Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.223
Peer-review started: February 1, 2015
First decision: March 20, 2015
Revised: May 11, 2015
Accepted: May 16, 2015
Article in press: May 18, 2015
Published online: August 26, 2015
The increasing incidence of obesity worldwide and its related cardiometabolic complications is an urgent public health problem. While weight gain results from a negative balance between the energy expenditure and calorie intake, recent research has demonstrated that several small organic molecules containing a four-carbon backbone can modulate this balance by favoring energy expenditure, and alleviating endoplasmic reticulum stress and oxidative stress. Such small molecules include the bacterially produced short chain fatty acid butyric acid, its chemically produced derivative 4-phenylbutyric acid, the main ketone body D-β-hydroxybutyrate - synthesized by the liver - and the recently discovered myokine β-aminoisobutyric acid. Conversely, another butyrate-related molecule, α-hydroxybutyrate, has been found to be an early predictor of insulin resistance and glucose intolerance. In this minireview, we summarize recent advances in the understanding of the mechanism of action of these molecules, and discuss their use as therapeutics to improve metabolic homeostasis or their detection as early biomarkers of incipient insulin resistance.
Core tip: Recent research demonstrated that the four-carbon molecule butyrate, and butyrate-related molecules (4-phenylbutyric acid, D-β-hydroxybutyrate and β-aminoisobutyric acid), act as modulators of metabolism, favoring energy expenditure. Conversely, another butyrate-related molecule, α-hydroxybutyrate, is an early predictor of insulin resistance and glucose intolerance. In this minireview, we summarize the recent progress in the understanding of the mechanism of action of these molecules and discuss their possible therapeutic use to improve metabolic homeostasis and their usefulness as early biomarkers for insulin resistance.
- Citation: Chriett S, Pirola L. Essential roles of four-carbon backbone chemicals in the control of metabolism. World J Biol Chem 2015; 6(3): 223-230
- URL: https://www.wjgnet.com/1949-8454/full/v6/i3/223.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i3.223
Accordingly to World Health Organisation figures, the increased incidence of overweight and obesity worldwide constitutes a major risk factor for global deaths in both developing and industrialized countries[1]. Obesity predisposes individuals to the development of several non-communicable diseases, including cardiovascular disease, dyslipidemia, insulin resistance and type 2 diabetes (T2D), and certain cancers[2]. Furthermore, obesity engenders psychological consequences in affected individuals, especially in the young[3], and recent research indicates that a predisposition to metabolic disease linked to obesity can be transmitted trans-generationally through epigenetic mechanisms[4].
Obesity is the most straightforward consequence of an energy imbalance. Indeed, body weight results from the difference in energy intake, determined by food consumption, and total energy expenditure required to sustain vital functions, including basal metabolism, thermoregulation, and exercise. If the balance is negative, stored fat is mobilized to provide calories, resulting in weight loss. Reciprocally, excessive food intake results in fat accumulation, and ultimately obesity.
Lifestyle interventions, including changes in diet and increased physical activity, are the simplest way to control body weight. Yet, these interventions are often limited by social and economic constraints, such as restricted access to healthy food and environments conducive to physical activity, especially for people already obese or overweight[5]. Consequently, extensive research has been conducted to discover therapeutic agents that might diminish or reverse the negative consequences of the western diet on body weight and associated health effects.
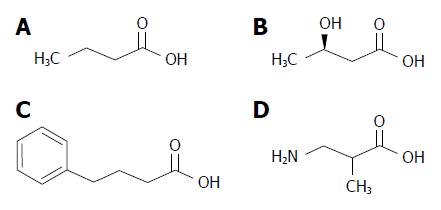
Interestingly, short chain fatty acids (SCFAs) and molecules related to the 4-carbon SCFA butyric acid (Figure 1) have been shown to exert beneficial effects on the control of body weight and metabolism. Here we summarize the current knowledge about these molecules, their biological source of production and physiological mode of action.
SCFAs, also known as volatile fatty acids, are carboxylic acids containing up to 6 carbons in their aliphatic chain. Accordingly to the number of carbons, SCFAs include acetic (C2), propionic (C3), butyric (C4), valeric (C5) and caproic (C6) acids. SCFAs are mainly produced by the gut microbiota using dietary fibers as the major substrate. Therefore, the amount of SCFAs produced is affected by environmental, dietary and microbiological factors[6], with a plant-based diet favoring the production of acetate and butyrate[7].
The gut microbiota is acquired postnatally and its composition is affected by both host genetics and dietary habits. For example, in mice, a high fat and high sugar diet administered to different inbred strains induced a strain-specific propensity to obesity and strain-specific effect on the composition of their gut microbial communities[8].
The dynamic composition of the gut microbiota is a reflection of the host’s physiological status[9]. A metabolic diseases like T2D, resulting from both genetic predisposition and environmental factors, is a good example of the interplay between the disease and the microbiota, as gut bacterial dysbiosis was observed in type 2 diabetic patients, with a decline in butyrate-producing bacteria[9,10].
It has been suggested that diets that favor the production of SCFAs by the microbiota may have multiple positive effects on host metabolic regulation. For example, the intake of dietary fibers, which contains indigestible and fermentable carbohydrates, exerts a positive action on the central regulation of satiety and appetite, leading to reduced weight gain and adiposity[11-13]. Some dietary fibers have been found to have a positive effect by decreasing weight gain and energy intake in mice when supplemented to a high fat diet[13], preventing insulin resistance and the development of T2D[14], and improving immune responses.
It has been shown that SCFA act on T cells to enhance the generation of Th1 and Th17 cell populations, boosting immunity to fight pathogens. At the same time SCFAs induce T cell production of IL-10 and favour the expansion of FOXP3-positive regulatory T cells, helping preventing inflammatory responses[15]. The intestinal epithelium, which separates the exterior environment from the inner organism, is constantly exposed to extrinsic pathogens: viruses, bacteria and their products, that interact with the host’s immune system[16]. The gut microbiota interacts with the immune system and, depending on its composition, promotes or limits inflammatory responses in the intestine. In fact, a mixture of Clostridia strains orally administered in a mouse model of colitis, was shown to induce T-regulatory (Treg) cells and promote the production of anti-inflammatory molecules including interleukin-10 (IL-10) and inducible T-cell co-stimulator (ICOS) in Treg cells[17]. The interplay between microbiota and immune system is mediated by bacterially produced metabolites, especially butyrate and other SCFAs. Indeed, SCFAs increase the number of Treg cells through the potentiation of their extrathymic differentiation[18]. Also, butyrate’s histone deacetylase (HDAC) inhibitor properties induce histone H3 acetylation on the Foxp3 promoter in Treg cells resulting in Foxp3 transcriptional upregulation and alleviation of inflammation in the intestine[18,19]. Another SCFA, propionate, exerts the same actions of butyrate on Treg cells, also through HDAC inhibition[18].
Inflammation and the development of obesity: The existence of a correlation between obesity and a sub-clinical inflammatory state is widely accepted[20]. The chronic inflammation observed in adipose tissue in the obese is attributed to the infiltration of immune cells, mainly monocytes - which subsequently differentiate into resident macrophages - but also T cells. Such immune cell infiltration results in a sustained local production of pro-inflammatory cytokines, secreted by the infiltrating immune cells, but also by adipocytes[21]. The establishment of a pro-inflammatory environment, in turn, renders the adipocyte insulin-resistant, predisposing the individual to development of T2D in the long term.
Biological actions of butyric acid: Butyric acid is one of the most well-studied SCFAs due to its presence in dairy products such as cheese and butter[12], and its endogenous production in the human gut, mainly by butyric bacteria[22]. Indeed, plasma butyrate results from its production in the gut, which is favored by foods high in fermentable fiber[23], and impaired with high fat diets[24].
When supplemented to mice diets, particularly in the context of a high fat diet, butyrate was shown to counteract the development of obesity[25], insulin resistance and the emergence of T2D. The mode of action of butyrate was attributed to a rise in energy expenditure. In skeletal muscle, butyrate caused an increase in type I muscle fibers along with an augmentation of ATP consumption. This effect seems to be associated with butyrate being an HDAC inhibitor and the fact that it favored type I oxidative muscle fibers and mitochondrial biogenesis[14]. Whether the beneficial actions of butyrate are due to a direct action on tissue metabolism will require further investigation, as a clinical study investigating the effects of perfusion of short-chain fatty acids - including butyrate - on glucose metabolism in healthy men failed to show beneficial changes of glucose metabolism[26]. On the other hand, a decreased population of gut butyric bacteria has been demonstrated in type 2 diabetic patients[9,10], indicating the prominent role of butyrate in the maintenance of a healthy metabolism.
Butyrate has been shown to act as an histone deacetylase (HDAC) inhibitor[27]. In fact, its presence leads to increased histone acetylation, giving butyrate a possible action on proliferation, differentiation and regulation of metabolism[27,28].
Recent research has suggested a link between inhibition of class I/II HDAC by butyrate and other SCFAs[29,30], and improved metabolic responses. In liver, class IIa HDACs dephosphorylate and activate FOXO, favouring the expression of gluconeogenic FOXO target genes. Thus, HDACs inhibition may provide a potential therapeutic approach to alleviate insulin resistance by decreasing gluconeogenesis[31].
Interestingly, butyric acid inhibits class I and class II HDACs in a competitive fashion[32], and dietary administration of sodium butyrate has been shown to improve systemic insulin sensitivity and increase energy expenditure in mice via up regulation of mitochondrial function in skeletal muscle and brown fat, through PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha) induction and elevation of AMPK (adenosine monophosphate-activated protein kinase) activity[14]. A further mechanism accounting for the beneficial metabolic effects of butyrate is the induction of hepatocyte- and adipocyte- produced fibroblast growth factor 21 (FGF-21), a hormone promoting fatty acid oxidation in mice. Increased FGF-21 production depends on acetylated and active PPARα (peroxisome proliferator-activated receptor alpha), and inhibition of the PPARα-deacetylating enzyme HDAC3 contributes to maintaining PPARα in its active state[33].
4-phenylbutyric acid (4-PBA) is a chemically produced derivative of butyric acid; 4-PBA is obtained by the reaction of benzene with butyrolactone in the presence of aluminum chloride, followed by neutralization with a base (Burzynsky SR and Musial L, US patent US6372938 B1, 2002). 4-PBA was first used in therapies for urea cycle enzyme deficiencies, due to its ability to act as a chaperone for other enzymes[34,35]. 4-PBA has also been shown to have wider therapeutic indications including alleviation of endoplasmic reticulum stress and associated pathological conditions such as inflammation, hypertension and diabetes[36-40]. The chaperone activity of 4-PBA prevents protein conformational abnormalities and protein aggregation occurring in neurodegenerative diseases[41,42] and in insulin resistance/T2D - as demonstrated by the capability of 4-PBA to restore glucose homeostasis in type 2 diabetic rodent models[43]. Recent preclinical research in zebrafish and mouse models demonstrated that 4-PBA is a PDK (pyruvate dehydrogenase kinase) inhibitor[44]. PDK, inhibits the pyruvate dehydrogenase complex (PDHC) catalytic activity via inhibitory phosphorylation and has been used to alleviate lactic acidosis caused by PDHC deficiency by restoring the conversion of glycolitic pyruvate into acetylCoA. Another use of 4-PBA is as an HDAC inhibitor, mostly in cancer studies[45-47]. Both 4-PBAs HDAC inhibitor activity and its chaperone-like activity might constitute upstream targets to regulate gene expression. Indeed, in metabolic diseases like insulin resistance, 4-PBA positively modulates energy expenditure by favoring the expression of key metabolic genes, including GLUT4[48]. Furthermore, in 3T3-L1 pre-adipocytes, administration of 4-PBA was shown to inhibit adipogenesis through the inhibition of the unfolded protein response, and nutritional supplementation of 4-PBA to mice lowered fat pad weight and resulted in smaller adipocytes in this tissue[49]. Considering its multiple modes of action, and in particular its activity as chemical chaperone, 4-PBA constitutes a very promising molecule to target metabolic states related to endoplasmic reticulum stress such as insulin resistance and fat over-accumulation in the adipose tissue[50]. Taken together, these studies suggest that 4-PBA favors the glycolytic utilization of glucose, while inhibiting adipogenesis and, by extension, fatty acid oxidation.
Ketone bodies (KB) are the organism’s main source of energy in periods of fasting or prolonged physical exercise, when glucose availability is scarce[51]. KB are mainly derived from free fatty acids, although a small percentage of KB can originate from the metabolism of amino acids[52].
Essentially produced by the liver, the two main KB are acetoacetate (AcAc) and D-β-hydroxybutyrate. KB are metabolically equivalent to fat yet are water-soluble and can be transported in blood to target tissues[53]. Once in the target tissues, KB are converted back to acetyl-coA, through the sequential action of D-β-hydroxybutyrate dehydrogenase, converting D-β-hydroxybutyrate to acetoacetate; 3-ketoacyl-CoA transferase, which produces acetoacetylCoA from acetoacetate, which is finally converted to acetyl-coA by acetoacetyl-CoA thiolase. Importantly, 3-ketoacyl-CoA transferase is only present in organs using KB, and not expressed in the liver. Thus, the flow of ketone bodies is unidirectional, from the liver to peripheral organs. For a long time the liver was thought to be the only organ to produce KB, but it appears the brain can also synthetize them to a certain extent for its own energetic needs[54].
α-hydroxybutyrate (α-HB), a D-β-hydroxybutyrate isomeric but metabolically unrelated molecule, is generated in the liver (1) in the cysteine formation pathway; (2) as a byproduct during the formation of α-ketobutyrate, - the final product of methionine and threonine catabolism[55]; and also (3) under increased oxidative stress, a physiological state associated to insulin resistance. Recently, α-HB has been proposed as a marker of oxidative stress, and increased α-HB circulating levels have been shown to be early biomarkers of insulin resistance in a non-diabetic population[56]. Therefore, α-hydroxybutyrate in the blood might be a predictive sign of incipient metabolic disease[57,58], and β pancreatic cells damage[59], thus representing an ideal biomarker for early detection of predisposition to insulin resistance and T2D.
D-β-hydroxybutyrate, which is present in the serum in the micromolar concentration range under postprandial or short-term fasting conditions, can increase up to millimolar concentrations in case of fasting or intense exercise and becomes the major energy source for the brain and muscles[60,61]. D-β-hydroxybutyrate is a central metabolic intermediate that is associated to decreased free fatty acids blood concentrations and at the same time prevents from the exhaustion of fat stocks during prolonged starvation[58,61] and might also regulate the aging process, as administration of D-β-hydroxybutyrate to Caenorhabditis elegans has been shown to increase the worm’s lifespan[62].
Recent research has demonstrated that the biological action of the ketone body D-β-hydroxybutyrate extends beyond its role as metabolite. Indeed, D-β-hydroxybutyrate has been shown to act both as a signaling molecule by activating G-protein coupled receptors (GPCRs), and as a transcriptional regulator by acting as a HDAC inhibitor.
Related to its properties as signaling intermediate, D-β-hydroxybutyrate has been shown to be an agonist for two GPCRs, PUMA-G (also named HCAR2, hydroxycarboxylic acid receptor 2, or Gpr109)[63] and the free fatty acids receptor 3 (FFA3, or Grp41)[64].
A novel and exciting research field has finally recently emerged with the discovery that D-β-hydroxybutyrate can be a key regulator of gene expression by acting as an endogenous HDACs inhibitor[60]. The HDACs inhibitory activity of D-β-hydroxybutyrate brings changes in histone acetylation and gene expression that seem to protect cells from oxidative stress[65].
β-aminoisobutyric acid (BAIBA) was first identified as a catabolite derived from the breakdown of pyrimidines[66] and branched chain amino acids[67]. More recent research has demonstrated that BAIBA can act to prevent weight gain in several ways. Through an action on lipid metabolism, BAIBA reduces plasma and liver triglycerides by increasing both liver fatty acid oxidation and ketogenesis, especially by inducing β-D-hydroxybutyrate synthesis[68]. A further mode of action of BAIBA may involve the action of leptin. Leptin is an adipokine expressed by adipose tissue which contributes to the central hypothalamic regulation of food intake and energy expenditure, therefore favoring weight loss[69]. Interestingly, ob/ob leptin deficient mice were not responsive to BAIBA, showing neither weight loss nor increased fatty oxidation upon BAIBA administration[68]. BAIBA, like leptin, appears to favor lipid metabolism, and at the same time, can act indirectly by promoting leptin expression in white adipose tissue[70].
Moreover, recent research has shown that BAIBA is produced in muscles and BAIBA plasmatic concentrations in rodents and humans are most likely representative of the amounts of BAIBA produced by skeletal muscle[71]. BAIBA derived from skeletal muscle can be considered as a myokine (e.g., a muscle-secreted molecule with hormonal-like properties on target organs) that mediates the metabolic crosstalk between skeletal muscle and other metabolically active tissues. BAIBA production is regulated by PGC-1α, which is a general co-activator overexpressed in response to exercise[71]. As a myokine, BAIBA is capable of inducing a transition in the adipose tissue from a white adipose tissue phenotype to a tissue endowed with brown adipose tissue characteristics, thereby favoring the expression of β-oxidation and thermogenic genes, including PPARα and its target mitochondrial uncoupling protein 1 (UCP1), rather than lipogenic and lipid-storage genes[71,72]. Although the direct mechanism of action of BAIBA upstream of PPARα has not yet been elucidated, it has been proposed that BAIBA could be a possible therapeutic molecule as an anti-obesity agent, by favoring a white fat to brown fat phenotypic transition[73].
Obesity and its metabolic consequences leading to increased morbidity and mortality can be currently considered as one of the major health burdens worldwide. Extensive preclinical and clinical research is thus necessary to identify novel agents that might be therapeutically useful - along with nutritional and physical activity approaches - to counteract this pandemic.
The four-carbon backbone chemicals described in this review, through their multiple mechanisms of actions summarized in Table 1, may provide a new class of potential pharmacologically effective candidates, to be used alone or in combination to effectively reverse or alleviate obesity and the insulin resistant state.
| Molecule | Putative molecular target or mechanism involved | Overall biological response | Ref. |
| Butyric acid | Increased PGC1α expression and AMPK activity | Increased type I muscle fibers, increased insulin sensitivity, reduced adiposity | [14] |
| Inhibition of HDACs | Decreased glycaemia in streptozotocin-induced diabetes. Decreased pancreatic β-cells apoptosis | [28] | |
| HDAC3 inhibition | Induction of FGF21, fatty acid oxidation and ketone bodies production | [33] | |
| D-β-hydroxybutyrate | Binds and activates GPCR PUMA-G/Gpr109 | Inhibition of adipocyte lipolysis | [63] |
| Binds and activates GPCR FFA3/Grp41 | Regulates energy consumption through FFA3/Grp41 activation in the sympathetic nervous system | [64] | |
| HDACs inhibition | Protection from oxidative stress | [65] | |
| 4-phenylbutyric acid | HDAC5 inhibition | Increased GLUT4 gene expression and promotion of glucose metabolism | [48] |
| Inhibition of oxidative stress and endoplasmic reticulum stress | Alleviation of diabetic nephropathy in streptozotocin-induced diabetes | [40] | |
| β-aminoisobutyric acid | Activates PPARα | Induction of hepatic β-oxidation and brown adipose tissue | [71] |
| Restores normal plasma leptin levels | Increase of fatty acid oxidation and alleviation of diet-induced obesity in ob/+ heterozygous mice | [69] | |
| Induces liver ketogenesis, increasing circulating D-β-hydroxybutyrate | Decreased body fat mass | [68] |
We wish to thank Professor Robert Ward for critically reading the manuscript.
P- Reviewer: Martinez-Costa OH, Socorro SC, Yeligar SM S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
| 1. | World Health Organisation. Obesity and overweight Fact sheet N°311. [updated 2015 Jan]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/. [Cited in This Article: ] |
| 2. | Gunter MJ, Leitzmann MF. Obesity and colorectal cancer: epidemiology, mechanisms and candidate genes. J Nutr Biochem. 2006;17:145-156. [PubMed] [Cited in This Article: ] |
| 3. | Harriger JA, Thompson JK. Psychological consequences of obesity: weight bias and body image in overweight and obese youth. Int Rev Psychiatry. 2012;24:247-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Vickers MH. Developmental programming and transgenerational transmission of obesity. Ann Nutr Metab. 2014;64 Suppl 1:26-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Casazza K, Fontaine KR, Astrup A, Birch LL, Brown AW, Bohan Brown MM, Durant N, Dutton G, Foster EM, Heymsfield SB. Myths, presumptions, and facts about obesity. N Engl J Med. 2013;368:446-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 6. | Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67-72. [PubMed] [Cited in This Article: ] |
| 7. | David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5625] [Cited by in F6Publishing: 5980] [Article Influence: 543.6] [Reference Citation Analysis (0)] |
| 8. | Parks BW, Nam E, Org E, Kostem E, Norheim F, Hui ST, Pan C, Civelek M, Rau CD, Bennett BJ. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 2013;17:141-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 386] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 9. | Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut. 2014;63:1513-1521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 10. | Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3971] [Cited by in F6Publishing: 4301] [Article Influence: 358.4] [Reference Citation Analysis (0)] |
| 11. | Arora T, Loo RL, Anastasovska J, Gibson GR, Tuohy KM, Sharma RK, Swann JR, Deaville ER, Sleeth ML, Thomas EL. Differential effects of two fermentable carbohydrates on central appetite regulation and body composition. PLoS One. 2012;7:e43263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Johansson EV, Nilsson AC, Östman EM, Björck IM. Effects of indigestible carbohydrates in barley on glucose metabolism, appetite and voluntary food intake over 16 h in healthy adults. Nutr J. 2013;12:46. [PubMed] [Cited in This Article: ] |
| 13. | Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 821] [Cited by in F6Publishing: 960] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 14. | Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509-1517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1337] [Cited by in F6Publishing: 1382] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 15. | Kim CH, Park J, Kim M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw. 2014;14:277-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 403] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 16. | Malago JJ. Contribution of microbiota to the intestinal physicochemical barrier. Benef Microbes. 2015;6:295-311. [PubMed] [Cited in This Article: ] |
| 17. | Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1875] [Cited by in F6Publishing: 1929] [Article Influence: 175.4] [Reference Citation Analysis (2)] |
| 18. | Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2516] [Cited by in F6Publishing: 2923] [Article Influence: 265.7] [Reference Citation Analysis (0)] |
| 19. | Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2951] [Cited by in F6Publishing: 3272] [Article Influence: 297.5] [Reference Citation Analysis (0)] |
| 20. | Kammoun HL, Kraakman MJ, Febbraio MA. Adipose tissue inflammation in glucose metabolism. Rev Endocr Metab Disord. 2014;15:31-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 509] [Cited by in F6Publishing: 522] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 22. | McOrist AL, Abell GC, Cooke C, Nyland K. Bacterial population dynamics and faecal short-chain fatty acid (SCFA) concentrations in healthy humans. Br J Nutr. 2008;100:138-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Jakobsdottir G, Jädert C, Holm L, Nyman ME. Propionic and butyric acids, formed in the caecum of rats fed highly fermentable dietary fibre, are reflected in portal and aortic serum. Br J Nutr. 2013;110:1565-1572 [. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 24. | Jakobsdottir G, Xu J, Molin G, Ahrné S, Nyman M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS One. 2013;8:e80476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 25. | Lin HV, Frassetto A, Kowalik EJ, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:e35240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 760] [Cited by in F6Publishing: 830] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 26. | Martin-Gronert MS, Tarry-Adkins JL, Cripps RL, Chen JH, Ozanne SE. Maternal protein restriction leads to early life alterations in the expression of key molecules involved in the aging process in rat offspring. Am J Physiol Regul Integr Comp Physiol. 2008;294:R494-R500. [PubMed] [Cited in This Article: ] |
| 27. | Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485S-2493S. [PubMed] [Cited in This Article: ] |
| 28. | Khan S, Jena GB. Protective role of sodium butyrate, a HDAC inhibitor on beta-cell proliferation, function and glucose homeostasis through modulation of p38/ERK MAPK and apoptotic pathways: study in juvenile diabetic rat. Chem Biol Interact. 2014;213:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 29. | Schilderink R, Verseijden C, de Jonge WJ. Dietary inhibitors of histone deacetylases in intestinal immunity and homeostasis. Front Immunol. 2013;4:226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem. 2008;19:587-593. [PubMed] [Cited in This Article: ] |
| 31. | Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG, Downes M, Evans RM, Montminy M, Shaw RJ. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145:607-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 401] [Cited by in F6Publishing: 435] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 32. | Sekhavat A, Sun JM, Davie JR. Competitive inhibition of histone deacetylase activity by trichostatin A and butyrate. Biochem Cell Biol. 2007;85:751-758. [PubMed] [Cited in This Article: ] |
| 33. | Li H, Gao Z, Zhang J, Ye X, Xu A, Ye J, Jia W. Sodium butyrate stimulates expression of fibroblast growth factor 21 in liver by inhibition of histone deacetylase 3. Diabetes. 2012;61:797-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 34. | Sodium phenylbutyrate for urea cycle enzyme deficiencies. Med Lett Drugs Ther. 1996;38:105-106. [PubMed] [Cited in This Article: ] |
| 35. | Cederbaum S, Lemons C, Batshaw ML. Alternative pathway or diversion therapy for urea cycle disorders now and in the future. Mol Genet Metab. 2010;100:219-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Zhu M, Guo M, Fei L, Pan XQ, Liu QQ. 4-phenylbutyric acid attenuates endoplasmic reticulum stress-mediated pancreatic β-cell apoptosis in rats with streptozotocin-induced diabetes. Endocrine. 2014;47:129-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Koyama M, Furuhashi M, Ishimura S, Mita T, Fuseya T, Okazaki Y, Yoshida H, Tsuchihashi K, Miura T. Reduction of endoplasmic reticulum stress by 4-phenylbutyric acid prevents the development of hypoxia-induced pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2014;306:H1314-H1323. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Kusaczuk M, Bartoszewicz M, Cechowska-Pasko M. Phenylbutyric Acid: simple structure - multiple effects. Curr Pharm Des. 2015;21:2147-2166. [PubMed] [Cited in This Article: ] |
| 39. | Zeng W, Guo YH, Qi W, Chen JG, Yang LL, Luo ZF, Mu J, Feng B. 4-Phenylbutyric acid suppresses inflammation through regulation of endoplasmic reticulum stress of endothelial cells stimulated by uremic serum. Life Sci. 2014;103:15-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Luo ZF, Feng B, Mu J, Qi W, Zeng W, Guo YH, Pang Q, Ye ZL, Liu L, Yuan FH. Effects of 4-phenylbutyric acid on the process and development of diabetic nephropathy induced in rats by streptozotocin: regulation of endoplasmic reticulum stress-oxidative activation. Toxicol Appl Pharmacol. 2010;246:49-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Nomura Y. [Pharmacological studies on neurodegenerative diseases focusing on refolding and degradation of unfolded proteins in the endoplasmic reticulum]. Yakugaku Zasshi. 2014;134:537-543. [PubMed] [Cited in This Article: ] |
| 42. | Mimori S, Ohtaka H, Koshikawa Y, Kawada K, Kaneko M, Okuma Y, Nomura Y, Murakami Y, Hamana H. 4-Phenylbutyric acid protects against neuronal cell death by primarily acting as a chemical chaperone rather than histone deacetylase inhibitor. Bioorg Med Chem Lett. 2013;23:6015-6018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137-1140. [PubMed] [Cited in This Article: ] |
| 44. | Ferriero R, Manco G, Lamantea E, Nusco E, Ferrante MI, Sordino P, Stacpoole PW, Lee B, Zeviani M, Brunetti-Pierri N. Phenylbutyrate therapy for pyruvate dehydrogenase complex deficiency and lactic acidosis. Sci Transl Med. 2013;5:175ra31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Kouraklis G, Theocharis S. Histone deacetylase inhibitors and anticancer therapy. Curr Med Chem Anticancer Agents. 2002;2:477-484. [PubMed] [Cited in This Article: ] |
| 46. | Stepulak A, Stryjecka-Zimmer M, Kupisz K, Polberg K. [Histone deacetylase inhibitors as a new generation of anti-cancer agents]. Postepy Hig Med Dosw (Online). 2005;59:68-74. [PubMed] [Cited in This Article: ] |
| 47. | Dovzhanskiy DI, Hartwig W, Lázár NG, Schmidt A, Felix K, Straub BK, Hackert T, Krysko DV, Werner J. Growth inhibition of pancreatic cancer by experimental treatment with 4-phenylbutyrate is associated with increased expression of Connexin 43. Oncol Res. 2012;20:103-111. [PubMed] [Cited in This Article: ] |
| 48. | Hu H, Li L, Wang C, He H, Mao K, Ma X, Shi R, Oh Y, Zhang F, Lu Y. 4-Phenylbutyric acid increases GLUT4 gene expression through suppression of HDAC5 but not endoplasmic reticulum stress. Cell Physiol Biochem. 2014;33:1899-1910. [PubMed] [Cited in This Article: ] |
| 49. | Basseri S, Lhoták S, Sharma AM, Austin RC. The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response. J Lipid Res. 2009;50:2486-2501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 50. | Gregor MF, Hotamisligil GS. Thematic review series: Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res. 2007;48:1905-1914. [PubMed] [Cited in This Article: ] |
| 51. | Balasse EO, Féry F. Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev. 1989;5:247-270. [PubMed] [Cited in This Article: ] |
| 52. | Thomas LK, Ittmann M, Cooper C. The role of leucine in ketogenesis in starved rats. Biochem J. 1982;204:399-403. [PubMed] [Cited in This Article: ] |
| 53. | Fukao T, Mitchell G, Sass JO, Hori T, Orii K, Aoyama Y. Ketone body metabolism and its defects. J Inherit Metab Dis. 2014;37:541-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 54. | Guzmán M, Blázquez C. Ketone body synthesis in the brain: possible neuroprotective effects. Prostaglandins Leukot Essent Fatty Acids. 2004;70:287-292. [PubMed] [Cited in This Article: ] |
| 55. | Landaas S. The formation of 2-hydroxybutyric acid in experimental animals. Clin Chim Acta. 1975;58:23-32. [PubMed] [Cited in This Article: ] |
| 56. | Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, Ryals JA, Milburn MV, Nannipieri M, Camastra S. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One. 2010;5:e10883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in F6Publishing: 497] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 57. | Ferrannini E, Natali A, Camastra S, Nannipieri M, Mari A, Adam KP, Milburn MV, Kastenmüller G, Adamski J, Tuomi T. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes. 2013;62:1730-1737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 262] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 58. | Xu F, Tavintharan S, Sum CF, Woon K, Lim SC, Ong CN. Metabolic signature shift in type 2 diabetes mellitus revealed by mass spectrometry-based metabolomics. J Clin Endocrinol Metab. 2013;98:E1060-E1065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 59. | Varvel SA, Pottala JV, Thiselton DL, Caffrey R, Dall T, Sasinowski M, McConnell JP, Warnick GR, Voros S, Graham TE. Serum α-hydroxybutyrate (α-HB) predicts elevated 1 h glucose levels and early-phase β-cell dysfunction during OGTT. BMJ Open Diabetes Res Care. 2014;2:e000038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Newman JC, Verdin E. β-hydroxybutyrate: much more than a metabolite. Diabetes Res Clin Pract. 2014;106:173-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 61. | Laeger T, Metges CC, Kuhla B. Role of beta-hydroxybutyric acid in the central regulation of energy balance. Appetite. 2010;54:450-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 62. | Edwards C, Canfield J, Copes N, Rehan M, Lipps D, Bradshaw PC. D-beta-hydroxybutyrate extends lifespan in C. elegans. Aging (Albany NY). 2014;6:621-644. [PubMed] [Cited in This Article: ] |
| 63. | Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem. 2005;280:26649-26652. [PubMed] [Cited in This Article: ] |
| 64. | Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA. 2011;108:8030-8035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 605] [Cited by in F6Publishing: 665] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 65. | Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 969] [Cited by in F6Publishing: 1107] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 66. | Fink K, Henderson RB, Fink RM. -Aminoisobutyric acid in rat urine following administration of pyrimidines. J Biol Chem. 1952;197:441-452. [PubMed] [Cited in This Article: ] |
| 67. | Nielsen HR, Borek E, Sjolin KE, Nyholm K. Dual origin of -aminoisobutyric acid, a thymine catabolite. Acta Pathol Microbiol Scand A. 1972;80:687-688. [PubMed] [Cited in This Article: ] |
| 68. | Maisonneuve C, Igoudjil A, Begriche K, Lettéron P, Guimont MC, Bastin J, Laigneau JP, Pessayre D, Fromenty B. Effects of zidovudine, stavudine and beta-aminoisobutyric acid on lipid homeostasis in mice: possible role in human fat wasting. Antivir Ther. 2004;9:801-810. [PubMed] [Cited in This Article: ] |
| 69. | Begriche K, Lettéron P, Abbey-Toby A, Vadrot N, Robin MA, Bado A, Pessayre D, Fromenty B. Partial leptin deficiency favors diet-induced obesity and related metabolic disorders in mice. Am J Physiol Endocrinol Metab. 2008;294:E939-E951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Begriche K, Massart J, Fromenty B. Effects of β-aminoisobutyric acid on leptin production and lipid homeostasis: mechanisms and possible relevance for the prevention of obesity. Fundam Clin Pharmacol. 2010;24:269-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 71. | Roberts LD, Boström P, O’Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014;19:96-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 418] [Cited by in F6Publishing: 429] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 72. | Ginter E, Simko V. Recent data on obesity research: β-aminoisobutyric acid. Bratisl Lek Listy. 2014;115:492-493. [PubMed] [Cited in This Article: ] |
| 73. | Kammoun HL, Febbraio MA. Come on BAIBA light my fire. Cell Metab. 2014;19:1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |