Revised: January 24, 2010
Accepted: January 25, 2010
Published online: January 26, 2010
AIM: To study if impaired renal function is associated with increased risk of peri-infarct heart failure (HF) in patients with preserved ejection fraction (EF).
METHODS: Patients with occluded infarct-related arteries (IRAs) between 1 to 28 d after myocardial infarction (MI) were grouped into chronic kidney disease (CKD) stages based on estimated glomerular filtration rate (eGFR). Rates of early post-MI HF were compared among eGFR groups. Logistic regression was used to explore independent predictors of HF.
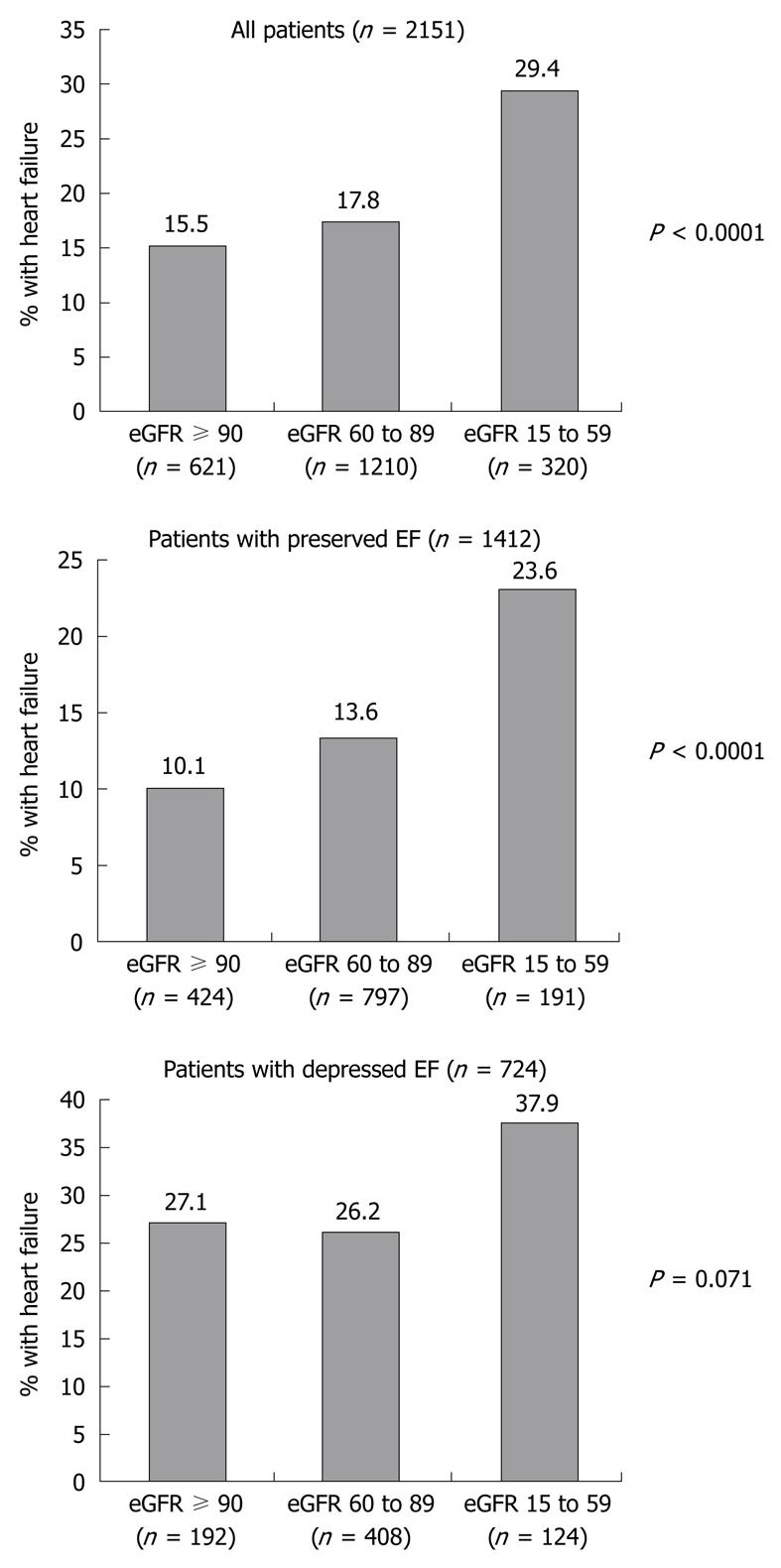
RESULTS: Reduced eGFR was present in 71.1% of 2160 patients, with significant renal impairment (eGFR < 60 mL/min every 1.73 m2) in 14.8%. The prevalence of HF was higher with worsening renal function: 15.5%, 17.8% and 29.4% in patients with CKD stages 1, 2 and 3 or 4, respectively (P < 0.0001), despite a small absolute difference in mean EF across eGFR groups: 48.2 ± 10.0, 47.9 ± 11.3 and 46.2 ± 12.1, respectively (P = 0.02). The prevalence of HF was again higher with worsening renal function among patients with preserved EF: 10.1%, 13.6% and 23.6% (P < 0.0001), but this relationship was not significant among patients with depressed EF: 27.1%, 26.2% and 37.9% (P = 0.071). Moreover, eGFR was an independent correlate of HF in patients with preserved EF (P = 0.003) but not in patients with depressed EF (P = 0.181).
CONCLUSION: A significant proportion of post-MI patients with occluded IRAs have impaired renal function. Impaired renal function was associated with an increased rate of early post-MI HF, the association being strongest in patients with preserved EF. These findings have implications for management of peri-infarct HF.
- Citation: Jorapur V, Lamas GA, Sadowski ZP, Reynolds HR, Carvalho AC, Buller CE, Rankin JM, Renkin J, Steg PG, White HD, Vozzi C, Balcells E, Ragosta M, Martin CE, Srinivas VS, Wharton III WW, Abramsky S, Mon AC, Kronsberg SS, Hochman JS. Renal impairment and heart failure with preserved ejection fraction early post-myocardial infarction. World J Cardiol 2010; 2(1): 13-18
- URL: https://www.wjgnet.com/1949-8462/full/v2/i1/13.htm
- DOI: https://dx.doi.org/10.4330/wjc.v2.i1.13
Impaired renal function is associated with an increased risk of early post-myocardial infarction (MI) heart failure (HF)[1-4], which in turn is a potent predictor of death[5]. However, this has not been well documented in patients with preserved left ventricular ejection fraction (EF).
The Occluded Artery Trial (OAT) was a randomized trial in 2201 patients with persistently occluded infarct-related arteries (IRAs) post-MI, a group at high risk of developing peri-infarct HF. Thus, the OAT population provided an opportunity to study the relationship between impaired renal function and peri-infarct HF in patients with a broad range of EFs.
The design and overall results of OAT have been published[6,7]. Briefly, OAT compared optimal medical therapy alone to optimal medical therapy and percutaneous coronary intervention in high-risk but stable patients with occluded IRAs over 24 h and up to 28 d post-MI. Eligibility criteria included confirmed index MI, occluded IRA and at least one of two high-risk criteria: EF < 50%, or proximal site of occlusion. Important exclusion criteria included significant angina, severe inducible ischemia, left main or triple vessel disease, serum creatinine > 2.5 mg/dL (221 μmol/L), severe valvular disease, New York Heart Association Class III or IV HF, or cardiogenic shock at the time of screening. The study was approved by institutional review committees at participating sites and subjects gave informed consent.
Data recorded included baseline clinical history, qualifying MI characteristics, EF and cardiac catheterization findings. All angiograms were independently reviewed at the angiographic core laboratory. Contrast ventriculograms recorded in the 30° right anterior oblique projection were used to calculate left ventricular volumes, EF, regional wall motion and sphericity index as described previously[8-10].
The Modification of Diet in Renal Disease equation was used to calculate estimated glomerular filtration rate (eGFR): eGFR (mL/min every 1.73 m2) = 186 × (Scr)-1.154× (Age)-0.203× (0.742 if female) × (1.210 if African American), where Scr is serum creatinine concentration in mg/dL and age is in years[11,12]. Patients were grouped into National Kidney Foundation chronic kidney disease (CKD) stages based on eGFR (mL/min every 1.73m2): CKD stage 1: ≥ 90; stage 2: 60-89; stage 3: 40-59; and stage 4: 15-39[13].
Early post-MI HF was defined as highest Killip class > I during index MI. Preserved and depressed EF was defined by the EF cut-off of 45%, which was the same as that used in the BNP and I-PRESERVE studies[14,15], and was intermediate between the cut-off points of 40% in the CHARM-PRESERVED study[16] and 50% in a community-based observational study[17].
Descriptive data were expressed as percentages and mean ± SD. Baseline variables were compared between eGFR groups using one-way analysis of variance for continuous variables and χ2 test for categorical variables. A forward stepwise logistic regression model was used to evaluate independent correlates of HF. Continuous variables (including eGFR) were entered as such in the multivariable model. Variables were selected based on P < 0.1 for comparison between eGFR groups. Subsequently, to test data-derived hypotheses, two separate logistic regression models were tested, restricted to patients with preserved and depressed EF, respectively. The pre-specified level of significance for all secondary analyses of OAT was P < 0.01. P≥ 0.01 and < 0.05 was considered to indicate a strong trend towards statistical significance.
Data on eGFR at the time of randomization, a median of 8 d post-MI, was available in 2160 of 2201 patients enrolled in OAT (98.1%). Of these, 71.1% had reduced eGFR, with 56.3% in stage 2, 14.5% in stage 3, and 0.3% in stage 4 (Table 1).
| eGFR≥90 (n = 624) | eGFR 60-89 (n = 1216) | eGFR 15-59 (n = 320) | P value | |
| eGFR mL/min every 1.73 m2 | 106.0 ± 16.6 | 75.5 ± 8.1 | 50.4 ± 7.7 | < 0.0001 |
| Age (yr) | 54.2 ± 9.7 | 58.7 ± 10.5 | 67.2 ± 9.6 | < 0.0001 |
| Female (%) | 14.6 | 20.3 | 43.4 | < 0.0001 |
| Diabetes (%) | 20.4 | 18.3 | 30.0 | 0.009 |
| Hypertension (%) | 39.3 | 48.4 | 69.1 | < 0.0001 |
| Hyperlipidemia (%) | 50.6 | 51.7 | 55.5 | 0.19 |
| Family history of CAD (%) | 42.6 | 40.8 | 32.8 | 0.01 |
| Current smoker (%) | 51.9 | 36.9 | 22.5 | < 0.0001 |
| Prior MI (%) | 9.5 | 12.2 | 10.9 | 0.28 |
| Thrombolytic use during initial 24 h of index MI (%) | 16.5 | 20.2 | 20.3 | 0.09 |
| Troponin I divided by ULN (ng/mL) (n = 1186) | 125.0 ± 213.9 | 186.3 ± 443.8 | 231.8 ± 516.6 | 0.01 |
| Troponin T divided by ULN (ng/mL) (n = 281) | 46.0 ± 73.4 | 69.6 ± 190.4 | 24.1 ± 28.3 | 0.2 |
| Days from index MI to randomization | 10.1 ± 7.4 | 11.2 ± 7.8 | 11.6 ± 7.6 | 0.004 |
| BMI (kg/m2) | 28.4 ± 5.1 | 28.6 ± 4.9 | 28.5 ± 5.3 | 0.77 |
| Heart rate (beats/min) | 72.1 ± 11.5 | 71.4 ± 11.9 | 72.7 ± 12.7 | 0.13 |
| Systolic BP (mmHg) | 119.4 ± 17.4 | 120.7 ± 17.7 | 123.9 ± 19.7 | 0.001 |
| Diastolic BP (mmHg) | 72.6 ± 11.1 | 72.3 ± 11.4 | 71.8 ± 11.6 | 0.63 |
| Discharge medications (%) | ||||
| Aspirin | 97.0 | 95.9 | 92.5 | 0.003 |
| Ticlopidine or clopidogrel | 73.6 | 73.2 | 70.2 | 0.33 |
| Beta blocker | 88.9 | 87.9 | 85.9 | 0.19 |
| ACE Inhibitor | 76.4 | 79.0 | 78.8 | 0.31 |
| Lipid lowering agent | 85.4 | 81.7 | 73.4 | < 0.0001 |
| IRA-LAD (%) | 32.9 | 36.8 | 38.4 | 0.19 |
| IRA-Circ (%) | 18.1 | 13.9 | 15.0 | |
| IRA-RCA (%) | 49.0 | 49.3 | 46.6 | |
| IRA TIMI Flow Grade 0-1 (%) | 99.8 | 99.5 | 99.4 | 0.41 |
| Two or three vessel disease (%) | 15.5 | 17.3 | 21.6 | 0.03 |
| Collaterals present (Grade 1 and 2) (%) | 89.2 | 88.5 | 86.7 | 0.29 |
| Mitral regurgitation (%) | ||||
| Grade 0 | 69.0 | 66.1 | 56.9 | < 0.0001b |
| Grade 1 | 27.3 | 28.3 | 30.6 | |
| Grade 2 | 2.7 | 4.4 | 7.4 | |
| Grade 3 | 1.0 | 1.2 | 5.1 | |
| End-diastolic volume (mL) (n = 201) | 137.4 ± 55.1 | 124.1 ± 52.8 | 130.4 ± 85.6 | 0.36 |
| End-systolic volume ± mL) (n = 201) | 70.7 ± 32.7 | 64.5 ± 32.2 | 71.7 ± 54.2 | 0.45 |
| Ejection fraction (n = 2145) | 48.2 ± 10.0 | 47.9 ± 11.3 | 46.2 ± 12.1 | 0.02 |
| Wall motion SD/Chord (n = 1677) | -2.9 ± 0.9 | -2.9 ± 0.9 | -3.0 ± 0.9 | 0.84 |
| Systolic sphericity index (n = 1677) | 23.3 ± 6.9 | 22.9 ± 6.6 | 24.7 ± 8.5 | 0.003 |
| Diastolic sphericity index (n = 1677) | 30.0 ± 6.7 | 30.6 ± 6.5 | 31.5 ± 7.4 | 0.02 |
Baseline clinical features associated with lower eGFR were older age, female sex, diabetes, hypertension, lower frequency of smokers, longer duration from MI to randomization, higher systolic blood pressure at randomization, and less frequent use of aspirin and lipid-lowering agents. In addition, patients with reduced eGFR showed a strong trend toward lower frequency of family history of coronary artery disease (CAD) and higher troponin I (Table 1).
Angiographic features associated with lower eGFR were higher systolic sphericity index and higher frequency and grade of mitral regurgitation. There was a strong trend toward significant association between lower eGFR and presence of multivessel CAD, lower EF and higher diastolic sphericity index (Table 1).
Of 2151 patients with data on eGFR and HF available, 406 (18.9%) had HF during the index MI. The prevalence of HF was higher with worsening renal function: 15.5% in patients with stage 1 CKD, 17.8% with stage 2 and 29.4% with stage 3 or 4 (P < 0.0001). The odds ratio (OR) for HF was 2.3 [95% confidence interval (CI): 1.6-3.1] in patients with stage 3 or 4 compared with stage 1, despite a small absolute difference in mean EF between eGFR groups (P = 0.02, Table 1). This prompted us to evaluate whether there was an association between eGFR group and HF in patients with preserved EF (Figure 1).
Of the study population of 2151 patients, 1412 had preserved EF and 724 had depressed EF. Of the 406 patients with HF, 196 (48.3%) had preserved EF, 206 (50.7%) had depressed EF, and EF was missing in four patients. Among patients with preserved EF, the prevalence of HF was again higher with worsening renal function: 10.1%, 13.6% and 23.6% (P < 0.0001). The OR for HF was 2.7 (95% CI: 1.7-4.3) in patients with stage 3 or 4 compared with stage 1. However, this relationship was not significant among patients with depressed EF: 27.1%, 26.2% and 37.9% (P = 0.071). The OR for HF was 1.6 (95% CI: 1.0-2.7) in patients with stage 3 or 4 compared with stage 1.
On multivariable analysis, HF was significantly associated with older age, higher heart rate at enrollment, lower EF, higher body mass index (BMI) and lower eGFR, while there was a strong trend towards significant association with absence of collaterals (Table 2).
| Covariates | P value | OR | 95% CI |
| All patients (n = 2096) | |||
| Decreasing EF1 | < 0.0001 | 1.554 | 1.398-1.727 |
| Increasing BMI | 0.001 | 1.037 | 1.015-1.059 |
| Decreasing eGFR1 | 0.006 | 1.088 | 1.025-1.156 |
| Increasing age1 | 0.007 | 1.171 | 1.044-1.315 |
| Increasing heart rate1 | 0.001 | 1.183 | 1.075-1.302 |
| Collaterals not present (Grade 0) | 0.045 | 1.397 | 1.007-1.939 |
| Patients with preserved EF (n = 1402) | |||
| Increasing BMI | 0.0001 | 1.060 | 1.029-1.093 |
| Decreasing eGFR1 | 0.003 | 1.140 | 1.044-1.245 |
| Prior MI | 0.021 | 1.701 | 1.082-2.673 |
| Increasing age1 | 0.031 | 1.185 | 1.015-1.383 |
| Increasing heart rate1 | 0.030 | 1.161 | 1.014-1.328 |
| Patients with depressed EF (n = 715) | |||
| Decreasing EF1 | < 0.0001 | 2.096 | 1.633-2.689 |
| Decreasing eGFR1 | 0.181 | 1.056 | 0.975-1.143 |
| Increasing heart rate1 | 0.006 | 1.210 | 1.055-1.388 |
| Collaterals not present (Grade 0) | 0.036 | 1.632 | 1.032-2.581 |
In the logistic regression model restricted to patients with preserved EF, lower eGFR and higher BMI were independently correlated with HF, and there was a trend toward significant association with prior MI, older age and higher heart rate at enrollment (Table 2). Conversely, in the model restricted to patients with depressed EF, lower eGFR was no longer correlated with HF, while lower EF and higher heart rate were strongly correlated, and there was a trend toward significant association with absence of collaterals (Table 2).
Decreased eGFR was associated with an increased risk of early post-MI HF, the association being strongest in patients with preserved EF, in whom it was an important independent predictor of HF.
Overall, we noted decreased eGFR (< 90 mL/min every 1.73 m2) in 71.1% and significant renal impairment (eGFR < 60 mL/min every 1.73 m2) in 14.8% of this large series of patients with persistently occluded IRAs post-MI. Prior studies in patients with MI reported significant renal impairment in 18.9%-33.5%[1,2,4,18]. Impaired renal function is associated with CAD risk factors such as older age, hypertension and diabetes[1-4], and has been found to be associated with a higher rate of MI in epidemiological studies[19,20].
Previous studies have demonstrated higher Killip class during acute MI in patients with impaired renal function[1-4]. We found that patients with lower eGFR had a higher prevalence of early post-MI HF, despite a small difference in EF between eGFR groups. Our multivariable models showed a significant continuous gradation of risk of post-MI HF with decreasing eGFR, even after adjusting for variables that differed between eGFR groups. The lower limits of the confidence intervals for the ORs probably reflect the wide spectrum of eGFR, with 624 of 2160 patients in the study having a normal eGFR (and consequently low risk of post-MI HF). Verma et al[21] found no significant difference in early post-MI EF between eGFR groups, but demonstrated higher left ventricular mass index in patients with lower eGFR. In addition to the association with diastolic dysfunction, higher left ventricular mass in patients with impaired renal function may be a marker for increased renin-angiotensin-aldosterone activity[22], which in turn, may contribute to HF through diverse mechanisms, including impaired sodium and water excretion and decreased venous capacitance[23,24].
The relationship between renal function and early post-MI HF was complex and was influenced by the degree of systolic dysfunction. Although, in general, patients with impaired renal function had a significantly higher prevalence of HF, this relationship was strongest in patients with preserved EF and weakest when EF was low.
Other studies have explored the potential influence of EF on the relationship between impaired renal function and clinical outcome. In a single-center study of non-ST elevation acute coronary syndrome, there was a step-wise increase in 1-year all-cause mortality with worsening renal function in patients with preserved as well as depressed EF[25]. However, rates of HF were not reported in this study and patients with ST elevation MI were excluded. In a pooled analysis of the three arms of the CHARM study, there was no interaction between eGFR and EF for a composite endpoint of cardiovascular death or HF hospitalization[26]. However, the entry criterion for CHARM was symptomatic HF of at least 4 wk duration and patients with recent MI (within 4 wk) were excluded.
Traditionally, assessment of a patient’s risk of post-MI HF has been based on knowledge of post-MI EF. However, there is a complex relationship between EF, renal function and post-MI HF. This study suggests that the use of EF for post-MI risk stratification in patients with impaired renal function has limitations. Furthermore, risk stratification can be improved by factoring in eGFR, particularly in patients with preserved EF. Of note, HF with preserved EF accounts for nearly half the cases of post-MI HF, and is associated with increased mortality following MI[27]. Renin-angiotensin-aldosterone blockade is well documented to reduce rates of late post-MI HF, particularly in patients with depressed EF. It is not known if intensive renin-angiotensin-aldosterone blockade during the acute phase of MI affects rates of early post-MI HF in patients with preserved EF and impaired renal function.
This study specifically analyzed the association of impaired renal function with HF and the influence of EF on this association in a large series of patients with occluded IRAs post-MI. A major strength of this study is that data were collected prospectively using pre-defined criteria, and important angiographic analyses were performed by a core laboratory blinded to clinical information. A potential limitation is that patients with creatinine > 2.5 mg/dL at randomization were excluded. Point measurements of serum creatinine were used to estimate GFR. The present study was not designed to address the possible mechanisms underlying our observations. Further studies are needed to address the precise mechanisms of post-MI HF in patients with impaired renal function, particularly in the presence of preserved EF.
In conclusion, a significant proportion of post-MI patients with persistently occluded IRAs have impaired renal function. Impaired renal function was associated with an increased rate of early post-MI HF, and the association was stronger in patients with preserved EF. The association between impaired renal function and HF in patients with preserved EF has important implications for management of peri-infarct HF and for post-MI risk stratification.
Patients with impaired renal function are at increased risk of heart failure (HF) early after a myocardial infarction (MI). HF after MI in turn is a potent predictor of death.
The relationship between impaired renal function and post-MI HF has been demonstrated mainly in patients with depressed ejection fraction (EF). However, this relationship has not been well studied in patients with preserved EF. In this study, the authors demonstrate that impaired renal function increases the risk of post-MI HF in patients with a wide range of EF.
This study furthermore shows that the relationship between impaired renal function and risk of post-MI HF is stronger in patients with preserved EF compared to patients with depressed EF.
Traditionally, estimation of EF has been used to identify patients who are at increased risk of adverse clinical outcome following a MI. By understanding the influence of impaired renal function on the risk of HF, we may include measures of renal function in the risk stratification of patients with MI, particularly in patients with preserved EF.
EF is the proportion of the blood volume in the left ventricle at the end of diastole that is ejected in systole. EF is expressed as a percentage and is a measure of left ventricular systolic function. Renal function in this study was assessed by an estimation of the glomerular filtration rate.
This is an interesting substudy from a well respected trial (Occluded Artery Trial). It can be published, however, the paper would gain a lot including echo and further scoring data (i.e. GRACE score) and revision.
Peer reviewers: Peter W Radke, Professor, Leitender Oberarzt, Medizinische Klinik 2, Kardiologie, Angiologie, Internistische Intensivmedizin, Universitätsklinikum Schleswig-Holstein Campus Lübeck, Ratzeburger Allee 160, D-23538 Lübeck, Germany; Pasquale Pagliaro, MD, PhD, Professor of Physiology, Department of Clinical and Biological Sciences, University of Turin, 10043 Orbassano, Italy; Paul Farand, MD, MSc, Assistant Professor, Cardiology Division, Centre hospitalier universitaire de Sherbrooke, Sherbrooke, J1H 5N4, Canada
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
| 1. | Tokmakova MP, Skali H, Kenchaiah S, Braunwald E, Rouleau JL, Packer M, Chertow GM, Moyé LA, Pfeffer MA, Solomon SD. Chronic kidney disease, cardiovascular risk, and response to angiotensin-converting enzyme inhibition after myocardial infarction: the Survival And Ventricular Enlargement (SAVE) study. Circulation. 2004;110:3667-3673. [Cited in This Article: ] |
| 2. | Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285-1295. [Cited in This Article: ] |
| 3. | Sørensen CR, Brendorp B, Rask-Madsen C, Køber L, Kjøller E, Torp-Pedersen C. The prognostic importance of creatinine clearance after acute myocardial infarction. Eur Heart J. 2002;23:948-952. [Cited in This Article: ] |
| 4. | Gibson CM, Pinto DS, Murphy SA, Morrow DA, Hobbach HP, Wiviott SD, Giugliano RP, Cannon CP, Antman EM, Braunwald E. Association of creatinine and creatinine clearance on presentation in acute myocardial infarction with subsequent mortality. J Am Coll Cardiol. 2003;42:1535-1543. [Cited in This Article: ] |
| 5. | Wu AH, Parsons L, Every NR, Bates ER. Hospital outcomes in patients presenting with congestive heart failure complicating acute myocardial infarction: a report from the Second National Registry of Myocardial Infarction (NRMI-2). J Am Coll Cardiol. 2002;40:1389-1394. [Cited in This Article: ] |
| 6. | Hochman JS, Lamas GA, Knatterud GL, Buller CE, Dzavik V, Mark DB, Reynolds HR, White HD. Design and methodology of the Occluded Artery Trial (OAT). Am Heart J. 2005;150:627-642. [Cited in This Article: ] |
| 7. | Hochman JS, Lamas GA, Buller CE, Dzavik V, Reynolds HR, Abramsky SJ, Forman S, Ruzyllo W, Maggioni AP, White H. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355:2395-2407. [Cited in This Article: ] |
| 8. | Sandler H, Dodge HT. The use of single plane angiocardiograms for the calculation of left ventricular volume in man. Am Heart J. 1968;75:325-334. [Cited in This Article: ] |
| 9. | Sheehan FH, Bolson EL, Dodge HT, Mathey DG, Schofer J, Woo HW. Advantages and applications of the centerline method for characterizing regional ventricular function. Circulation. 1986;74:293-305. [Cited in This Article: ] |
| 10. | Lamas GA, Vaughan DE, Parisi AF, Pfeffer MA. Effects of left ventricular shape and captopril therapy on exercise capacity after anterior wall acute myocardial infarction. Am J Cardiol. 1989;63:1167-1173. [Cited in This Article: ] |
| 11. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [Cited in This Article: ] |
| 12. | Levey AS, Greene T, Kusek J, Beck GJ, Group MS. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:A0828. [Cited in This Article: ] |
| 13. | National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-S266. [Cited in This Article: ] |
| 14. | Maisel AS, McCord J, Nowak RM, Hollander JE, Wu AH, Duc P, Omland T, Storrow AB, Krishnaswamy P, Abraham WT. Bedside B-Type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational Study. J Am Coll Cardiol. 2003;41:2010-2017. [Cited in This Article: ] |
| 15. | Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456-2467. [Cited in This Article: ] |
| 16. | Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777-781. [Cited in This Article: ] |
| 17. | Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251-259. [Cited in This Article: ] |
| 18. | Januzzi JL, Cannon CP, DiBattiste PM, Murphy S, Weintraub W, Braunwald E. Effects of renal insufficiency on early invasive management in patients with acute coronary syndromes (The TACTICS-TIMI 18 Trial). Am J Cardiol. 2002;90:1246-1249. [Cited in This Article: ] |
| 19. | Beddhu S, Allen-Brady K, Cheung AK, Horne BD, Bair T, Muhlestein JB, Anderson JL. Impact of renal failure on the risk of myocardial infarction and death. Kidney Int. 2002;62:1776-1783. [Cited in This Article: ] |
| 20. | Meisinger C, Döring A, Löwel H. Chronic kidney disease and risk of incident myocardial infarction and all-cause and cardiovascular disease mortality in middle-aged men and women from the general population. Eur Heart J. 2006;27:1245-1250. [Cited in This Article: ] |
| 21. | Verma A, Anavekar NS, Meris A, Thune JJ, Arnold JM, Ghali JK, Velazquez EJ, McMurray JJ, Pfeffer MA, Solomon SD. The relationship between renal function and cardiac structure, function, and prognosis after myocardial infarction: the VALIANT Echo Study. J Am Coll Cardiol. 2007;50:1238-1245. [Cited in This Article: ] |
| 22. | Schunkert H, Hense HW, Muscholl M, Luchner A, Kürzinger S, Danser AH, Riegger GA. Associations between circulating components of the renin-angiotensin-aldosterone system and left ventricular mass. Heart. 1997;77:24-31. [Cited in This Article: ] |
| 23. | Gabrielsen A, Bie P, Holstein-Rathlou NH, Christensen NJ, Warberg J, Dige-Petersen H, Frandsen E, Galatius S, Pump B, Sørensen VB. Neuroendocrine and renal effects of intravascular volume expansion in compensated heart failure. Am J Physiol Regul Integr Comp Physiol. 2001;281:R459-R467. [Cited in This Article: ] |
| 24. | Rietzschel E, Duprez DA, De Buyzere ML, Clement DL. Inverse relation between aldosterone and venous capacitance in chronically treated congestive heart failure. Am J Cardiol. 2000;85:977-980. [Cited in This Article: ] |
| 25. | Kontos MC, Garg R, Anderson FP, Tatum JL, Ornato JP, Jesse RL. Predictive power of ejection fraction and renal failure in patients admitted for chest pain without ST elevation in the troponin era. Am Heart J. 2005;150:666-673. [Cited in This Article: ] |
| 26. | Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671-678. [Cited in This Article: ] |
| 27. | Møller JE, Brendorp B, Ottesen M, Køber L, Egstrup K, Poulsen SH, Torp-Pedersen C. Congestive heart failure with preserved left ventricular systolic function after acute myocardial infarction: clinical and prognostic implications. Eur J Heart Fail. 2003;5:811-819. [Cited in This Article: ] |