Published online Feb 26, 2017. doi: 10.4330/wjc.v9.i2.139
Peer-review started: July 16, 2016
First decision: September 30, 2016
Revised: November 20, 2016
Accepted: December 1, 2016
Article in press: December 3, 2016
Published online: February 26, 2017
Non-valvular atrial fibrillation is associated with a significantly increased risk of embolic stroke due to blood clot forming predominantly in the left atrial appendage (LAA). Preventive measures to avoid embolic events are permanent administration of anticoagulants or surgical closure of the LAA. Various clinical trials provide evidence about safety, effectiveness and therapeutic success of LAA occlusion using various cardiac occluder devices. The use of such implants for interventional closure of the LAA is likely to become a valuable alternative for stroke prevention, especially in patients with contraindication for oral anticoagulation as safety, clinical benefit and cost-effectiveness of LAA occlusion has recently been demonstrated.
Core tip: Non-valvular atrial fibrillation is associated with increased risk of embolic stroke. To date, risk-based anticoagulation is the cornerstore to avoid this. However, several patients have got absolute or relative contraindication to this and thus are undertreated. For these patient population the implantation of a local left atrial appendage occluder might be an alternative.
- Citation: Akin I, Nienaber CA. Left atrial appendage occlusion: A better alternative to anticoagulation? World J Cardiol 2017; 9(2): 139-146
- URL: https://www.wjgnet.com/1949-8462/full/v9/i2/139.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i2.139
The left atrial appendage (LAA) is an external protrusion of the left atrium located next to the pulmonary trunk[1,2]. Compelling evidence points to the LAA as the primary origin of thrombus formation particular in the presence of non-valvular atrial fibrillation (AF); since the major risk of non-valvular AF to suffer from ischemic stroke, the LAA has drawn much attention in the context of stroke prevention[3-5] considering missing awareness and unrecognized AF prior to strokes[6]. Thus, the current approach for stroke prevention in patients with non-valvular AF of risk-adjusted prevention via oral anticoagulants (OAC) or antiplatelet agents[7,8] may be challenged by elective LAA occlusion in selective patients[3,4,9-12]. In the past, physical LAA closure required either surgical excision or exclusion by suture or stapler[10,13]. With the introduction of cardiac occluder devices open surgery is not required any more and redundant for this purpose[14-16]. This review summarizes current knowledge of LAA occlusion as an emerging alternative to chronic OAC therapy for non-valvular AF patients at risk for embolic strokes.
The function of the LAA is not fully understood but it has been linked to secretion of the hormone “atrial natriuretic factor” (ANF) and, hence, could be involved in the regulation and homoeostatic control of water, salt and fat[17,18]. Regardless, the anatomy of the LAA is highly diverse and was classified into four different morphological types; “chicken wing” was the most frequently identified type (48%) followed by “cactus” (30%), “windsock” (19%), and “cauliflower” (3%)[19]. The “chicken wing” type has a dominant lobe, which may have secondary lobes or twigs, and is bent in the proximal or middle part or even folds back on itself at some distance from the orifice. The “cactus” type has a dominant central lobe with secondary lobes extending in both superior and inferior directions, whereas the primary structure of the “windsock” type is a dominant lobe with variation in the location and number of secondary or even tertiary lobes. Lastly, the “cauliflower” type has a short overall length with more complex internal characteristics, lacks a dominant lobe but has variable number of lobes and a more irregular shape of the LAA orifice. Previous studies indicate that the “chicken wing” type poses the lowest risk for embolism in contrast to the “cauliflower” type, which, notably, exhibits the highest degree of structural complexity[20]. However, due to the complex anatomy of the LAA, it is difficult to correctly assess length, branches and courses, as well as thrombus formation by transesophageal echocardiography (TEE) and it was demonstrated that the outcome of this visualization is dependent on the selection of the imaging plane[21].
The current gold standard for stroke prevention in patients with non-valvular AF is the oral administration of anticoagulants to reduce the risk of thrombus formation and prevent any embolic events[7,8]. Chronic anticoagulation is carried out by traditional and novel oral anticoagulants (NOACs) also called directly acting oral anticoagulants (DOACs). Traditional anticoagulants include heparins and coumarins (vitamin K antagonists) of which warfarin is the most common. NOACs are inhibitors of coagulation factors such as factors IIa (e.g., dabigatran) or factor Xa (e.g., rivaroxaban, apixaban and edoxaban)[22-25]. However, chronic OAC therapy is not recommended if contraindications are present or potential interference with other therapies. Moreover, difficulties to adjust treatment, dietary restriction, low compliance or even refusal of the patient to follow treatment protocol are considered contraindication for OAC[10,22-26]. Therefore, alternative strategies for stroke prevention in patients with AF are required.
There are two fundamental approaches beyond anticoagulation to avoid emboli in patients with non-valvular AF, e.g., surgical excision of the LAA or exclusion by suture line, stapler or cardiac plug[10,13]. Before introduction of cardiac occluder for LAA closure, surgical excision was the superior method while LAA closure requires either suture line[10,13]. However, a surgical excision is not risk free and may cause bleeding[27]. Nonetheless, surgical excision is still an option mostly in conjunction with other cardiac surgery[10,27]. In recent years, an alternative approach for LAA closure was established by sealing the orifice of the LAA with an occluder. Such a LAA occlusion was performed 2001 for the very first time using the PLAATO system which has been taken off market[28,29]. The current generation of occluders are the Amplatzer™ Cardiac Plug (ACP), Amplatzer Amulet™ from St. Jude Medical and the Watchman™ device from Boston Scientific[29,30]. In addition, a small number of novel devices have been mentioned and applied in the last few years such as the WaveCrest™ device and the Lariat™ device[31,32] (Table 1). The ACP-originally used for closure of atrial septal defects[33] - consists of a self-expanding flexible nitinol mesh with a distal lobe filled with polyester and is equipped with fixation barbs to adhere of the LAA. The distal lobe is connected via a small waist to a proximal disc sealing the orifice of the LAA[34]. Similar to the ACP, the Watchman™ device consists of a self-expanding nitinol mesh with fixation barbs and a polyester coating covering the surface facing the left atrium[35].
| Device name | Company | Design |
| PLAATO | Appriva Medical Inc. | Single-lobe occluder; nitinol cage; ePTFE membrane hooks |
| WATCHMAN | Boston Scientific | Single-lobe occluder;nitinol frame; PET membrane; hooks |
| ACP | St. Jude Medical | Lobe and disc (polyester mesh); nitinol mesh structure; stabilizing wires |
| Amulet | St. Jude Medical | Lobe and disk (polyester mesh in both); nitinol mesh structure; stabilizing wires |
| WaveCrest | Coherex Medical | Single-lobe occluder; nitinol frame, polyurethane foam and ePTFE membrane; retractable anchors |
| Occlutech LAA | Occlutech | Single-lobe occluder; nitinol wire mesh; stabilizing loops; nanomaterial covering |
| Sideris Patch | Custom Medical Devices | Frameless detachable latex balloon covered with polyurethane |
| Lambre | Lifetech | Lobe and disk; nitinol; PET membrane; distal barbs anchors |
| Pfm | PFM Medical | Dual disk (distal anchor, variable middle connector, proximal disk); nitinol frame |
| Ultrasept | Cardia | Lobe and disk; nitinol frame; Ivalon covering; distal anchors |
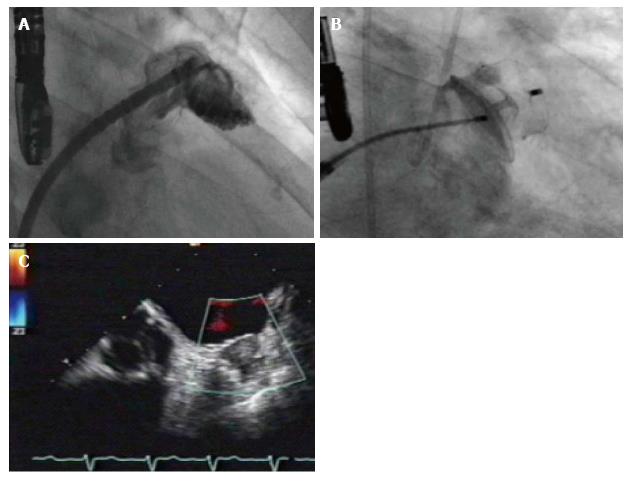
Implantation of both Watchman™ device and ACP can be performed under local anesthesia and is introduced via catheter through the femoral vein by transseptal passage[33,36-39]. TEE guiding or intracardiac echocardiography (ICE) during implantation procedure is used to rule out intracardiac thrombus and to facilitate transseptal puncture. After transseptal puncture heparin is administered to achieve an active clotting time of > 250 s. The LAA is fluoroscopically illustrated in at least 2 standard angulations (RAO 30°, RAO 30°/10° caudal) and sized by TEE measurements and cine angiography. Device size will be chosen according to manufacturer’s recommendations 20% larger than the landing zone (measured from left circumflex coronary artery to the ridge delineating the LAA from the left upper pulmonary vein). Optimally, the device should not protrude more than 5 mm beyond the LAA ostium and should cover the entire ostium with no or minimal (less than 5 mm by colour Doppler) residual flow and a compression grade of 8%-30%. After device releasing and sheath removal the puncture site is dealed with a Z-suture or with a pressure band. Periprocedural anticoagulation is managed by heparine or bivalirudine[14,28,33] (Figure 1). The implanted device becomes initially coated by fibrin and subsequently covered by endothelial cells forming an endocardial lining, which consequently excludes the LAA from circulating blood[40]. In order to allow the process of endothelialization patients have to take warfarin after the intervention for at least 45 d. Warfarin is then replaced by clopidogrel and aspirin for half a year, while aspirin administration is continued life-long[15]. According to newer data a dual antiplatelet therapy is more efficacious than the use of anticoagulants[37,41,42]. In addition to the general risks of catheter-based interventions including air or blood embolism, an incomplete closure of the orifice, pericardial perforation, dislodgement of the implant or the formation of blood clots on the surface of the device leading to prolonged OAC treatment may occur[14,28,33,43]. Finally, since ANF is secreted in the LAA, the LAA closure could interfere with thirst regulation and water retention in the patient, but this theoretical concept has been scarcely investigated so far[17,18].
For several years great effort has been devoted to the use of cardiac plugs in the prevention of AF-related strokes. A number of studies have been published about the PLAATO system including a five-year follow-up study[28,44]. In addition to the PLAATO system, the ACP system which has been used for closure of atrial septal defects for more than 20 years and its features, design and applicability are very well studied and has been reported to be successful for LAA closure[33,34]. It is worth mentioning that a large randomized clinical trial to study the ACP for LAA occlusion was recently halted, probably due to the approval of a competitive product (i.e., Watchman device) by the United States Food and Drug Administration (FDA)[45]. The successful use of the Watchman device for LAA occlusion has been shown in two large, prospective randomized clinical trials-the PROTECT-AF and the PREVAIL study-in which this implant was compared to chronic OAC therapy using warfarin[14,15]. Over five years, the PROTECT-AF study examined about 800 patients, of which 463 had received a Watchman™ device and 244 were left on warfarin[14] and demonstrated that the implant is non-inferior to OAC therapy in patients with non-valvular AF being eligible for OAC. In comparison to the OAC treatment group, the incidence of an embolic event was reduced by approximately 30% in the implant group; the overall mortality showed a reduction of the same magnitude. While the per-protocol analysis was in favor of LAA occlusion, the intention-to-treat results were neutral. Furthermore, the safety data also favored warfarin over LAA occlusion, but this was explained by the learning curve phenomenon. The highest risk from LAA occlusion arises from complications associated with the one-time interventional treatment, while the risks from chronic OAC therapy accumulates during time especially with increasing age of the patient. Recently, a 45-mo follow-up of the PROTECT-AF study demonstrated that LAA occlusion is not only as efficient as warfarin treatment but even superior in term of stroke and cardiovascular mortality[46]. Further, the safety data showed a considerable reduction in the risk for complications during intervention, likely due to increasing experience of the surgeons. However, the FDA criticized the patient selection and raised questions about the safety of LAA occlusion[47]. To address these limitations, a confirmatory randomized trial (PRE-VAIL) comparing LAA occlusion with the Watchman device to warfarin, which mandated inclusion of new operators, slight modifications in inclusion criteria, and elimination of clopidogrel 7 d before implant. In PREVAIL more than 400 patients were randomized to either warfarin (n = 138) or occluder device (n = 269)[15]. This study showed that LAA occlusion achieved non-inferiority in stroke prevention compared to warfarin; the difference between both groups was low and not significant. Most importantly, the number of early safety events (e.g., pericardial effusions) was significantly reduced compared to the PROTECT-AF study and, hence, satisfied the predefined goal. Therefore, the PREVAIL study addressed the concerns rose by the FDA and demonstrated the safety of this intervention for LAA occlusion[15]. As a result of this study the Watchman™ device was approved by the FDA in 2015[48]. Results from the real-world EWOLUTION registry consisting of 1021 patients being implanted with the Watchman device revealed a procedural success rate of 98.5%[49]. During 30-d follow-up 28 subjects experienced serious adverse events with an overall 30-d mortality rate of 0.7%. Serious procedure related complication rates, defined as stroke, pericardial effusion, device embolism and death, were present in 8.7% in PROTECT-AF, 4.1% in CAP registry, 4.2% in PREVAIL and 2.7% in EWOLUTION. However, the average CHADS2 score of 2.8 and CHA2DS2-VASc score of 4.5 in EWOLUTION indicate a relatively higher risk of stroke than either the PROTECT AF (average CHADS2 of 2.2 and CHA2DS2-VASc of 3.4) or PREVAIL (CHADS2 score of 2. 6 and CHA2DS2-VASc of 4.0) studies. In addition, 40% of EWOLUTION subjects had a HAS-BLED score of ≥ 3, compared with only 20% of PROTECT AF subjects and 30% of PREVAIL subjects (Table 2). Similar results were obtained in a registry with the ACP device[50] and a large meta-analysis[51] including 2406 patients from the PROTECT AF, PREVAIL, CAP I and CAP II registries with a mean follow-up of 2.69 years. Patients receiving LAA occlusion with the Watchman device had significantly fewer hemorrhagic strokes [0.15 vs 0.96 events/100 patient-years (PY); hazard ratio (HR): 0.22; P < 0.004], cardiovascular/unexplained death (1.1 vs 2.3 events/100 PY; HR: 0.48; P < 0.006), and nonprocedural bleeding (6.0% vs 11.3%; HR: 0.51; P < 0.006) compared with warfarin. All-cause stroke or systemic embolism was similar between both strategies (1.75 vs 1.87 events/100 PY; HR: 1.02; 95%CI: 0.62 to 1.7; P = 0.94). There were more ischemic strokes in the device group (1.6 vs 0.9 and 0.2 vs 1.0 events/100 PY; HR: 1.95 and 0.22, respectively; P = 0.05 and 0.004, respectively)[51].
| PROTECT-AF[46] | CAP[43] | ASAP[42] | EWOLUTION[49] | ACP[50] | |
| Patients (n) | 463 | 460 | 150 | 1021 | 1047 |
| Follow-up | 4 yr | 16 mo | 14 mo | 30 d | 13 mo |
| CHADS-score | 2.2 | 2.4 | 2.8 | 2.8 | n.a. |
| CHA2DS2-Vasc score | n.a. | n.a. | 4.4 | 4.5 | 4.5 |
| Procedural success | 88.00% | 95.00% | 94.70% | 98.50% | 97.30% |
| Procedural stroke | 1.30% | 0 | 0.70% | n.a. | 0.90% |
| Pericardial effusion | 4.80% | 2.20% | 1.30% | 0.50% | 1.20% |
| Device embolization | 0.60% | 0 | 1.30% | 0.20% | n.a. |
| Major bleeding | 3.50% | n.a. | n.a. | 1.60% | 1.50% |
| Long-term stroke | 2.30% | 1.50% | 0.70% | 0.30% | 2.30% |
An analysis of Panikker et al[16] on 110 patients being suitable and unsuitable for long-term OAC and outcome analysis from the PROTECT-AF trial and registry study compared warfarin, dabigatran, rivaroxaban, apıxaban, aspirin and no treatment using a network meta-analysis. They revealed that stroke and bleeding rates were significantly lower than PROTECT-AF results. Additionally, LAA occlusion achieved cost parity between 4.9 years vs dabigatran 110 mg and 8.4 years vs warfarin and at 10 years, occlusion was cost-saving against all therapies. Similarly, another analysis evaluated the cost effectiveness in patients suffering from AF and absolute contraindication for OAC[52]. For this purpose the ASAP study evaluating the Watchman device, the ACTIVE-A trial evaluating aspirin and clopidogrel and the AVERROES trial evaluating apixaban were compared in a cost-effectiveness analysis. At 5 years, LAA occlusion was cost effective compared with aspirin with an incremental cost-effectiveness ratio of 16971 Euro. As compared with apixaban, it was also cost-effective at 7 years with an incremental cost-effectiveness ratio of 9040 Euro. Apart from the population having an absolute contraindication for OAC similar analysis were performed for patient being eligible for OAC, where LAA occlusion was cost-effective at 7 years and novel oral anticoagulation was cost-effective at 16 years. However, LAA occlusion was superior to novel oral anticoagulation by year 5 and to warfarin by year 10 with respect to cost-effectiveness and cost saving for stroke prevention[53].
In 2012, the European Society of Cardiology (ESC) released a focused update of the guidelines for the management of AF[8]. Interestingly, the ESC also commented on LAA occlusion for prevention of stroke (class IIb recommendation, level of evidence B). Due to insufficient amount of data demonstrating efficacy and safety, the ESC did not recommend an LAA occlusion at this point as a routinely alternative therapy to replace chronic OAC therapy in order to reduce AF-related stroke risk, but recommended to consider this approach for patients with an increased for stroke and contraindications for OAC treatment[8]. However, the references cited as evidence for the recommendation are the PROTECT AF study and the CAP registry. Importantly, neither of these studies included patients who had contraindications to long-term anticoagulation, and both enrolled a majority of patients with relatively low estimated stroke risk. A growing body of evidence suggests that LAA occlusion with the Watchman™ device is an important alternative for OAC therapy using warfarin, yet, a perspective randomized study comparing LAA occlusion to the NOAC is still missing and therefore a final conclusion about the general applicability of LAA occlusion using the Watchman implant cannot be drawn at this point[49,50,54]. Additionally, there are not enough data analyzing different patient cohorts (e.g., sex, age, race, renal insufficiency) as there are data revealing a direct correlation between elevated adiponectin levels and the degree of left atrial blood stasis in men but not in women, and there are more extensive left atrial remodeling and deterioration in LAA function in women than in men[55-57]. There are some subanalyses revealing higher bleeding events in patients older than 75 years after LAA Occluder implantations compared to younger ones (4.4% vs 1.4%), as well as in males as compared to females (3.0% vs 1.8%)[58]. In accordance with the latest recommendations from the ESC, LAA occlusion should definitely be considered if complications with OAC therapy arise or a high bleeding risk exist, regardless if the patient is treated with traditional or novel anticoagulants. This therapeutic approach is even more justified if the patient undergoing chronic OAC therapy suffers a stroke. Under this circumstance and under the light of recent studies about the safety and efficacy of LAA occlusion, this interventional treatment could be a better choice and advisable for this with a CHA2DS2Vasc Score ≥ 2 (Table 3). Summing up the current data, LAA occlusion is a very promising treatment to prevent AF-related strokes due to its safety, cost-effectiveness and therapeutic success.
| Pro | Contra |
| Non-inferiority to oral anticoagulation | Evaluation of other atherothrombotic sources |
| Alternative in patients with contraindication for anticoagulation | Unknown hemodynamic impact |
| Cost-effective | Postprocedural medical treatment not well defined |
| Reduced cumulative bleeding events during follow-up | No comparison between different devices |
| Good results in real-world registries | Undefined impact of residual leaks |
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kirali K, Petretta M S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Wang Y, Di Biase L, Horton RP, Nguyen T, Morhanty P, Natale A. Left atrial appendage studied by computed tomography to help planning for appendage closure device placement. J Cardiovasc Electrophysiol. 2010;21:973-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 2. | Douglas YL, Jongbloed MR, Gittenberger-de Groot AC, Evers D, Dion RA, Voigt P, Bartelings MM, Schalij MJ, Ebels T, DeRuiter MC. Histology of vascular myocardial wall of left atrial body after pulmonary venous incorporation. Am J Cardiol. 2006;97:662-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Manning WJ, Silverman DI, Katz SE, Riley MF, Come PC, Doherty RM, Munson JT, Douglas PS. Impaired left atrial mechanical function after cardioversion: relation to the duration of atrial fibrillation. J Am Coll Cardiol. 1994;23:1535-1540. [PubMed] [Cited in This Article: ] |
| 4. | Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1072] [Cited by in F6Publishing: 1108] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 5. | Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart. 1999;82:547-554. [PubMed] [Cited in This Article: ] |
| 6. | Lamassa M, Di Carlo A, Pracucci G, Basile AM, Trefoloni G, Vanni P, Spolveri S, Baruffi MC, Landini G, Ghetti A. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital-based registry (The European Community Stroke Project). Stroke. 2001;32:392-398. [PubMed] [Cited in This Article: ] |
| 7. | Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010;12:1360-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 953] [Cited by in F6Publishing: 1016] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 8. | Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC)Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESCEndorsed by the European Stroke Organisation (ESO). Eur Heart J. 2016;37:2893-2962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4706] [Cited by in F6Publishing: 4663] [Article Influence: 582.9] [Reference Citation Analysis (0)] |
| 9. | Investigators SP in AF. Stroke Prevention in Atrial Fibrillation Study. Final results. Circulation. 1991;84:527-539. [PubMed] [Cited in This Article: ] |
| 10. | Crystal E, Lamy A, Connolly SJ, Kleine P, Hohnloser SH, Semelhago L, Abouzhar L, Cybulsky I, Shragge B, Teoh K. Left Atrial Appendage Occlusion Study (LAAOS): a randomized clinical trial of left atrial appendage occlusion during routine coronary artery bypass graft surgery for long-term stroke prevention. Am Heart J. 2003;145:174-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Wehinger C, Stöllberger C, Länger T, Schneider B, Finsterer J. Evaluation of risk factors for stroke/embolism and of complications due to anticoagulant therapy in atrial fibrillation. Stroke. 2001;32:2246-2252. [PubMed] [Cited in This Article: ] |
| 12. | Brass LM, Krumholz HM, Scinto JM, Radford M. Warfarin use among patients with atrial fibrillation. Stroke. 1997;28:2382-2389. [PubMed] [Cited in This Article: ] |
| 13. | Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol. 2008;52:924-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 376] [Cited by in F6Publishing: 382] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 14. | Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P; PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1498] [Cited by in F6Publishing: 1483] [Article Influence: 98.9] [Reference Citation Analysis (0)] |
| 15. | Holmes DR, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1027] [Cited by in F6Publishing: 1176] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 16. | Panikker S, Lord J, Jarman JW, Armstrong S, Jones DG, Haldar S, Butcher C, Khan H, Mantziari L, Nicol E. Outcomes and costs of left atrial appendage closure from randomized controlled trial and real-world experience relative to oral anticoagulation. Eur Heart J. 2016;37:3470-3482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Zimmerman MB, Blaine EH, Stricker EM. Water intake in hypovolemic sheep: effects of crushing the left atrial appendage. Science. 1981;211:489-491. [PubMed] [Cited in This Article: ] |
| 18. | Yoshihara F, Nishikimi T, Kosakai Y, Isobe F, Matsuoka H, Takishita S, Kawashima Y, Saito Y, Matsuo H, Kangawa K. Atrial natriuretic peptide secretion and body fluid balance after bilateral atrial appendectomy by the maze procedure. J Thorac Cardiovasc Surg. 1998;116:213-219. [PubMed] [Cited in This Article: ] |
| 19. | Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, Horton R, Sanchez JE, Bai R, Mohanty S. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. 2012;60:531-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 515] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 20. | Beigel R, Wunderlich NC, Ho SY, Arsanjani R, Siegel RJ. The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging. 2014;7:1251-1265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 275] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 21. | Stöllberger C, Ernst G, Bonner E, Finsterer J, Slany J. Left atrial appendage morphology: comparison of transesophageal images and postmortem casts. Z Kardiol. 2003;92:303-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7917] [Cited by in F6Publishing: 7703] [Article Influence: 513.5] [Reference Citation Analysis (0)] |
| 23. | Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6519] [Cited by in F6Publishing: 6472] [Article Influence: 497.8] [Reference Citation Analysis (2)] |
| 24. | Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6075] [Cited by in F6Publishing: 6088] [Article Influence: 468.3] [Reference Citation Analysis (0)] |
| 25. | Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3447] [Cited by in F6Publishing: 3474] [Article Influence: 315.8] [Reference Citation Analysis (0)] |
| 26. | Albertsen IE, Rasmussen LH, Overvad TF, Graungaard T, Larsen TB, Lip GY. Risk of stroke or systemic embolism in atrial fibrillation patients treated with warfarin: a systematic review and meta-analysis. Stroke. 2013;44:1329-1336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 27. | Healey JS, Crystal E, Lamy A, Teoh K, Semelhago L, Hohnloser SH, Cybulsky I, Abouzahr L, Sawchuck C, Carroll S. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J. 2005;150:288-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 340] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 28. | Sievert H, Lesh MD, Trepels T, Omran H, Bartorelli A, Della Bella P, Nakai T, Reisman M, DiMario C, Block P. Percutaneous Left Atrial Appendage Transcatheter Occlusion to Prevent Stroke in High-Risk Patients With Atrial Fibrillation Early Clinical Experience. Circulation. 2002;105:1887-1899. [PubMed] [Cited in This Article: ] |
| 29. | Aryana A, Saad EB, d’Avila A. Left atrial appendage occlusion and ligation devices: what is available, how to implement them, and how to manage and avoid complications. Curr Treat Options Cardiovasc Med. 2012;14:503-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Khattab AA, Meier B. Transcatheter left atrial appendage closure for stroke prevention among atrial fibrillation patients. Expert Rev Cardiovasc Ther. 2012;10:819-821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Saw J, Lempereur M. Percutaneous left atrial appendage closure: procedural techniques and outcomes. JACC Cardiovasc Interv. 2014;7:1205-1220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 32. | De Backer O, Arnous S, Ihlemann N, Vejlstrup N, Jørgensen E, Pehrson S, Krieger TD, Meier P, Søndergaard L, Franzen OW. Percutaneous left atrial appendage occlusion for stroke prevention in atrial fibrillation: an update. Open Heart. 2014;1:e000020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Meier B, Palacios I, Windecker S, Rotter M, Cao QL, Keane D, Ruiz CE, Hijazi ZM. Transcatheter left atrial appendage occlusion with Amplatzer devices to obviate anticoagulation in patients with atrial fibrillation. Catheter Cardiovasc Interv. 2003;60:417-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 164] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 34. | Freixa X, Chan JL, Tzikas A, Garceau P, Basmadjian A, Ibrahim R. The Amplatzer™ Cardiac Plug 2 for left atrial appendage occlusion: novel features and first-in-man experience. EuroIntervention. 2013;8:1094-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Fountain RB, Holmes DR, Chandrasekaran K, Packer D, Asirvatham S, Van Tassel R, Turi Z. The PROTECT AF (WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial. Am Heart J. 2006;151:956-961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 36. | Onalan O, Crystal E. Left atrial appendage exclusion for stroke prevention in patients with nonrheumatic atrial fibrillation. Stroke. 2007;38:624-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Sharma D, Reddy VY, Sandri M, Schulz P, Majunke N, Hala P, Wiebe J, Mraz T, Miller MA, Neuzil P. Left Atrial Appendage Closure in Patients With Contraindications to Oral Anticoagulation. J Am Coll Cardiol. 2016;67:2190-2192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Nietlispach F, Gloekler S, Krause R, Shakir S, Schmid M, Khattab AA, Wenaweser P, Windecker S, Meier B. Amplatzer left atrial appendage occlusion: single center 10-year experience. Catheter Cardiovasc Interv. 2013;82:283-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 39. | Guérios EE, Schmid M, Gloekler S, Khattab AA, Wenaweser PM, Windecker S, Meier B. Left atrial appendage closure with the Amplatzer cardiac plug in patients with atrial fibrillation. Arq Bras Cardiol. 2012;98:528-536. [PubMed] [Cited in This Article: ] |
| 40. | Schwartz RS, Holmes DR, Van Tassel RA, Hauser R, Henry TD, Mooney M, Matthews R, Doshi S, Jones RM, Virmani R. Left atrial appendage obliteration: mechanisms of healing and intracardiac integration. JACC Cardiovasc Interv. 2010;3:870-877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 41. | Chun KR, Bordignon S, Urban V, Perrotta L, Dugo D, Fürnkranz A, Nowak B, Schmidt B. Left atrial appendage closure followed by 6 weeks of antithrombotic therapy: a prospective single-center experience. Heart Rhythm. 2013;10:1792-1799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | Reddy VY, Möbius-Winkler S, Miller MA, Neuzil P, Schuler G, Wiebe J, Sick P, Sievert H. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol. 2013;61:2551-2556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 481] [Cited by in F6Publishing: 529] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 43. | Sick PB, Schuler G, Hauptmann KE, Grube E, Yakubov S, Turi ZG, Mishkel G, Almany S, Holmes DR. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2007;49:1490-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 243] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 44. | Block PC, Burstein S, Casale PN, Kramer PH, Teirstein P, Williams DO, Reisman M. Percutaneous left atrial appendage occlusion for patients in atrial fibrillation suboptimal for warfarin therapy: 5-year results of the PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) Study. JACC Cardiovasc Interv. 2009;2:594-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 45. | Price MJ, Valderrábano M. Left atrial appendage closure to prevent stroke in patients with atrial fibrillation. Circulation. 2014;130:202-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 46. | Reddy VY, Sievert H, Halperin J, Doshi SK, Buchbinder M, Neuzil P, Huber K, Whisenant B, Kar S, Swarup V. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 633] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 47. | Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011;123:417-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 592] [Cited by in F6Publishing: 647] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 48. | Waksman R, Pendyala LK. Overview of the Food and Drug Administration circulatory system devices panel meetings on WATCHMAN left atrial appendage closure therapy. Am J Cardiol. 2015;115:378-384. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | Boersma LV, Schmidt B, Betts TR, Sievert H, Tamburino C, Teiger E, Pokushalov E, Kische S, Schmitz T, Stein KM. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37:2465-2474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 358] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 50. | Tzikas A, Shakir S, Gafoor S, Omran H, Berti S, Santoro G, Kefer J, Landmesser U, Nielsen-Kudsk JE, Cruz-Gonzalez I. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention. 2016;11:1170-1179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 376] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 51. | Holmes DR, Doshi SK, Kar S, Price MJ, Sanchez JM, Sievert H, Valderrabano M, Reddy VY. Left Atrial Appendage Closure as an Alternative to Warfarin for Stroke Prevention in Atrial Fibrillation: A Patient-Level Meta-Analysis. J Am Coll Cardiol. 2015;65:2614-2623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 366] [Cited by in F6Publishing: 386] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 52. | Reddy VY, Akehurst RL, Armstrong SO, Amorosi SL, Brereton N, Hertz DS, Holmes DR. Cost effectiveness of left atrial appendage closure with the Watchman device for atrial fibrillation patients with absolute contraindications to warfarin. Europace. 2016;18:979-986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 53. | Reddy VY, Akehurst RL, Armstrong SO, Amorosi SL, Beard SM, Holmes DR. Time to Cost-Effectiveness Following Stroke Reduction Strategies in AF: Warfarin Versus NOACs Versus LAA Closure. J Am Coll Cardiol. 2015;66:2728-2739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 54. | Koifman E, Lipinski MJ, Escarcega RO, Didier R, Kiramijyan S, Torguson R, Waksman R. Comparison of Watchman device with new oral anti-coagulants in patients with atrial fibrillation: A network meta-analysis. Int J Cardiol. 2016;205:17-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Cohoon KP, Mazur M, McBane RD, Ketha S, Ammash N, Wysokinski WE. The impact of gender and left atrial blood stasis on adiponectin levels in non-valvular atrial fibrillation. Int J Cardiol. 2015;181:207-212. [PubMed] [DOI] [Cited in This Article: ] |
| 56. | Yu HT, Lee JS, Kim TH, Uhm JS, Joung B, Hong GR, Lee MH, Shim CY, Pak HN. Advanced Left Atrial Remodeling and Appendage Contractile Dysfunction in Women Than in Men Among the Patients With Atrial Fibrillation: Potential Mechanism for Stroke. J Am Heart Assoc. 2016;5:pii: e003361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Gafoor S, Franke J, Bertog S, Boehm P, Heuer L, Gonzaga M, Bauer J, Braut A, Lam S, Vaskelyte L. Left atrial appendage occlusion in octogenarians: short-term and 1-year follow-up. Catheter Cardiovasc Interv. 2014;83:805-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | Price MJ, Reddy VY, Valderrábano M, Halperin JL, Gibson DN, Gordon N, Huber KC, Holmes DR. Bleeding Outcomes After Left Atrial Appendage Closure Compared With Long-Term Warfarin: A Pooled, Patient-Level Analysis of the WATCHMAN Randomized Trial Experience. JACC Cardiovasc Interv. 2015;8:1925-1932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |