Published online Apr 26, 2021. doi: 10.4330/wjc.v13.i4.82
Peer-review started: December 8, 2020
First decision: February 15, 2021
Revised: February 21, 2021
Accepted: April 13, 2021
Article in press: April 13, 2021
Published online: April 26, 2021
Most of the randomized clinical trials that led to the wide use of non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with atrial fibrillation (AF) originated from western countries.
To systematically review and quantitatively synthesize the real-world data regarding the efficacy and safety of dabigatran, rivaroxaban, and apixaban compared to warfarin for stroke prevention in Asian patients with non-valvular AF.
Medline, Cochrane, and ClinicalTrial.gov databases were reviewed. A random-effect model meta-analysis was used and I-square was utilized to assess the heterogeneity. The primary outcome was ischemic stroke. The secondary outcomes were all-cause mortality, major bleeding, intracranial hemorrhage, and gastrointestinal bleeding.
Twelve studies from East Asia or Southeast Asia and 441450 patients were included. Dabigatran, rivaroxaban, and apixaban were associated with a significant reduction in the incidence of ischemic stroke [hazard ratio (HR) = 0.78, 95% confidence interval (CI): 0.65-0.94; HR = 0.79, 95%CI: 0.74-0.85, HR = 0.70, 95%CI: 0.62-0.78; respectively], all-cause mortality (HR = 0.68, 95%CI: 0.56-0.83; HR = 0.66, 95%CI: 0.52-0.84; HR = 0.66, 95%CI: 0.49-0.90; respectively), and major bleeding (HR = 0.61, 95%CI: 0.54-0.69; HR = 0.70, 95%CI: 0.54-0.90; HR = 0.58, 95%CI: 0.43-0.78; respectively) compared to warfarin.
Dabigatran, rivaroxaban, and apixaban appear to be superior to warfarin in both efficacy and safety in Asians with non-valvular AF.
Core Tip: Dabigatran, rivaroxaban, apixaban have better efficacy (reduction in ischemic stroke and all-cause mortality) and are safer (reduction in major bleeding) than warfarin. Dabigatran is associated with a lower rate of intracranial hemorrhage than warfarin, while rivaroxaban and apixaban appear to have a trend towards reduced intracranial hemorrhage, without statistical significance. Dabigatran, rivaroxaban, and apixaban are associated with a lower rate of gastrointestinal bleeding.
- Citation: Li WJ, Archontakis-Barakakis P, Palaiodimos L, Kalaitzoglou D, Tzelves L, Manolopoulos A, Wang YC, Giannopoulos S, Faillace R, Kokkinidis DG. Dabigatran, rivaroxaban, and apixaban are superior to warfarin in Asian patients with non-valvular atrial fibrillation: An updated meta-analysis. World J Cardiol 2021; 13(4): 82-94
- URL: https://www.wjgnet.com/1949-8462/full/v13/i4/82.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i4.82
Stroke is the second leading cause of death and a major cause of disability globally[1,2]. It was estimated that more than 5.5 million people died secondary to stroke in 2016[3]. One third of all ischemic strokes are attributed to atrial fibrillation (AF), which leads to cardioembolism of large cerebral arteries[4]. As a consequence, AF-related strokes are more likely to be fatal or debilitating[4]. AF is the most common sustained cardiac arrhythmia with an estimated global prevalence of 2.8% and an estimated life time risk of about 25%[5]. The presence of AF is associated with a fivefold increase in stroke risk[6]. AF and stroke are independently associated with dementia onset in this population[7]. Therefore, anticoagulation therapy has been the mainstay of primary and secondary stroke prevention in patients with diagnosed AF for decades[8,9]. Warfarin was the standard of care until 2009, when dabigatran, a non-vitamin K oral anticoagulant (NOAC) was found to be non-inferior to warfarin with regards to stroke prevention in patients with AF[10]. Since then, three other NOACs have been approved, namely rivaroxaban, apixaban, and edoxaban, all of which have shown substantial clinical benefits in randomized clinical trials[10-13]. The relative ease of prescribing and adhering to NOAC therapy has resulted in a more widespread use of NOACs as opposed to warfarin[14]. However, most of the randomized clinical trials, which led to this substantial change in clinical practice, originated from western countries with underrepresentation of Asian population; yet a number of real-world studies have assessed the use of NOACs in Asians with AF. With this study we aimed to systematically review and quantitatively analyze the existing observational studies regarding the efficacy and safety of dabigatran, rivaroxaban, and apixaban compared to warfarin for stroke prevention in Asians with non-valvular AF.
The study was performed in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[15]. The study protocol was submitted for registration at the International Prospective Register for Systematic Reviews.
Medline, Cochrane Library, and ClinicalTial.gov were systematically searched from their inception to April 27, 2020. Two independent investigators (Li WJ and Archontakis-Barakakis P) independently searched for eligible studies. In cases where there was a disagreement regarding the eligibility of a study, a third investigator (Kokkinidis DG) was involved in order consensus to be reached.
The search algorithm was (“novel oral anticoagulants” OR “direct oral anticoagulants” OR “non-vitamin K antagonist oral anticoagulants” OR NOAC OR DOAC OR dabigatran OR rivaroxaban OR apixaban OR warfarin OR coumadin OR “vitamin K antagonist”) AND (atrial fibrillation OR AF OR AFIB) AND (real-world OR “real world” OR observational OR cohort OR post-approval). The reference list of pertinent reviews and observational studies was also manually searched for further potentially eligible studies. Duplicate publications were removed at the end of the literature search.
The pre-specified inclusion criteria were: (1) Retrospective or prospective observational studies; (2) Studies where the majority or all study participants were of Asian race; (3) Studies comparing warfarin to one of the NOACs; and (4) Studies examining at least one of the following outcomes: Thromboembolic stroke, intracranial hemorrhage, any bleeding, major bleeding, gastrointestinal bleeding. We excluded randomized control trials and studies that presented comparisons between different NOACs. Studies on valvular AF (i.e., diagnosis of mitral stenosis or mitral valve replacement or repair) were also excluded.
Data extraction was performed based on a pre-defined data extraction form by two independent investigators (Li WJ and Kokkinidis DG) blinded to each other. Disagreements were resolved following discussion and final decision was reached by consensus and as needed with the addition of a third reviewer (Archontakis-Barakakis P). The primary outcome was ischemic stroke (as defined by the individual studies) occurring during study follow up period. The secondary outcomes were all-cause mortality, major bleeding (as defined by the included studies), intracranial hemorrhage, and gastrointestinal bleeding.
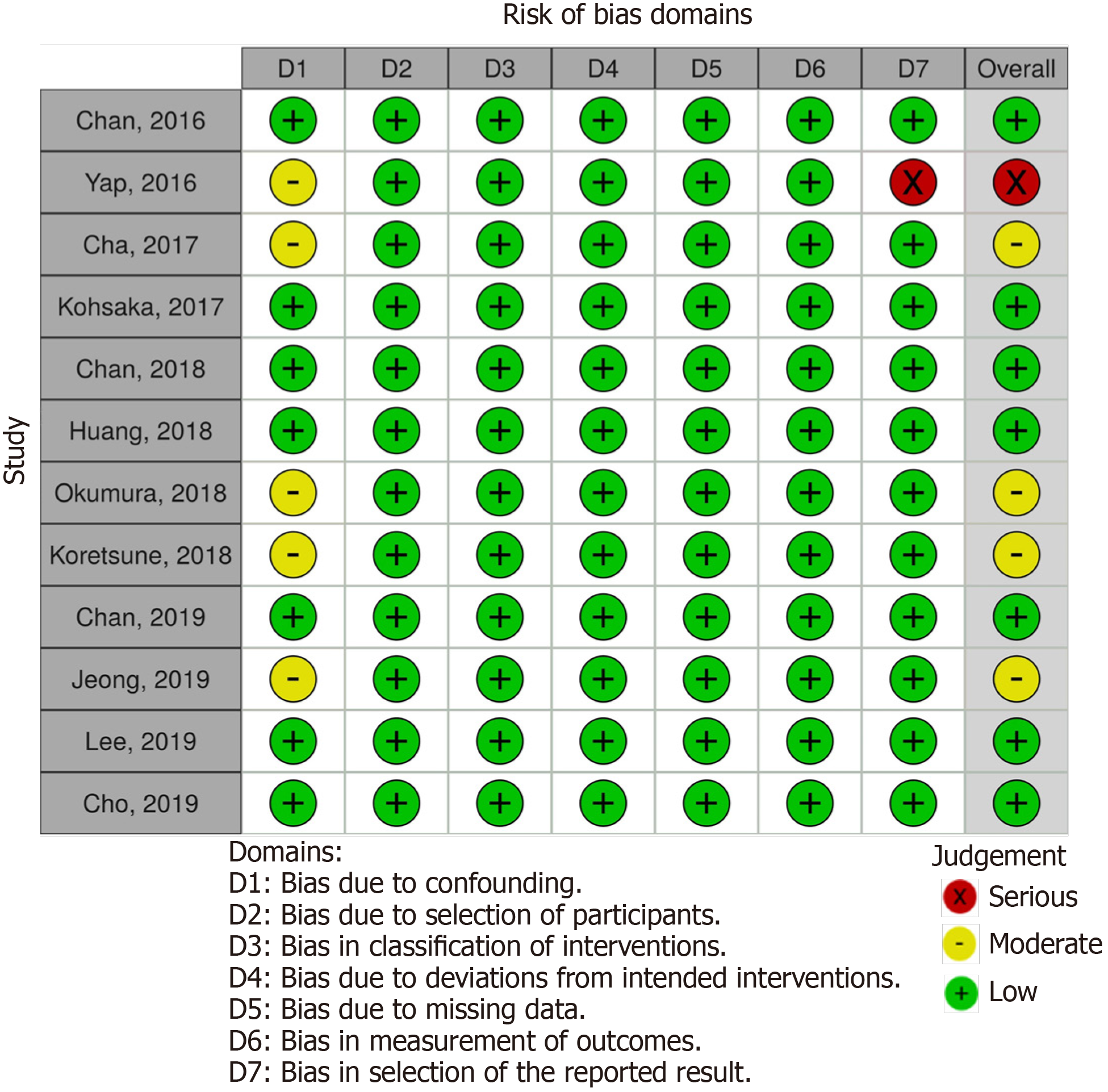
Two independent reviewers (Palaiodimos L and Tzelves L) assessed the risk of bias of the included studies with the Risk of Bias in Non-Randomized Studies - of Interventions tool[16]. Studies were assessed as having low, moderate, serious or critical risk of bias for the following domains: Confounding measurement and account, selection of participants, classification of interventions, deviations from intended interventions, missing data, outcome measurement, and results reporting.
When potentially duplicated populations were encountered, studies were not analyzed together under the same comparison and outcome. The larger one of the two studies was the one that was prioritized. Hazard ratios (HRs) with the corresponding 95% confidence interval (CI) were used for evaluation of outcomes. A random effects model was used to account for heterogeneity among studies. Heterogeneity was assessed with the Higgins I-square (I2) statistic. I2 > 75% indicated significant heterogeneity. Forest plots were used to graphically display the effect size in each study and the pooled estimates. Funnel plots and the Egger’s test were used for assessment of publication bias. A P value < 0.05 was considered significant. R 3.4 version (RStudio software, RStudio, Inc.) was used as statistical software.
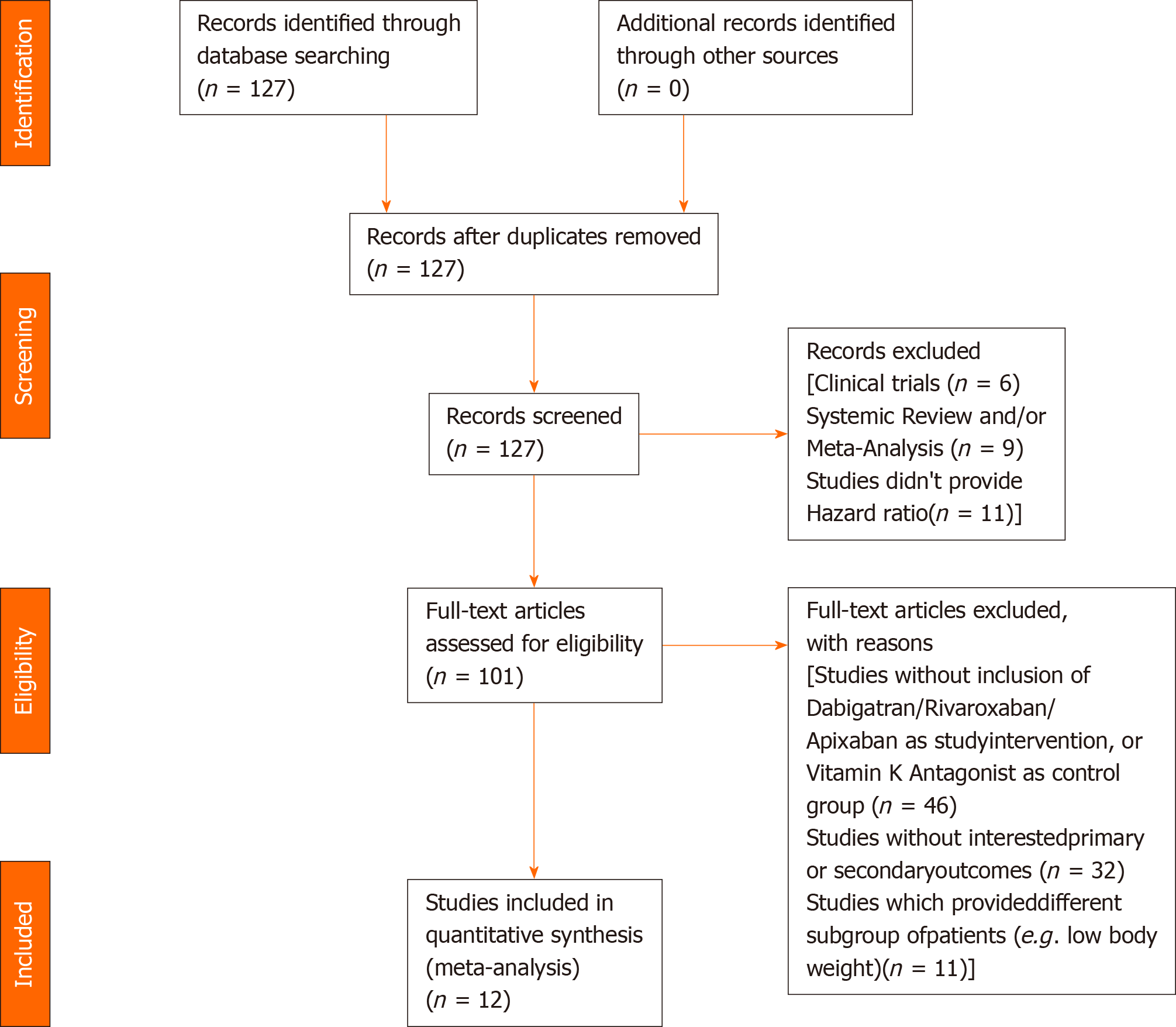
Our literature search has yielded 127 potentially relevant records after duplicates were removed. After excluding studies for multiple reasons (Figure 1), twelve studies were included in our analysis. All of the studies were conducted in countries or regions located in East Asia or Southeast Asia with the majority of their study participants being Asians. Most of the studies were products of large databases/registries (9 out of 12). In ClinicalTrials.gov database, we only found DARING-AF trial (comparison of efficacy and safety among dabigatran, rivaroxaban, and apixaban in non-valvular AF) which attempted to evaluate the efficacy and safety of NOACs in Asian patients but no results were posted. The baseline study characteristics are presented in Table 1. The patient characteristics are presented in Table 2. The risk of bias assessment is presented in Figure 2.
| Ref. | Design | Setting/data source | Country/region | Study period | Major eligibility criteria | Major exclusion criteria | OAC comparison groups | Outcomes of interest |
| Chan et al[25], 2016 | Database, retrospective cohort study | National Health Insurance Research Database | Taiwan | June 1, 2012 to December 31, 2013 | NVAF patients, age ≥ 30 years old | PE or DVT, joint replacement or valvular surgery within 6 mo before AF was diagnosed, ESRD, switcher from dabigatran to warfarin | Dabigatran vs warfarin | Ischemic stroke, intracranial hemorrhage, major GI bleeding, all-cause mortality |
| Yap et al[26], 2016 | Single-center, retrospective cohort study | Malaysia’s National Heart Institute | Malaysia | January, 2009 to December, 2013 | NVAF patients | Not mentioned | Dabigatran vs warfarin | Ischemic CVA, major bleeding |
| Cha et al[27], 2017 | Database, retrospective cohort study | Korean National Health Insurance Service database | Korea | January, 2014 to December, 2015 | NVAF patients with CHA2DS2-VASc score ≥ 2 taking anticoagulants for primary prevention of stroke/systemic embolism | Thromboembolic event/TIA or ICH, patients received joint replacement, medications change between warfarin and NOACs | Dabigatran vs warfarin, rivaroxaban vs warfarin, apixaban vs warfarin | Ischemic stroke, intracranial hemorrhage, or all-cause mortality |
| Kohsaka et al[28], 2017 | Database, retrospective cohort study | 275 acute care hospitals across Japan | Japan | March 1, 2011 to March 31, 2016 | NVAF patients 18 yr or older without use of any OAC within 180 d before the index date | Valvular AF, post-operative AF, mechanical-valvular AF, rheumatic AF | Dabigatran vs warfarin, rivaroxaban vs warfarin, apixaban vs warfarin | Major bleeding |
| Chan et al[29], 2018 | Database, retrospective cohort study | National HealthInsurance Research Database | Taiwan | June 1, 2012 to December 31, 2016 | NVAF patients ≥ 30 years old | More than 1 NOAC use during treatment course, diagnosis of valvular AF, VTE or joint replacement therapy, ESRD requiring renal replacement therapy within 6 mo before the index date | Dabigatran vs warfarin, rivaroxaban vs warfarin, apixaban vs warfarin | All-cause mortality, intracranial hemorrhage, GI bleeding, major bleeding |
| Huang et al[30], 2018 | Database, retrospective cohort study | National Health Insurance Research Database | Taiwan | June 1, 2012 to December 31, 2015 | NVAF patients ≥ 20 years old, at least 1 inpatient or 2 separate outpatient diagnoses of AF | Prosthetic heart valve or MV disease during the study period, pregnant, cancer, or chronic dialysis within 12 months prior to index date | Rivaroxaban vs warfarin | Ischemic stroke, intracranial hemorrhage, GI bleeding |
| Okumura et al[31], 2018 | Multi-center, prospective cohort study | 63 institutions in the Tokyo area | Japan | September 1, 2013 to December 31, 2015 | NVAF patients, age ≥ 20 years old, treatment with any anticoagulant drug for stroke prophylaxis | Not mentioned | Dabigatran vs warfarin, rivaroxaban vs warfarin, apixaban vs warfarin | Intracranial hemorrhage, major bleeding, all-cause mortality |
| Koretsune et al[32], 2018 | Database, retrospective cohort study | Hospital Information systems and administration database by Medical Data Vision | Japan | March 14, 2011 to June 30, 2016 | NVAF patients, age ≥ 18, new user of either dabigatran or warfarin | Dialysis, kidney transplant, atrial flutter, valvular AF, mechanical valve replacement, rheumatic AF or MV prolapse/regurgitation or stenosis; or DVT or PE < 6 months before the AF diagnosis | Dabigatran vs warfarin | Major bleeding, intracranial hemorrhage, GI bleeding |
| Chan et al[33], 2019 | Database, retrospective cohort study | National Health Insurance Research Database | Taiwan | June 1, 2012 to December 31, 2017 | NVAF patients | More than one NOAC use, ESRD, DVT, PE, joint replacement therapy up to 6 mo prior to the index date. | Dabigatran vs warfarin, rivaroxaban vs warfarin, apixaban vs warfarin | Ischemic stroke, intracranial hemorrhage, major bleeding, major GI bleeding |
| Jeong et al[34], 2019 | Single-center, retrospective cohort study | Chonnam National University Hospital | Korea | January, 2014 to December, 2016 | NVAF patients, CHA2DS2-VASc > 2 | Valvular AF, or any OAC class change | Rivaroxaban vs warfarin | Ischemic stroke, major bleeding, GI bleeding, intracranial bleeding, all-cause mortality |
| Lee et al[35], 2019 | Database, retrospective cohort study | Korean Health Insurance Review Database | Korea | January, 2015 to December, 2017 | NVAF patients naïve to OAC treatment | Not mentioned | Dabigatran vs warfarin, rivaroxaban vs warfarin, apixaban vs warfarin | Ischemic stroke, intracranial hemorrhage, GI bleeding, major bleeding |
| Cho et al[36], 2019 | Database, retrospective cohort study | Korean National Health Insurance Service | Korea | July 1, 2015 to December 31, 2016 | NVAF patients with new prescription of anticoagulants, age ≥ 18 | Less than 30 d of anticoagulants use, two or more types of anticoagulants user, CHA2DS2-VASc score < 2, prior PE or DVT, underwent joint replacement surgery, dialysis patients | Dabigatran vs warfarin, rivaroxaban vs warfarin, apixaban vs warfarin | All-cause mortality, major bleeding |
| Ref. | Patient Number (Api) | Patient Number (Riv) | Patient Number (Dab) | Patient Number (War) | Age (Api) | Age (Riv) | Age (Dab) | Age (War) | HAS-BLED (Api) | HAS-BLED (Riv) | HAS-BLED (Dab) | HAS-BLED (War) | CHA2DS2-VASc (Api) | CHA2DS2-VASc (Riv) | CHA2DS2-VASc (Dab) | CHA2DS2-VASc (War) |
| Chan et al[25], 2016 | N/A | N/A | 9940 | 9913 | N/A | N/A | 75 ± 10 | 76 ± 10 | N/A | N/A | 2.57 ± 1.01 | 2.58 ± 1.06 | N/A | N/A | 4.13 ± 1.59 | 4.16 ± 1.75 |
| Yap et al[26], 2016 | N/A | N/A | 500 | 500 | N/A | N/A | 65.3 ± 11.3 | 66.8 ± 11.3 | N/A | N/A | 1.57 ± 0.96 | 1.67 ± 0.94 | N/A | N/A | 2.69 ± 1.54 | 3.40 ± 1.54 |
| Cha et al[27], 2017 | 2189 | 5681 | 3741 | 23222 | 70.3 ± 10.0 | 70.5 ± 9.9 | 69.3 ± 10.0 | 68.82 ± 11.1 | N/A | N/A | N/A | N/A | 3.57 ± 1.29 | 3.60 ± 1.32 | 3.51 ± 1.28 | 3.57 ± 1.31 |
| Kohsaka et al[28], 2017 | 5977 | 5090 | 6726 | 6726 (War-Dab matched); 5090 (War-Riv matched); 5977 (War-Api matched) | 77.4 ± 10.0 | 75.8 ± 10.0 | 73.1 ± 9.9 | 73.3 ± 10.5 (War-Dab matched); 76.2 ± 10.5 (War-Riv matched); 77.7 ± 10.0 (War-Api matched) | N/A | N/A | N/A | N/A | 3.5 ± 1.6 | 3.3 ± 1.6 | 3.0 ± 1.6 | 3.0 ± 1.6 (War-Dab matched); 3.4 ± 1.6 (War-Riv matched); 3.5 ± 1.5 (War-Api matched) |
| Chan et al[29], 2018 | 5843 | 27777 | 20079 | 19375 | 76 ± 10 | 76 ± 10 | 76 ± 10 | 76 ± 10 | 2.96 ± 1.12 | 2.96 ± 0.51 | 2.96 ± 0.59 | 2.97 ± 0.61 | 3.89 ± 1.56 | 3.89 ± 0.71 | 3.88 ± 0.82 | 3.89 ± 0.88 |
| Huang et al[30], 2018 | N/A | 9637 | N/A | 9637 | N/A | 75.2 ± 10.24 | N/A | 74.98 ± 10.6 | N/A | 2.21 ± 1.46 | N/A | 2.33 ± 1.49 | N/A | 4.02 ± 1.92 | N/A | 4.11 ± 2 |
| Okumura et al[31], 2018 | 428 | 761 | 456 | 1561 | 73.2 ± 10.1 | 71.5 ± 9.1 | 70.9 ± 9.5 | 72.2 ± 9.3 | 1.42 ± 0.81 | 1.32 ± 0.77 | 1.07 ± 0.71 | 1.61 ± 0.88 | 3.12 ± 1.47 | 2.87 ± 1.45 | 2.83 ± 1.46 | 3.08 ± 1.51 |
| Koretsune et al[32], 2018 | N/A | N/A | 4606 | 4606 | N/A | N/A | 74 ± 10 | 73 ± 11 | N/A | N/A | 2.1 ± 1.0 | 2.1 ± 1.1 | N/A | N/A | 3.3 ± 1.7 | 3.3 ± 1.7 |
| Chan et al[33], 2019 | 9952 | 33022 | 22371 | 19761 | 76 ± 10.5 | 75.3 ± 10.6 | 74.2 ± 10.4 | 70.6 ± 13.4 | 2.9 ± 1.1 | 2.9 ± 1.1 | 2.8 ± 1.1 | 2.6 ± 1.3 | 3.9 ± 1.6 | 3.8 ± 1.6 | 3.7 ± 1.5 | 3.2 ± 1.8 |
| Jeong et al[34], 2019 | N/A | 804 | N/A | 804 | N/A | 71.4 ± 10.5 | N/A | 70.4 ± 10.2 | N/A | N/A | N/A | N/A | N/A | 3.3 ± 1.8 | N/A | 3.4 ± 1.8 |
| Lee et al[35], 2019 | 22177 | 35965 | 17745 | 25420 | 72.7 ± 10.2 | 72.0 ± 9.9 | 70.8 ± 9.9 | 67.3 ± 12.6 | 2.75 ± 1.04 | 2.77 ± 1.02 | 2.67 ± 1.01 | 2.58 ± 1.14 | 3.76 ± 1.41 | 3.63 ± 1.40 | 3.55 ± 1.37 | 3.18 ± 1.61 |
| Cho et al[36], 2019 | 12502 | 21000 | 12593 | 10409 | 74.3 ± 8.9 | 73.8 ± 8.8 | 72.9 ± 8.9 | 70.8 ± 11 | 2.5 ± 0.9 | 2.5 ± 0.9 | 2.5 ± 0.9 | 2.6 ± 1.0 | 3.7 ± 1.2 | 3.6 ± 1.2 | 3.5 ± 1.2 | 3.5 ± 1.2 |
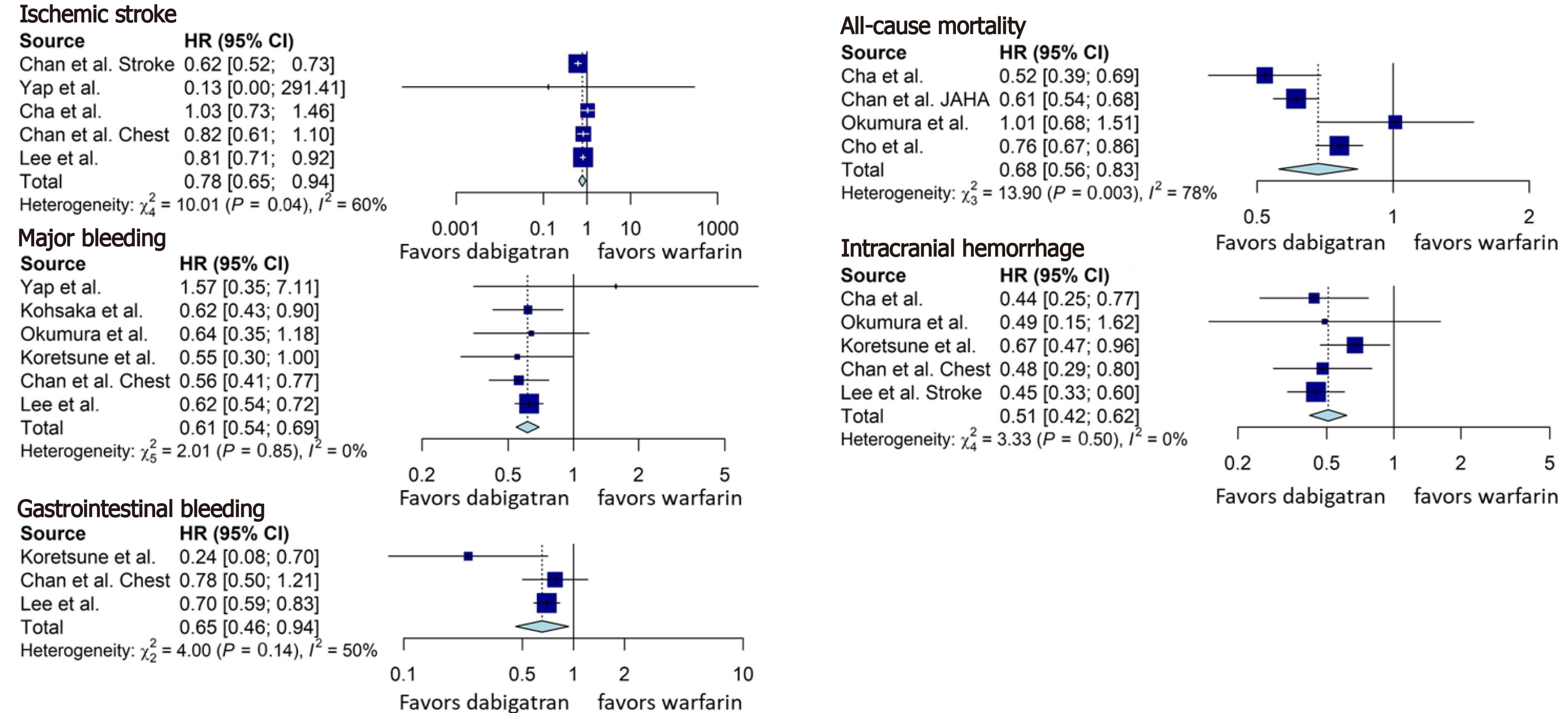
Dabigatran was associated with a significant reduction in the incidence of ischemic stroke (HR = 0.78, 95%CI: 0.65-0.94, Figure 3), all-cause mortality (HR = 0.68, 95%CI: 0.56-0.83, Figure 3), major bleeding (HR = 0.61, 95%CI: 0.54-0.69, Figure 3), intracranial hemorrhage (HR = 0.51, 95%CI: 0.42-0.62, Figure 3), and gastrointestinal bleeding (HR = 0.65, 95%CI: 0.46-0.94, Figure 3), compared to warfarin.
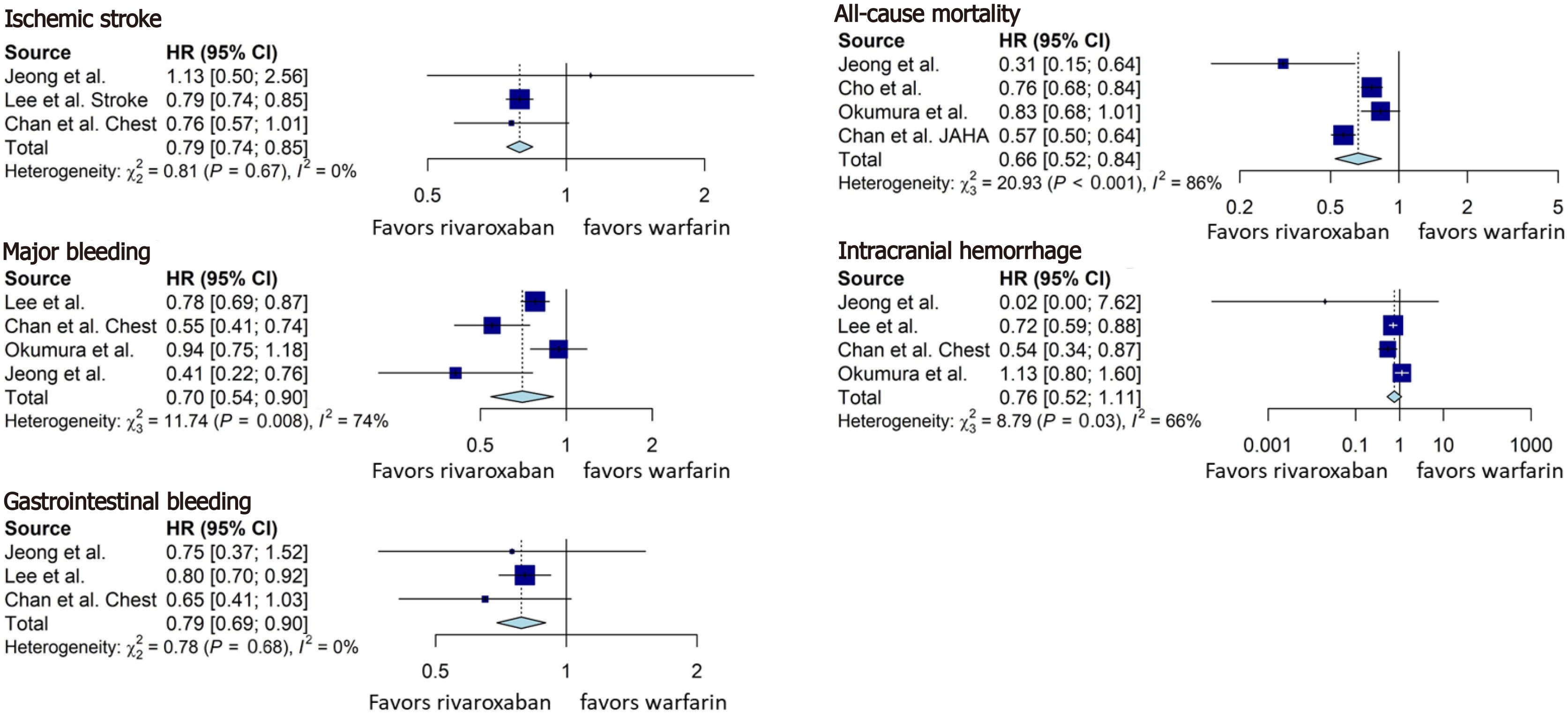
Rivaroxaban was associated with a statistically significant lower rate of ischemic stroke (HR = 0.79, 95%CI: 0.74-0.85, Figure 4), all-cause mortality (HR = 0.66, 95%CI: 0.52-0.84, Figure 4), major bleeding (HR = 0.70, 95%CI: 0.54-0.90, Figure 4), and gastrointestinal bleeding (HR = 0.79, 95%CI: 0.69-0.90, Figure 4), compared with the warfarin group. Although there was a tendency towards reduction in the rate of intracranial hemorrhage (HR = 0.76, 95%CI: 0.52-1.11, Figure 4) in rivaroxaban group, the result was not statistically significant.
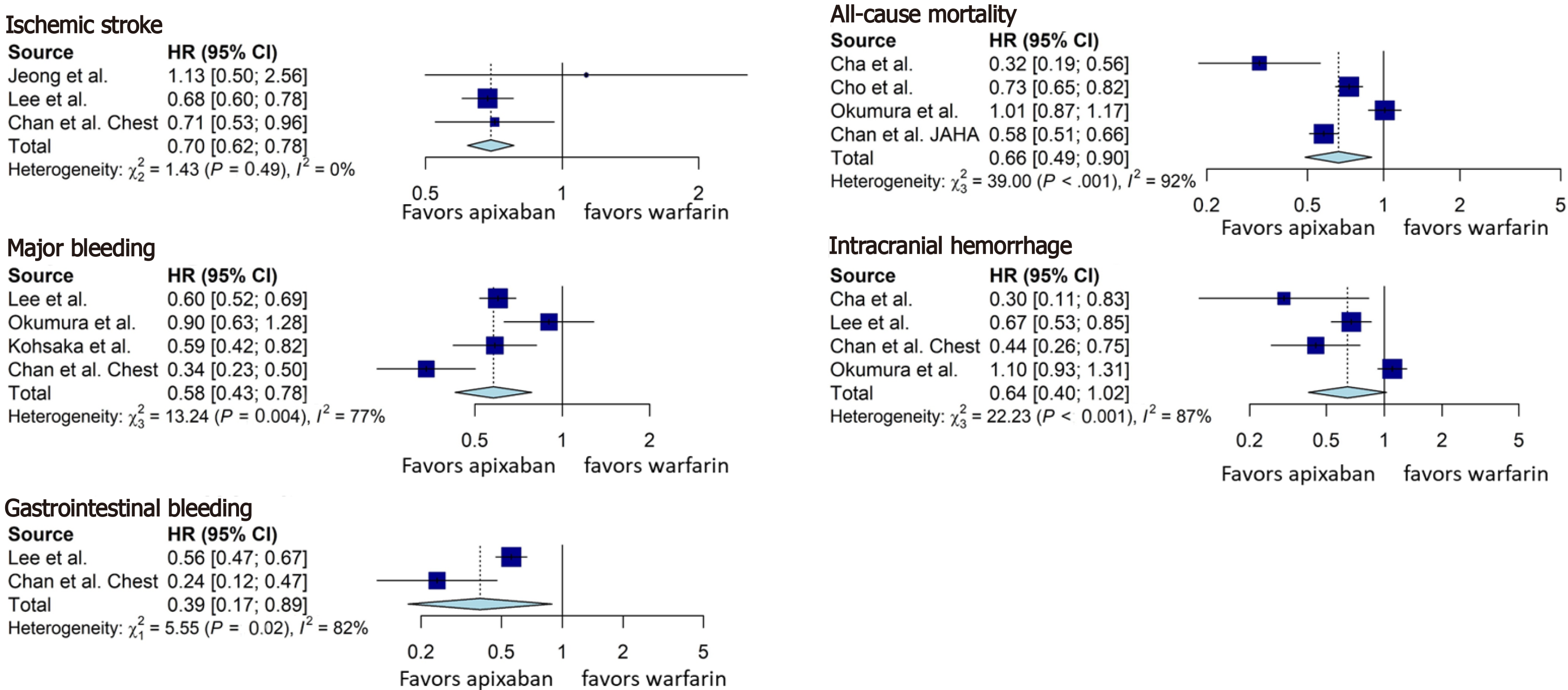
Apixaban was associated with a lower rate of ischemic stroke (HR = 0.70, 95%CI: 0.62-0.78, Figure 5), all-cause mortality (HR = 0.66, 95%CI: 0.49-0.90, Figure 5), major bleeding (HR = 0.58, 95%CI: 0.43-0.78, Figure 5), and gastrointestinal bleeding (HR = 0.39, 95%CI: 0.17-0.89, Figure 5). However, there was no difference in the intracranial hemorrhage, although a clear trend was noticed (HR = 0.64, 95%CI: 0.40-1.02, Figure 5) towards apixaban superiority.
This study was a systematic review and meta-analysis of observational studies comparing the efficacy and safety of NOACs to warfarin in Asians with non-valvular AF. To the best of our knowledge, this is the first real-world data meta-analysis on this topic that is focused on Asian patient population. Our findings can be summarized as following: (1) Dabigatran, rivaroxaban, and apixaban were associated with significantly lower incidence of ischemic stroke and all-cause mortality compared to warfarin; (2) NOACs were associated with significantly fewer major bleeding events and gastrointestinal bleeding events compared to warfarin; and (3) Dabigatran was associated with significantly fewer intracranial bleeding events compared to warfarin; no significant difference in intracranial bleeding between the other two studied NOACs and warfarin was found, but a trend favoring rivaroxaban and apixaban was noted.
Dabigatran is a direct thrombin inhibitor that prevents the conversion of fibrinogen to fibrin and has been used for stroke and systemic embolism prevention in non-valvular AF since 2010[8]. The landmark trial that led to the approval and wide use of dabigatran, the randomized evaluation of long-term anticoagulation therapy (RE-LY) study, demonstrated that patients in the dabigatran arm (dose 150 mg twice daily) had significantly lower risk for stroke or systemic embolism [relative risk (RR) = 0.66, 95%CI: 0.53-0.82] and intracranial bleeding (RR = 0.40, 95%CI: 0.27-0.60) compared to warfarin arm in the two-year follow-up[10]; these findings are consistent with the results of our meta-analysis. The RE-LY trial did not show a significant difference between dabigatran and warfarin in terms of all-cause mortality and major bleeding but revealed higher gastrointestinal bleeding event rates in the dabigatran arm[10]. Real-world studies, which mainly included non-Asians, have confirmed that dabigatran has an overall better efficacy and safety profile compared to warfarin, however superiority of dabigatran in each of the individual outcomes was not consistent across studies[17-19]. In contrast, our findings demonstrated that dabigatran was associated with 32% lower all-cause mortality, 39% lower risk for major bleeding, and 35% lower risk for gastrointestinal bleeding compared to warfarin. This meta-analysis focused on Asian population reveals consistent superiority of dabigatran compared to warfarin for all important outcomes.
Rivaroxaban acts by inhibiting activated factor Xa (FXa), a key molecule in the coagulation cascade[8]. Rivaroxaban once daily oral direct FXa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in AF (ROCKET AF) trial was published in 2011 paving the way for approval of rivaroxaban for stroke prevention in patients with non-valvular AF[11]. The rivaroxaban group was associated with significantly fewer stroke events compared to warfarin group (HR = 0.79, 95%CI: 0.66-0.96) which is in agreement with the findings of our meta-analysis but failed to demonstrate significant result in intention-to-treat population. No difference in major bleeding events between rivaroxaban and warfarin groups was demonstrated in ROCKET-AF (HR = 1.04, 95%CI: 0.90-1.20)[11]. However, an increased risk of decrease in hemoglobin more than or equal to 2 g/dL in rivaroxaban group was seen. This is different from our study that found that Asian patients on rivaroxaban had 30% lower risk for major bleeding events occurrence compared to patients receiving warfarin. The findings from a large real-world study from Canada and the United States revealed no significant difference for stroke and bleeding outcomes between the rivaroxaban and warfarin groups[20]. A propensity score matching analysis from Denmark demonstrated that patients on rivaroxaban had lower stroke rates but similar bleeding rates to patients on warfarin[21]. Observational data from Coleman et al[22] showed that rivaroxaban reduced stroke rates in patients with non-valvular AF compared to warfarin without any significant difference in bleeding rates. While another study from the United States showed higher risk of gastrointestinal bleeding in the rivaroxaban group[23], our study yielded favorable result pointing toward reduction of gastrointestinal bleeding of rivaroxaban in Asian patients. In summary, our meta-analysis showed that Asian patients with non-valvular AF on rivaroxaban had less stroke and bleeding rates compared to those on warfarin.
Apixaban is another FXa inhibitor approved for stroke prevention in patients with non-valvular AF[8]. The publication of the results of the apixaban for reduction in stroke and other thromboembolic events in AF (ARISTOTLE) trial was the milestone after which apixaban became one of the most widely prescribed oral anticoagulants[12]. In ARISTOTLE trial, patients with non-valvular AF were randomized in the apixaban and warfarin groups. The apixaban group had significantly lower stroke and major bleeding events rates, and lower mortality compared to warfarin group. Large real-world studies mainly in non-Asians confirmed the randomized trial data[18,24]. With this meta-analysis, we demonstrate that apixaban is more efficacious and safer than warfarin in our Asian population, as well.
The main strengths of our study are its strict methodology, robust analysis, and the relatively large number of included studies and overall patient sample. Our study has a number of limitations, mainly associated with the observational nature of the included studies. The patient populations of the individual studies were heterogeneous despite the fact that the vast majority was of Asian race. We were unable to adjust for baseline comorbidities because we did not have access to patient level data. For some of the outcomes, the number of eligible studies was limited which decreases the certainty in our findings.
In conclusion, the present systematic review and meta-analysis revealed that dabigatran, rivaroxaban, and apixaban, appear to be superior to warfarin in terms of efficacy (stroke prevention, all-cause mortality) and safety outcomes (bleeding events) in Asians with non-valvular AF.
Stroke is the second leading cause of mortality and disability worldwide with one-third of all ischemic stroke cases attributed to atrial fibrillation (AF).
Warfarin was the standard of care of stroke prevention in patients with nonvalvular AF but novel oral anticoagulants including dabigatran, rivaroxaban, and apixaban have demonstrated better efficacy and safety than warfarin in randomized clinical trials but they originated from Western countries where the Asian population was underrepresented.
We aimed to systematically review and quantitatively analyze the existing real-world observational studies regarding the efficacy and safety of dabigatran, rivaroxaban, and apixaban when they are compared with warfarin for stroke prevention in Asian patients with non-valvular AF.
The study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Medline, Cochrane, and ClinicalTrial.gov databases were systematically searched. A random-effect model meta-analysis was used and I2 was utilized to assess heterogeneity. Risk of bias assessment was performed for every study included.
Twelve studies were qualified for our study. Dabigatran, rivaroxaban, and apixaban were all associated with significantly reduced incidence of ischemic stroke, all-cause mortality, major bleeding, and gastrointestinal bleeding. Dabigatran was also found associated with a lower risk of intracranial hemorrhage.
Dabigatran, rivaroxaban, and apixaban have better efficacy and safety profile than warfarin for stroke prevention in Asian patients with non-valvular AF.
Future research which conducts a head-to-head comparison between different novel oral anticoagulants is necessary to determine the best option for Asian patients with non-valvular AF.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang XQ S-Editor: Zhang H L-Editor: A P-Editor: Li JH
| 1. | Katan M, Luft A. Global Burden of Stroke. Semin Neurol. 2018;38:208-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 657] [Cited by in F6Publishing: 946] [Article Influence: 157.7] [Reference Citation Analysis (0)] |
| 2. | Palaiodimos L, Kokkinidis DG, Faillace RT, Foley TR, Dangas GD, Price MJ, Mastoris I. Percutaneous closure of patent foramen ovale vs. medical treatment for patients with history of cryptogenic stroke: A systematic review and meta-analysis of randomized controlled trials. Cardiovasc Revasc Med. 2018;19:852-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | GBD 2016 Stroke Collaborators. . Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:439-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1626] [Cited by in F6Publishing: 1612] [Article Influence: 322.4] [Reference Citation Analysis (0)] |
| 4. | Freedman B, Potpara TS, Lip GY. Stroke prevention in atrial fibrillation. Lancet. 2016;388:806-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 5. | Ball J, Carrington MJ, McMurray JJ, Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol. 2013;167:1807-1824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 397] [Cited by in F6Publishing: 434] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 6. | Amin HP, Schindler JL. Epidemiology and Risk Factors. In: Vascular Neurology Board Review. Cham: Springer, 2017: 37-42. [Cited in This Article: ] |
| 7. | Kokkinidis DG, Zareifopoulos N, Theochari CA, Arfaras-Melainis A, Papanastasiou CA, Uppal D, Giannakoulas G, Kalogeropoulos AP, Fontes JDT. Association Between Atrial Fibrillation and Cognitive Impairment in Individuals With Prior Stroke: A Meta-Analysis and Meta-Regression Analysis. Stroke. 2020;51:1662-1666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Palaiodimos L, Miles J, Kokkinidis DG, Barkolias C, Jonnalagadda AK, Papaconstantinou D, Frountzas M, Misiakos EP, Schizas D. Reversal of Novel Anticoagulants in Emergent Surgery and Trauma: A Comprehensive Review and Proposed Management Algorithm. Curr Pharm Des. 2018;24:4540-4553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Snow V, Weiss KB, LeFevre M, McNamara R, Bass E, Green LA, Michl K, Owens DK, Susman J, Allen DI, Mottur-Pilson C; AAFP Panel on Atrial Fibrillation; ACP Panel on Atrial Fibrillation. Management of newly detected atrial fibrillation: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Intern Med. 2003;139:1009-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7917] [Cited by in F6Publishing: 7707] [Article Influence: 513.8] [Reference Citation Analysis (0)] |
| 11. | Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6519] [Cited by in F6Publishing: 6475] [Article Influence: 498.1] [Reference Citation Analysis (2)] |
| 12. | Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6075] [Cited by in F6Publishing: 6091] [Article Influence: 468.5] [Reference Citation Analysis (0)] |
| 13. | Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3447] [Cited by in F6Publishing: 3477] [Article Influence: 316.1] [Reference Citation Analysis (0)] |
| 14. | Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National Trends in Ambulatory Oral Anticoagulant Use. Am J Med 2015; 128: 1300-5. e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 421] [Cited by in F6Publishing: 451] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 15. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18463] [Cited by in F6Publishing: 16653] [Article Influence: 1110.2] [Reference Citation Analysis (0)] |
| 16. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7683] [Cited by in F6Publishing: 7773] [Article Influence: 971.6] [Reference Citation Analysis (2)] |
| 17. | Larsen TB, Rasmussen LH, Skjøth F, Due KM, Callréus T, Rosenzweig M, Lip GY. Efficacy and safety of dabigatran etexilate and warfarin in "real-world" patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol. 2013;61:2264-2273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 355] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 18. | Yao X, Abraham NS, Sangaralingham LR, Bellolio MF, McBane RD, Shah ND, Noseworthy PA. Effectiveness and Safety of Dabigatran, Rivaroxaban, and Apixaban Versus Warfarin in Nonvalvular Atrial Fibrillation. J Am Heart Assoc. 2016;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 294] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 19. | Villines TC, Schnee J, Fraeman K, Siu K, Reynolds MW, Collins J, Schwartzman E. A comparison of the safety and effectiveness of dabigatran and warfarin in non-valvular atrial fibrillation patients in a large healthcare system. Thromb Haemost. 2015;114:1290-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Laliberté F, Cloutier M, Nelson WW, Coleman CI, Pilon D, Olson WH, Damaraju CV, Schein JR, Lefebvre P. Real-world comparative effectiveness and safety of rivaroxaban and warfarin in nonvalvular atrial fibrillation patients. Curr Med Res Opin. 2014;30:1317-1325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 21. | Gorst-Rasmussen A, Lip GY, Bjerregaard Larsen T. Rivaroxaban versus warfarin and dabigatran in atrial fibrillation: comparative effectiveness and safety in Danish routine care. Pharmacoepidemiol Drug Saf. 2016;25:1236-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Coleman CI, Peacock WF, Bunz TJ, Alberts MJ. Effectiveness and Safety of Apixaban, Dabigatran, and Rivaroxaban Versus Warfarin in Patients With Nonvalvular Atrial Fibrillation and Previous Stroke or Transient Ischemic Attack. Stroke. 2017;48:2142-2149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Norby FL, Bengtson LGS, Lutsey PL, Chen LY, MacLehose RF, Chamberlain AM, Rapson I, Alonso A. Comparative effectiveness of rivaroxaban versus warfarin or dabigatran for the treatment of patients with non-valvular atrial fibrillation. BMC Cardiovasc Disord. 2017;17:238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Li XS, Deitelzweig S, Keshishian A, Hamilton M, Horblyuk R, Gupta K, Luo X, Mardekian J, Friend K, Nadkarni A, Pan X, Lip GYH. Effectiveness and safety of apixaban versus warfarin in non-valvular atrial fibrillation patients in "real-world" clinical practice. A propensity-matched analysis of 76,940 patients. Thromb Haemost. 2017;117:1072-1082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 25. | Chan YH, Yen KC, See LC, Chang SH, Wu LS, Lee HF, Tu HT, Yeh YH, Kuo CT. Cardiovascular, Bleeding, and Mortality Risks of Dabigatran in Asians With Nonvalvular Atrial Fibrillation. Stroke. 2016;47:441-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Yap LB, Eng DT, Sivalingam L, Rusani BI, Umadevan D, Muhammad Z, Koh KW, Aisha B, Hashim MI, Rebo R, Hussin A, Kaur S, Shanmugam R, Omar R. A Comparison of Dabigatran With Warfarin for Stroke Prevention in Atrial Fibrillation in an Asian Population. Clin Appl Thromb Hemost. 2016;22:792-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Cha MJ, Choi EK, Han KD, Lee SR, Lim WH, Oh S, Lip GYH. Effectiveness and Safety of Non-Vitamin K Antagonist Oral Anticoagulants in Asian Patients With Atrial Fibrillation. Stroke. 2017;48:3040-3048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 28. | Kohsaka S, Murata T, Izumi N, Katada J, Wang F, Terayama Y. Bleeding risk of apixaban, dabigatran, and low-dose rivaroxaban compared with warfarin in Japanese patients with non-valvular atrial fibrillation: a propensity matched analysis of administrative claims data. Curr Med Res Opin. 2017;33:1955-1963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Chan YH, See LC, Tu HT, Yeh YH, Chang SH, Wu LS, Lee HF, Wang CL, Kuo CF, Kuo CT. Efficacy and Safety of Apixaban, Dabigatran, Rivaroxaban, and Warfarin in Asians With Nonvalvular Atrial Fibrillation. J Am Heart Assoc. 2018;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 30. | Huang HY, Lin SY, Cheng SH, Wang CC. Effectiveness and Safety of Different Rivaroxaban Dosage Regimens in Patients with Non-Valvular Atrial Fibrillation: A Nationwide, Population-Based Cohort Study. Sci Rep. 2018;8:3451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Okumura Y, Yokoyama K, Matsumoto N, Tachibana E, Kuronuma K, Oiwa K, Matsumoto M, Kojima T, Hanada S, Nomoto K, Arima K, Takahashi F, Kotani T, Ikeya Y, Fukushima S, Itou S, Kondo K, Chiku M, Ohno Y, Onikura M, Hirayama A; SAKURA AF Registry Investigators. Three-Year Clinical Outcomes Associated With Warfarin vs. Direct Oral Anticoagulant Use Among Japanese Patients With Atrial Fibrillation - Findings From the SAKURA AF Registry. Circ J. 2018;82:2500-2509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 32. | Koretsune Y, Yamashita T, Yasaka M, Ono Y, Hirakawa T, Ishida K, Kuroki D, Sumida T, Urushihara H. Comparative effectiveness and safety of warfarin and dabigatran in patients with non-valvular atrial fibrillation in Japan: A claims database analysis. J Cardiol. 2019;73:204-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Chan YH, Lee HF, See LC, Tu HT, Chao TF, Yeh YH, Wu LS, Kuo CT, Chang SH, Lip GYH. Effectiveness and Safety of Four Direct Oral Anticoagulants in Asian Patients With Nonvalvular Atrial Fibrillation. Chest. 2019;156:529-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 34. | Jeong HK, Lee KH, Park HW, Yoon NS, Kim MC, Lee N, Kim JS, Ahn Y, Jeong MH, Park JC, Cho JG. Real World Comparison of Rivaroxaban and Warfarin in Korean Patients with Atrial Fibrillation: Propensity Matching Cohort Analysis. Chonnam Med J. 2019;55:54-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 35. | Lee SR, Choi EK, Kwon S, Han KD, Jung JH, Cha MJ, Oh S, Lip GYH. Effectiveness and Safety of Contemporary Oral Anticoagulants Among Asians With Nonvalvular Atrial Fibrillation. Stroke. 2019;50:2245-2249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 36. | Cho MS, Yun JE, Park JJ, Kim YJ, Lee J, Kim H, Park DW, Nam GB. Outcomes After Use of Standard- and Low-Dose Non-Vitamin K Oral Anticoagulants in Asian Patients With Atrial Fibrillation. Stroke. 2018;STROKEAHA118023093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |