Published online Sep 27, 2020. doi: 10.4331/wjbc.v11.i2.30
Peer-review started: June 17, 2020
First decision: July 21, 2020
Revised: July 24, 2020
Accepted: August 25, 2020
Article in press: August 25, 2020
Published online: September 27, 2020
The airway innate immune system maintains the first line of defense against respiratory infections. The airway epithelium and associated immune cells protect the respiratory system from inhaled foreign organisms. These cells sense pathogens via activation of receptors like toll-like receptors and taste family 2 receptors (T2Rs) and respond by producing antimicrobials, inflammatory cytokines, and chemokines. Coordinated regulation of fluid secretion and ciliary beating facilitates clearance of pathogens via mucociliary transport. Airway cells also secrete antimicrobial peptides and radicals to directly kill microorganisms and inactivate viruses. The phosphoinositide-3-kinase/protein kinase B (Akt) kinase pathway regulates multiple cellular targets that modulate cell survival and proliferation. Akt also regulates proteins involved in innate immune pathways. Akt phosphorylates endothelial nitric oxide synthase (eNOS) enzymes expressed in airway epithelial cells. Activation of eNOS can have anti-inflammatory, anti-bacterial, and anti-viral roles. Moreover, Akt can increase the activity of the transcription factor nuclear factor erythroid 2 related factor-2 that protects cells from oxidative stress and may limit inflammation. In this review, we summarize the recent findings of non-cancerous functions of Akt signaling in airway innate host defense mechanisms, including an overview of several known downstream targets of Akt involved in innate immunity.
Core Tip: The human respiratory epithelium is continuously exposed to pathogens during each inhalation. Protection of the lung depends on complex signaling networks that activate host defense mechanisms. The kinase protein kinase B (Akt) interacts with numerous cellular proteins involved in airway innate immunity. In this review, we discuss the Akt pathway and known downstream targets involved in airway innate immunity.
- Citation: Gopallawa I, Lee RJ. Targeting the phosphoinositide-3-kinase/protein kinase B pathway in airway innate immunity. World J Biol Chem 2020; 11(2): 30-51
- URL: https://www.wjgnet.com/1949-8454/full/v11/i2/30.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v11.i2.30
The respiratory epithelium is the first-point of defense in the respiratory system, which is continuously exposed to a wide variety of inhaled pathogens. The classic role of the respiratory epithelium in airway defense is clearance of inhaled microbes via ciliary beating and mucociliary clearance (MCC)[1]. However, the respiratory epithelium is complex and contains not only epithelial cells but also resident macrophages, dendritic cells, and other leukocytes. All of these cells sense infection through protein receptors like toll-like receptors (TLRs) and taste family 2 receptors (T2R) bitter taste receptors[2] which activate production of a wide variety of antimicrobial peptides and radicals as well as inflammatory cytokines and chemokines. For example, stimulation of TLRs on airway epithelial cells regulates the expression of genes encoding multiple cytokines and antimicrobial peptides[3] via nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) signaling[4]. Activation of the innate immune system thus stimulates adaptive immunity and is also associated with apoptosis and other signal transduction pathways.
One understudied protein in airway innate immunity is protein kinase B, also known as Akt, a widely expressed serine/threonine kinase[5]. Activation of Akt by upstream kinases such as phosphoinositide-3-kinase (PI3K) stimulates phosphorylation of downstream targets involved in cell proliferation, apoptosis, and/or cell growth depending on the signaling context. Akt has also recently been suggested to play a role in innate immunity by regulating immune cell development, survival, and function[6].
Akt also has other targets important for innate immune responses of the airway epithelial cells themselves. Akt phosphorylates and activates endothelial (e) nitric oxide synthase (NOS), an enzyme that produces nitric oxide (NO)[7]. NO has many biological functions in the airway, including activating smooth muscle relaxation, increasing ciliary beat frequency, and having direct bactericidal and anti-viral effects[8]. Another target activated by Akt is nuclear factor erythroid 2 related factor-2 (Nrf-2), a transcriptional factor that drives the expression of antioxidant genes that can protect against oxidative stress-induced by microbes as well as over-active inflammatory pathways[9].
Abnormalities of innate immunity are linked with numerous airway diseases including chronic rhinosinusitis[10-12] and cystic fibrosis (CF)[13]. This review focuses on the Akt-dependent regulation of innate immunity in the lung and the potential role of Akt in protecting the airways against infection. Emphasis will be placed on novel directions for drug development. Furthermore, we summarize the current understanding of the role of the airway epithelium in Akt-dependent innate immunity and host defenses against bacterial infections in CF.
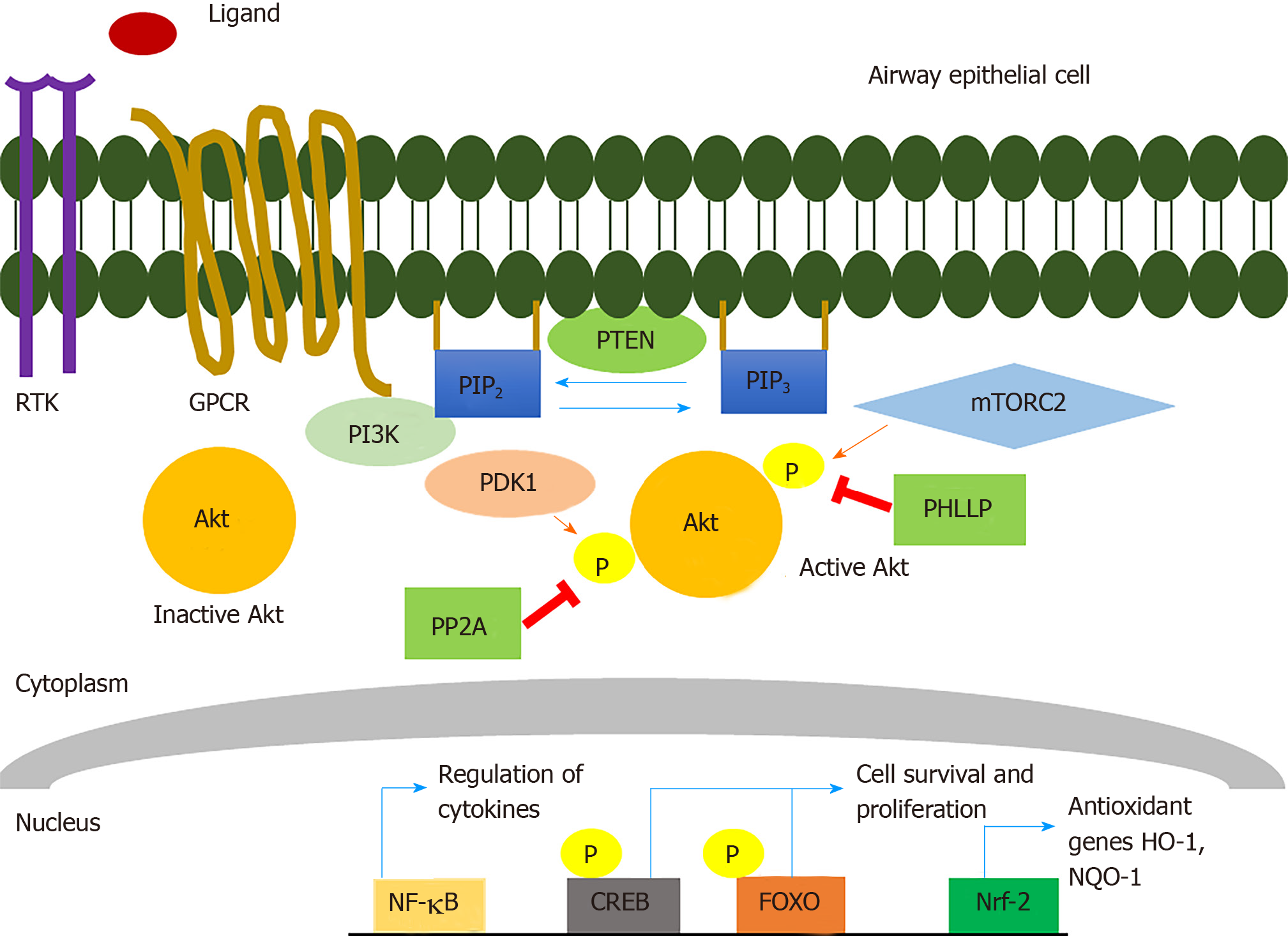
Akt was identified almost 30 years ago by its homology to the v-akt retroviral oncogene[5] and subsequently found to be a 57 kDa serine and threonine protein kinase that plays a pivotal role in cell proliferation, survival, and death[14,15] (Figure 1). There are three conserved mammalian Akt isoforms: Akt1 (PKBa), Akt2 (PKBb), and Akt3 (PKBg). Some Akt isoform knockout studies conducted in mice have suggested there may be specific functions for certain isoforms in growth, metabolism, and development, though this may be in large part due to differences in the tissue distributions of the isoforms[15,16]. All three isoforms of Akt contain an N-terminal Pleckstrin Homology (PH) domain, a kinase domain, and a C-terminal regulatory domain. Activation of some receptor tyrosine kinases (RTKs) and/or some G-protein-coupled-receptors (GPCRs) by growth factors such as insulin-like growth factor-1 can activate the Akt pathway via plasma membrane recruitment and activation of class I PI3K isoforms[15,17]. Activated PI3K can phosphorylate phosphatidylinositol 4,5-bisphosphate (PI4, 5P2) to generate phosphatidylinositol (3,4,5)-trisphosphate (PIP3). Inactive cytosolic Akt gets subsequently recruited to the membrane via the interaction of PIP3 with the Akt PH domain. Akt can also be recruited to the membrane by PI3, 4P2 produced by class II PI3K phosphorylation of PI4P[15].
Akt localization to the plasma membrane induces conformational changes that allow phosphoinositide-dependent protein kinase-1 (PDK-1) to phosphorylate threonine (T) 308 within the activation loop of the Akt1 kinase domain (corresponding to T309 and T305 in Akt2 and Akt3, respectively) and mTOR Complex 2 (mTORC-2) to phosphorylate serine (S) 473 within the hydrophobic C-terminal Akt regulatory domain (corresponding to S474 and S471 in Akt2 and Akt3, respectively)[15]. Maximal activation of the kinase requires phosphorylation of both residues[15]. Multiple other phosphorylation sites exist in Akt that can be phosphorylated by kinase complexes like mammalian target of rapamycin (mTORC2) (T450 in Akt1), CK2 (S129 in Akt1) GSK-3a (T312 in Akt1), cyclin A-CDK2 (S477 and T479 in Akt1), though how they modulate Akt activity is less clear[15].
Once Akt is activated, it can phosphorylate multiple downstream targets and/or redistribute to many cellular compartments, including the nucleus[18]. A study done with a genetically-encoded fluorescent biosensor for Akt activity showed that its activity in the nucleus was less rapid but more sustained compared with cytosolic Akt activity[19], suggesting that Akt can be regulated differently in the cytosol vs nucleus or other organelles. Activation of Akt is also negatively regulated by phosphatases, including phosphatase and tensin homolog (PTEN) that antagonizes PI3K signaling by dephosphorylating PIP3 and converting it back to PI4, 5P2. Protein phosphatase 2 (PP2A) and PH domain and Leucine-rich repeat Protein Phosphatases (PHLLP) also reduce Akt activation by dephosphorylation at T308 and S473, respectively[15].
Dysregulation of the PI3K/Akt pathway is associated with diabetes, cancer neurological disorders, and cardiovascular diseases[15]. Numerous studies have reported that components of the Akt signaling pathway are frequently mutated in multiple types of cancer; in some cases, this is associated with tumor aggressiveness[18]. In many tumors, Akt activity is upregulated via one or more mechanisms including loss of PTEN, mutations in the PI3K catalytic subunit, or loss of expression of phosphatases such as leucine rich repeat protein phosphatases (PHLPP)1 and PHLPP2 that dephosphorylate Akt[15,20].
Beyond the well-known functions of Akt in cell proliferation and survival and, consequently, the pathophysiology of cancer, the Akt pathway has several roles in the immune system. Akt signaling is important for the maturation and survival of dedicated immune cells. Activation of the Akt pathway is a necessity for the development of human dendritic cells (DCs)[21] and survival of activated B cells[22]. Furthermore, within airway epithelial cells, there are many known downstream targets of Akt that have important innate immune functions. The below sections will describe the innate immune system of the respiratory epithelium and importance of some known Akt targets in airway innate immunity.
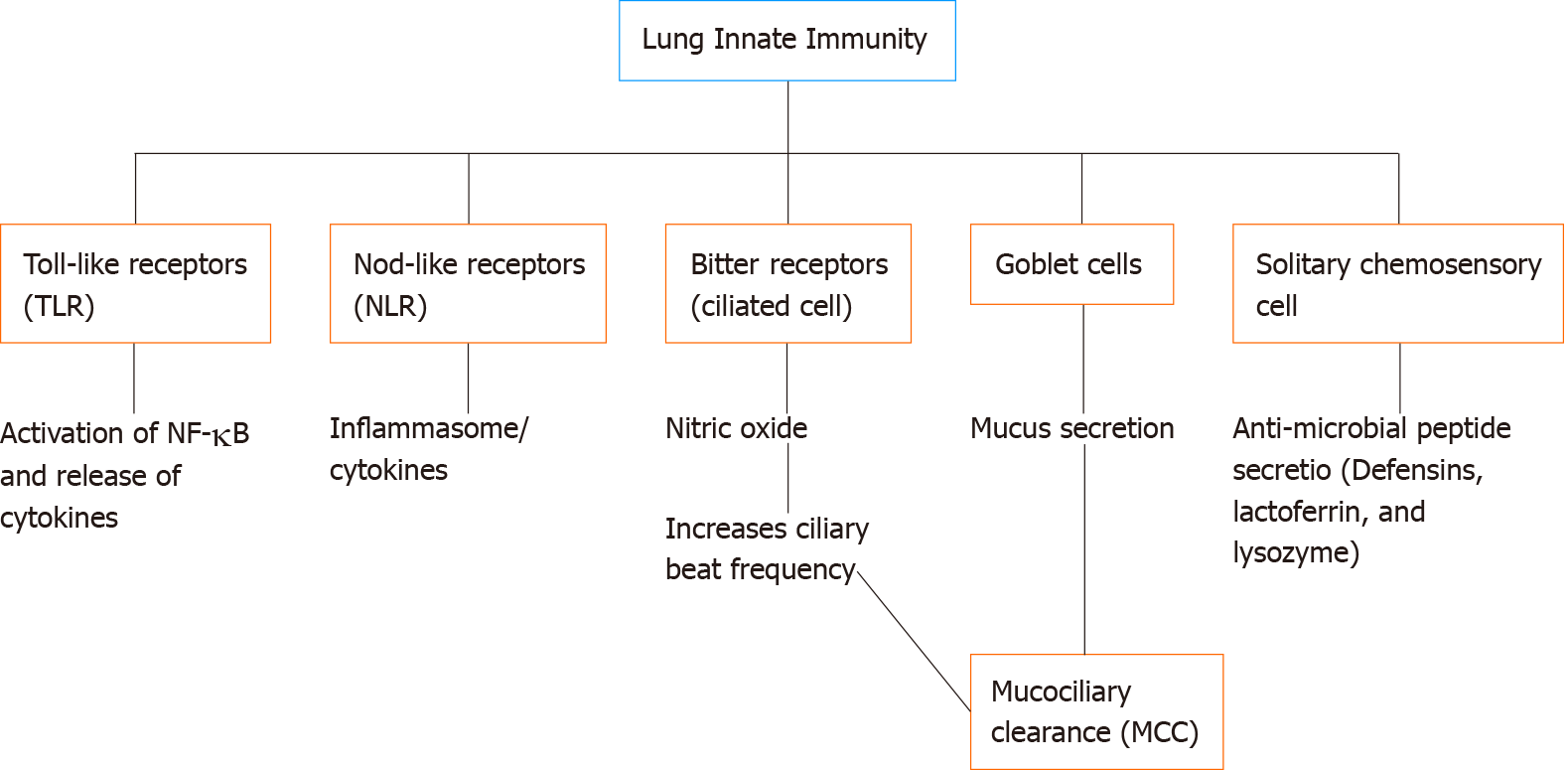
The innate immune system is the first line of defense against potentially dangerous microbes, and its main role is to recognize pathogens and initiate fast defensive responses. Because the human respiratory tracts are exposed to a myriad of pathogens daily, the immune system needs to recognize and initiate host defenses against these pathogens[11]. Akt may regulate multiple points of the airway innate immune system as well as the airway’s ability to detect pathogens.
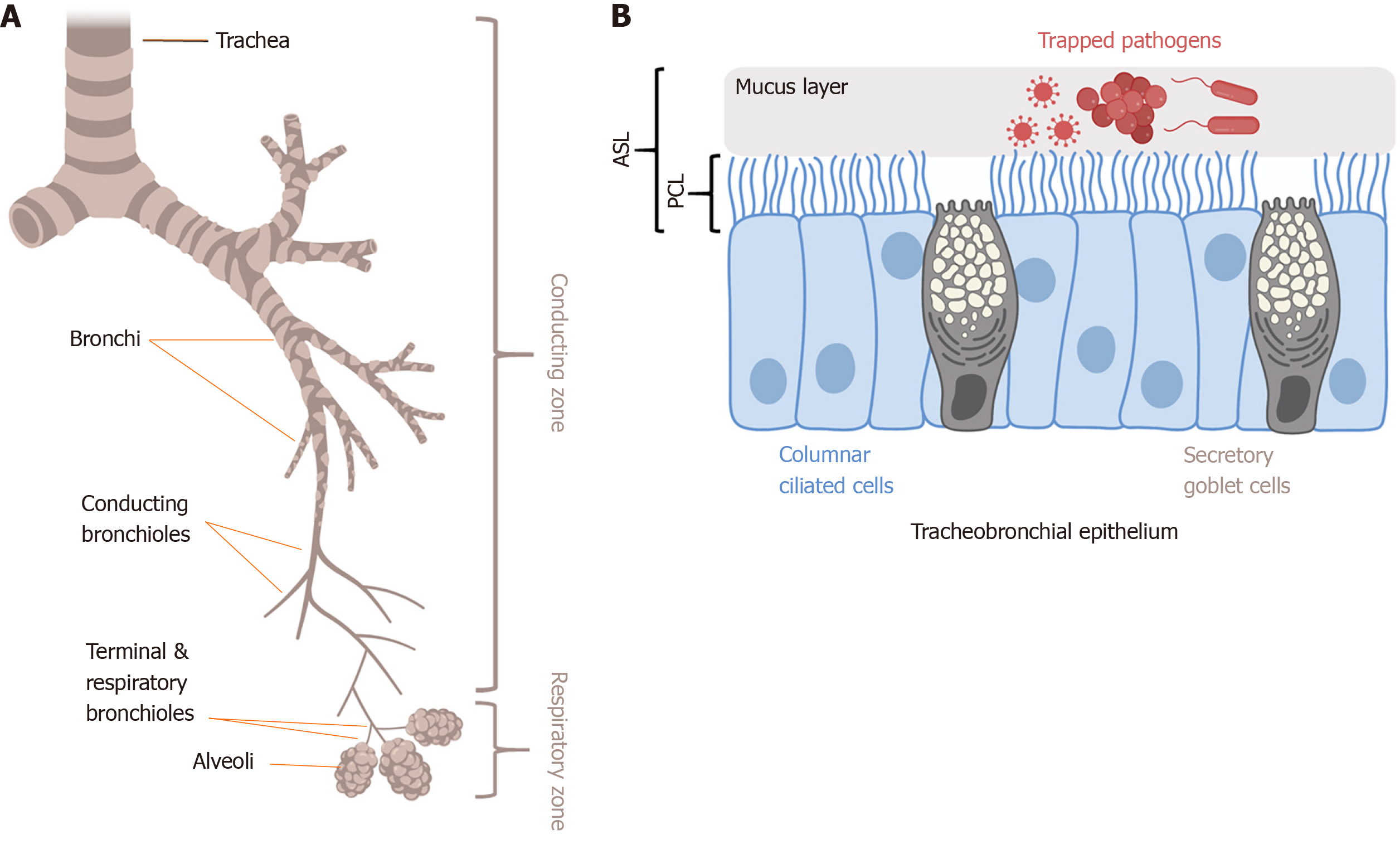
The primary physical innate defense mechanism of the airways is mucociliary clearance (MCC) (Figure 2). The main functional components of MCC are mucus production by airway secretory cells[23] and mucus transport by airway ciliated cells[11,24-26]. Cilia are specialized organelles lining airway epithelial cells. Mucus traps inhaled particulates and pathogens, and coordinated ciliary beating drives debris-laden mucus toward the pharynx, where it is swallowed or expectorated[27]. The airway surface liquid (ASL) is composed of the mucus layer that rides on top of the periciliary liquid (PCL) that surrounds the cilia. The composition of the PCL (volume, viscosity, and pH) mainly depends on epithelial ion channels[28]. Dysregulation of epithelial ion channels in CF is associated with increased mucus viscosity and PCL depletion[29] that impairs MCC. Direct cilia motor protein defects in primary ciliary dyskinesia (PCD) also impair MCC. Both CF and PCD patients are more susceptible to airway infections[30-32], supporting the importance of effective MCC to airway defense. A reduction of ciliated cells is also observed in patients with inflammatory diseases like chronic rhinosinusitis[32,33] as well as after exposure to compounds in cigarette smoke[24].
The normal mucus layer is composed of mainly water, mucins, proteins, lipids, and salts. However, the gel properties of mucus are produced by mucins, large cross-linked glycoproteins, including mucin 5AC (MUC5AC) produced by surface goblet cells[34] and MUC5B produced by mucus cells of submucosal glands[35]. Elevated MUC5AC levels are linked to asthma and may contribute to airway obstruction[36-38]. Akt has been suggested to be linked to MUC5AC production, though the data are conflicting. In human bronchial epithelial cells, direct inhibition of Akt upregulates MUC5AC production[39]. Activation of the PI3K/Akt pathway may also significantly reduced influenza-induced MUC5AC overproduction via negative cross-talk with the mitogen-activated protein kinase (MAPK) pathway[40]. In contrast, other studies showed that inhibition of Akt reduces MUC5AC levels[41,42]. The discrepancies in these studies might be due to different experimental models used. However, because Akt may play a role in regulating MCC by controlling MUC5AC levels, Akt inhibitors or activators may be a novel therapeutic strategy to manipulate MUC5AC levels to reduce mucus hypersecretion in asthma or chronic obstructive pulmonary disease (COPD).
Beyond the airway’s physical defenses, Akt is also involved in immune surveillance in the airway. The airway utilizes a gamut of receptors such as toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), and T2R bitter receptors to detect invading pathogens[10,43-45]. TLRs are pattern recognition receptors (PRRs) initially discovered based on homology to Drosophila toll receptors[46]. TLRs recognize pathogen-associated microbial patterns (PAMPs) and activate signaling pathways that can lead to increased transcription of cytokines as well as production of antimicrobial peptides and iNOS[47]. Dysfunction of TLR signaling has been linked to COPD, acute lung injury, CF, and CRS[10,48-50].
In humans, 11 TLRs have been identified and are involved in the innate sensing of microbial products[51]. These TLRs are found in dedicated immune cells such as macrophages and dendritic cells. TLRs are also found in fibroblasts, epithelial cells in the lung, intestine, and many other cell types[10,11,52,53]. Primary and immortalized airway cells express TLRs 2 through 10 at varying expression levels[50,54-57]. Lung epithelial cell TLRs respond to a variety of factors such as Pseudomonas aeruginosa flagellin (via TLR5), gram-negative bacterial lipopolysaccharide (LPS; via TLR4), unmethylated CpG from prokaryotic DNA (via TLR9), bacterial peptidoglycan (via TLR2), gram-positive bacterial lipoteichoic acid (via TLR2), viral double-stranded RNA (via TLR3), and fungal zymosan/beta-glucan (via TLR2)[43,50,57].
The broad principles of TLR signaling are already described by several excellent reviews[58,59]. Briefly, binding of PAMPs to TLRs activate their intracellular Toll/IL-1 receptor (TIR) domains[2] and recruits one or more TIR domain-containing adaptor proteins, including myeloid differentiation primary response protein 88 (MyD88), TIR-domain-containing adaptor protein (TIRAP), TIR-domain-containing adaptor protein inducing interferon-β (TRIF), and TRIF-related adaptor molecule (TRAM)[59]. Signaling then proceeds through a serious of adapter proteins. Association of MyD88 recruits IL-1R-associated kinase (IRAK)[60] through interactions of N-terminal death domains in both proteins[61]. Phosphorylation of IRAK activates tumor necrosis factor receptor-associated factor-6 (TRAF6) which in turn activates transcription factors such as NF-kB and JNK to promote the production of cytokines or initiate apoptosis signaling pathways, respectively[62]. Some TLRs, like TLR3, can also activate MyD88-independent signaling pathways leading to NF-kB activation[63].
In epithelial and immune cells, experimental studies have identified both positive and negative cross-talk between TLR activation and the PI3K/Akt pathway[64-66]. It is not yet fully understood how Akt is linked to TLR signaling, and these links maybe cell type-dependent or even TLR-isoform-dependent. PI3K, upstream of Akt, is often activated by TLRs in many cells[67], with Akt phosphorylation peaking at approximately 20 min and decreasing by approximately 1 h after stimulation[68]. Activation of Akt via TLR stimulation may increase NF-kB signaling and cytokine expression in macrophages[65], while other studies showed that the PI3K/Akt pathway suppresses TLR-induced cytokine secretion in monocytes via inhibition of NF-kB[69-71]. One study suggested that binding of vasoactive intestinal peptide (VIP) to GPCRs reduced TLR4 expression via Akt in macrophages and regulatory T cells[52,72,73]. Another group demonstrated the activation of PI3K/Akt after stimulation of TLR4 is crucial for B cell survival[22]. The role of Akt in airway TLR signaling is relatively unexplored, but data suggest that pharmacological manipulation of PI3K or Akt signaling may be a mechanism by which NF-kB activity could be controlled during bacterial or viral infection and the resulting activation of TLRs in the airway.
Cross-talk between TLRs and Akt maybe particularly important during cellular hyperoxia in the lung. Oxygen therapy is commonly used to reduce tissue hypoxia in patients with pulmonary disease. However, hyperoxia can induce lung damage that may be tied to a reduction of Akt signaling. Expression of a constitutively active form of Akt protected mouse lungs from hyperoxic injury[74]. In a rat model of bronchopulmonary dysplasia (BPD), exposure of neonatal lungs to high (95%) oxygen reduced the expression of Akt, while overexpression of Akt was protective against lung damage[75]. TLR4-deficient mice showed increased lung injury, higher mortality, and reduced levels of phospho (p)-Akt after hyperoxia. Expression of anti-apoptotic BCL-2 and activation of p-Akt significantly attenuated hyperoxia-induced lung injury in these TLR4-deficient mice[76]. Thus, activating the Akt pathway with receptor ligands or direct activators like SC-79[77] may be useful for treatment of lung injury during hyperoxia.
Other PRRs exist beyond TLRs. NLRs are PRRs that activate signaling pathways leading to activation of the inflammasome. Unlike the transmembrane TLRs, NLRs are cytosolic. NLRs can respond to microbial pathogens and stimulate the production of cytokines. Depending on the domains that are expressed, NLRs can be categorized as NOD receptors, NLRP, NLRC, or NLRB, and have been extensively reviewed[78,79]. NOD1 and NOD2 are expressed in lung epithelial cells, endothelial cells, alveolar macrophages, and airway smooth muscle cells[78]. Binding of NOD1 and NOD2 to secreted bacterial moieties results in activation of NF-kB, and polymorphisms of these receptors may increase susceptibility to respiratory infections[80]. NLRP3 may play a major role in recruiting neutrophils and dendritic cells during Mycoplasma pneumoniae lung infection in mice[81]. Because NLRs are relatively novel compared with TLRs, the knowledge of NLRs/Akt/PI3K/NF-kB in the lung immunity field is still rapidly developing.
Two decades ago, the GPCRs for bitter taste (known as taste family 2 receptors or T2Rs) were discovered in taste bud type II cells on the tongue[82]. There are 25 T2R isoforms in humans[82,83] that detect bitter compounds in food. However, in recent years, the discovery of the T2Rs in extraoral tissues has suggested other roles for these receptors beyond taste, including immune surveillance[83]. A variety of bitter receptors are expressed in the motile cilia in human airway epithelial cells[44] and macrophages[84] which are stimulated by bitter molecules such as denatonium benzoate[85], thujone from the wormwood plant[85], sodium thiocyanate[12], phenylthiocarbamide (PTC)[12], and bitter plant flavonoids[25]. These T2Rs also recognize gram-negative bacterial products such as acyl-homoserine lactone (AHL)[12] and quinolone[86] quorum-sensing molecules, suggesting they may play a role in sensing developing biofilms.
Stimulation of bitter receptors in sinonasal epithelial cell cilia activates Ca2+-dependent nitric oxide (NO) production which is bactericidal[12]. Additionally, NO can act as a second messenger to stimulate soluble guanylyl cyclase (sGC) and protein kinase G (PKG) to phosphorylate downstream effector proteins within the cilia and increase the ciliary beat frequency and thereby MCC[87]. One T2R isoform expressed in respiratory cilia is T2R38. Common polymorphisms in the TAS2R38 gene that render the T2R38 receptor nonfunctional are associated with increased susceptibility to upper respiratory infection[12,88], susceptibility to chronic rhinosinusitis[89-94], and surgical outcomes after functional endoscopic sinus surgery[95].
T2Rs also play other roles in the airway. A different subset of T2R isoforms in non-ciliated solitary chemosensory cells (SCCs), sometimes called tuft cells[44,96], leads to the propagation of Ca2+ to neighboring ciliated cells via gap junctions, triggering the neighboring cells to release anti-microbial peptides such as beta-defensin 1 and 2[96,97], which can permeabilize fungi and both gram-positive and negative bacteria[44]. Moreover, in mouse asthma models, bitter receptor agonists are effective in reducing airway smooth muscle contraction by modulating Ca2+ signaling[98-100].
Such studies of primary cells in vitro and patients in vivo suggest that T2Rs may contribute to the recognition of bacterial products similarly to TLR signaling[45,86]. Since T2Rs activate endothelial nitric oxide synthase (eNOS) to acutely produce NO in ciliated cells, targeting this pathway through Akt, which phosphorylates and activates eNOS[101,102] independently of Ca2+, as described below, is possibly a way to activate these innate immune responses in patients with polymorphisms that render specific T2Rs like T2R38 nonfunctional. Akt also has many other downstream targets, including Nrf-2[103] that play a role in the above innate immune processes. Several of these targets are reviewed below.
Nitric oxide synthase (NOS) enzymes catalyze the production of NO. L-arginine and NAD(P)H are converted to NO, NAD(P), and
There are three mammalian NOS isoforms; endothelial (eNOS, or NOS3), neuronal (nNOS or NOS1), and inducible (iNOS or NOS2) isoforms, named after the tissue where they were originally discovered. The main NOS isoform in neurons is nNOS[112], but nNOS has been detected in epithelial cells of various organs, pancreatic islets, and vascular smooth muscle, and exocrine acinar cells[113]. The dominant isoform in endothelial cells that maintains vascular tone and blood pressure is eNOS[101]. The airway epithelium generally expresses eNOS at baseline, while iNOS expression can be up-regulated during inflammation[11,25,101,106,110,114]. Pollutants and cigarette smoke can downregulate eNOS expression and subsequently reduce the production of NO[115].
Both eNOS (NOS3) and nNOS (NOS1) are generally constitutively expressed and are regulated acutely via binding of Ca2+-bound calmodulin as well as phosphorylation, described below. Activation of airway epithelial cells or immune cells by inflammatory mediators can cause transcription of iNOS (NOS2) via NFkB[101,116]. Like eNOS and nNOS, iNOS requires Ca2+ for function, but the affinity is so high that iNOS is maximally activated at resting Ca2+ levels, and iNOS can output high levels of NO in the cell microenvironment, ranging from 10 nmol/L to μmol/L amounts[117]. These high levels of NO can be involved in immune cell killing of bacteria[117]. In contrast, nNOS and eNOS produce lower levels of NO often associated with cellular signaling pathways that regulate a variety of physiological endpoints like ciliary beating and vascular tone, as described above, and also macrophage phagocytosis[84]. However, Ca2+-dependent activation of eNOS in sinonasal airway epithelial cells can directly kill bacteria like P. aeruginosa in the airway surface liquid[12,118]. The sinuses are thought to be sites of high NO production, important for immune function in the airways, and reduced fractional exhaled NO (FeNO) is correlated with several airway diseases[10,11,119].
Beyond Ca2+-calmodulin binding to eNOS, it can also be activated by Ca2+- independent mechanisms. Akt is an important regulator of eNOS function. Akt increases eNOS-mediated NO production by phosphorylation at Ser-1177 in humans and S1176 in mice[101]. Akt inhibitors such as wortmannin and LY294002 reduce NO production and PKG activity in platelets[120], while Akt co-immunoprecipitates with eNOS, suggesting the two proteins physically interact[121]. In mice in vivo, defective angiogenesis in Akt1 knockout mice can be rescued by a phospho-mimetic (S1176D) mutation in eNOS rendering the enzyme constitutively active[122]. This demonstrates the physiological importance of the regulation of eNOS by Akt.
Other proteins such as heat shock protein 90 (HSP90) can also associate with eNOS and modify its activity. Biochemical studies have shown a synergetic activation of eNOS by HSP90 and Akt in a calcium-independent manner in response to physiological agonist like insulin[123-126]. However, HSP90 also enhances Ca2+-calmodulin activation of eNOS[124].
An important role for eNOS has been demonstrated in various models of lung injury. In the lungs of male C57BL/J6 wild-type or eNOS knockout mice exposed to mechanical ventilation, reduced phospho-Akt, phospho-eNOS, and NO leads to increased epithelial permeability. The authors concluded that the PI3K/Akt/eNOS pathway exerts significant protective effects against ventilation-induced lung injury[127]. Production of NO by eNOS may also be important for protection against neonatal hypoxia in mice[119]. Thus, data presented above suggest that activating eNOS by directly targeting PI3K/Akt signaling may have several beneficial effects in lung disease, including protection against bacterial infections, reduced damage during mechanical ventilation, or reduced inflammation.
Another downstream target of Akt is Nrf-2, a transcription factor that serves as a master regulator of cellular responses against oxidative stress. Nrf-2 belongs to the cap “n” collar (CNC) family of transcription factors. Nrf-2 counteracts oxidative stress and inflammation by initiating transcription of genes encoding antioxidant proteins such as NADP(H): quinone oxidoreductase (NQO1) and heme oxygenase (HO-1)[128,129]. Nrf-2 binds to a specific approximately 41 base pair consensus enhancer sequence known as the antioxidant response element (ARE) to promote transcription of antioxidant and other genes[130-132]. Nrf-2 is regulated by Kelch-like ECH- associated protein-1 (Keap1), which binds to and sequesters Nrf-2 in the cytosol and targets Nrf-2 for ubiquitination and proteasomal degradation[133,134]. Nrf-2 is rapidly turned over, with a half-life of approximately 20 min in many cells[135-137]. Keap-1 facilitates the interaction of Nrf-2 with its E3 ubiquitin ligase. However, when the Nrf-2-Keap1 interaction is disrupted, Nrf-2 can escape ubiquitination and translocate to the nucleus[134]. Disruption of the Nrf-2-Keap-1 interaction can occur by oxidative modification of cysteine thiols on Keap-1, binding of heavy metal oxidants like Cd2+ or Cr6+ to Keap-1, or activation of Akt signaling[135,138].
Activation of antioxidant gene transcription by Nrf-2 may be protective in multiple tissues against injury and inflammation in a variety of conditions such as autoimmune and neurodegenerative diseases[139,140]. Nrf-2 induction may counterbalance excess mitochondrial production of ROS, and Nrf-2 levels may be decreased in mitochondria-related neurodegenerative diseases such as Alzheimer's and Parkinson's diseases[141,142]. Nrf-2 activators are in clinical development for cancer, although, due to Nrf-2’s role in promoting cell survival, there is controversy over whether activating or inhibiting Nrf-2 will be useful in different types of cancer[143,144]. In head and neck cancer, high levels of Nrf-2 may be associated with poorer patient outcomes[145]. Multiple mechanisms for aberrant activation of Nrf-2 in cancer have been reported, including Keap-1 mutations, epigenetic factors, and genetic changes[146]. Thus, while Nrf-2 is cytoprotective against oxidative stress, hyperactive Nrf-2 may be deleterious in some cancers.
Induction of Nrf-2 reduces the expression of pro-inflammatory cytokines such as IL6, IL1β, and COX2 in mice exposed to UV radiation; the same study showed that in healthy human subjects, Nrf-2 activator sulforaphane reduced solar-stimulated UV radiation-induced skin erythema[147]. Nrf-2 may interfere with lipopolysaccharide (LPS)-induced production of IL6 and IL1β in murine macrophages[148]. In the lung specifically, Nrf-2 activation may attenuate airway inflammation linked to allergy[149,150] or COPD and emphysema[151-155]. In most studies using mouse models of airway disease, the deletion of Nrf-2 results in increased inflammation and injury. Nrf-2 deficient mice are more susceptible to cigarette-smoke induced emphysema[156,157], and when cigarette-smoke-exposed Nrf-2 deficient and Wt. mice were exposed to influenza virus, Nrf-2-deficient mice exhibited higher mortality[158]. Nrf-2 may also directly modulate TLR4 signaling[159], though most studies of inflammation point to downstream effects of Nrf-2 on NF-kB-induced cytokine secretion. Nrf-2 knockout mice exhibit more lung inflammation in response to LPS or TNFa compared with Wt. mice[9,160,161], likely via enhanced NF-kB signaling.
Nrf-2 is also likely important during oxidative stress induced by airway hypoxia or hyperoxia. Nrf-2-dependent reduction of alveolar growth inhibition caused by hyperoxia increases survival in newborn mice[162]. Pharmacological inhibition of Akt resulted in higher levels of inflammation and lower expression levels of antioxidant genes in mice exposed to hyperoxia, likely via reduced Nrf-2 signaling[163]. However, this study also found that PI3K/Akt signaling promoted inflammation after hyperoxic injury in a Nrf-2-independent manner[163]. These studies suggest that activating PI3K/Akt/Nrf-2 signaling may reduce inflammation in lung diseases where oxidative stress is an important component of the pathophysiology, though more work is needed to understand the relationship of Akt and Nrf-2 to initial injury and subsequent sustained inflammatory responses after injury.
Many experimental studies in the airway have focused on the beneficial effects of Nrf-2 activation against commonly seen oxidative stressors in lung diseases. Activation of Nrf-2 (either via endogenous receptors, overexpression, or activators like curcumin or sulforaphane) is protective against oxidative stress-induced lung damage caused by exposure to compounds in cigarette smoke[164-173] or H2O2[174]. While Nrf-2 activators have shown benefit in animal models, we hypothesize that activation of upstream PI3K/Akt signaling may also be beneficial and requires more investigation, as it would combine Nrf-2 activation with the activation of other beneficial pathways, like eNOS.
Only a limited number of studies exist on protective effects of Akt-dependent Nrf-2 activation. In prostate cancer, increased Akt and Nrf-2 activity correlated with cell survival[175]. Another study reported that raw garlic can reduce cardiac hypertrophy in fructose-fed type 2 diabetic mice through activation of the PI3K/Akt pathway; this study showed that activation of Akt increased Nrf-2 activity that protected mouse hearts from oxidative stress[176]. Similarly, overexpression of constitutively active Akt increased Nrf-2 activation in retinal pigment epithelium[177]. In this study, both the induced levels of Nrf-2 and basal levels were reduced by PI3K inhibitors wortmannin and LY294002, confirming the Nrf-2 is activated downstream of PI3K/Akt[177].
Inositol trisphosphate receptors (IP3Rs) are endoplasmic reticulum (ER)-resident Ca2+ channels that contribute to Ca2+ release downstream of GPCR activation and other stimuli that activate phospholipase C[178]. Phospholipase C catalyzes hydrolysis of membrane phosphatidylinositol 4,5-bisphosphate (PIP2) to release inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. While diacylglycerol can activate protein kinase C, IP3 can bind to the IP3 receptor and sensitize it to resting cytosolic Ca2+ levels to cause the channel to open and promote Ca2+ release from endoplasmic reticulum stores[178]. The IP3 sensitivity and Ca2+ release activity of IP3Rs can be regulated by IP3R phosphorylation by multiple kinases[179]. A consensus motif for Akt phosphorylation is contained within the C-terminal tail of all three IP3R isoforms[180].
Phosphorylation of IP3Rs by Akt has been suggested to reduce Ca2+ efflux from the ER in response to apoptotic stimuli, thus protecting cells from apoptosis[180-182]. The activity of Akt2 in lymphocytes can reduce the duration of Ca2+ signaling and reduce activation of the NFAT transcription factor[183,184]. However, one study suggested that the effects of Akt on IP3Rs is specific to the type III IP3R, while Akt activation does not affect type I IP3R[184]. As cytokine secretion can also be driven by Ca2+, reduction of Ca2+ signaling may reduce inflammation. However, the ability of Akt to inhibit apoptosis or inflammation may depend on the predominant subtype of IP3R expressed in a specific cell type.
However, further research needs to be done on the role of Akt in Ca2+ release in airway cells. In some cells, Akt signaling may enhance Ca2+ release. In neurons, progesterone was shown to potentiate IP3R-dependent Ca2+ release via Akt signaling[185,186]. Akt activity may also regulate the expression of IP3Rs through multiple pathways. Akt2 activation of the ETS1 transcription factor may increase the expression of type II IP3R expression in dendritic cells[187]. Additionally, Nrf-2 was shown to bind to the promoter of the gene encoding the type III IP3R and reduce its expression in cholangiocytes, resulting in reduced Ca2+ signaling and reduced secretion from the bile duct[188]. Pharmacological targeting of the Akt pathway may modulate airway cell cytokine release and apoptosis through alteration of Ca2+ signaling, but it remains to be determined if inhibition or activation of Akt would be more beneficial.
Cystic fibrosis (CF) is an autosomal recessive disease caused by nearly 2000 different known mutations in the CFTR gene, which encodes the CF transmembrane conductance regulator (CFTR) protein. Although the life expectancy of CF patients is increasing with current small molecule therapies[189], CF affects approximately 75000 people in North America, Australia, and Europe[190]. The CFTR protein is expressed in the apical membranes of airway surface epithelial cells[191], airway submucosal gland serous cells[23,192,193], and a recently discovered rare cell type termed the ionocyte[194,195]. CFTR functions as chloride (Cl-) and bicarbonate (HCO3-) anion channel[196] to regulate salt and fluid homeostasis and control the volume and pH of the airway surface liquid[96]. Dehydration of the ASL caused by defective CFTR function leads to thickened mucus that impairs mucociliary clearance and increases susceptibility to respiratory pathogens[31], particularly the gram-negative opportunistic bacterium P. aeruginosa[197]. Respiratory failure is responsible for > 95% of CF patient deaths[198]. However, the reduced flux of Cl- and HCO3- ions through CFTR also affects multiple other organs where CFTR is expressed, including the exocrine pancreas, male reproductive tract, and sweat glands[31].
CFTR belongs to ATP binding cassette (ABC) superfamily of proteins and consists of two membrane-spanning domains (MSD), two nucleotide-binding domains (NBD), and a regulatory (R) domain[199]. The R domain consists of charged amino acids and several sites for phosphorylation by cAMP-dependent protein kinase A (PKA) as well as protein kinase C (PKC)[200]. Phosphorylation of the R domain enhances the association of adenosine triphosphate (ATP) to the NBDs, allowing a conformational change that results in the opening of the CFTR channel pore[201]. Subsequent hydrolysis of the ATP leads to channel closing[202]. Maturation of CFTR protein requires proper domain folding, glycosylation, and trafficking from the endoplasmic reticulum (ER) to the Golgi apparatus and eventually the plasma membrane. Dysregulation of any point in this complex multiple-step process can create a non-functional protein[203]. The most common mutation occurring in CF patients is the deletion of phenylalanine at position 508 (termed ΔF508 or F508del)[204].
In the CF lung, numerous studies have suggested the thickened mucus that is the hallmark of CF is accompanied by increased inflammation. The accumulation of neutrophils may increase inflammation and damage bronchial walls[205], while increased levels of pro-inflammatory cytokines and chemokines such as IL8 and TNF-α may also contribute to the destruction of lung tissue[206,207]. However, loss of CFTR may also confer hyper-inflammatory cellular properties, suggesting intrinsic cellular signaling defects caused by loss of CFTR function beyond the inflammation secondary to defective mucociliary clearance and bacterial infection[192,205,208-213]. CFTR itself has been linked to TLR4[214] and Akt[215,216] signaling via its proposed role as a signaling scaffold[217]. Thus, defective CFTR function may likely result in dysregulation of innate immunity beyond just loss of MCC[218]. All of these mechanisms may contribute to airflow obstruction, increased risk for bacterial infection, and damage to the microenvironment of the lung. It is not yet fully clear how small molecule CFTR corrector and potentiator therapies may suppress hyper-inflammation phenotypes in CF lungs[219].
Exhaled air from CF patients also contains less NO compared to non-CF individuals, possibly via decreased production of NO, increased metabolism of NO, downregulation of NOS enzymes, or polymorphisms in NOS genes[220-223]. Small molecule CFTR modulators have been shown to restore airway NO production, and the fraction of exhaled NO (FeNO) has been proposed to be a biomarker of pharmacological restoration of CFTR[224,225]. Moreover, boosting NO signaling may also increase the effect of corrector/potentiator modulator therapies[226], suggesting multiple levels of feedback may exist between CFTR and the NO signaling pathway.
As described above, eNOS is one target of Akt. Totani and colleagues showed that inhibition of CFTR in pulmonary endothelial cells reduced NO levels via reducing levels of activated phosphorylated Akt and activated phosphorylated eNOS[216]. This was associated with an increase in IL8 levels. In mice, CFTR knockout macrophages had a significant reduction of Akt phosphorylation at S473 compared with control mice; this same study showed Celecoxib, an FDA-approved COX-2 inhibitor for osteoarthritis, activated the PI3K/Akt pathway and reduced inflammation in this mouse model[227]. Thus, directly targeting Akt using small molecule activators or activating upstream PI3K may enhance NO production in CF lungs and alleviate inflammation. It may also have anti-bacterial effects similar to the activation of T2R bitter taste receptors, which drive eNOS-mediated NO production via Ca2+ rather than Akt. Of note, P. aeruginosa, the most common pathogen in CF lungs, is more susceptible to NO-induced killing than some other airway bacteria like Staphylococcus aureus[118]. A lack of efficient NO production in CF cells, possibly due to intrinsic defects in Akt signaling, may partly contribute to why these bacteria are so prevalent in CF lungs while almost never causing infection in non-CF patients unless non-CF patients are otherwise immunocompromised[228].
NO itself has been suggested to activate CFTR via PKG in some studies[229-231], while NO has also been reported in other studies to have no effect on CFTR[232], inhibit CFTR trafficking and/or activation[233,234], or activate non-CFTR Cl- currents[235]. Part of the discrepancy may be that most studies use different NO donor compounds at different concentrations, as well as occasionally more physiological ways to induce NO production (e.g., receptor activation). While no one has thoroughly examined the activation of CFTR downstream of specific Akt activation in the airway, targeting Akt would increase NO production to a more physiological level than NO donor compounds. Akt activation would stimulate endogenous eNOS, the major NOS isotype in uninflamed airway cells[114].
As indicated earlier, Nrf-2 is also a downstream target of Akt that plays a cytoprotective role against oxidative stress. Nrf-2 may convey resistance to pyocyanin, a bacterial product from P. aeruginosa that causes oxidative stress. The PI3K/Akt pathway is activated in lung epithelial cells during pyocyanin exposure, and the increased transcription of antioxidants may protect these cells from death[236]. It has been suggested that defective Nrf-2 in CF cells causes enhanced oxidative stress that increases inflammatory cytokine production[237], and Nrf-2 function is restored when mutant CFTR function is enhanced by small molecule therapeutics[238]. Alterations of Nrf-2 signaling in CF may also be tied to alterations in cAMP signaling and the CREB binding protein[239]. Boosting Nrf-2 function by targeting the PI3K/Akt pathway may have beneficial effects in CF lungs.
Furthermore, Nrf-2 may also regulate expression of CFTR itself[240]. We hypothesize that a direct Nrf-2 activator such as curcumin[241], dimethyl fumarate[242] or andrographolide[165] and/or activating Nrf-2 via Akt may be useful in combination with small molecule CFTR correctors and potentiators[189]. Such a strategy may further increase the number of functional CFTR channels at the plasma membrane by boosting CFTR gene transcription. This may be useful in patients where specific CFTR mutations reduce the efficiency of small molecule correction or potentiation.
The respiratory epithelia are in constant contact with bacteria, viruses, and pathogens during every breath. Airway innate immunity is the first line of host defense against these challenges[243]. Some of the strategies of the innate immune system of the airways include mucociliary-clearance, antimicrobial peptide secretion, NO production, cytokine secretion, and antioxidant gene production (Figure 3)[244]. PI3K/Akt signaling is one of the major signaling pathways regulating multiple components of these processes. Akt signaling maybe altered in airway diseases like CF. Together, the above studies discussed in this review suggest that therapeutic strategies to enhance the PI3K/Akt pathway and increase NO production, boost antioxidant transcription via Nrf-2, or activate other anti-inflammatory pathways might be particularly beneficial in CF patients. These strategies may also benefit patients with other inflammatory airway diseases like CRS, asthma, and/or COPD. Because pharmacological tools to inhibit PI3K[245,246], inhibit Akt[247-249], or even directly activate Akt[77] are available, exploring the effects of Akt signaling in airway cells may yield druggable targets that can be translated to human therapeutics.
Manuscript source: Invited manuscript
Specialty type: Respiratory System
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee SH S-Editor: Wang DM L-Editor: A P-Editor: Li X
| 1. | Hiemstra PS, McCray PB, Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J. 2015;45:1150-1162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 266] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 2. | Hartl D, Tirouvanziam R, Laval J, Greene CM, Habiel D, Sharma L, Yildirim AÖ, Dela Cruz CS, Hogaboam CM. Innate Immunity of the Lung: From Basic Mechanisms to Translational Medicine. J Innate Immun. 2018;10:487-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 3. | Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23:327-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 410] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 4. | Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc. 2005;2:403-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 231] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 592] [Cited by in F6Publishing: 650] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 6. | Zhang Y, Wang X, Yang H, Liu H, Lu Y, Han L, Liu G. Kinase AKT controls innate immune cell development and function. Immunology. 2013;140:143-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2723] [Cited by in F6Publishing: 2678] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 8. | Antosova M, Mokra D, Pepucha L, Plevkova J, Buday T, Sterusky M, Bencova A. Physiology of nitric oxide in the respiratory system. Physiol Res. 2017;66:S159-S172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984-995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 744] [Cited by in F6Publishing: 782] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 10. | Stevens WW, Lee RJ, Schleimer RP, Cohen NA. Chronic rhinosinusitis pathogenesis. J Allergy Clin Immunol. 2015;136:1442-1453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 221] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 11. | Hariri BM, Cohen NA. New insights into upper airway innate immunity. Am J Rhinol Allergy. 2016;30:319-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Lee RJ, Xiong G, Kofonow JM, Chen B, Lysenko A, Jiang P, Abraham V, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Beauchamp GK, Doulias PT, Ischiropoulos H, Kreindler JL, Reed DR, Cohen NA. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122:4145-4159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 405] [Article Influence: 33.8] [Reference Citation Analysis (1)] |
| 13. | Hartl D, Gaggar A, Bruscia E, Hector A, Marcos V, Jung A, Greene C, McElvaney G, Mall M, Döring G. Innate immunity in cystic fibrosis lung disease. J Cyst Fibros. 2012;11:363-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 14. | Staal SP, Hartley JW, Rowe WP. Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma. Proc Natl Acad Sci U S A. 1977;74:3065-3067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 180] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Manning BD, Toker A. AKT/PKB Signaling: Navigating the Network. Cell. 2017;169:381-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1732] [Cited by in F6Publishing: 2172] [Article Influence: 310.3] [Reference Citation Analysis (0)] |
| 16. | Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans. 2007;35:231-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 17. | Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905-2927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3190] [Cited by in F6Publishing: 3199] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 18. | Toker A, Marmiroli S. Signaling specificity in the Akt pathway in biology and disease. Adv Biol Regul. 2014;55:28-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 19. | Kunkel MT, Ni Q, Tsien RY, Zhang J, Newton AC. Spatio-temporal dynamics of protein kinase B/Akt signaling revealed by a genetically encoded fluorescent reporter. J Biol Chem. 2005;280:5581-5587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Nitulescu GM, Van De Venter M, Nitulescu G, Ungurianu A, Juzenas P, Peng Q, Olaru OT, Grădinaru D, Tsatsakis A, Tsoukalas D, Spandidos DA, Margina D. The Akt pathway in oncology therapy and beyond (Review). Int J Oncol. 2018;53:2319-2331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 21. | Weichhart T, Hengstschläger M, Linke M. Regulation of innate immune cell function by mTOR. Nat Rev Immunol. 2015;15:599-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 539] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 22. | Vivarelli MS, McDonald D, Miller M, Cusson N, Kelliher M, Geha RS. RIP links TLR4 to Akt and is essential for cell survival in response to LPS stimulation. J Exp Med. 2004;200:399-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Lee RJ, Foskett JK. cAMP-activated Ca2+ signaling is required for CFTR-mediated serous cell fluid secretion in porcine and human airways. J Clin Invest. 2010;120:3137-3148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Carey RM, Freund JR, Hariri BM, Adappa ND, Palmer JN, Lee RJ. Polarization of protease-activated receptor 2 (PAR-2) signaling is altered during airway epithelial remodeling and deciliation. J Biol Chem. 2020;295:6721-6740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Hariri BM, McMahon DB, Chen B, Freund JR, Mansfield CJ, Doghramji LJ, Adappa ND, Palmer JN, Kennedy DW, Reed DR, Jiang P, Lee RJ. Flavones modulate respiratory epithelial innate immunity: Anti-inflammatory effects and activation of the T2R14 receptor. J Biol Chem. 2017;292:8484-8497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 26. | McMahon DB, Workman AD, Kohanski MA, Carey RM, Freund JR, Hariri BM, Chen B, Doghramji LJ, Adappa ND, Palmer JN, Kennedy DW, Lee RJ. Protease-activated receptor 2 activates airway apical membrane chloride permeability and increases ciliary beating. FASEB J. 2018;32:155-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Bustamante-Marin XM, Ostrowski LE. Cilia and Mucociliary Clearance. Cold Spring Harb Perspect Biol. 2017;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 343] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 28. | Bartoszewski R, Matalon S, Collawn JF. Ion channels of the lung and their role in disease pathogenesis. Am J Physiol Lung Cell Mol Physiol. 2017;313:L859-L872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 847] [Cited by in F6Publishing: 795] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 30. | Munkholm M, Mortensen J. Mucociliary clearance: pathophysiological aspects. Clin Physiol Funct Imaging. 2014;34:171-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 474] [Cited by in F6Publishing: 436] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 32. | Tilley AE, Walters MS, Shaykhiev R, Crystal RG. Cilia dysfunction in lung disease. Annu Rev Physiol. 2015;77:379-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 33. | Gudis D, Zhao KQ, Cohen NA. Acquired cilia dysfunction in chronic rhinosinusitis. Am J Rhinol Allergy. 2012;26:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 34. | Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233-2247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1049] [Cited by in F6Publishing: 1041] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 35. | Widdicombe JH, Wine JJ. Airway Gland Structure and Function. Physiol Rev. 2015;95:1241-1319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 36. | Widdicombe JH. Regulation of the depth and composition of airway surface liquid. J Anat. 2002;201:313-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med. 2009;15:4-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 38. | Groneberg DA, Eynott PR, Lim S, Oates T, Wu R, Carlstedt I, Roberts P, McCann B, Nicholson AG, Harrison BD, Chung KF. Expression of respiratory mucins in fatal status asthmaticus and mild asthma. Histopathology. 2002;40:367-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 39. | Iwashita J, Ito Y, Yokoo M, Takahashi S, Murata J. Akt induces down regulation of MUC5AC production in NCI-H292 human airway epithelial cells cultured on extracellular matrix. Biosci Biotechnol Biochem. 2014;78:212-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Wang B, Lim DJ, Han J, Kim YS, Basbaum CB, Li JD. Novel cytoplasmic proteins of nontypeable Haemophilus influenzae up-regulate human MUC5AC mucin transcription via a positive p38 mitogen-activated protein kinase pathway and a negative phosphoinositide 3-kinase-Akt pathway. J Biol Chem. 2002;277:949-957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Yang J, Li Q, Zhou XD, Kolosov VP, Perelman JM. Naringenin attenuates mucous hypersecretion by modulating reactive oxygen species production and inhibiting NF-κB activity via EGFR-PI3K-Akt/ERK MAPKinase signaling in human airway epithelial cells. Mol Cell Biochem. 2011;351:29-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Kitazaki T, Soda H, Doi S, Nakano H, Nakamura Y, Kohno S. Gefitinib inhibits MUC5AC synthesis in mucin-secreting non-small cell lung cancer cells. Lung Cancer. 2005;50:19-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Parker LC, Prince LR, Sabroe I. Translational mini-review series on Toll-like receptors: networks regulated by Toll-like receptors mediate innate and adaptive immunity. Clin Exp Immunol. 2007;147:199-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 44. | Carey RM, Lee RJ. Taste Receptors in Upper Airway Innate Immunity. Nutrients. 2019;11. [PubMed] [Cited in This Article: ] |
| 45. | Freund JR, Lee RJ. Taste receptors in the upper airway. World J Otorhinolaryngol Head Neck Surg. 2018;4:67-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Imler JL, Hoffmann JA. Toll receptors in Drosophila: a family of molecules regulating development and immunity. Curr Top Microbiol Immunol. 2002;270:63-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:189-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 312] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 48. | Lafferty EI, Qureshi ST, Schnare M. The role of toll-like receptors in acute and chronic lung inflammation. J Inflamm (Lond). 2010;7:57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 964] [Cited by in F6Publishing: 1028] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 50. | Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A, Prince A. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2004;30:777-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 51. | Lin YT, Verma A, Hodgkinson CP. Toll-like receptors and human disease: lessons from single nucleotide polymorphisms. Curr Genomics. 2012;13:633-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Arranz A, Androulidaki A, Zacharioudaki V, Martinez C, Margioris AN, Gomariz RP, Tsatsanis C. Vasoactive intestinal peptide suppresses toll-like receptor 4 expression in macrophages via Akt1 reducing their responsiveness to lipopolysaccharide. Mol Immunol. 2008;45:2970-2980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Majewska M, Szczepanik M. [The role of Toll-like receptors (TLR) in innate and adaptive immune responses and their function in immune response regulation]. Postepy Hig Med Dosw (Online). 2006;60:52-63. [PubMed] [Cited in This Article: ] |
| 54. | Armstrong L, Medford AR, Uppington KM, Robertson J, Witherden IR, Tetley TD, Millar AB. Expression of functional toll-like receptor-2 and -4 on alveolar epithelial cells. Am J Respir Cell Mol Biol. 2004;31:241-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 55. | Mayer AK, Muehmer M, Mages J, Gueinzius K, Hess C, Heeg K, Bals R, Lang R, Dalpke AH. Differential recognition of TLR-dependent microbial ligands in human bronchial epithelial cells. J Immunol. 2007;178:3134-3142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 56. | Platz J, Beisswenger C, Dalpke A, Koczulla R, Pinkenburg O, Vogelmeier C, Bals R. Microbial DNA induces a host defense reaction of human respiratory epithelial cells. J Immunol. 2004;173:1219-1223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 57. | Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 369] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 58. | Sabroe I, Parker LC, Dower SK, Whyte MK. The role of TLR activation in inflammation. J Pathol. 2008;214:126-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 59. | Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6034] [Cited by in F6Publishing: 6115] [Article Influence: 305.8] [Reference Citation Analysis (0)] |
| 60. | Li D, Shirakami G, Zhan X, Johns RA. Regulation of ciliary beat frequency by the nitric oxide-cyclic guanosine monophosphate signaling pathway in rat airway epithelial cells. Am J Respir Cell Mol Biol. 2000;23:175-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Kawai T, Akira S. Toll-like receptor downstream signaling. Arthritis Res Ther. 2005;7:12-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 62. | Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1617] [Cited by in F6Publishing: 1692] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 63. | Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979-87; quiz 988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 607] [Cited by in F6Publishing: 637] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 64. | Pourrajab F, Yazdi MB, Zarch MB, Zarch MB, Hekmatimoghaddam S. Cross talk of the first-line defense TLRs with PI3K/Akt pathway, in preconditioning therapeutic approach. Mol Cell Ther. 2015;3:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 65. | Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 259] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 66. | Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K Pathway in Human Disease. Cell. 2017;170:605-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1604] [Cited by in F6Publishing: 1491] [Article Influence: 213.0] [Reference Citation Analysis (0)] |
| 67. | Troutman TD, Bazan JF, Pasare C. Toll-like receptors, signaling adapters and regulation of the pro-inflammatory response by PI3K. Cell Cycle. 2012;11:3559-3567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 68. | Rhee SH, Kim H, Moyer MP, Pothoulakis C. Role of MyD88 in phosphatidylinositol 3-kinase activation by flagellin/toll-like receptor 5 engagement in colonic epithelial cells. J Biol Chem. 2006;281:18560-18568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124-32132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 614] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 70. | Hildebrand D, Sahr A, Wölfle SJ, Heeg K, Kubatzky KF. Regulation of Toll-like receptor 4-mediated immune responses through Pasteurella multocida toxin-induced G protein signalling. Cell Commun Signal. 2012;10:22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 71. | la Sala A, Gadina M, Kelsall BL. G(i)-protein-dependent inhibition of IL-12 production is mediated by activation of the phosphatidylinositol 3-kinase-protein 3 kinase B/Akt pathway and JNK. J Immunol. 2005;175:2994-2999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 72. | Arranz A, Gutiérrez-Cañas I, Carrión M, Juarranz Y, Pablos JL, Martínez C, Gomariz RP. VIP reverses the expression profiling of TLR4-stimulated signaling pathway in rheumatoid arthritis synovial fibroblasts. Mol Immunol. 2008;45:3065-3073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Arranz A, Juarranz Y, Leceta J, Gomariz RP, Martínez C. VIP balances innate and adaptive immune responses induced by specific stimulation of TLR2 and TLR4. Peptides. 2008;29:948-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 74. | Lu Y, Parkyn L, Otterbein LE, Kureishi Y, Walsh K, Ray A, Ray P. Activated Akt protects the lung from oxidant-induced injury and delays death of mice. J Exp Med. 2001;193:545-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 75. | Alphonse RS, Vadivel A, Coltan L, Eaton F, Barr AJ, Dyck JR, Thébaud B. Activation of Akt protects alveoli from neonatal oxygen-induced lung injury. Am J Respir Cell Mol Biol. 2011;44:146-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 76. | Zhang X, Shan P, Qureshi S, Homer R, Medzhitov R, Noble PW, Lee PJ. Cutting edge: TLR4 deficiency confers susceptibility to lethal oxidant lung injury. J Immunol. 2005;175:4834-4838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 77. | Jo H, Mondal S, Tan D, Nagata E, Takizawa S, Sharma AK, Hou Q, Shanmugasundaram K, Prasad A, Tung JK, Tejeda AO, Man H, Rigby AC, Luo HR. Small molecule-induced cytosolic activation of protein kinase Akt rescues ischemia-elicited neuronal death. Proc Natl Acad Sci U S A. 2012;109:10581-10586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 78. | Chaput C, Sander LE, Suttorp N, Opitz B. NOD-Like Receptors in Lung Diseases. Front Immunol. 2013;4:393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 79. | Kim YK, Shin JS, Nahm MH. NOD-Like Receptors in Infection, Immunity, and Diseases. Yonsei Med J. 2016;57:5-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 255] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 80. | Leiva-Juárez MM, Kolls JK, Evans SE. Lung epithelial cells: therapeutically inducible effectors of antimicrobial defense. Mucosal Immunol. 2018;11:21-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 81. | Segovia JA, Chang TH, Winter VT, Coalson JJ, Cagle MP, Pandranki L, Bose S, Baseman JB, Kannan TR. NLRP3 Is a Critical Regulator of Inflammation and Innate Immune Cell Response during Mycoplasma pneumoniae Infection. Infect Immun. 2018;86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 82. | Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell. 2000;100:703-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 980] [Cited by in F6Publishing: 938] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 83. | Kinnamon SC. Taste receptor signalling - from tongues to lungs. Acta Physiol (Oxf). 2012;204:158-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 84. | Gopallawa I, Freund JR, Lee RJ. Bitter taste receptors stimulate phagocytosis in human macrophages through calcium, nitric oxide, and cyclic-GMP signaling. Cell Mol Life Sci. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 85. | Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 515] [Cited by in F6Publishing: 506] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 86. | Freund JR, Mansfield CJ, Doghramji LJ, Adappa ND, Palmer JN, Kennedy DW, Reed DR, Jiang P, Lee RJ. Activation of airway epithelial bitter taste receptors by Pseudomonas aeruginosa quinolones modulates calcium, cyclic-AMP, and nitric oxide signaling. J Biol Chem. 2018;293:9824-9840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 87. | Salathe M. Regulation of mammalian ciliary beating. Annu Rev Physiol. 2007;69:401-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 306] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 88. | Rom DI, Christensen JM, Alvarado R, Sacks R, Harvey RJ. The impact of bitter taste receptor genetics on culturable bacteria in chronic rhinosinusitis. Rhinology. 2017;55:90-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 89. | Adappa ND, Howland TJ, Palmer JN, Kennedy DW, Doghramji L, Lysenko A, Reed DR, Lee RJ, Cohen NA. Genetics of the taste receptor T2R38 correlates with chronic rhinosinusitis necessitating surgical intervention. Int Forum Allergy Rhinol. 2013;3:184-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 90. | Adappa ND, Zhang Z, Palmer JN, Kennedy DW, Doghramji L, Lysenko A, Reed DR, Scott T, Zhao NW, Owens D, Lee RJ, Cohen NA. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int Forum Allergy Rhinol. 2014;4:3-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 91. | Adappa ND, Workman AD, Hadjiliadis D, Dorgan DJ, Frame D, Brooks S, Doghramji L, Palmer JN, Mansfield C, Reed DR, Cohen NA. T2R38 genotype is correlated with sinonasal quality of life in homozygous ΔF508 cystic fibrosis patients. Int Forum Allergy Rhinol. 2016;6:356-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 92. | Dżaman K, Zagor M, Sarnowska E, Krzeski A, Kantor I. The correlation of TAS2R38 gene variants with higher risk for chronic rhinosinusitis in Polish patients. Otolaryngol Pol. 2016;70:13-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 93. | Mfuna Endam L, Filali-Mouhim A, Boisvert P, Boulet LP, Bossé Y, Desrosiers M. Genetic variations in taste receptors are associated with chronic rhinosinusitis: a replication study. Int Forum Allergy Rhinol. 2014;4:200-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 94. | Purnell PR, Addicks BL, Zalzal HG, Shapiro S, Wen S, Ramadan HH, Setola V, Siderovski DP. Single Nucleotide Polymorphisms in Chemosensory Pathway Genes GNB3, TAS2R19, and TAS2R38 Are Associated with Chronic Rhinosinusitis. Int Arch Allergy Immunol. 2019;180:72-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 95. | Adappa ND, Farquhar D, Palmer JN, Kennedy DW, Doghramji L, Morris SA, Owens D, Mansfield C, Lysenko A, Lee RJ, Cowart BJ, Reed DR, Cohen NA. TAS2R38 genotype predicts surgical outcome in nonpolypoid chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:25-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 96. | Lee RJ, Kofonow JM, Rosen PL, Siebert AP, Chen B, Doghramji L, Xiong G, Adappa ND, Palmer JN, Kennedy DW, Kreindler JL, Margolskee RF, Cohen NA. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. 2014;124:1393-1405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 286] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 97. | Lee RJ, Hariri BM, McMahon DB, Chen B, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Jiang P, Margolskee RF, Cohen NA. Bacterial d-amino acids suppress sinonasal innate immunity through sweet taste receptors in solitary chemosensory cells. Sci Signal. 2017;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 98. | Devillier P, Naline E, Grassin-Delyle S. The pharmacology of bitter taste receptors and their role in human airways. Pharmacol Ther. 2015;155:11-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 99. | Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299-1304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 452] [Cited by in F6Publishing: 451] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 100. | Zhang CH, Lifshitz LM, Uy KF, Ikebe M, Fogarty KE, ZhuGe R. The cellular and molecular basis of bitter tastant-induced bronchodilation. PLoS Biol. 2013;11:e1001501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 101. | Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829-837, 837a-837d. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2678] [Cited by in F6Publishing: 2500] [Article Influence: 208.3] [Reference Citation Analysis (0)] |
| 102. | Li CJ, Elsasser TH, Kahl S. AKT/eNOS signaling module functions as a potential feedback loop in the growth hormone signaling pathway. J Mol Signal. 2009;4:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 103. | Saha S, Sadhukhan P, Sinha K, Agarwal N, Sil PC. Mangiferin attenuates oxidative stress induced renal cell damage through activation of PI3K induced Akt and Nrf-2 mediated signaling pathways. Biochem Biophys Rep. 2016;5:313-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 104. | Weinberger B, Heck DE, Laskin DL, Laskin JD. Nitric oxide in the lung: therapeutic and cellular mechanisms of action. Pharmacol Ther. 1999;84:401-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 105. | Wyatt TA, Spurzem JR, May K, Sisson JH. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am J Physiol. 1998;275:L827-L835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 106. | Stout SL, Wyatt TA, Adams JJ, Sisson JH. Nitric oxide-dependent cilia regulatory enzyme localization in bovine bronchial epithelial cells. J Histochem Cytochem. 2007;55:433-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 107. | Sisson JH, May K, Wyatt TA. Nitric oxide-dependent ethanol stimulation of ciliary motility is linked to cAMP-dependent protein kinase (PKA) activation in bovine bronchial epithelium. Alcohol Clin Exp Res. 1999;23:1528-1533. [PubMed] [Cited in This Article: ] |
| 108. | Sisson JH. Ethanol stimulates apparent nitric oxide-dependent ciliary beat frequency in bovine airway epithelial cells. Am J Physiol. 1995;268:L596-L600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 109. | Jain B, Rubinstein I, Robbins RA, Leise KL, Sisson JH. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem Biophys Res Commun. 1993;191:83-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 252] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 110. | Uzlaner N, Priel Z. Interplay between the NO pathway and elevated [Ca2+]i enhances ciliary activity in rabbit trachea. J Physiol. 1999;516:179-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 111. | Simet SM, Pavlik JA, Sisson JH. Proteomic analysis of bovine axonemes exposed to acute alcohol: role of endothelial nitric oxide synthase and heat shock protein 90 in cilia stimulation. Alcohol Clin Exp Res. 2013;37:609-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 112. | Tricoire L, Vitalis T. Neuronal nitric oxide synthase expressing neurons: a journey from birth to neuronal circuits. Front Neural Circuits. 2012;6:82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 113. | Förstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, Kleinert H. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension. 1994;23:1121-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 756] [Cited by in F6Publishing: 736] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 114. | German Z, Chambliss KL, Pace MC, Arnet UA, Lowenstein CJ, Shaul PW. Molecular basis of cell-specific endothelial nitric-oxide synthase expression in airway epithelium. J Biol Chem. 2000;275:8183-8189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 115. | Barberà JA, Peinado VI, Santos S, Ramirez J, Roca J, Rodriguez-Roisin R. Reduced expression of endothelial nitric oxide synthase in pulmonary arteries of smokers. Am J Respir Crit Care Med. 2001;164:709-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 140] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 116. | Kleinert H, Schwarz PM, Förstermann U. Regulation of the expression of inducible nitric oxide synthase. Biol Chem. 2003;384:1343-1364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 289] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 117. | Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton CA, Harris CC, Roberts DD, Wink DA. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008;45:18-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 704] [Cited by in F6Publishing: 637] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 118. | Workman AD, Carey RM, Kohanski MA, Kennedy DW, Palmer JN, Adappa ND, Cohen NA. Relative susceptibility of airway organisms to antimicrobial effects of nitric oxide. Int Forum Allergy Rhinol. 2017;7:770-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 119. | Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, Abman SH. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L119-L127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 120. | Stojanovic A, Marjanovic JA, Brovkovych VM, Peng X, Hay N, Skidgel RA, Du X. A phosphoinositide 3-kinase-AKT-nitric oxide-cGMP signaling pathway in stimulating platelet secretion and aggregation. J Biol Chem. 2006;281:16333-16339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 121. | Michell BJ, Griffiths JE, Mitchelhill KI, Rodriguez-Crespo I, Tiganis T, Bozinovski S, de Montellano PR, Kemp BE, Pearson RB. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr Biol. 1999;9:845-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 386] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 122. | Schleicher M, Yu J, Murata T, Derakhshan B, Atochin D, Qian L, Kashiwagi S, Di Lorenzo A, Harrison KD, Huang PL, Sessa WC. The Akt1-eNOS axis illustrates the specificity of kinase-substrate relationships in vivo. Sci Signal. 2009;2:ra41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 123. | Takahashi S, Mendelsohn ME. Synergistic activation of endothelial nitric-oxide synthase (eNOS) by HSP90 and Akt: calcium-independent eNOS activation involves formation of an HSP90-Akt-CaM-bound eNOS complex. J Biol Chem. 2003;278:30821-30827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 124. | Takahashi S, Mendelsohn ME. Calmodulin-dependent and -independent activation of endothelial nitric-oxide synthase by heat shock protein 90. J Biol Chem. 2003;278:9339-9344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 125. | García-Cardeña G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821-824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 783] [Cited by in F6Publishing: 761] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 126. | Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, Sessa WC. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ Res. 2002;90:866-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 272] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 127. | Peng XQ, Damarla M, Skirball J, Nonas S, Wang XY, Han EJ, Hasan EJ, Cao X, Boueiz A, Damico R, Tuder RM, Sciuto AM, Anderson DR, Garcia JG, Kass DA, Hassoun PM, Zhang JT. Protective role of PI3-kinase/Akt/eNOS signaling in mechanical stress through inhibition of p38 mitogen-activated protein kinase in mouse lung. Acta Pharmacol Sin. 2010;31:175-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 128. | Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304-1309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1139] [Cited by in F6Publishing: 1199] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 129. | Cho HY, Kleeberger SR. Nrf2 protects against airway disorders. Toxicol Appl Pharmacol. 2010;244:43-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 130. | Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2966] [Cited by in F6Publishing: 3046] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 131. | Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 824] [Cited by in F6Publishing: 851] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 132. | Rushmore TH, Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990;265:14648-14653. [PubMed] [Cited in This Article: ] |
| 133. | Furukawa M, Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol Cell Biol. 2005;25:162-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 597] [Cited by in F6Publishing: 589] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 134. | Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2794] [Cited by in F6Publishing: 2709] [Article Influence: 108.4] [Reference Citation Analysis (0)] |
| 135. | He X, Chen MG, Lin GX, Ma Q. Arsenic induces NAD(P)H-quinone oxidoreductase I by disrupting the Nrf2 x Keap1 x Cul3 complex and recruiting Nrf2 x Maf to the antioxidant response element enhancer. J Biol Chem. 2006;281:23620-23631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 136. | Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941-10953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 893] [Cited by in F6Publishing: 973] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 137. | Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 655] [Cited by in F6Publishing: 684] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 138. | He X, Lin GX, Chen MG, Zhang JX, Ma Q. Protection against chromium (VI)-induced oxidative stress and apoptosis by Nrf2. Recruiting Nrf2 into the nucleus and disrupting the nuclear Nrf2/Keap1 association. Toxicol Sci. 2007;98:298-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 139. | Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 761] [Cited by in F6Publishing: 800] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 140. | Dinkova-Kostova AT, Kostov RV, Kazantsev AG. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018;285:3576-3590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 141. | Esteras N, Dinkova-Kostova AT, Abramov AY. Nrf2 activation in the treatment of neurodegenerative diseases: a focus on its role in mitochondrial bioenergetics and function. Biol Chem. 2016;397:383-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 142. | Dinkova-Kostova AT, Fahey JW, Kostov RV, Kensler TW. KEAP1 and Done? Targeting the NRF2 Pathway with Sulforaphane. Trends Food Sci Technol. 2017;69:257-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 143. | Zimta AA, Cenariu D, Irimie A, Magdo L, Nabavi SM, Atanasov AG, Berindan-Neagoe I. The Role of Nrf2 Activity in Cancer Development and Progression. Cancers (Basel). 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 144. | Wu S, Lu H, Bai Y. Nrf2 in cancers: A double-edged sword. Cancer Med. 2019;8:2252-2267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 258] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 145. | Kitamura H, Motohashi H. NRF2 addiction in cancer cells. Cancer Sci. 2018;109:900-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 146. | Konstantinopoulos PA, Spentzos D, Fountzilas E, Francoeur N, Sanisetty S, Grammatikos AP, Hecht JL, Cannistra SA. Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res. 2011;71:5081-5089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 147. | Knatko EV, Ibbotson SH, Zhang Y, Higgins M, Fahey JW, Talalay P, Dawe RS, Ferguson J, Huang JT, Clarke R, Zheng S, Saito A, Kalra S, Benedict AL, Honda T, Proby CM, Dinkova-Kostova AT. Nrf2 Activation Protects against Solar-Simulated Ultraviolet Radiation in Mice and Humans. Cancer Prev Res (Phila). 2015;8:475-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 148. | Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi H, Nakayama K, Yamamoto M. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 827] [Cited by in F6Publishing: 1068] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 149. | Hsu WH, Lee BH, Huang YC, Hsu YW, Pan TM. Ankaflavin, a novel Nrf-2 activator for attenuating allergic airway inflammation. Free Radic Biol Med. 2012;53:1643-1651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 150. | Ci X, Zhong W, Ren H, Wen Z, Li D, Peng L. Esculentoside A Attenuates Allergic Airway Inflammation via Activation of the Nrf-2 Pathway. Int Arch Allergy Immunol. 2015;167:280-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 151. | Cui W, Zhang Z, Zhang P, Qu J, Zheng C, Mo X, Zhou W, Xu L, Yao H, Gao J. Nrf2 attenuates inflammatory response in COPD/emphysema: Crosstalk with Wnt3a/β-catenin and AMPK pathways. J Cell Mol Med. 2018;22:3514-3525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 152. | Boutten A, Goven D, Artaud-Macari E, Boczkowski J, Bonay M. NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease. Trends Mol Med. 2011;17:363-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 153. | Boutten A, Goven D, Boczkowski J, Bonay M. Oxidative stress targets in pulmonary emphysema: focus on the Nrf2 pathway. Expert Opin Ther Targets. 2010;14:329-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 154. | Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol. 2003;8:39-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 155. | Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 1992;119:598-620. [PubMed] [Cited in This Article: ] |
| 156. | Iizuka T, Ishii Y, Itoh K, Kiwamoto T, Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A, Sakamoto T, Shimura M, Yoshida A, Yamamoto M, Sekizawa K. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes Cells. 2005;10:1113-1125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 157. | Gebel S, Diehl S, Pype J, Friedrichs B, Weiler H, Schüller J, Xu H, Taguchi K, Yamamoto M, Müller T. The transcriptome of Nrf2-/- mice provides evidence for impaired cell cycle progression in the development of cigarette smoke-induced emphysematous changes. Toxicol Sci. 2010;115:238-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 158. | Yageta Y, Ishii Y, Morishima Y, Masuko H, Ano S, Yamadori T, Itoh K, Takeuchi K, Yamamoto M, Hizawa N. Role of Nrf2 in host defense against influenza virus in cigarette smoke-exposed mice. J Virol. 2011;85:4679-4690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 159. | Yan J, Li J, Zhang L, Sun Y, Jiang J, Huang Y, Xu H, Jiang H, Hu R. Nrf2 protects against acute lung injury and inflammation by modulating TLR4 and Akt signaling. Free Radic Biol Med. 2018;121:78-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 160. | Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2529] [Cited by in F6Publishing: 2699] [Article Influence: 158.8] [Reference Citation Analysis (0)] |
| 161. | Wei J, Chen G, Shi X, Zhou H, Liu M, Chen Y, Feng D, Zhang P, Wu L, Lv X. Nrf2 activation protects against intratracheal LPS induced mouse/murine acute respiratory distress syndrome by regulating macrophage polarization. Biochem Biophys Res Commun. 2018;500:790-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 162. | McGrath-Morrow S, Lauer T, Yee M, Neptune E, Podowski M, Thimmulappa RK, O'Reilly M, Biswal S. Nrf2 increases survival and attenuates alveolar growth inhibition in neonatal mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2009;296:L565-L573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 163. | Reddy NM, Potteti HR, Vegiraju S, Chen HJ, Tamatam CM, Reddy SP. PI3K-AKT Signaling via Nrf2 Protects against Hyperoxia-Induced Acute Lung Injury, but Promotes Inflammation Post-Injury Independent of Nrf2 in Mice. PLoS One. 2015;10:e0129676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 164. | Lin XX, Yang XF, Jiang JX, Zhang SJ, Guan Y, Liu YN, Sun YH, Xie QM. Cigarette smoke extract-induced BEAS-2B cell apoptosis and anti-oxidative Nrf-2 up-regulation are mediated by ROS-stimulated p38 activation. Toxicol Mech Methods. 2014;24:575-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 165. | Guan SP, Tee W, Ng DS, Chan TK, Peh HY, Ho WE, Cheng C, Mak JC, Wong WS. Andrographolide protects against cigarette smoke-induced oxidative lung injury via augmentation of Nrf2 activity. Br J Pharmacol. 2013;168:1707-1718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 166. | Sakurai H, Morishima Y, Ishii Y, Yoshida K, Nakajima M, Tsunoda Y, Hayashi SY, Kiwamoto T, Matsuno Y, Kawaguchi M, Yamamoto M, Hizawa N. Sulforaphane ameliorates steroid insensitivity through an Nrf2-dependent pathway in cigarette smoke-exposed asthmatic mice. Free Radic Biol Med. 2018;129:473-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 167. | Kubo H, Asai K, Kojima K, Sugitani A, Kyomoto Y, Okamoto A, Yamada K, Ijiri N, Watanabe T, Hirata K, Kawaguchi T. Astaxanthin Suppresses Cigarette Smoke-Induced Emphysema through Nrf2 Activation in Mice. Mar Drugs. 2019;17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 168. | Tan WSD, Liao W, Peh HY, Vila M, Dong J, Shen HM, Wong WSF. Andrographolide simultaneously augments Nrf2 antioxidant defense and facilitates autophagic flux blockade in cigarette smoke-exposed human bronchial epithelial cells. Toxicol Appl Pharmacol. 2018;360:120-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 169. | Müller T, Hengstermann A. Nrf2: friend and foe in preventing cigarette smoking-dependent lung disease. Chem Res Toxicol. 2012;25:1805-1824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 170. | Sekine T, Hirata T, Ishikawa S, Ito S, Ishimori K, Matsumura K, Muraki K. Regulation of NRF2, AP-1 and NF-κB by cigarette smoke exposure in three-dimensional human bronchial epithelial cells. J Appl Toxicol. 2019;39:717-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 171. | Posso SV, Quesnot N, Moraes JA, Brito-Gitirana L, Kennedy-Feitosa E, Barroso MV, Porto LC, Lanzetti M, Valença SS. AT-RVD1 repairs mouse lung after cigarette smoke-induced emphysema via downregulation of oxidative stress by NRF2/KEAP1 pathway. Int Immunopharmacol. 2018;56:330-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 172. | Jiao Z, Chang J, Li J, Nie D, Cui H, Guo D. Sulforaphane increases Nrf2 expression and protects alveolar epithelial cells against injury caused by cigarette smoke extract. Mol Med Rep. 2017;16:1241-1247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 173. | Liu Q, Gao Y, Ci X. Role of Nrf2 and Its Activators in Respiratory Diseases. Oxid Med Cell Longev. 2019;2019:7090534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 174. | Hsu WH, Lee BH, Pan TM. Monascin attenuates oxidative stress-mediated lung inflammation via peroxisome proliferator-activated receptor-gamma (PPAR-γ) and nuclear factor-erythroid 2 related factor 2 (Nrf-2) modulation. J Agric Food Chem. 2014;62:5337-5344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 175. | Bellezza I, Scarpelli P, Pizzo SV, Grottelli S, Costanzi E, Minelli A. ROS-independent Nrf2 activation in prostate cancer. Oncotarget. 2017;8:67506-67518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 176. | Padiya R, Chowdhury D, Borkar R, Srinivas R, Pal Bhadra M, Banerjee SK. Garlic attenuates cardiac oxidative stress via activation of PI3K/AKT/Nrf2-Keap1 pathway in fructose-fed diabetic rat. PLoS One. 2014;9:e94228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 177. | Wang L, Chen Y, Sternberg P, Cai J. Essential roles of the PI3 kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Invest Ophthalmol Vis Sci. 2008;49:1671-1678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 178. | Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 858] [Cited by in F6Publishing: 907] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 179. | Vanderheyden V, Devogelaere B, Missiaen L, De Smedt H, Bultynck G, Parys JB. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim Biophys Acta. 2009;1793:959-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 180. | Khan MT, Wagner L 2nd, Yule DI, Bhanumathy C, Joseph SK. Akt kinase phosphorylation of inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2006;281:3731-3737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 181. | Szado T, Vanderheyden V, Parys JB, De Smedt H, Rietdorf K, Kotelevets L, Chastre E, Khan F, Landegren U, Söderberg O, Bootman MD, Roderick HL. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc Natl Acad Sci U S A. 2008;105:2427-2432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 182. | Marchi S, Rimessi A, Giorgi C, Baldini C, Ferroni L, Rizzuto R, Pinton P. Akt kinase reducing endoplasmic reticulum Ca2+ release protects cells from Ca2+-dependent apoptotic stimuli. Biochem Biophys Res Commun. 2008;375:501-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 183. | Martin VA, Wang WH, Lipchik AM, Parker LL, He Y, Zhang S, Zhang ZY, Geahlen RL. Akt2 inhibits the activation of NFAT in lymphocytes by modulating calcium release from intracellular stores. Cell Signal. 2012;24:1064-1073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 184. | Marchi S, Marinello M, Bononi A, Bonora M, Giorgi C, Rimessi A, Pinton P. Selective modulation of subtype III IP3R by Akt regulates ER Ca2+ release and apoptosis. Cell Death Dis. 2012;3:e304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 185. | Hwang JY, Duncan RS, Madry C, Singh M, Koulen P. Progesterone potentiates calcium release through IP3 receptors by an Akt-mediated mechanism in hippocampal neurons. Cell Calcium. 2009;45:233-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 186. | Koulen P, Madry C, Duncan RS, Hwang JY, Nixon E, McClung N, Gregg EV, Singh M. Progesterone potentiates IP(3)-mediated calcium signaling through Akt/PKB. Cell Physiol Biochem. 2008;21:161-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 187. | Yang W, Nurbaeva MK, Schmid E, Russo A, Almilaji A, Szteyn K, Yan J, Faggio C, Shumilina E, Lang F. Akt2- and ETS1-dependent IP3 receptor 2 expression in dendritic cell migration. Cell Physiol Biochem. 2014;33:222-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 188. | Weerachayaphorn J, Amaya MJ, Spirli C, Chansela P, Mitchell-Richards KA, Ananthanarayanan M, Nathanson MH. Nuclear Factor, Erythroid 2-Like 2 Regulates Expression of Type 3 Inositol 1,4,5-Trisphosphate Receptor and Calcium Signaling in Cholangiocytes. Gastroenterology. 2015;149:211-222.e10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 189. | Gentzsch M, Mall MA. Ion Channel Modulators in Cystic Fibrosis. Chest. 2018;154:383-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 190. | Pranke I, Golec A, Hinzpeter A, Edelman A, Sermet-Gaudelus I. Emerging Therapeutic Approaches for Cystic Fibrosis. From Gene Editing to Personalized Medicine. Front Pharmacol. 2019;10:121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 191. | Rich DP, Anderson MP, Gregory RJ, Cheng SH, Paul S, Jefferson DM, McCann JD, Klinger KW, Smith AE, Welsh MJ. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990;347:358-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 480] [Cited by in F6Publishing: 455] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 192. | McMahon DB, Carey RM, Kohanski MA, Tong CCL, Papagiannopoulos P, Adappa ND, Palmer JN, Lee RJ. Neuropeptide regulation of secretion and inflammation in human airway gland serous cells. Eur Respir J. 2020;55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 193. | Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, Cohn JA, Wilson JM. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet. 1992;2:240-248. [PubMed] [Cited in This Article: ] |
| 194. | Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, Yuan F, Chen S, Leung HM, Villoria J, Rogel N, Burgin G, Tsankov AM, Waghray A, Slyper M, Waldman J, Nguyen L, Dionne D, Rozenblatt-Rosen O, Tata PR, Mou H, Shivaraju M, Bihler H, Mense M, Tearney GJ, Rowe SM, Engelhardt JF, Regev A, Rajagopal J. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560:319-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 785] [Cited by in F6Publishing: 684] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 195. | Plasschaert LW, Žilionis R, Choo-Wing R, Savova V, Knehr J, Roma G, Klein AM, Jaffe AB. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 2018;560:377-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 575] [Cited by in F6Publishing: 608] [Article Influence: 101.3] [Reference Citation Analysis (0)] |
| 196. | Linsdell P. Functional architecture of the CFTR chloride channel. Mol Membr Biol. 2014;31:1-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 197. | Bhagirath AY, Li Y, Somayajula D, Dadashi M, Badr S, Duan K. Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm Med. 2016;16:174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 220] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 198. | Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229-1256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 702] [Cited by in F6Publishing: 653] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 199. | Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992-2001. [PubMed] [Cited in This Article: ] |
| 200. | Hegedus T, Aleksandrov A, Mengos A, Cui L, Jensen TJ, Riordan JR. Role of individual R domain phosphorylation sites in CFTR regulation by protein kinase A. Biochim Biophys Acta. 2009;1788:1341-1349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 201. | Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem. 2008;77:701-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 445] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 202. | Vergani P, Lockless SW, Nairn AC, Gadsby DC. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature. 2005;433:876-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 336] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 203. | Varga K, Jurkuvenaite A, Wakefield J, Hong JS, Guimbellot JS, Venglarik CJ, Niraj A, Mazur M, Sorscher EJ, Collawn JF, Bebök Z. Efficient intracellular processing of the endogenous cystic fibrosis transmembrane conductance regulator in epithelial cell lines. J Biol Chem. 2004;279:22578-22584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 204. | Mall M, Kreda SM, Mengos A, Jensen TJ, Hirtz S, Seydewitz HH, Yankaskas J, Kunzelmann K, Riordan JR, Boucher RC. The DeltaF508 mutation results in loss of CFTR function and mature protein in native human colon. Gastroenterology. 2004;126:32-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 205. | Nichols DP, Chmiel JF. Inflammation and its genesis in cystic fibrosis. Pediatr Pulmonol. 2015;50 Suppl 40:S39-S56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 206. | Dhooghe B, Noël S, Huaux F, Leal T. Lung inflammation in cystic fibrosis: pathogenesis and novel therapies. Clin Biochem. 2014;47:539-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 207. | Kim Chiaw P, Eckford PD, Bear CE. Insights into the mechanisms underlying CFTR channel activity, the molecular basis for cystic fibrosis and strategies for therapy. Essays Biochem. 2011;50:233-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 208. | Ribeiro CM, Paradiso AM, Schwab U, Perez-Vilar J, Jones L, O'neal W, Boucher RC. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J Biol Chem. 2005;280:17798-17806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 209. | Tabary O, Zahm JM, Hinnrasky J, Couetil JP, Cornillet P, Guenounou M, Gaillard D, Puchelle E, Jacquot J. Selective up-regulation of chemokine IL-8 expression in cystic fibrosis bronchial gland cells in vivo and in vitro. Am J Pathol. 1998;153:921-930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 210. | Veit G, Bossard F, Goepp J, Verkman AS, Galietta LJ, Hanrahan JW, Lukacs GL. Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Mol Biol Cell. 2012;23:4188-4202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 211. | Zhang YL, Chen PX, Guan WJ, Guo HM, Qiu ZE, Xu JW, Luo YL, Lan CF, Xu JB, Hao Y, Tan YX, Ye KN, Lun ZR, Zhao L, Zhu YX, Huang J, Ko WH, Zhong WD, Zhou WL, Zhong NS. Increased intracellular Cl- concentration promotes ongoing inflammation in airway epithelium. Mucosal Immunol. 2018;11:1149-1157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 212. | Turcios NL. Cystic Fibrosis Lung Disease: An Overview. Respir Care. 2020;65:233-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 213. | Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol Cell Physiol. 2006;291:C218-C230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 214. | Di Pietro C, Zhang PX, O'Rourke TK, Murray TS, Wang L, Britto CJ, Koff JL, Krause DS, Egan ME, Bruscia EM. Ezrin links CFTR to TLR4 signaling to orchestrate anti-bacterial immune response in macrophages. Sci Rep. 2017;7:10882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 215. | Molina SA, Moriarty HK, Infield DT, Imhoff BR, Vance RJ, Kim AH, Hansen JM, Hunt WR, Koval M, McCarty NA. Insulin signaling via the PI3-kinase/Akt pathway regulates airway glucose uptake and barrier function in a CFTR-dependent manner. Am J Physiol Lung Cell Mol Physiol. 2017;312:L688-L702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 216. | Totani L, Plebani R, Piccoli A, Di Silvestre S, Lanuti P, Recchiuti A, Cianci E, Dell'Elba G, Sacchetti S, Patruno S, Guarnieri S, Mariggiò MA, Mari VC, Anile M, Venuta F, Del Porto P, Moretti P, Prioletta M, Mucilli F, Marchisio M, Pandolfi A, Evangelista V, Romano M. Mechanisms of endothelial cell dysfunction in cystic fibrosis. Biochim Biophys Acta Mol Basis Dis. 2017;1863:3243-3253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 217. | Kunzelmann K, Mehta A. CFTR: a hub for kinases and crosstalk of cAMP and Ca2+. FEBS J. 2013;280:4417-4429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 218. | Bals R, Weiner DJ, Wilson JM. The innate immune system in cystic fibrosis lung disease. J Clin Invest. 1999;103:303-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 161] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 219. | Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, Sagel SD, Khan U, Mayer-Hamblett N, Van Dalfsen JM, Joseloff E, Ramsey BW; GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190:175-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 389] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 220. | Kelley TJ, Drumm ML. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J Clin Invest. 1998;102:1200-1207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 194] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 221. | Grasemann H, Knauer N, Büscher R, Hübner K, Drazen JM, Ratjen F. Airway nitric oxide levels in cystic fibrosis patients are related to a polymorphism in the neuronal nitric oxide synthase gene. Am J Respir Crit Care Med. 2000;162:2172-2176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 222. | Dötsch J, Puls J, Klimek T, Rascher W. Reduction of neuronal and inducible nitric oxide synthase gene expression in patients with cystic fibrosis. Eur Arch Otorhinolaryngol. 2002;259:222-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 223. | Thomas SR, Kharitonov SA, Scott SF, Hodson ME, Barnes PJ. Nasal and exhaled nitric oxide is reduced in adult patients with cystic fibrosis and does not correlate with cystic fibrosis genotype. Chest. 2000;117:1085-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 224. | Grasemann H, Klingel M, Avolio J, Prentice C, Gonska T, Tullis E, Ratjen F. Long-term effect of CFTR modulator therapy on airway nitric oxide. Eur Respir J. 2020;55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 225. | Kotha K, Szczesniak RD, Naren AP, Fenchel MC, Duan LL, McPhail GL, Clancy JP. Concentration of fractional excretion of nitric oxide (FENO): A potential airway biomarker of restored CFTR function. J Cyst Fibros. 2015;14:733-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 226. | Wu YS, Jiang J, Ahmadi S, Lew A, Laselva O, Xia S, Bartlett C, Ip W, Wellhauser L, Ouyang H, Gonska T, Moraes TJ, Bear CE. ORKAMBI-Mediated Rescue of Mucociliary Clearance in Cystic Fibrosis Primary Respiratory Cultures Is Enhanced by Arginine Uptake, Arginase Inhibition, and Promotion of Nitric Oxide Signaling to the Cystic Fibrosis Transmembrane Conductance Regulator Channel. Mol Pharmacol. 2019;96:515-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 227. | Zhang PX, Cheng J, Zou S, D'Souza AD, Koff JL, Lu J, Lee PJ, Krause DS, Egan ME, Bruscia EM. Pharmacological modulation of the AKT/microRNA-199a-5p/CAV1 pathway ameliorates cystic fibrosis lung hyper-inflammation. Nat Commun. 2015;6:6221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 228. | Langan KM, Kotsimbos T, Peleg AY. Managing Pseudomonas aeruginosa respiratory infections in cystic fibrosis. Curr Opin Infect Dis. 2015;28:547-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 229. | Dong YJ, Chao AC, Kouyama K, Hsu YP, Bocian RC, Moss RB, Gardner P. Activation of CFTR chloride current by nitric oxide in human T lymphocytes. EMBO J. 1995;14:2700-2707. [PubMed] [Cited in This Article: ] |
| 230. | Chen L, Bosworth CA, Pico T, Collawn JF, Varga K, Gao Z, Clancy JP, Fortenberry JA, Lancaster JR, Matalon S. DETANO and nitrated lipids increase chloride secretion across lung airway cells. Am J Respir Cell Mol Biol. 2008;39:150-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 231. | Oliynyk I, Hussain R, Amin A, Johannesson M, Roomans GM. The effect of NO-donors on chloride efflux, intracellular Ca(2+) concentration and mRNA expression of CFTR and ENaC in cystic fibrosis airway epithelial cells. Exp Mol Pathol. 2013;94:474-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 232. | Rückes-Nilges C, Lindemann H, Klimek T, Glanz H, Weber WM. Nitric oxide has no beneficial effects on ion transport defects in cystic fibrosis human nasal epithelium. Pflugers Arch. 2000;441:133-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 233. | Skinn AC, MacNaughton WK. Nitric oxide inhibits cAMP-dependent CFTR trafficking in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G739-G744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 234. | Jilling T, Haddad IY, Cheng SH, Matalon S. Nitric oxide inhibits heterologous CFTR expression in polarized epithelial cells. Am J Physiol. 1999;277:L89-L96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 235. | Kamosinska B, Radomski MW, Duszyk M, Radomski A, Man SF. Nitric oxide activates chloride currents in human lung epithelial cells. Am J Physiol. 1997;272:L1098-L1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 236. | Xu Y, Duan C, Kuang Z, Hao Y, Jeffries JL, Lau GW. Pseudomonas aeruginosa pyocyanin activates NRF2-ARE-mediated transcriptional response via the ROS-EGFR-PI3K-AKT/MEK-ERK MAP kinase signaling in pulmonary epithelial cells. PLoS One. 2013;8:e72528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 237. | Chen J, Kinter M, Shank S, Cotton C, Kelley TJ, Ziady AG. Dysfunction of Nrf-2 in CF epithelia leads to excess intracellular H2O2 and inflammatory cytokine production. PLoS One. 2008;3:e3367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 238. | Borcherding DC, Siefert ME, Lin S, Brewington J, Sadek H, Clancy JP, Plafker SM, Ziady AG. Clinically-approved CFTR modulators rescue Nrf2 dysfunction in cystic fibrosis airway epithelia. J Clin Invest. 2019;129:3448-3463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 239. | Ziady AG, Sokolow A, Shank S, Corey D, Myers R, Plafker S, Kelley TJ. Interaction with CREB binding protein modulates the activities of Nrf2 and NF-κB in cystic fibrosis airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1221-L1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 240. | Zhang Z, Leir SH, Harris A. Oxidative stress regulates CFTR gene expression in human airway epithelial cells through a distal antioxidant response element. Am J Respir Cell Mol Biol. 2015;52:387-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 241. | Lin JK. Molecular targets of curcumin. Adv Exp Med Biol. 2007;595:227-243. [PubMed] [Cited in This Article: ] |
| 242. | Scannevin RH, Chollate S, Jung MY, Shackett M, Patel H, Bista P, Zeng W, Ryan S, Yamamoto M, Lukashev M, Rhodes KJ. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther. 2012;341:274-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 346] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 243. | Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. 2015;16:27-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 451] [Cited by in F6Publishing: 463] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 244. | Bartlett JA, Fischer AJ, McCray PBJ. Innate immune functions of the airway epithelium. Contrib Microbiol. 2008;15:147-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 245. | Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem. 1994;269:5241-5248. [PubMed] [Cited in This Article: ] |
| 246. | Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1152] [Cited by in F6Publishing: 1133] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 247. | Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, Kotani H. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956-1967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 661] [Cited by in F6Publishing: 706] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 248. | Luo Y, Shoemaker AR, Liu X, Woods KW, Thomas SA, de Jong R, Han EK, Li T, Stoll VS, Powlas JA, Oleksijew A, Mitten MJ, Shi Y, Guan R, McGonigal TP, Klinghofer V, Johnson EF, Leverson JD, Bouska JJ, Mamo M, Smith RA, Gramling-Evans EE, Zinker BA, Mika AK, Nguyen PT, Oltersdorf T, Rosenberg SH, Li Q, Giranda VL. Potent and selective inhibitors of Akt kinases slow the progress of tumors in vivo. Mol Cancer Ther. 2005;4:977-986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 249. | Davies BR, Greenwood H, Dudley P, Crafter C, Yu DH, Zhang J, Li J, Gao B, Ji Q, Maynard J, Ricketts SA, Cross D, Cosulich S, Chresta CC, Page K, Yates J, Lane C, Watson R, Luke R, Ogilvie D, Pass M. Preclinical pharmacology of AZD5363, an inhibitor of AKT: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther. 2012;11:873-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |