measured using a commercially available specific radioimmunoassay for human BNP (Shiono RIA BNP assay kit, Shionogi Co Ltd, Tokyo, Japan). The analytical ranges, and intra- and inter-assay coefficients of variation for the H-FABP and BNP assays were, 1.1-250 ng/mL, 3% and 3.5%, and 4.0-2000 pg/mL, 10.9% and 10.6%, respectively.
Patients were prospectively followed for a median period of 534 d (range 203-1014). Patients were followed in our hospital outpatient clinic every month. The other patients were followed by telephone twice a year until 2555 d after discharge. The end points were cardiac death, defined as death due to progressive heart failure, myocardial infarction or sudden cardiac death, and progressive heart failure requiring rehospitalization. Sudden cardiac death was defined as death without definite premonitory symptoms or signs, and was established by the attending physician. The study was approved by the Institutional Ethics Committee, and all patients gave written informed consent prior to participating. The study was performed in accordance with the Helsinki Declaration.
Results are presented as the mean values ± SD for continuous variables and as percentages of the total number of patients for categorical variables. The independent samples t test and χ2 test or linear regression analysis were used for comparison of continuous and categorical variables, respectively. A Cox proportional hazard analysis was performed to assess the independent predictors for cardiac events in the entire population. Statistical significance was defined as P < 0.05. Variables identified as significant by univariate analysis were entered into the multivariate analysis. The cardiac event-free curve was computed according to the Kaplan-Meier method, and comparison of cardiac event-free survival between subgroups was performed using the log-rank test. Receiver operating characteristic (ROC) curve analysis, as well as area under the curve (AUC) was used as measures of the predictive accuracy of traditional prognostic factors for cardiac events. In addition, the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were calculated in order to quantify the improvement for the corrected re-classification and sensitivity after inclusion of high H-FABP levels and QRS prolongation in the model. Statistical analyses were performed using a standard software package (JMP version 8; SAS Institute Inc., Cary, NC, United States) or R 3.0.2 with additional packages (Rcmdr, Epi, pROC and PredictABEL).
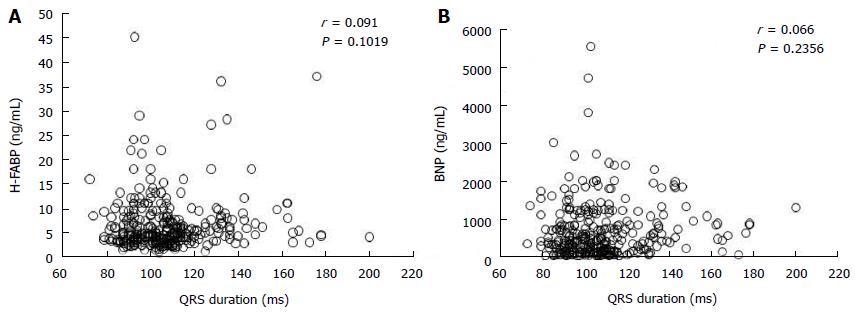
Table 1 shows that clinical characteristics of the study patients. The mean age of the patients was 69 ± 13 years. There were 175 patients in NYHA functional class II, 105 in NYHA class III, and 42 in NYHA class IV. Diabetes mellitus, dyslipidemia, and hypertension were identified in 117 (36%), 87 (26%), and 217 (67%) of the CHF patients, respectively. The etiology of heart failure was dilated cardiomyopathy in 80 (25%) patients, hypertensive heart disease in 14 (4%), hypertrophic cardiomyopathy in 21 (7%), ischemic heart disease in 65 (20%), valvular heart disease in 80 (25%), arrhythmia in 24 (7%), and other etiologies in 38 (12%) patients. The median H-FABP and BNP levels were 4.7 (3.3-7.6) ng/mL and 397 (135-853) pg/mL, respectively. The mean QRS duration was 107 ± 20 ms and 61 patients (19%) showed QRS prolongation. Simple linear regression analysis showed that QRS duration was not correlated with H-FABP level (r = 0.091, P = 0.1019) or BNP level (r = 0.066, P = 0.2356) as shown in Figure 1.
| All patients (n = 322) | Event-free (n = 205) | Cardiac event (n = 117) | P value | |
| Age, yr | 69 ± 13 | 67 ± 14 | 72 ± 11 | 0.0041 |
| Female, n (%) | 140 (43) | 92 (45) | 48 (41) | 0.5024 |
| NYHA functional class, II/III/IV | 175/105/42 | 125/53/27 | 50/52/15 | 0.002 |
| Etiology, n (%) | 0.5273 | |||
| Dilated cardiomyopathy | 80 (25) | 56 (27) | 24 (21) | |
| Hypertensive heart disease | 14 (4) | 10 (5) | 4 (3) | |
| Hypertrophic cardiomyopathy | 21 (7) | 15 (7) | 6 (5) | |
| Ischemic heart disease | 65 (20) | 36 (18) | 29 (25) | |
| Valvular heart disease | 80 (25) | 52 (25) | 28 (24) | |
| Arrhythmia | 24 (7) | 14 (7) | 10 (8) | |
| Others | 38 (12) | 22 (11) | 16 (14) | |
| Atrial fibrillation, n (%) | 109 (34) | 64 (31) | 45 (38) | 0.1866 |
| Diabetes mellitus, n (%) | 117 (36) | 71 (35) | 44 (38) | 0.5923 |
| Dyslipidemia, n (%) | 87 (26) | 56 (26) | 31 (27) | 0.8732 |
| Hypertension, n (%) | 217 (67) | 137 (67) | 80 (68) | 0.7758 |
| Blood biomarkers | ||||
| BNP, pg/mL (IQR) | 397 (135-853) | 314 (101-710) | 625 (280-1147) | 0.0326 |
| H-FABP, ng/mL (IQR) | 4.7 (3.3-7.6) | 4.0 (2.9-6.3) | 6.0 (4.2-10.0) | < 0.0001 |
| eGFR, mL/min per 1.73 m2 | 65 ± 22 | 69 ± 23 | 58 ± 19 | < 0.0001 |
| Echocardiographic data | ||||
| LV end-diastolic diameter, mm | 55 ± 10 | 54 ± 9 | 55 ± 12 | 0.6018 |
| LV ejection fraction, % | 49 ± 18 | 50 ± 18 | 47 ± 18 | 0.1472 |
| Electrocardiogram | ||||
| Heart rate, beat/min | 77 ± 22 | 78 ± 21 | 74 ± 19 | 0.0841 |
| QRS duration, ms | 107 ± 20 | 106 ± 18 | 109 ± 22 | 0.0989 |
| QRS prolongation, n (%) | 61 (19) | 28 (17) | 33 (28) | 0.0014 |
| Medications, n (%) | ||||
| ACE inhibitors and/or ARBs, n (%) | 213 (66) | 138 (67) | 75 (64) | 0.5577 |
| β-blockers, n (%) | 170 (53) | 106 (52) | 64 (55) | 0.6048 |
| Ca channel blockers, n (%) | 66 (21) | 41 (21) | 25 (20) | 0.77 |
| Diuretics, n (%) | 202 (63) | 111 (54) | 91 (78) | < 0.0001 |
| Statins, n (%) | 83 (26) | 54 (26) | 29 (25) | 0.759 |
During the follow-up period, there were 117 primary events, including 27 cardiac deaths and 90 re-admissions for worsening CHF. Among 27 cardiac deaths, there were 21 deaths from worsening CHF, 2 fatal acute myocardial infarction, and 4 sudden cardiac deaths. The patients with cardiac events were older and had a more severe NYHA functional class compared to those who did not (Table 1). Furthermore, higher BNP and H-FABP levels, and a higher prevalence of QRS prolongation were observed in patients who experienced cardiac events, compared with those who did not. Patients who experienced cardiac events also had a lower estimated GFR (eGFR) compared with those who did not. There was no difference in gender, prevalence of atrial fibrillation, hypertension, diabetes mellitus or hyperlipidemia between CHF patients with and without cardiac events. Patients who experienced cardiac events took loop diuretics more frequently than patients who were event-free.
To investigate the risk factors for cardiac events, Cox proportional hazards regression analyses were performed (Table 2). In the univariate analysis, high H-FABP levels and QRS prolongation were significantly associated with cardiac events. Further, age, NYHA functional class, BNP levels, and eGFR were significantly associated with cardiac events. In the multivariate analysis, NYHA functional class, eGFR, high serum H-FABP levels, and prolonged QRS duration were independently associated with cardiac events.
| HR | 95%CI | P value | |
| Univariate analysis | |||
| Age, per 10-yr increase | 1.297 | 1.105-1.524 | 0.0016 |
| Female gender | 0.829 | 0.573-1.199 | 0.3183 |
| NYHA functional class II and III vs IV | 1.960 | 1.381-2.747 | 0.0003 |
| Atrial fibrillation | 1.256 | 0.865-1.824 | 0.2304 |
| Diabetes mellitus | 1.103 | 0.758-1.605 | 0.6062 |
| Dyslipidemia | 0.958 | 0.635-1.447 | 0.8417 |
| Hypertension | 0.986 | 0.667-1.457 | 0.9459 |
| BNP, per 1SD increase | 1.166 | 1.019-1.334 | 0.0249 |
| eGFR, per 1SD increase | 0.589 | 0.467-0.733 | < 0.0001 |
| LV end-diastolic diameter, per 1SD increase | 1.062 | 0.877-1.280 | 0.5272 |
| LV ejection fraction, per 1SD increase | 0.881 | 0.734-1.074 | 0.1998 |
| Heart rate, per 1SD increase | 0.869 | 0.724-1.062 | 0.1724 |
| High H-FABP (> 4.5 ng/mL) | 2.994 | 1.996-4.504 | < 0.0001 |
| QRS prolongation (≥ 120 ms) | 1.897 | 1.264-2.832 | 0.0019 |
| Multivariate analysis | |||
| Age, per 10-yr increase | 1.093 | 0.921-1.298 | 0.3055 |
| NYHA functional class II and III vs IV | 1.55 | 1.055-2.309 | 0.0262 |
| BNP, per 1SD increase | 0.948 | 0.811-1.151 | 0.7003 |
| eGFR, per 1SD increase | 0.733 | 0.571-0.938 | 0.0144 |
| High H-FABP (> 4.5 ng/mL) | 1.745 | 1.088-2.793 | 0.0210 |
| QRS prolongation (≥ 120 ms) | 1.612 | 1.060-2.451 | 0.0258 |
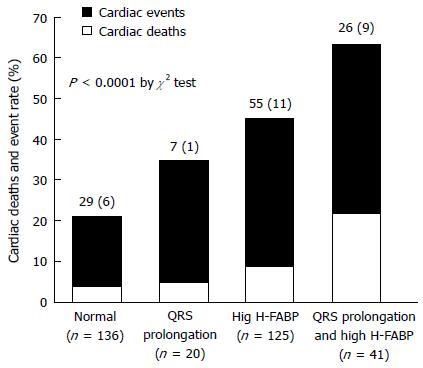
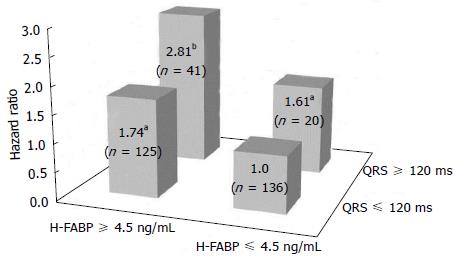
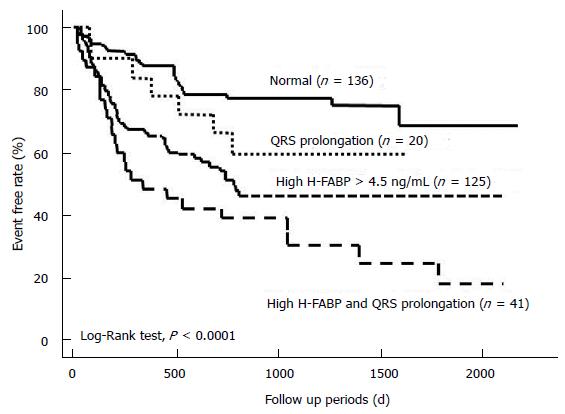
Simple linear analysis demonstrated that QRS duration was not correlated with H-FABP or BNP levels in patients with CHF (Figure 1). The patients were divided into four groups based on QRS prolongation and H-FABP cutoff values as shown in Figure 2: (1) normal group (n = 136), H-FABP ≤ 4.5 ng/mL, QRS duration < 120 ms; (2) QRS prolongation group (n = 20), H-FABP ≤ 4.5 ng/mL, QRS ≥ 120 ms; (3) high H-FABP group (n = 125), H-FABP > 4.5 ng/mL, QRS duration < 120 ms; and (4) high H-FABP + QRS prolongation group (n = 41), H-FABP > 4.5 ng/mL, QRS duration ≥ 120 ms. High serum H-FABP + QRS prolongation group showed the highest rates of cardiac deaths and cardiac events (P < 0.001). Multivariate Cox hazard analysis revealed that after adjustment for age, NYHA functional class, BNP levels and eGFR, the QRS prolongation, high H-FABP, and high H-FABP + QRS prolongation groups had 1.61-fold (P < 0.05), 1.74-fold (P < 0.05), and 2.81-fold higher risks of cardiac events (P < 0.01), respectively, compared with the normal group (Figure 3). The characteristics of these four groups are presented in Table 3. The QRS prolongation group had lower BNP levels than the high H-FABP and high H-FABP + QRS prolongation groups. The QRS prolongation group also had the lowest left ventricular (LV) ejection fraction and largest LV end-diastolic diameter among 4 groups. Kaplan-Meier analysis demonstrated that the high H-FABP + QRS prolongation group had a significantly higher rate of cardiac events than the other groups (Figure 4). In order to examine whether model fit and discrimination improved with the addition of high H-FABP levels and QRS prolongation to the traditional prognostic factors of age, BNP level, NYHA functional class and eGFR, the differences in area under the ROC curves, and the improvement in NRI and IDI were evaluated for two models: With (group 2) or without (group 1) a high H-FABP level and QRS prolongation. The area under the ROC curve for predicted cardiac events was significantly greater for group 2 than group 1 (Table 4). Further, the group 2 model improved the NRI and IDI values for predicting cardiac events compared with the group 1 model.
| Normal (n = 136) | QRS prolongation (n = 20) | High H-FABP (n = 125) | High H-FABP and QRS prolongation (n = 41) | |
| Age, yr | 65 ± 13 | 59 ± 11 | 74 ± 11ab | 71 ± 13b |
| Female, n (%) | 58 (42) | 10 (50) | 55 (45) | 17 (41) |
| NYHA functional class, II/III/IV | 97/30/9 | 3/4/2013 | 51/54/20 | 14/18/9e |
| Etiology, n (%) | ||||
| Dilated cardiomyopathy | 33 (24) | 8 (40) | 24 (19) | 15 (37) |
| Hypertensive heart disease | 8 (6) | 1 (5) | 5 (4) | 1 (2) |
| Hypertrophic cardiomyopathy | 11 (8) | 3 (15) | 6 (5) | 0 (0) |
| Ischemic heart disease | 21 (15) | 3 (15) | 31 (24) | 10 (24) |
| Valvular heart disease | 40 (30) | 3 (15) | 29 (24) | 8 (20) |
| Arrhythmia | 12 (9) | 0 (0) | 8 (7) | 4 (10) |
| Others | 11 (8) | 2 (10) | 22 (17) | 3 (7) |
| Atrial fibrillation, n (%) | 48 (35) | 7 (35) | 41 (33) | 13 (32) |
| Diabetes mellitus, n (%) | 46 (33) | 6 (28) | 46 (37) | 17 (41) |
| Dyslipidemia, n (%) | 39 (28) | 4 (20) | 32 (26) | 12 (29) |
| Hypertension, n (%) | 92 (67) | 11 (55) | 89 (72) | 25 (61) |
| Blood biomarkers | ||||
| BNP, pg/mL (IQR) | 347 (69-453) | 389 (213-855) | 700 (311-1257)a | 628 (328-1075)a |
| H-FABP, ng/mL (IQR) | 3.2 (2.4-3.9) | 3.6 (2.8-4.2) | 7.6 (5.7-11.0)ab | 7.6 (5.7-9.8)ab |
| eGFR, mL/min per 1.73 m2 | 75 ± 20 | 71 ± 26 | 57 ± 20a | 52 ± 17ad |
| Echocardiographic data | ||||
| LV end-diastolic diameter, mm | 52 ± 10 | 65 ± 9ad | 54 ± 9b | 60 ± 10ac |
| LV ejection fraction, % | 55 ± 18 | 35 ± 15a | 49 ± 17b | 38 ± 14ad |
| Electrocardiogram | ||||
| Heart rate, beat/min | 78 ± 19 | 72 ± 13 | 79 ± 22 | 72 ± 20 |
| QRS duration, ms | 100 ± 10 | 143 ± 23ad | 100 ± 10 | 138 ± 14ad |
| Medications, n (%) | ||||
| ACE inhibitors and/or ARBs, n (%) | 86 (62) | 13 (65) | 85 (69) | 29 (71) |
| β-blockers, n (%) | 65 (47) | 15 (75) | 64 (52) | 26 (63) |
| Ca channel blockers, n (%) | 36 (26) | 0 (0) | 24 (20) | 6 (15) |
| Diuretics, n (%) | 72 (52) | 14 (70) | 82 (67) | 34 (83)e |
| Statins, n (%) | 40 (29) | 5 (25) | 28 (23) | 10 (24) |
| Group 1 | Group 2 | P value | |
| AUC of ROC curve | 0.668 | 0.706 | 0.029 |
| NRI (95%CI) | Ref | 0.223 (0.073-0.372) | 0.003 |
| IDI (95%CI) | Ref | 0.036 (0.015-0.056) | 0.016 |
In the present study, we demonstrated that QRS prolongation as a marker of electrical disturbance, and high H-FABP levels as a marker of ongoing myocardial damage are significantly related to cardiac events in CHF patients. The inclusion of high H-FABP level and QRS prolongation with BNP level, NYHA functional class and eGFR in the model for predicting cardiac events improved the NRI and IDI values, indicating effective reclassification and discrimination. Therefore, a combined measurement of H-FABP levels and QRS duration is a promising strategy for risk stratification for future cardiac events in CHF patients.
There are several markers of myocardial damage, including troponin T, troponin I and H-FABP[9,22]. Since H-FABP is a small cytosolic protein, it is readily released into the circulation when cardiomyocytes are injured. The mechanism by which serum levels of H-FABP are increased in CHF has been reported to be related to cardiomyocyte necrosis, apoptosis, chronic inflammation and microcirculatory disorder[8,23]. In this study, elevated levels of H-FABP were significantly associated with cardiac events, which are consistent with previous reports[19,24].
QRS duration reflects LV conduction disturbance, LV systolic dysfunction and LV dilation[25]. In this study, the QRS prolongation group had the lowest LV ejection fraction and the greatest LV end-diastolic diameter compared with the other groups. Since left bundle branch block is an unfavorable prognostic marker in CHF patients[26,27], patients with bundle branch block were excluded from the present study. Therefore, QRS prolongation is an independent risk factor for cardiac events in patients with CHF, irrespective of bundle branch block. Recently, it was reported that cardiac resynchronization therapy (CRT) can improve the cardiac prognosis in patients with QRS prolongation[28,29] and measurement of QRS duration has attracted widespread interest.
The present study showed that there was no correlation between QRS duration and H-FABP or BNP levels in patients with CHF. These results suggest that H-FABP and BNP levels and QRS duration reflect different pathophysiological backgrounds. In the multivariate analysis, high H-FABP levels and QRS prolongation were independent predictors of cardiac events. In addition, multivariate Cox hazard analysis revealed that the combination of elevated H-FABP levels and QRS prolongation was associated with the highest increase in risk for cardiac events (2.81-fold) compared with the normal group.
Taniguchi et al[30] reported that the combined measurement of BNP levels and QRS duration can be used to predict cardiac events in heart failure patients. We recently determined that the AUC for prediction of cardiac events in heart failure was greater for H-FABP level than for BNP level[10]. Both the sensitivity and the specificity for predicting cardiac events were significantly greater for H-FABP level than for BNP level, indicating that H-FABP level is superior to BNP level for predicting cardiac events in CHF patients[10]. In this study, BNP level was not associated with cardiac events in the multivariate analysis. A weak correlation between H-FABP levels and BNP levels was observed (data not shown), which was consistent with the results from a previous study[10]. H-FABP and BNP reflect different pathophysiological backgrounds as markers of left ventricular overload. Combined assessment of H-FABP as a biochemical marker of myocardial damage and QRS prolongation as an electrophysiological marker of myocardial impairment is a potentially useful method for risk-stratification in CHF patients.
This study has several limitations. The effect of changes in QRS duration and H-FABP level between the time of hospitalization and discharge were not evaluated. However, it was reported that QRS duration in patients with CHF did not change significantly over two years[31]. On the other hand, although H-FABP level is usually decreased at discharge, persistently elevated H-FABP levels were reported to be associated with adverse outcomes in patients with CHF[32]. Therefore, further research is needed to elucidate whether the combined assessment of H-FABP level at discharge and QRS prolongation can be used to more precisely predict the cardiac prognosis of patients with CHF.
In conclusion, the combined assessment of markers of ongoing myocardial damage and electrical disturbance can be used to risk-stratify patients with CHF.
The authors would like to express their gratitude to the staff at the Department of Cardiology, Pulmonology, and Nephrology, Yamagata University School of Medicine, Yamagata, Japan, for their cooperation while we conducted this study.
Despite advancing medical therapy, chronic heart failure (CHF) is a major health problem with high morbidity and mortality. It is important to risk-stratify patients with CHF.
Prolonged QRS duration reflects intraventricular conduction disturbance caused by left ventricular fibrosis and cardiac myocyte loss, and is associated with cardiac prognosis in patients with CHF. However, there are CHF patients with narrow QRS duration showing poor prognosis. Biochemical myocardial damage markers are also useful for predicting prognosis in addition to electrophysiological myocardial impairment markers in CHF patients.
The combined assessment of markers of ongoing myocardial damage and electrical disturbance can risk-stratify patients with CHF.
It may be difficult to predict prognosis of CHF patients using a single biomarker precisely. The combined assessment of commonly used biomarkers is easily applicable to clinical practice.
Since heart-type fatty acid binding protein (H-FABP) is a low molecular weight protein and abundant in the cytosolic fraction of cardiomyocytes, it is rapidly released into the circulation from damaged myocardium. Therefore, H-FABP is a potential marker of ongoing myocardial damage.
The manuscript was very easy to follow and well written.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Boos CJ, Liu T, Sochman J S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293-302. [PubMed] [Cited in This Article: ] |
| 2. | Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3634] [Cited by in F6Publishing: 3289] [Article Influence: 156.6] [Reference Citation Analysis (0)] |
| 3. | McMurray JJ, Pfeffer MA. Heart failure. Lancet. 2005;365:1877-1889. [PubMed] [Cited in This Article: ] |
| 4. | Sharma R, Coats AJ, Anker SD. The role of inflammatory mediators in chronic heart failure: cytokines, nitric oxide, and endothelin-1. Int J Cardiol. 2000;72:175-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Ahmad T, Fiuzat M, Felker GM, O’Connor C. Novel biomarkers in chronic heart failure. Nat Rev Cardiol. 2012;9:347-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Glatz JF, Paulussen RJ, Veerkamp JH. Fatty acid binding proteins from heart. Chem Phys Lipids. 1985;38:115-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Schaap FG, van der Vusse GJ, Glatz JF. Fatty acid-binding proteins in the heart. Mol Cell Biochem. 1998;180:43-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Panteghini M. Standardization activities of markers of cardiac damage: the need of a comprehensive approach. Eur Heart J. 1998;19 Suppl N:N8-11. [PubMed] [Cited in This Article: ] |
| 9. | Niizeki T, Takeishi Y, Arimoto T, Takabatake N, Nozaki N, Hirono O, Watanabe T, Nitobe J, Harada M, Suzuki S. Heart-type fatty acid-binding protein is more sensitive than troponin T to detect the ongoing myocardial damage in chronic heart failure patients. J Card Fail. 2007;13:120-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Niizeki T, Takeishi Y, Arimoto T, Takahashi T, Okuyama H, Takabatake N, Nozaki N, Hirono O, Tsunoda Y, Shishido T. Combination of heart-type fatty acid binding protein and brain natriuretic peptide can reliably risk stratify patients hospitalized for chronic heart failure. Circ J. 2005;69:922-927. [PubMed] [Cited in This Article: ] |
| 11. | Setsuta K, Seino Y, Ogawa T, Ohtsuka T, Seimiya K, Takano T. Ongoing myocardial damage in chronic heart failure is related to activated tumor necrosis factor and Fas/Fas ligand system. Circ J. 2004;68:747-750. [PubMed] [Cited in This Article: ] |
| 12. | Cygankiewicz I, Zareba W, Vazquez R, Bayes-Genis A, Pascual D, Macaya C, Almendral J, Fiol M, Bardaji A, Gonzalez-Juanatey JR. Risk stratification of mortality in patients with heart failure and left ventricular ejection fraction & gt; 35%. Am J Cardiol. 2009;103:1003-1010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Park SJ, On YK, Byeon K, Kim JS, Choi JO, Choi DJ, Ryu KH, Jeon ES. Short- and long-term outcomes depending on electrical dyssynchrony markers in patients presenting with acute heart failure: clinical implication of the first-degree atrioventricular block and QRS prolongation from the Korean Heart Failure registry. Am Heart J. 2013;165:57-64.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Galinier M, Albenque JP, Afchar N, Fourcade J, Massabuau P, Doazan JP, Legoanvic C, Fauvel JM, Bounhoure JP. Prognostic value of late potentials in patients with congestive heart failure. Eur Heart J. 1996;17:264-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Iuliano S, Fisher SG, Karasik PE, Fletcher RD, Singh SN. QRS duration and mortality in patients with congestive heart failure. Am Heart J. 2002;143:1085-1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 16. | Wang NC, Maggioni AP, Konstam MA, Zannad F, Krasa HB, Burnett JC, Grinfeld L, Swedberg K, Udelson JE, Cook T. Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA. 2008;299:2656-2666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Bleeker GB, Schalij MJ, Molhoek SG, Verwey HF, Holman ER, Boersma E, Steendijk P, Van Der Wall EE, Bax JJ. Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol. 2004;15:544-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 279] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 18. | Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4276] [Cited by in F6Publishing: 4827] [Article Influence: 321.8] [Reference Citation Analysis (0)] |
| 19. | Arimoto T, Takeishi Y, Shiga R, Fukui A, Tachibana H, Nozaki N, Hirono O, Nitobe J, Miyamoto T, Hoit BD. Prognostic value of elevated circulating heart-type fatty acid binding protein in patients with congestive heart failure. J Card Fail. 2005;11:56-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Ohkaru Y, Asayama K, Ishii H, Nishimura S, Sunahara N, Tanaka T, Kawamura K. Development of a sandwich enzyme-linked immunosorbent assay for the determination of human heart type fatty acid-binding protein in plasma and urine by using two different monoclonal antibodies specific for human heart fatty acid-binding protein. J Immunol Methods. 1995;178:99-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 72] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Setsuta K, Seino Y, Kitahara Y, Arau M, Ohbayashi T, Takano T, Mizuno K. Elevated levels of both cardiomyocyte membrane and myofibril damage markers predict adverse outcomes in patients with chronic heart failure. Circ J. 2008;72:569-574. [PubMed] [Cited in This Article: ] |
| 22. | Mair J. Cardiac troponin I and troponin T: are enzymes still relevant as cardiac markers? Clin Chim Acta. 1997;257:99-115. [PubMed] [Cited in This Article: ] |
| 23. | Goto T, Takase H, Toriyama T, Sugiura T, Sato K, Ueda R, Dohi Y. Circulating concentrations of cardiac proteins indicate the severity of congestive heart failure. Heart. 2003;89:1303-1307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Setsuta K, Seino Y, Ogawa T, Arao M, Miyatake Y, Takano T. Use of cytosolic and myofibril markers in the detection of ongoing myocardial damage in patients with chronic heart failure. Am J Med. 2002;113:717-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Sandhu R, Bahler RC. Prevalence of QRS prolongation in a community hospital cohort of patients with heart failure and its relation to left ventricular systolic dysfunction. Am J Cardiol. 2004;93:244-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Baldasseroni S, Opasich C, Gorini M, Lucci D, Marchionni N, Marini M, Campana C, Perini G, Deorsola A, Masotti G. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian network on congestive heart failure. Am Heart J. 2002;143:398-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 534] [Cited by in F6Publishing: 554] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 27. | Hawkins NM, Wang D, McMurray JJ, Pfeffer MA, Swedberg K, Granger CB, Yusuf S, Pocock SJ, Ostergren J, Michelson EL. Prevalence and prognostic impact of bundle branch block in patients with heart failure: evidence from the CHARM programme. Eur J Heart Fail. 2007;9:510-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-2150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4324] [Cited by in F6Publishing: 4021] [Article Influence: 201.1] [Reference Citation Analysis (0)] |
| 29. | Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539-1549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4673] [Cited by in F6Publishing: 4416] [Article Influence: 232.4] [Reference Citation Analysis (0)] |
| 30. | Taniguchi T, Kawasaki T, Miyai N, Kamitani T, Kawasaki S, Sugihara H. [Brain natriuretic peptide and QRS duration as a predictor for cardiac events in patients with heart failure]. J Cardiol. 2006;47:277-283. [PubMed] [Cited in This Article: ] |
| 31. | Hofmann M, Bauer R, Handrock R, Weidinger G, Goedel-Meinen L. Prognostic value of the QRS duration in patients with heart failure: a subgroup analysis from 24 centers of Val-HeFT. J Card Fail. 2005;11:523-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Niizeki T, Takeishi Y, Arimoto T, Nozaki N, Hirono O, Watanabe T, Nitobe J, Miyashita T, Miyamoto T, Koyama Y. Persistently increased serum concentration of heart-type fatty acid-binding protein predicts adverse clinical outcomes in patients with chronic heart failure. Circ J. 2008;72:109-114. [PubMed] [Cited in This Article: ] |