INTRODUCTION
Berberine (BBR) is a natural alkaloid isolated from the Coptis Chinensis. This plant has been used for medicinal purposes for more than 2500 years in Ayurvedic and Chinese medicine. Although routinely prescribed in Asian countries for its antimicrobial activity in the treatment of gastrointestinal infections and diarrhoea, and usually used for the treatment of diabetes mellitus, an interest in its beneficial effects in metabolic and cardiovascular diseases has been growing in the Western world over the last decade. Recent literature suggests BBR is a drug with multiple target characteristics, which are already known in traditional medicine. Its activity in carbohydrate and lipid metabolisms, diabetes mellitus treatment, endothelial function and the cardiovascular system has been investigated in the last decade with interesting results both in animals and clinical studies. This review analyzes the scientific literature on the effects and the underlying mechanisms of BBR on carbohydrate and lipid metabolism, endothelial function and the cardiovascular system.
GLUCIDIC METABOLISM
BBR’s effects on glucidic metabolism are well known in China, where it has been used as an oral hypoglycemic agent in the treatment of type 2 diabetes mellitus for many years. There are many clinical reports on the hypoglycaemic action of BBR in the Chinese literature, which were confirmed by controlled clinical trials[1].
Several studies have only recently investigated how it may exert its action on glucose metabolism and insulin sensitivity. Insulin resistance is a major metabolic abnormality leading not only to type 2 diabetes, but also to a group of metabolic disorders known as the metabolic syndrome[2].
In 2006, Lee et al[3] investigated the mechanisms underlying the effects of BBR in the treatment of diabetes and obesity and on insulin resistance. Their experiments in vivo and in vitro paved the way to future understanding. They focused interest on a heterotrimeric protein that plays a key role in the regulation of whole-body energy homeostasis; i.e. adenosine mono-phosphate kinase (AMPK), showing that part of the effects exerted by BBR on diabetes and obesity was due to the stimulation of this protein kinase. The administration of BBR to db/db mice led to a significant body weight reduction with both a significant reduction in fasting blood glucose and improvement in glucose tolerance. Similar effects were also observed in high fat fed Whistar rats, in which BBR administration reduced triglycerides (TG), body weight and improved insulin action in comparison to chow fed rats. The mechanisms that were the basis of these results were detected in the expression of genes involved in energy metabolism. BBR downregulated the expression of genes involved in lipogenesis and upregulated those involved in energy expenditure in adipose tissue and in muscle. Particularly, 11β-hydroxysteroid dehydrogenase, a key enzyme linked to visceral obesity and metabolic syndrome, decreased, and the expression of most genes involved in carbohydrate metabolism was also reduced. In contrast, the transcript level of enzymes related to energy dissipation, including glycerol kinase and acyl-CoA dehydrogenase, increased. These results implied that BBR treatment in vivo resulted in a modulation of the gene expression profile that would promote catabolism of high energy intermediates[3].
Others mechanisms involved in BBR actions were clarified by Zhou et al[4] and subsequently confirmed by other authors. They reported that BBR promotes glucose uptake in 3T3-L1 preadipocytes through a mechanism distinct from insulin. Insulin increases cellular glucose uptake by promoting GLUT4 expression on the cell surface through the activation of phosphatidylinositol 3-kinase (PI3K). On the contrary, BBR’s effect on glucose uptake was insensitive to wortmannin, an inhibitor of PI3K. It seemed that BBR could induce glucose transport by activating GLUT1; particularly BBR increases glucose transport by enhancing GLUT1 gene expression[5]. These effects are mediated by the activation of AMPK, which coordinates both short and long term metabolic changes, leading to an improvement in energy production and a reduction of energy storage. Specifically, its activation results in an increase in the uptake of glucose from the blood to target organs. Further, AMPK inhibits the accumulation of fat by modulating down-stream-signaling components like acetil CoA carbossilase (ACC). AMPK inhibits ACC activity by direct phosphorylation, which leads to a blockage of fatty acid synthesis pathways[6,7]. AMPK phosphorylation and ACC phosphorylation were increased in myoblasts and adipocytes in vitro after short-term treatment with BBR, and in liver after long-term BBR treatment of db/db mice[3].
The results of various recent studies have pointed out a possible mechanism of activation of AMPK, mediated by BBR. It was observed that BBR reduced oxygen-dependent glucose oxidation through inhibition of the respirator mitochondrial complex I. To compensate for the reduction in aerobic respiration, it was observed that there was an increase in glycolysis, a biochemical pathway that requires more glucose than aerobic respiration for the production of the same amount of ATP. As a consequence, glucose uptake and its utilization were increased, and associated with a persistent elevation in the AMP/ATP ratio, which induced the activation of AMPK. Also metformin and rosiglitazone, in a dose-dependent manner, inhibited respiration; rosiglitazone displayed similar potency to BBR, while metformin was substantially less potent. These data highlighted the importance of complex I of the mitochondrial respiratory chain as a major target for BBR in order to obtain an activation of AMPK[8,9].
In obese hyperinsulinemic rats, BBR treatment significantly decreased lipid levels, plasma glucose and insulin levels. Oral glucose tolerance tests revealed a decrease of plasma glucose and insulin levels; in addition, the results of insulin tolerance suggested a marked improvement in insulin resistance. According to these in vivo results, it was observed that BBR acutely decreased glucose-stimulated insulin secretion in pancreatic β-cells isolated from rats through the AMPK signalling pathway[10].
This evidence was clinically confirmed by a double-blind, placebo-controlled trial, in which BBR administration decreased fasting and postprandial plasma glucose with slightly decreasing postprandial insulin and body weight reduction in type 2 diabetic patients[11].
Another important mechanism underlying the effects of BBR on insulin sensitivity is increases insulin-receptor (InsR) expression in a dose and time-dependent manner. BBR enhancement of InsR expression improves cellular glucose consumption only in the presence of insulin. Silencing the InsR gene reduces this effect. BBR induces InsR gene expression, with a mechanism of transcriptional regulation through protein kinase C (PKC). In type 2 diabetic mice, treatment with BBR lowered fasting blood glucose and fasting serum insulin, increased insulin sensitivity and elevated InsR mRNA, as well as PKC activity in the liver. In addition, it was observed that BBR did not lower blood glucose in type 1 diabetic mice, because of their insulin deficiency[12]. The same results were obtained in a variety of human cell lines and were confirmed in a randomized clinical trial, in which BBR treatment significantly lowered fasting blood glucose, hemoglobin A1c, TG and insulin levels in patients with type 2 diabetes mellitus. In this study, metformin and rosiglitazone were used as references. The effects of BBR on fasting glucose and hemoglobin A1c were similar to those of metformin and rosiglitazone; moreover, BBR showed an important activity in reducing the serum levels of TG. Serum insulin levels declined significantly. Consistent with the in vitro experiments, the mean percentage of peripheral blood lymphocytes that express InsR on the surface, isolated from the patients treated with BBR, was significantly elevated in comparison to that before BBR treatment. These finding confirmed the activity of BBR on the up-regulation of InsR in type 2 diabetes mellitus patients and its relationship with the glucose-lowering effect[13].
Contrary to thiazolidinediones (TZDs), it has been shown that BBR reduces the expression levels of peroxisome proliferator activated receptor γ, suppresses the differentiation of preadipocytes and reduces the accumulation of lipid droplets[14-16]. Thus, unlike TZDs, which may lead to weight gain, BBR may be more suitable for insulin-resistant and diabetic patients with obesity. The insulin-sensitizing and glucose-lowering mechanisms of BBR are of great interest in this field.
In conclusion, we can summarize that, although all the mechanisms underlying BBR’s action on glucidic metabolism are not yet completely clarified and continue to be under investigation, its properties, namely reducing fasting blood glucose, hemoglobin A1c, and insulin levels in patients with type 2 diabetes mellitus, reduction of fat mass and TG, improvement of insulin resistance, and reduction of body weight, make BBR a promising molecule for future development in the treatment of glucidic disorders.
LIPID METABOLISM
The main interest of the Western world for BBR properties was initially concentrated on lipid metabolism. In fact, it is an approved nutraceutical substance for treatment of hyperlipidemia in many countries. In 2004, Kong et al[17] defined BBR as “a new cholesterol-lowering drug”. They demonstrated in vitro and in vivo the efficacy of this substance in lipid lowering, which was comparable to that of statins. The in vitro studies on human hepatocellular liver carcinoma cell lines (HepG2) showed that BBR increases the expression of the liver low-density lipoprotein receptor (LDLR) gene at a post-transcriptional level. The increase was dose and time dependent and was obtained, by stabilizing its mRNA, through the activation of the extracellular signal-regulated kinase pathway. This mechanism is distinct from statins, and indeed this activity is totally independent of intracellular cholesterol levels and has no effects on the activation process of the sterol-regulatory element binding protein (SREBP) or the activity of hydroxymethylglutaryl CoA reductase. These findings translated into clinical results, and, in fact, BBR administration in hypercholesterolemic patients led to a significant cholesterol reduction, which was also evident in animal studies[17]. The same research group subsequently elucidated the mechanism observed by Brusq et al[18]. This latter group demonstrated that BBR via AMPK activation inhibited cholesterol and TG synthesis in hepatic cells. He assumed that LDLR upregulation, AMPK activation and lipid synthesis inhibition were abolished when the MAPK/extracellular signal-regulated kinase (ERK) pathway was blocked, but he did not identify the exact mechanisms[18]. Some years later, Abidi et al[19] clarified that the BBR-induced stabilization of LDLR mRNA is mediated by the ERK signalling pathway through interactions of the cis-regulatory sequences of the 3-untranslated region of the LDLR mRNA and mRNA binding proteins that are downstream effectors of this signalling cascade[19]. Moreover, they identified the LDLR mRNA untranslated region responsible for the rapid mRNA turnover, as the target of BBR action leading to LDLR-mRNA stabilization[20].
An additional mechanism acting on the pro-protein convertase subtilisin/kexin type 9 (PCSK9) has only been recently clarified. PCSK9 downregulates, post-trascriptionally, LDLR by shuttling it to the lysosomes for degradation, thus increasing the level of circulating LDL-cholesterol (LDLc). BBR decreases PCSK9 mRNA and protein levels in a type and dose dependent manner, likely due to a decreased transcription of the PCSK9 gene[21]. Thus, BBR could have dual actions on LDLR metabolism by prolonging its mRNA half-life as well as directly increasing protein abundance through the blockage of PCSK9-mediated degradation. Differently from BBR, statins induce PCSK9 transcription[22], which makes BBR an attractive candidate for enhancing statin efficacy. The mechanisms underlying the transcriptional suppression of PCSK9 by BBR have been recently clarified in an in vitro study on HepG2 cells. Li et al[23] identified a highly conserved hepatocyte nuclear factor 1 (HNF1) binding site as a critical sequence motif for PCSK9 transcription. BBR reduction of HNF1 and nuclear SREBP2 led to a strong suppression of PCSK9.
This observed synergy would be beneficial with regard to LDLR expression, because SREBP2 is absolutely required for LDLR transcription, and a strong inhibition of SREBP2 would eventually shut down LDLR expression. The fact that BBR treatment increases LDLR protein levels in HepG2 cells, and in livers of hyperlipidemic hamsters in vivo, suggests that the balanced effects of BBR are in favour of LDLR expression and stability[12,17].
Since BBR counteracts the inducing effects of statins on PCSK9 transcription, their combination may translate into a synergistic efficacy. In fact the combination of BBR with simvastatin (SIMVA) increased LDLR gene expression to a level significantly higher than that in monotherapies. The rats treated with a combination of BBR and SIMVA showed a significantly reduced serum LDLc by 46.2%, which was more effective than that of the SIMVA (28.3%) or BBR (26.8%) monotherapy. More effective reduction of serum TG was also achieved with the combination as compared with either monotherapy. In addition, a significant reduction of liver fat storage was found after combination therapy[24]. These observations translated into clinical results; in fact, the evaluation of the therapeutic efficacy of the combination in 63 hypercholesterolemic patients showed an improved lipid-lowering effect, with 31.8% reduction of serum LDLc compared with 23.8% with BBR and 14.3% with SIMVA monotherapies. Similar effects were observed in the reduction of total cholesterol as well as TG[24]. What is really intriguing in all these mechanisms is the activation of AMPK, which has been proposed to play a key role in the regulation not only of glucidic metabolism, as above clarified, but also of lipid one[12]. As observed for LDLR up-regulation, BBR effects on lipid synthesis are mediated by the ERK pathway. AMPK phosphorylates and inactivates ACC, a key enzyme involved in fatty acid synthesis, leading to an increase in fatty acid oxidation, decrease in fatty acid synthesis and TG synthesis inhibition. This finding could be more interesting because AMPK activation has been proposed as a valuable approach to target lipid disorders[25] and because antidiabetic drugs, such as rosiglitazone and metformin, have been described to act, at least partially, through AMPK activation[26].
A more recent study about combination strategy investigated the efficacy in lipid lowering of BBR combined with other nutraceutical compounds. In vivo study in hamsters showed that combination with plant sterol improves cholesterol lowering efficacy through a synergistic action on cholesterol absorption in addition to synergistically reducing plasma TG[27]. Some clinical trials confirmed the effectiveness of a combination strategy. Cicero et al[28] demonstrated not only the efficacy of BBR alone but also its enhanced potency when combined with other recognized hypolipidemic nutraceutical agents, namely policosanols and red yeast rice (RYR). The efficacy of the same nutraceutical combination in dislipidemic patients has been confirmed recently by Affuso et al[29] with a double blind placebo controlled clinical trial, in which a significant reduction of total LDLc and TG was shown in a group of patients with hypercholesterolemia. The intriguing mechanisms involved in glucidic and lipidic metabolism support BBR efficacy in treating metabolic disorders. The sharing of metabolic pathways and the increasing prevalence of metabolic syndrome have focused interest on this molecule. The safety profile demonstrated in the clinical studies and the favourable results in combination therapy support its use not only in mild hyperlipidemia but also in patients that do not tolerate statins or do not achieve therapeutic goals[30] with single therapy.
EFFECTS ON ENDOTHELIUM AND THE HEART
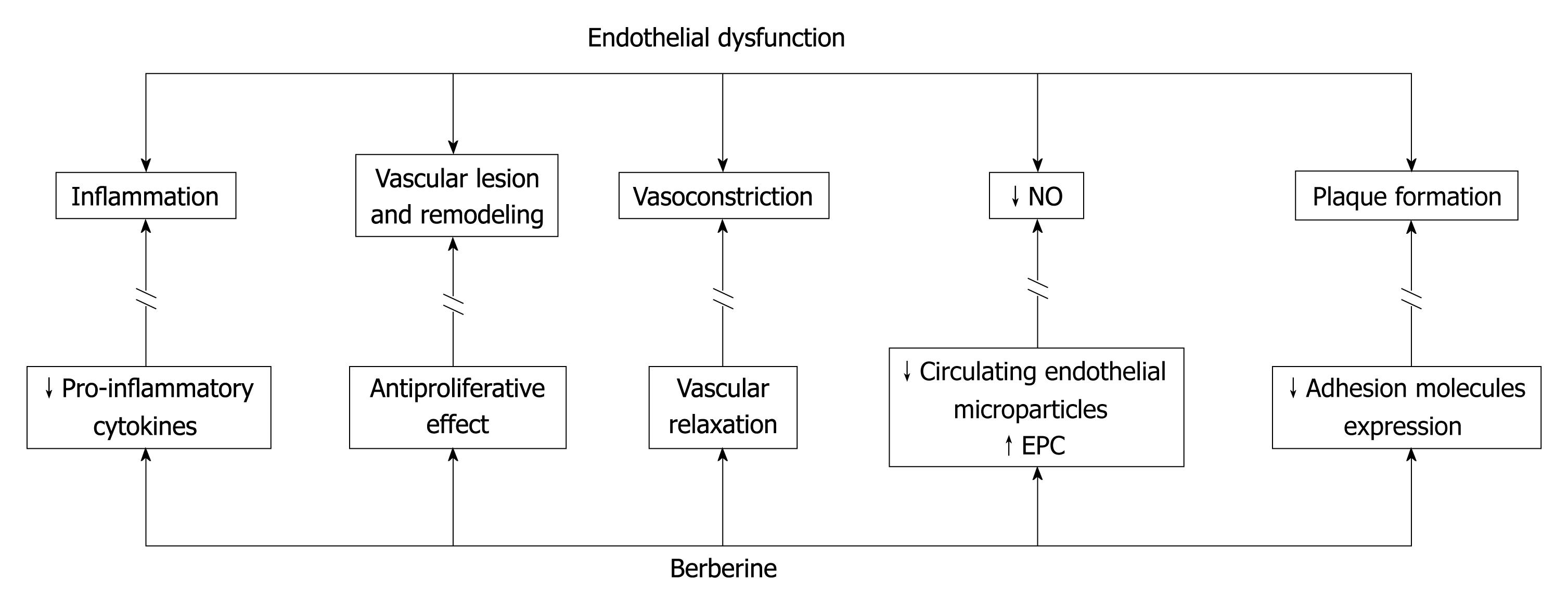
Endothelial dysfunction is an important early event in the pathogenesis of atherosclerosis. The mechanisms underlying endothelial injury are numerous and linked to metabolic alteration. In obesity and insulin resistance, the increased secretion of proinflammatory cytokines and decreased secretion of adiponectine, the increased circulating levels of free fatty acids, and hyperglycaemia may alter gene expression and cell signalling in the vascular endothelium, contributing to changes in the release of endothelium derived factors. Dysfunctional endothelium is characterized by activation of NADPH oxidase, uncoupling of endothelial nitric oxide synthase (eNOS), increased expression of endothelin-1, an imbalance between the production of vasodilators and vasoconstrictor mediators, and induction of adhesion molecules[31]. The altered endothelial homeostasis, in turn, contributes to plaque initiation and progression. It is associated with most cardiovascular disease, such as hypertension, coronary artery disease, chronic heart failure, peripheral artery disease, diabetes and chronic renal failure[32]. Endothelial cells exposed to hypercholesterolemia show a reduced capacity to release endothelium-derived relaxing factors, because of LDLc promotion of endothelial eNOS downregulation[33]. Lowering cholesterol levels appears to improve endothelial function[34]. In diabetes and insulin resistance, other mechanisms may trigger endothelial dysfunction. Insulin signalling is altered in these two conditions, and affects the pathway leading to phosphorylation and activation of eNOS, which is also, in this case, dramatically downregulated[35]. eNOS represents a major weapon of endothelial cells to fight vascular disease. It generates nitric oxide (NO), whose role is to dilate blood vessels and maintain vascular homeostasis by stimulating cGMP[36]. Several studies have suggested a central role of endothelial AMPK in maintaining physiological functions, such as mediation of eNOS activation in response to shear stress[37], modulation of endothelial cell energy supply[38], protection from apoptosis[39] and regulation of inflammation, angiogenesis, and maintenance of perfusion[40,41]. Impairment of endothelium dependent relaxation (EDR) represents reduced eNOS derived NO bioavailability, and is the first step in endothelial injury. It is present also in the absence of vessel damage. In 2000, Ko et al[42], by in vitro investigation, demonstrated that BBR has not only vasorelaxant but also antiproliferative effects. According to their results, BBR could act both on the endothelium and on the underlying vascular smooth muscle cells to induce relaxation (Figure 1). NO, is likely involved in the EDR. More recently, this mechanism has been clarified in endothelial cells isolated from rat. It was confirmed that the vasodilatant effect of BBR was mediated by eNOS leading to NO production through activation of the AMPK cascade. Moreover, BBR counteracts several adverse effects of hyperglycemia on the endothelium, including the inhibition of high glucose-induced reactive species intracellular accumulation and cellular apoptosis and inflammation, which characterize vascular injury[31,43]. Another recognized effect of BBR is the significant decrease in the number of adherent monocytes on endothelial cells, which is a key event in the early stages of atherosclerosis. Furthermore, BBR suppresses the activation of the nuclear factor-κB (NF-κB), the expression of adhesion molecules (VCAM-1 and ICAM-1) induced by hyperglycemia and the high glucose-induced elevation of several pro-inflammatory cytokines and chemokines, including tumor necrosis factor-α, IL1-β, IL8 and MCP1, which are other targets of NF-κB involved in the development of atherosclerotic plaques[43] (Figure 1).
Figure 1 Counteracting effects of Berberine (BBR) on endothelial dysfunction.
eNOS: Endothelial nitric oxide synthase; AMPK: Adenosine mono-phosphate kinase.
Others mechanisms as the basis of BBR effects on the endothelium have been clarified by Xu et al[44]. Recently they demonstrated, in healthy subjects, that BBR induces an up regulation of endothelial progenitor cells (EPC-CFUs) through NO production. The number of EPC-CFUs was increased after BBR treatment and the functions, particularly proliferation, adhesion and migration, were enhanced. In addition, the same research group observed that the circulating endothelial microparticles (EMPs), usually associated with endothelial dysfunction, declined after BBR therapy (Figure 2)[45]. This observation was associated with an improvement of flow mediated vasoldilation (FMD) in healthy subjects, underlying a strong relationship with EMPs decline. Moreover the EMPs led to diminished eNOS protein expression in vitro, a detrimental effect inhibited by BBR[45]. The clinical effects of BBR treatment on FMD have also been recently demonstrated in a clinical, double blind placebo controlled study, in which BBR was administered combined together with policosanols and RYR. This study demonstrated that the treatment produced a significant improvement of FMD in a population of hypercholesterolemic subjects[29].
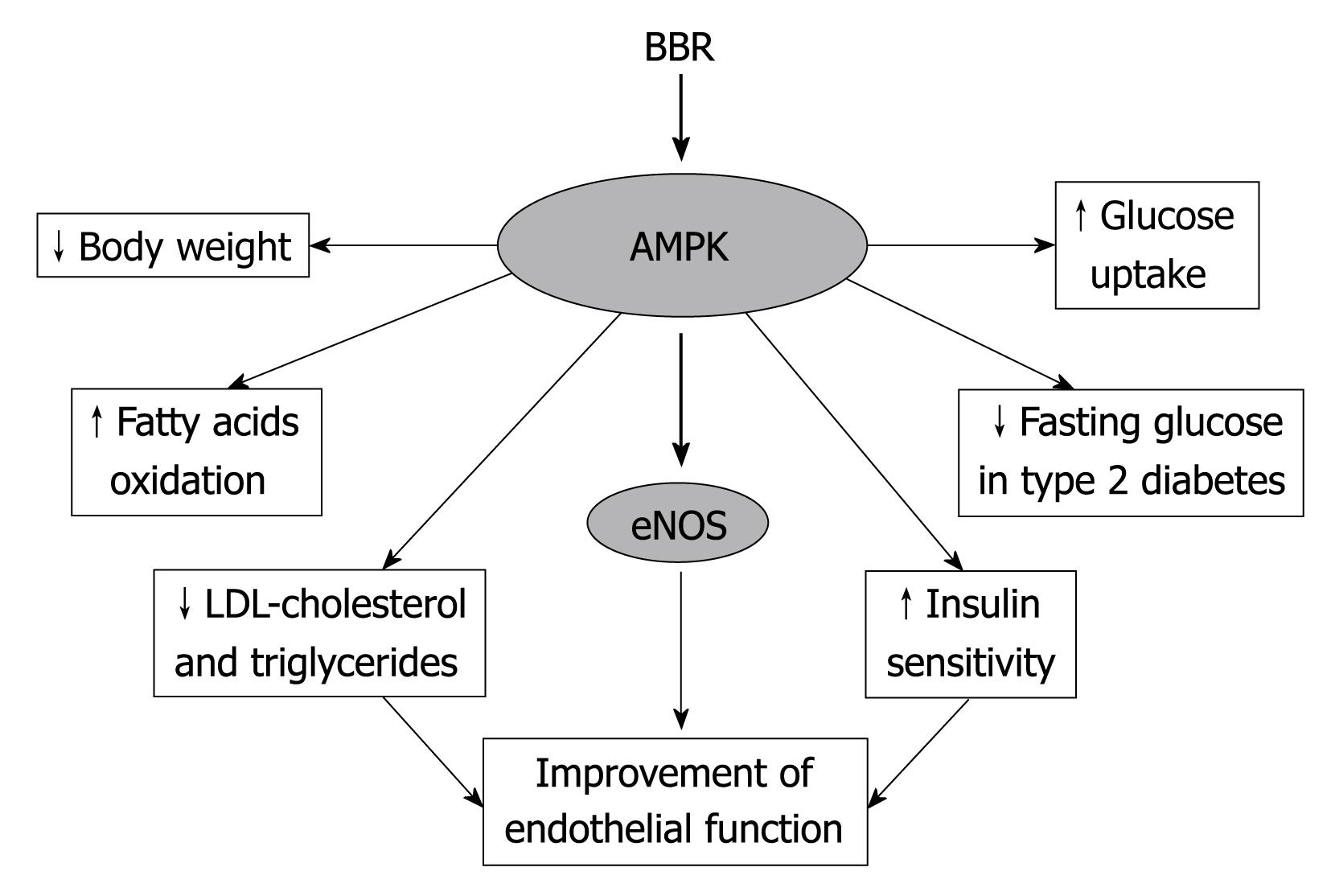
Figure 2 Most metabolic and vascular effects exerted by BBR are mediated by the activation of the AMPK cascade.
Besides the effects on endothelial function, several animal and clinical studies have demonstrated the therapeutic potential of BBR as supportive in the treatment of hypertension, atherosclerosis and heart disease, including left ventricular remodelling[44,46,47]. Specifically, BBR seems to have an inhibitory effect on cardiac hypertrophy, experimentally induced. Hong et al[48] demonstrated, in Sprague-Dawley rats with a supra-renal abdominal aorta constriction, that 8 wk of treatment with BBR led to cardiac growth inhibition. Both whole heart and left ventricular weight were notably decreased, and the parameters of cardiac contractility and relaxation improved. Subsequently they investigated the effects of BBR on catecholamine levels, in rats with experimental cardiac hypertrophy, demonstrating that BBR decreased plasma noradrenaline levels and the adrenaline levels both in plasma and in left ventricular tissue[49]. These findings showed the efficacy of BBR in modulating the sympathetic nervous activity of rats with experimental cardiac hypertrophy, and may support the therapeutic potentials of BBR in patients with cardiac hypertrophy and chronic heart failure. In fact, Zeng et al[46] investigated the efficacy and safety of BBR administration in patients with congestive heart failure (CHF) secondary to ischemic or idiopathic dilated cardiomyopathy. The addition of BBR to standard therapy showed a significant improvement of left ventricular function, exercise capacity and dyspnea-fatigue index in patients treated with BBR compared with a control group. These positive effects on heart function and symptoms were also associated with an important anti-arrhythmic action. During long-term follow-up, total mortality was significantly lower in the BBR treated patients than in the placebo group. This was due to an apparent decrease in both sudden cardiac death and death due to CHF[46].
The antiarrhythmic effect of BBR and its metabolites (tetra-hydro-berberin and 8-oxo-berberin) is due to the modulation of multiple ion channels both in sarcolemma and the sarcoplasmatic reticulum. Complex mechanisms as the basis of the antiarrhythmic activity have been demonstrated in vivo in several animal models[50]. These observations make BBR a promising antiarrhythmic agent with the potential to prevent sudden cardiac death.
CONCLUSION
Although BBR is usually thought of as a traditional Chinese medicine, recent discoveries have provided novel evidence that it may be considered a promising tool to counteract metabolic and cardiovascular (CV) disorders. Particularly, its demonstrated effects on the AMPK cascade, involved in CV disorders and metabolic pathways, propose BBR as a new therapeutic agent in the treatment of type 2 diabetes and metabolic syndrome (Figure 2). In fact, widely used drugs, including statins, metformin and rosiglitazone, execute CV protective effects though the activation of AMPK. The demonstrated efficacy in dyslipidemia and the promising antiarrhythmic activity make BBR a candidate for further study to provide new insights for therapeutic purpose.
Peer reviewer: Dr. Thomas Hellmut Schindler, PD, Department of Cardiology, Internal Medecine, University Hospitals of Geneva, Geneva 1211, Switzerland
S- Editor Cheng JX L- Editor Lutze M E- Editor Zheng XM