Published online Feb 26, 2017. doi: 10.4330/wjc.v9.i2.147
Peer-review started: September 13, 2016
First decision: December 13, 2016
Revised: December 15, 2016
Accepted: January 11, 2017
Article in press: January 13, 2017
Published online: February 26, 2017
Although the incidence of pediatric heart failure is low, the mortality is relatively high, with severe clinical symptoms requiring repeated hospitalization or intensive care treatment in the surviving patients. Cardiac biopsy specimens have revealed a higher number of resident human cardiac progenitor cells, with greater proliferation and differentiation capacity, in the neonatal period as compared with adults, demonstrating the regeneration potential of the young heart, with rising interest in cardiac regeneration therapy in critically ill pediatric patients. We review here the available literature data, searching the MEDLINE, Google Scholar and EMBASE database for completed, and www.clinicaltrials.gov homepage for ongoing studies involving pediatric cardiac regeneration reports. Because of difficulties conducting randomized blinded clinical trials in pediatric patients, mostly case reports or cohort studies with a limited number of individuals have been published in the field of pediatric regenerative cardiology. The majority of pediatric autologous cell transplantations into the cardiac tissue have been performed in critically ill children with severe or terminal heart failure. Congenital heart disease, myocarditis, and idiopathic hypertrophic or dilated cardiomyopathy leading to congestive heart failure are some possible areas of interest for pediatric cardiac regeneration therapy. Autologous bone marrow mononuclear cells, progenitor cells, or cardiospheres have been applied either intracoronary or percutaneously intramyocardially in severely ill children, leading to a reported clinical benefit of cell-based cardiac therapies. In conclusion, compassionate use of autologous stem cell administration has led to at least short-term improvement in heart function and clinical stability in the majority of the critically ill pediatric patients.
Core tip: This review summarizes the available literature data involving pediatric cardiac regeneration reports. Due to lack of randomized blinded clinical trials in pediatric cardiology patients, mostly case reports with limited number of individuals have been published in the pediatric regenerative cardiology. The majority of pediatric autologous cell transplantations into the cardiac tissue have been performed in children with severe or terminal heart failure, and led to the conclusion, that compassionate use of autologous stem cell administration may lead to at least short-term improvement in heart function and clinical stability in the majority of the critically ill pediatric patients.
- Citation: Pavo IJ, Michel-Behnke I. Clinical cardiac regenerative studies in children. World J Cardiol 2017; 9(2): 147-153
- URL: https://www.wjgnet.com/1949-8462/full/v9/i2/147.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i2.147
The overall prevalence of pediatric heart failure is largely unknown because of the non-unique definition and classification of this disease. According to statistical estimations and pediatric registries, 2.5 million children annually are born with congenital heart disease (CHD) worldwide, and among these children, 15%-25% eventually develop heart failure[1-4].
The incidence of pediatric dilated cardiomyopathy with consequent heart failure is low, calculated as 0.57-2.6 per 100000 children under age 18 years[5,6]. In this group, approximately two thirds of cases are idiopathic, and the remaining involve postmyocarditis syndrome or musculoskeletal diseases[7]. Dilated cardiomyopathy dominates myocardial disease-related heart failure, followed by hypertrophic cardiomyopathy, with restrictive cardiomyopathy identified least frequently[8]. The median age of the patients with dilated cardiomyopathy is approximately 1.8 years when the initial diagnosis is made[8].
The mortality of pediatric heart failure is high, and approximately one third of patients die in the first year following diagnosis[9,10]. The surviving children develop progressive heart failure requiring intensive medical care and heart transplantation[7]. For those surviving at least 2 years after the diagnosis, mortality and the need for heart transplantation are somewhat lower (13.6%)[6]. Approximately 18 of every 100000 children are hospitalized annually because of heart failure, with 0.87 new cases per 100000 children per year[11]. The hospital mortality of these pediatric patients is 7%, and numbers are much higher compared to the adult population (4%)[11,12]. After the first hospitalization, only 21% of pediatric patients remain free from serious adverse events (rehospitalization, death, or heart transplantation)[13]. The lack of sufficient numbers of young donor organs and the relatively high post-transplantation mortality limit the incidence and success of pediatric heart transplantation.
In addition, the cost of hospital treatment for pediatric heart failure is usually extremely high, exceeding 135000 USD per patient. Underlying CHD involving a single ventricle, for example, expands the costs of in-hospital treatment for heart failure to over 200000 USD[14].
The medical therapy for pediatric heart failure includes the whole armamentarium used in adults; however, the benefit cannot be clearly demonstrated for all interventions in children[15]. Some established methods for adult cardiology, such as diverse regenerative therapies or left ventricular assist devices, are rarely available for young patients because of incompatibilities of implant size in growing children. Medical treatment might be insufficient because, as noted, many children end up requiring heart transplantation[16].
Newborn mice can regenerate the cardiac apex after resection but only if the resection occurs within the first 7 d after birth[17]. Lineage tracing investigations have revealed that cell cycle entry of pre-existing cardiomyocytes in mice is responsible for this regeneration. Gene expression analysis indicates that neonatal cardiomyocytes maintain proliferation capacity only up to 7 d post-birth, this regeneration property is then lost[17]. Mishra et al[18] investigated the prevalence and proliferation capacity of different stem cell-like cells acquired from cardiac biopsy specimens of children undergoing open heart surgery. They showed that plenty of resident human cardiac progenitor cells (hCPCs, a subpopulation of cardiospheres, CDCs) can be found in the neonatal period but that the number of these cells decreases rapidly with advancing age, from 8.9% to 3.2% in the right atrium and from 0.4% to 0.1% in the right ventricle. In addition, c-kit+ hCPCs were three times more frequently found in neonates than in children over age 2 years. The proliferation and differentiation potential of the hCPCs was also greater in neonates, as shown by the higher expression levels of c-kit and Ki67, as well as the expression of NKX2, NOTCH1, and NUMB, the genes responsible for proliferation and differentiation. Furthermore, heart tissue samples of children with CHD contained an increased number of c-kit+ hCPCs and CD133+ cells, and these cells expressed cardiac lineage and endothelial transcription factors during differentiation under in vitro conditions[19]. CDCs are a rich source of secreted regenerative substances, such as cytokines and growth factors, e.g., vascular endothelial growth factor, hepatocyte growth factor, or insulin-like growth factor, and exert anti-apoptotic and proangiogenic effects in the myocardium[20,21]. CDCs found in infant hearts have higher telomerase activity compared with those of adults.
Together, these data suggest that the regenerative capacity of the heart in children is much greater than that of adults. Additional evidence comes from clinical observations that the younger heart can exhibit morphological changes after volume unloading by surgical correction of CHD[22]. Additionally, pressure overload from a single right ventricle leads to an increase in the number of cardiac stem cells (0.41% ± 0.24%) compared to dilated cardiomyopathy (0.15% ± 0.09%)[23].
To establish standardized therapy and guidelines for treatment of diseases, randomized double-blinded clinical studies delivering evidence-based medicine are necessary. In contrast with the huge number of adult clinical trials, in pediatric cardiology, especially for cardiac regenerative therapy, large randomized trials are lacking. In addition to the understandable ethical reasons, other factors also preclude such trials: The relative rarity of heart failure with a limited number of pediatric patients in the stable clinical condition necessary for randomization, a divergence in terminology, proprietary and often incompatible informatics platforms, and variability in data standards in growing children[24]. In 2012, the United States Food and Drug Administration Safety and Innovation Act intensified pediatric product development, also enhancing the number of pediatric clinical trials. In Europe, the Pediatric Regulation and Pediatric Therapeutics programs have strengthened the applications of new medicines in evidence-based pediatric clinical studies. In contrast with the very spare pediatric regenerative cardiology studies, pediatric cancer and HIV/AIDS treatment networks have already been successfully established and developed with standardized data validity and consistency[24]. We review here the available literature data, searching the Medline, Google Scholar and Embase database for completed, and http://www.clinicaltrials.gov homepage for ongoing studies involving pediatric cardiac regeneration reports.
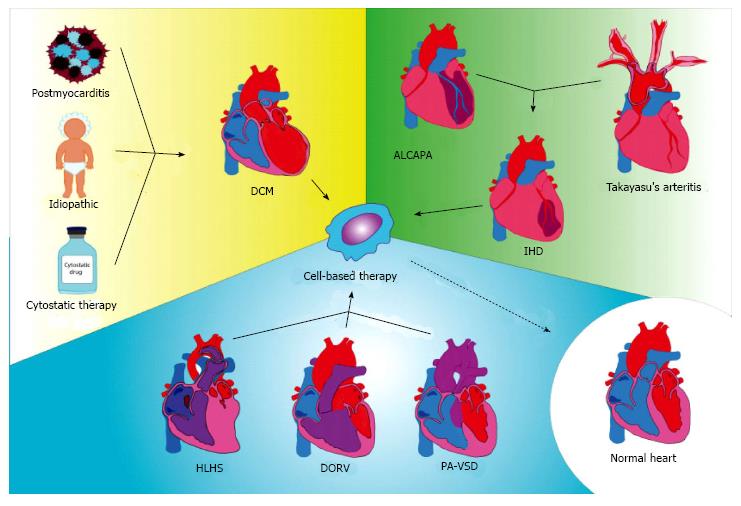
In most cases, cardiac cell-based therapy has been applied in children with severe heart failure caused by diverse diseases, predominantly idiopathic dilated cardiomyopathy, post-myocarditis, or chemotherapy-induced dilated cardiomyopathy (Table 1 and Figure 1). Severe heart failure has been described also with post-myocardial infarction in cases of an anomalous origin of the left coronary artery from the pulmonary artery or Takayasu’s arteritis, treated with different kinds of reparative cells. Other congenital diseases such as double outlet right ventricle, pulmonary atresia with ventricular septal defect, or hypoplastic left heart syndrome (HLHS) causing severely depressed heart function, have been considered for treatment with non-committed cells. Table 2 lists the pediatric diseases for which cardiac cell-based regenerative studies might be considered.
| Cell-based cardiac regenerative treatment | Ongoing studies |
| Dilated cardiomyopathy (Dil. CMP) | Dilated cardiomyopathy (Dil. CMP) |
| Idiopathic dilated CMP | |
| Cytostatics-induced dilated CMP | |
| Postmyocarditis dilated CMP | |
| Ischemic heart failure (myocardial infarction) | |
| Anomalous origin of the left coronary arteries | |
| Takayasu arteritis | |
| Congenital heart disease | |
| DORV after surgical correction | |
| Pulmonary atresia with ventricular septal defect | |
| HLHS | Hypoplastic left heart syndrome (HLHS) |
| Ref. | Study type | Diagnosis | No. of children | Mean age of children (m) | Sex | Type of stem cell | Cell application | FUP | Main results |
| Lacis et al[30] | Case report | Dil. CMP | 1 | 3.5 mo | F | BM-MNC | IM | 4 mo | LV EF from 20% to 41% |
| Rupp et al[31] | Case report | Dil. CMP | 9 | 4 mo-16 yr | NA | BM-MNCs | IC | 1-52 mo | 3 patients HTX, 1 patient died, others improved |
| Ishigami et al[32] (TICAP study) | Controlled study | HLHS | 7 treated and 7 controls | < 6 yr | NA | CDCs | IC | 18 mo | Increase in RV EF from 46.9% to 52.1% in treated patients |
| Rupp et al[33] | Case report | HLHS | 1 | 11 mo | M | BMC | IC | 14 mo | RV EF from 22% to 44% |
| Rupp et al[34] | Case report | Dil. CMP | 1 | 2 year | M | BMC | IC | 6 mo | EF from 24% to 45%, |
| BNP and NYHA decreased | |||||||||
| De Lezo et al[35] | Case Report | Post-AMI | 1 | 7 mo | NA | BM-MNCs | IC | 14 mo | LV EF from 20% to 43% |
| Olguntürk et al[36] | Case report | Dil. CMP | 2 | 6 and 9 yr | M, F | PBSC after GCSF treatment | IC | 8 wk, and 6 mo | 1st patient LV EF from: 16% to 39%; 2nd patient LV EF from 34% to 54% |
| Limsuwan et al[37] | Case report | HF post-AMI | 1 | 9 yr | F | BMC after GCSF treatment | IC | 3 mo | LV EF form 30% to 47% |
| Zeinaloo et al[38] | Case report | Dil. CMP | 1 | 11 yr | M | BM-MSC | IC | 1 yr | LV EF from 20% to 42% |
| Rivas et al[39] | Case report | Dil. CMP | 2 | 3 and 4 mo | M | PBSC after G-CSF treatment | IC | 4 mo | EF from < 30% to > 40% |
| Bergmane et al[40] | Case report | Dil. CMP | 7 | 4 mo-17 yr | NA | BMC | IM | 1 yr | 6 patients controlled, LV EF from 33.5% to 54% |
| Burkhart et al[41] | Case report | HLHS | 1 | 3 m | NA | Umbilical cord blood derived cells | IM | 3 mo | EF increased to 45% |
For the reasons described, to date, only two randomized clinical cardiac regenerative trials with a low number of included children have been conducted. Both have revealed benefits of cardiac cell-based therapy[25-29]. In addition to these currently finished trials, case reports or pilot trial results have been published, mainly based on an indication of compassionate use in severely ill pediatric patients. The majority of children receiving cardiac cell-based therapy were in a critical or terminal status of cardiac decompensation, as evidenced by the fact that some of the children had to undergo heart transplants afterwards[22].
Different types of cells have been used for cardiac regenerative cell therapy in children, such as bone marrow-derived mononuclear cells, cells from leukocyte apheresis, and mesenchymal stem cells. In all cases, autologous cells were used.
Most of the children received the reparative cells via intracoronary injections. To ensure retention of the injected cells, echocardiography-guided transcutaneous intramyocardial delivery was also used, or a transapical delivery mode was applied[30].
The evidence for pediatric cardiac regeneration is mostly anecdotal, deriving from case reports or cohort studies including very limited number of patients (max. nine treated children in Rupp et al[31]). In addition, the only comparative study, published by Ishigami et al[32] allocated 14 children with HLHS to receive either autologous CDCs (n = 7) or standard therapy (n = 7) without randomization. Because of these significant limitations of the available literature, a usual review or meta-analysis of cardiac regenerative studies in children is not reasonable. Thus, this review summarizes the published cases and their conclusions.
Autologous stem cell administration has led to at least short-term improvement in heart function and clinical stability in the majority of patients. Because of the lack of randomization and control groups, an unambiguous interpretation of the results is not possible. At the least, the outcomes indicate a compassionate use of cell-based cardiac regeneration in critically ill patients.
Rupp et al[33,34] reported two cases of bone marrow-origin progenitor cell intracoronary injection, one involving a 2-year-old boy with dilated cardiomyopathy and the other an 11-mo-old infant with HLHS; both of them were in a critical clinical condition of heart failure. The bone marrow progenitor cells were injected into the left anterior descending and left circumflex coronary arteries in the first case and into the dominant right coronary artery in the second case, using a stop-flow technique. The cardiac cell therapy led to an increase in the left ventricular ejection fraction from 24% to 45% at 6 mo of follow-up in the first case, and to reverse remodeling and marked improvement in clinical status in the second case.
In further work, Rupp et al[34] published a somewhat larger cohort study of nine pediatric patients receiving intracoronary injections of autologous bone marrow mononuclear cells (BM-MNCs). The reasons for terminal heart failure in these children were anthracycline-induced dilated cardiomyopathy; post-myocarditis, idiopathic, or congenital cardiomyopathy; CHD with poor ventricular function, such as hypoplastic left heart or double outlet right ventricle; and pulmonary atresia with ventricular septal defect after surgical corrections. Three of the nine patients received a heart transplant and one patient died after cell treatment. The surviving children showed an improvement in clinical status during the 24 to 52 mo of follow-up.
De Lezo et al[35] presented a case of a 5-mo-old infant with severe heart failure due to extensive myocardial infarction because of an anomalous origin of the left coronary artery. After surgical re-implantation of the left coronary artery to the aorta, the artery was occluded, then stented, then dilated after stent occlusion. Because of the critical clinical situation, during the second percutaneous procedure, autologous bone marrow-origin mononuclear cells were injected into the left main branch, which led to a gradual improvement in clinical status and allowed the discharge of the patient.
After mobilizing stem cells from the bone marrow with granulocyte colony-stimulating factor (G-CSF), Olguntürk et al[36] selected peripheral blood-origin stem cells and performed intracoronary injections of these cells into both the left and right coronary arteries in two patients both with dilated cardiomyopathy and severe congestive heart failure. At the 4-mo follow-up, both children showed impressive improvement, and one of them could be removed from the heart transplantation list.
Similarly, Limsuwan et al[37] applied the first daily injections of G-CSF, followed by bone marrow aspiration and selection of CD133+/CD34+ cells in an 8.5-year-old girl who had had an acute extensive anterior myocardial infarction related to Takayasu arteritis one year earlier. The selected stem cells were injected into the left anterior descending artery with the stop-flow technique. The 3-mo follow-up showed an increase in ejection fraction from 30% to 47.8% by cardiac magnetic resonance imaging.
Zeinaloo et al[38] selected autologous bone marrow mesenchymal stem cells in an 11-year-old boy with a diagnosis of dilated cardiomyopathy and injected them into the left and right coronary arteries. The one-year clinical check-up revealed an improvement of the left ventricular ejection fraction from 20% to 42%.
Lacis et al[30] treated a 3-mo-old child, who was in critical clinical condition with dilated cardiomyopathy, with autologous BM-MNCs via echocardiography-guided transcutaneous transapical intramyocardial injections. The ejection fraction increased from 20% to 41% at the 4-mo follow-up, and the child’s clinical well-being was obvious.
Rivas et al[39] treated two children who both had dilated cardiomyopathy and were ages 3 and 4 mo, respectively, by administering peripheral blood progenitor cells, mobilized by G-CSF treatment. One month later, both children presented improvement, but one child developed progression later. This article described a temporary effect of the cell-based cardiac regenerative therapy.
Ishigami et al[32] published a nonrandomized prospective cohort study comparing data for seven patients treated with intracoronary injection of cardiosphere-derived cells and seven controls treated with standard therapy. All children had HLHS with planned stage 2 or 3 surgical palliation, which allowed the collection of autologous tissue for selection of CDCs in the treated group. The intracoronary injection of CDCs proved to be safe, and the right ventricle ejection fraction increased and remained constant at the 18 mo follow-up.
Bergmane et al[40] treated seven children with dilated cardiomyopathy with autologous bone marrow cells administered transcutaneously and intramyocardially by subxyphoid needle puncture under echocardiographic guidance. Six of the seven patients showed dramatically increased left ventricular ejection fraction at one year after the treatment, paralleled by a decrease in N-terminal proBNP and improved clinical status.
Burkhart et al[41] injected autologous umbilical cord blood-derived cells directly into the right ventricle during a second palliative operation of a child with HLHS. Three months later, the ejection fraction had increased to 45% with a marked decrease in plasma pro-BNP. Ongoing registered clinical studies are listed in Table 3.
| Clinicaltrials.gov ID | Diagnosis | Intervention | Study design | No. of patients to enroll | Age eligible | Status |
| NCT01504594 | Dilated CMP | Intracoronary autologous stem cell infusion | Single Group Assignment | 10 | 1 to 16 | Suspended |
| NCT02256501 | CMP | Intracoronary | Randomized | 32 | 1 to 16 | Recruiting |
| NCT02398604 | HLHS | intramyocardial injection of allogeneic mesenchymal cells during the Bi-Directional | Randomized | 30 | to 28 d | Study is not yet open |
| Cavopulmonary Anastomosis | ||||||
| NCT01883076 | HLHS | injections of autologous umbilical cord blood cells into the right ventricle of | Safety Study | 10 | < 18 mo | Recruiting |
| HLHS children undergoing a scheduled Glenn surgical procedure. | ||||||
| NCT01829750 | HLHS | efficacy of intracoronary infusion of cardiac progenitor cells in patients with univentricular heart disease | Randomized | 34 | < 20 yr | Recruiting |
Cell-based cardiac regeneration therapy in pediatric patients has led to at least transient improvement of heart function and improvement of heart failure symptoms in a limited number of pediatric patients included in mostly non-randomized studies or case reports.
The majority of pediatric autologous cell transplantations into the cardiac tissue have been performed in critically ill children with severe or terminal heart failure, indicating that at the moment, this treatment strategy is a supplement after standard therapies have been exhausted. Whether specific age groups or those with structural heart diseases may benefit more than others has to be elucidated.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Austria
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Soliman EZ, Teragawa H, Ueda H S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Bernstein HS, Srivastava D. Stem cell therapy for cardiac disease. Pediatr Res. 2012;71:491-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1440] [Cited by in F6Publishing: 1417] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 3. | Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 578] [Cited by in F6Publishing: 553] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 4. | Madriago E, Silberbach M. Heart failure in infants and children. Pediatr Rev. 2010;31:4-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Kaushal S, Jacobs JP, Gossett JG, Steele A, Steele P, Davis CR, Pahl E, Vijayan K, Asante-Korang A, Boucek RJ. Innovation in basic science: stem cells and their role in the treatment of paediatric cardiac failure--opportunities and challenges. Cardiol Young. 2009;19 Suppl 2:74-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Lipshultz SE, Sleeper LA, Towbin JA, Lowe AM, Orav EJ, Cox GF, Lurie PR, McCoy KL, McDonald MA, Messere JE. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647-1655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 581] [Cited by in F6Publishing: 511] [Article Influence: 24.3] [Reference Citation Analysis (1)] |
| 7. | Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867-1876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 684] [Cited by in F6Publishing: 618] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 8. | Selem SM, Kaushal S, Hare JM. Stem cell therapy for pediatric dilated cardiomyopathy. Curr Cardiol Rep. 2013;15:369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Alvarez JA, Wilkinson JD, Lipshultz SE. Outcome Predictors for Pediatric Dilated Cardiomyopathy: A Systematic Review. Prog Pediatr Cardiol. 2007;23:25-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Arola A, Tuominen J, Ruuskanen O, Jokinen E. Idiopathic dilated cardiomyopathy in children: prognostic indicators and outcome. Pediatrics. 1998;101:369-376. [PubMed] [Cited in This Article: ] |
| 11. | Burns KM, Byrne BJ, Gelb BD, Kühn B, Leinwand LA, Mital S, Pearson GD, Rodefeld M, Rossano JW, Stauffer BL. New mechanistic and therapeutic targets for pediatric heart failure: report from a National Heart, Lung, and Blood Institute working group. Circulation. 2014;130:79-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Rossano JW, Kim JJ, Decker JA, Price JF, Zafar F, Graves DE, Morales DL, Heinle JS, Bozkurt B, Towbin JA. Prevalence, morbidity, and mortality of heart failure-related hospitalizations in children in the United States: a population-based study. J Card Fail. 2012;18:459-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Hollander SA, Bernstein D, Yeh J, Dao D, Sun HY, Rosenthal D. Outcomes of children following a first hospitalization for dilated cardiomyopathy. Circ Heart Fail. 2012;5:437-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Rossano JW, Goldberg DJ, Mott AR, Lin KY, Shaddy RE, Kaufman BD, J . Rychik. Heart failure related hospitalizations in children with single ventricle heart disease in the United States: costly and getting more expensive. J Card Fail. 2012;18:S73. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, Ross RD, Pahl E, Blume ED, Dodd DA. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298:1171-1179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 349] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 16. | Lipshultz SE. Ventricular dysfunction clinical research in infants, children and adolescents. Prog Pediatr Cardiol. 2000;12:1-28. [PubMed] [Cited in This Article: ] |
| 17. | Polizzotti BD, Ganapathy B, Walsh S, Choudhury S, Ammanamanchi N, Bennett DG, dos Remedios CG, Haubner BJ, Penninger JM, Kühn B. Neuregulin stimulation of cardiomyocyte regeneration in mice and human myocardium reveals a therapeutic window. Sci Transl Med. 2015;7:281ra45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 18. | Mishra R, Vijayan K, Colletti EJ, Harrington DA, Matthiesen TS, Simpson D, Goh SK, Walker BL, Almeida-Porada G, Wang D. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation. 2011;123:364-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 19. | Ghazizadeh Z, Vahdat S, Fattahi F, Fonoudi H, Omrani G, Gholampour M, Aghdami N. Isolation and characterization of cardiogenic, stem-like cardiac precursors from heart samples of patients with congenital heart disease. Life Sci. 2015;137:105-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Tarui S, Sano S, Oh H. Stem cell therapies in patients with single ventricle physiology. Methodist Debakey Cardiovasc J. 2014;10:77-81. [PubMed] [Cited in This Article: ] |
| 21. | Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marbán E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 495] [Cited by in F6Publishing: 492] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 22. | Rupp S, Schranz D. Cardiac regeneration in children. Pediatr Cardiol. 2015;36:713-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Rupp S, Bauer J, von Gerlach S, Fichtlscherer S, Zeiher AM, Dimmeler S, Schranz D. Pressure overload leads to an increase of cardiac resident stem cells. Basic Res Cardiol. 2012;107:252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Connor EM, Smoyer WE, Davis JM, Zajicek A, Ulrich L, Purucker M, Hirschfeld S. Meeting the demand for pediatric clinical trials. Sci Transl Med. 2014;6:227fs11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Patel P, Mital S. Stem cells in pediatric cardiology. Eur J Pediatr. 2013;172:1287-1292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 26. | Yang Q, Zhang J, Jiang J. Intracoronary transplantation of genetically modified mesenchymal stem cells, a novel method to close muscular ventricular septal defects. Med Hypotheses. 2011;77:505-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Pillekamp F, Reppel M, Brockmeier K, Hescheler J. Stem cells and their potential relevance to paediatric cardiology. Cardiol Young. 2006;16:117-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Pillekamp F, Khalil M, Emmel M, Brockmeier K, Hescheler J. Stem cells in pediatric heart failure. Minerva Cardioangiol. 2008;56:335-348. [PubMed] [Cited in This Article: ] |
| 29. | Tobita K. Autologous cellular cardiomyoplasty for pediatric dilated cardiomyopathy patients: new therapeutic option for children with failing heart? Pediatr Transplant. 2010;14:151-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Lacis A, Erglis A. Intramyocardial administration of autologous bone marrow mononuclear cells in a critically ill child with dilated cardiomyopathy. Cardiol Young. 2011;21:110-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Rupp S, Jux C, Bönig H, Bauer J, Tonn T, Seifried E, Dimmeler S, Zeiher AM, Schranz D. Intracoronary bone marrow cell application for terminal heart failure in children. Cardiol Young. 2012;22:558-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Ishigami S, Ohtsuki S, Tarui S, Ousaka D, Eitoku T, Kondo M, Okuyama M, Kobayashi J, Baba K, Arai S. Intracoronary autologous cardiac progenitor cell transfer in patients with hypoplastic left heart syndrome: the TICAP prospective phase 1 controlled trial. Circ Res. 2015;116:653-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 33. | Rupp S, Zeiher AM, Dimmeler S, Tonn T, Bauer J, Jux C, Akintuerk H, Schranz D. A regenerative strategy for heart failure in hypoplastic left heart syndrome: intracoronary administration of autologous bone marrow-derived progenitor cells. J Heart Lung Transplant. 2010;29:574-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Rupp S, Bauer J, Tonn T, Schächinger V, Dimmeler S, Zeiher AM, Schranz D. Intracoronary administration of autologous bone marrow-derived progenitor cells in a critically ill two-yr-old child with dilated cardiomyopathy. Pediatr Transplant. 2009;13:620-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | de Lezo JS, Pan M, Herrera C. Combined percutaneous revascularization and cell therapy after failed repair of anomalous origin of left coronary artery from pulmonary artery. Catheter Cardiovasc Interv. 2009;73:833-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Olguntürk R, Kula S, Sucak GT, Ozdoğan ME, Erer D, Saygili A. Peripheric stem cell transplantation in children with dilated cardiomyopathy: preliminary report of first two cases. Pediatr Transplant. 2010;14:257-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Limsuwan A, Pienvichit P, Limpijankit T, Khowsathit P, Hongeng S, Pornkul R, Siripornpitak S, Boonbaichaiyapruk S. Transcoronary bone marrow-derived progenitor cells in a child with myocardial infarction: first pediatric experience. Clin Cardiol. 2010;33:E7-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Zeinaloo A, Zanjani KS, Bagheri MM, Mohyeddin-Bonab M, Monajemzadeh M, Arjmandnia MH. Intracoronary administration of autologous mesenchymal stem cells in a critically ill patient with dilated cardiomyopathy. Pediatr Transplant. 2011;15:E183-E186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Rivas J, Menéndez JJ, Arrieta R, Alves J, Romero MP, García-Guereta L, Álvarez-Doforno R, Parrón M, González A, Ruza F. [Usefulness of intracoronary therapy with progenitor cells in patients with dilated cardiomyopathy: Bridge or alternative to heart transplantation?]. An Pediatr (Barc). 2011;74:218-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Bergmane I, Lacis A, Lubaua I, Jakobsons E, Erglis A. Follow-up of the patients after stem cell transplantation for pediatric dilated cardiomyopathy. Pediatr Transplant. 2013;17:266-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Burkhart HM, Qureshi MY, Peral SC, O‘Leary PW, Olson TM, Cetta F, Nelson TJ. Regenerative therapy for hypoplastic left heart syndrome: first report of intraoperative intramyocardial injection of autologous umbilical-cord blood-derived cells. J Thorac Cardiovasc Surg. 2015;149:e35-e37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |