Published online Jul 27, 2016. doi: 10.4240/wjgs.v8.i7.513
Peer-review started: January 29, 2016
First decision: March 9, 2016
Revised: April 3, 2016
Accepted: April 14, 2016
Article in press: April 18, 2016
Published online: July 27, 2016
AIM: To evaluate accuracy of three-dimensional endoanal ultrasound (3D-EAUS) as compared to 2D-EAUS and physical examination (PE) in diagnosis of perianal fistulas and correlate with intraoperative findings.
METHODS: A prospective observational consecutive study was performed with patients included over a two years period. All patients were studied and operated on by the Colorectal Unit surgeons. The inclusion criteria were patients over 18, diagnosed with a criptoglandular perianal fistula. The PE, 2D-EAUS and 3D-EAUS was performed preoperatively by the same colorectal surgeon at the outpatient clinic prior to surgery and the fistula anatomy was defined and they were classified in intersphincteric, high or low transsphincteric, suprasphincteric and extrasphincteric. Special attention was paid to the presence of a secondary tract, the location of the internal opening (IO) and the site of external opening. The results of these different examinations were compared to the intraoperative findings. Data regarding location of the IO, primary tract, secondary tract, and the presence of abscesses or cavities was analysed.
RESULTS: Seventy patients with a mean age of 47 years (range 21-77), 51 male were included. Low transsphincteric fistulas were the most frequent type found (33, 47.1%) followed by high transsphincteric (24, 34.3%) and intersphincteric fistulas (13, 18.6%). There are no significant differences between the number of IO diagnosed by the different techniques employed and surgery (P > 0.05) and, there is a good concordance between intraoperative findings and the 2D-EAUS (k = 0.67) and 3D-EAUS (k = 0.75) for the diagnosis of the primary tract. The ROC curves for the diagnosis of transsphincteric fistulas show that both ultrasound techniques are adequate for the diagnosis of low transsphincteric fistulas, 3D-EAUS is superior for the diagnosis of high transsphincteric fistulas and PE is weak for the diagnosis of both types.
CONCLUSION: 3D-EAUS shows a higher accuracy than 2D-EAUS for assessing height of primary tract in transsphincteric fistulas. Both techniques show a good concordance with intraoperative finding for diagnosis of primary tracts.
Core tip: The authors think that this paper provides new information regarding the diagnosis of perianal fistulas with three-dimensional endoanal ultrasound when compared with the results obtained from two-dimensional endoanal ultrasound, physical examination, and examination under anesthesia. This allows us to validate the technique.
- Citation: Garcés-Albir M, García-Botello SA, Espi A, Pla-Martí V, Martin-Arevalo J, Moro-Valdezate D, Ortega J. Three-dimensional endoanal ultrasound for diagnosis of perianal fistulas: Reliable and objective technique. World J Gastrointest Surg 2016; 8(7): 513-520
- URL: https://www.wjgnet.com/1948-9366/full/v8/i7/513.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v8.i7.513
Management of perianal fistulas continues to be a challenge for the surgeon. The correct classification of the fistulas and their relationship with the anal sphincters is fundamental in choosing the adequate treatment.
Magnetic Resonance Imaging (MRI) and endoanal ultrasound (EAUS) have become the most valuable tools for diagnosing anal pathology. Two dimensional EAUS (2D-EAUS) affords sufficient information to be able to make adequate decisions in the management of these patients[1-4]. It does not however measure volumes of the elements of the anal canal, and gives less information regarding the anatomic structures involved[5-7]. These limitations have been overcome with the introduction of three dimensional EAUS (3D-EAUS).
For many authors, the characteristics of 3D-EAUS (easy access, cost and accuracy) have made it the first choice for the diagnosis of perianal fistulas. To date, examination under anesthesia has been considered the gold standard. There are some groups that now believe MRI is better as it can diagnose fistulas and secondary tracts which are not seen during surgery[8], though there are no studies as yet which investigate the value of 3D-EAUS for this purpose.
The objective of this study was to evaluate the diagnostic accuracy of 3D-EAUS vs 2D-EAUS vs physical examination (PE) for the diagnosis of perianal fistulas and correlate the results with the intraoperative findings.
A prospective observational consecutive study was performed with patients included over a two year period. All patients were studied and operated on by surgeon from the Colorectal Unit, Hospital Clínico Universitario, Valencia. The study protocol was approved by the hospital ethics committee and all patients signed an informed consent form.
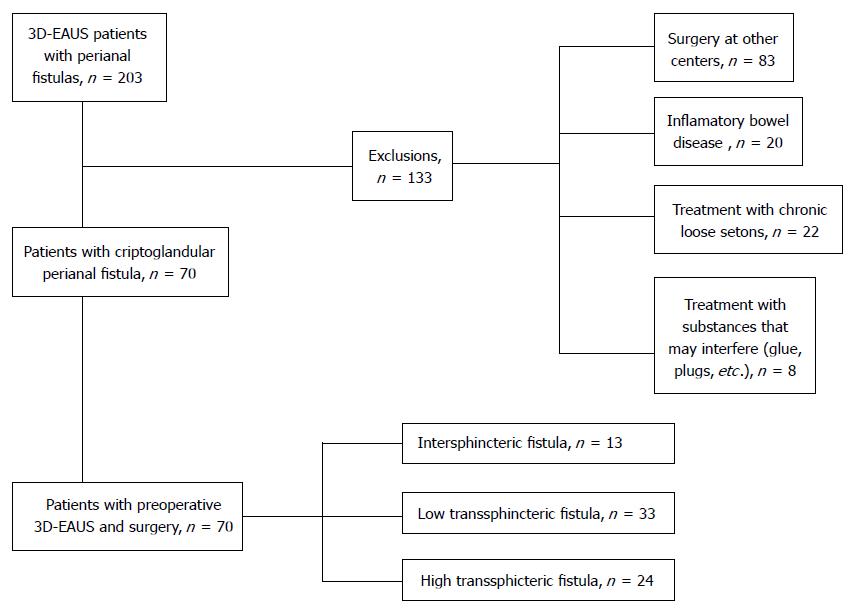
The inclusion criteria were patients 18 years and over, diagnosed with a cryptoglandular perianal fistula. The exclusion criteria were patients operated in other centres, patients with chronic inflammatory bowel disease, suprasphincteric or extrasphincteric fistulas, and patients who were already receiving nonsurgical treatment which could affect the results such as plugs, biological glues or stem cell therapy, etc.
History and PE: A meticulous history was taken during the first consultation. PE performed in the prone jack-knife position included palpation of the perianal region and a digital rectal exam. The fistula anatomy was defined and they were classified as intersphincteric, high or low transsphincteric, suprasphincteric and extrasphincteric. Special attention was paid to the presence of a secondary tract, the location of the internal opening (IO) and the site of the external opening (EO).
EAUS: All ultrasounds were performed by the same surgeon with over 10 years’ experience in EAUS using the B and K Medical Systems Pro Focus 2202® scanner and B-K 2050 probe (B-K Medical, Herlev, Denmark).
Ultrasound evaluation was carried out at a frequency of 10 MHz initially in 2D and followed by 3D using 0.2 mm slices throughout the length of the anal canal and producing 300 sequential images that were automatically reconstructed as a cube, which could be worked on later. This automated reconstruction of the images reduces human error as the ultrasound probe does not need to be moved throughout the examination and can be subsequently saved allowing post examination analysis of the 3D-EAUS scan in coronal, sagittal or axial planes as deemed necessary.
All patients were examined in the prone jack-knife position. The ultrasound was systematically performed from the upper to the lower third of the anal canal. When examining the inferior third of the anal canal it is important to keep the buttocks separated because the subcutaneous tissue can be confused with images of the external anal sphincter (EAS) and therefore overestimate the length of the anal canal and of the EAS. When the EO was open the examination was repeated after instilling 10% hydrogen peroxide solution through a cannula.
2D-EAUS: The IO was classified with or without the instillation of hydrogen peroxide according to the criteria proposed by Cho D-Y[9], distance from the anal margin and radial location. The primary fistulous tract was classified as: (1) Not seen; (2) Intersphincteric: Crosses the intersphincteric space without crossing the EAS; (3) Low transsphincteric: Crosses both sphincters or the EAS in the lower two thirds of the anal canal; (4) High transsphincteric: Crosses both sphincters in the upper third of the anal canal; (5) Suprasphincteric: Crosses the intersphincteric space and courses above the upper border of the puborectalis muscle; and (6) Extrasphincteric: Lies external to the sphincteric apparatus.
Other data obtained with this technique were the presence of secondary tracts (hypoechoic tracts which join the primary tract at some point) and the presence of cavities and perianal abscesses.
3D-EAUS: Sagittal, oblique, transverse and coronal images can be obtained and recorded in video format to be reviewed later if necessary. The location and distance of the IO from the anal margin are recorded together with possible secondary tracts and abscesses, confirming or improving the information obtained from the 2D-EAUS.
Once the examination is finalized, the images can be recovered and reviewed, taking meticulous measurements. There are a series of endosonography images that can lead to error in the measurements if the examination is not performed by someone experienced in the field. The separation of the EAS from the puborectalis muscle can be seen on sagittal section as a hypoechoic line, which when combined with the transverse axial image, perfectly defines the proximal limit of the EAS. The proximal limit of the internal anal sphincter (IAS) is defined as the anorrectal junction and quantitative measurements are taken in mm. The following measurements were taken in all patients: Total length of the anal canal, length of the puborectalis muscle, total length of the EAS, total length of the IAS, length of the IAS and EAS involved by the fistula and percentage of sphincter involved by the fistula with respect to the total sphincter length.
According to the measurements obtained, the fistulas were classified by 3D-EAUS as: (1) Unidentified; (2) Intersphincteric: Crosses the intersphincteric space without crossing the EAS; (3) Low transsphincteric: Involves less than 66% of the EAS; (4) High transsphincteric: Involves over 66% of the EAS; (5) Suprasphincteric: Crosses the intersphincteric space and courses above the upper border of the puborectalis muscle; and (6) Extrasphincteric: lies external to the sphincteric apparatus.
Surgery: All patients were operated in the prone jack-knife position with locoregional anesthesia. Surgery is started with a PE under anesthesia using a Hill Ferguson retractor and the EO is probed up to the IO. The presence of secondary tracts and other pathology, which could modify the surgery, is ruled out. If the IO is not seen, 10% hydrogen peroxide is instilled through the EO. At this point, data regarding the site, type, and distance from the anal marginal are taken. The type of surgery to be performed is then chosen.
Data from the PE, 2D-EAUS and 3D-EAUS are compared with data from the examination under anesthesia considered the gold standard. The concordance rate and Kappa coefficient (degree of non-random agreement between different measurements of the same variable) are calculated. The Kappa coefficient varies between -1 and 1, considering: k = -1, agreement due to chance; k < 0.2, poor agreement; k = 0.2-0.4, low; k = 0.4-0.6, moderate; k = 0.6-0.8, good; k = 0.8-1, very good. Furthermore, the sensitivity, specificity and predictive values were calculated for each test. The chi-square test was used to compare differences between percentages. The ROC curves (curves for the receiver operating characteristics) have been determined for the diagnosis of transsphincteric fistulas by PE, 2D-EAUS and 3D-EAUS. The ideal diagnostic test has sensitivity and specificity equal to 1 (upper left corner of the curve) and will be poorer the closer it is to the diagonal (area under the curve = 0.50). Therefore, the minimum requirement for a diagnostic method would be an area under the curve greater than 0.50.
In all cases a value of P < 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS version 19.0 for Windows (SPSS, Chicago, IL, United States).
Seventy patients with a diagnosis of perianal fistula of criptoglandular origin were eventually included (Figure 1). The most frequent type were low transsphincteric fistulas (33, 47.1%), followed by high transsphincteric fistulas (24, 34.3%) and finally intersphincteric fistulas (13, 18.6%). Gynaecological history and past perianal surgeries can be seen in Table 1. Median duration of symptoms at first consultation was 12 mo (range 1-120).
| n = 70 (n females = 19) | % | |
| Females with vaginal deliveries | 9 | 47.3 |
| Episiotomy | 4 | 21.0 |
| Hysterectomy | 2 | 10.5 |
| Perianal abscesses drained | 42 | 60.0 |
| Seton | 22 | 31.4 |
| Fistulotomy | 6 | 8.6 |
| Fistulectomy | 3 | 4.3 |
| LIS | 7 | 10.0 |
| Hemorroidectomy | 3 | 4.3 |
| Rectal mucosal advancement flap | 3 | 4.3 |
Findings for PE, 2D-EAUS, 3D-EAUS and surgery are in Table 2.
| PE | 2D-EAUS | 3D-EAUS | Surgery | |
| IO identified | 3 (75.7) | 67 (95.7) | 67 (95.7) | 67 (95.7) |
| Primary tract | ||||
| Intersphincteric | 19 (27.1) | 14 (20) | 10 (14.3) | 13 (18.6) |
| Transsphincteric | ||||
| Low | 22 (31.4) | 25 (35.7) | 34 (48.6) | 33 (47.1) |
| High | 19 (27.1) | 30 (42.9) | 25 (35.7) | 24 (34.3) |
| Unclassified | 10 (14.3) | 1 (1.4) | 1 (1.4) | 0 (0) |
| Secondary tract | 6 (8.6) | 15 (21.4) | 16 (22.9) | 11 (15.7) |
| Adjacent abscesses | 12 (17.1) | 17 (24.3) | 19 (27.1) | 8 (11.4) |
Internal opening: Sixty-seven IOs were found in 70 patients intraoperative. The majority of IO were found by digital rectal examination (n = 53; 75.7%). Both 2D-EAUS and 3D-EAUS diagnosed 67 IO in 70 patients (95.7%). Both examinations failed to find the IO in 3 patients despite the instillation of hydrogen peroxide. Two of the patients not diagnosed by EAUS do not coincide with those not found during surgery. There are no significant differences between the number of IO diagnosed between the different techniques employed and surgery (P > 0.05) (Table 3).
| PE | 2D-EAUS | 3D-EAUS | ||||
| Concordance | k | Concordance | k | Concordance | k | |
| IO identified | 51/70 (72.8%) | 1 | 68/70 (97.1%) | 1 | 68/70 (97.1%) | 1 |
| Primary tract | 37/70 (52.9%) | 0.33 | 55/70 (78.6%) | 0.67 | 58/70 (82.8%) | 0.75 |
| Secondary tract | 61/70 (87.1%) | 0.44 | 64/70 (91.4%) | 0.66 | 65/70 (92.8%) | 0.60 |
| Adjacent abscesses | 58/70 (82.8%) | 0.30 | 61/70 (87.1%) | 0.57 | 60/70 (85.7%) | 0.54 |
Thirteen intersphincteric fistulas, 33 low transsphincteric and 24 high transsphincteric fistulas were diagnosed intraoperatively. PE could not classify 10 patients due to pain, or because the tract could not be palpated during the examination. Thirty-seven patients were correctly diagnosed (52.9%). 55 (78.6%) and 58 (82.8%) were diagnosed by 2D-EAUS and 3D-EAUS respectively as shown in Table 3. One patient could not be classified by 2D-EAUS or 3D-EAUS due to the difficulty in differentiating the fistulous tract from fibrosis secondary to prior anal surgeries. There is a good concordance between intraoperative and ultrasound diagnosis of primary tract, the highest concordance was with 3D-EAUS (k = 0.67 and k = 0.75, respectively). There is a tendency to overestimate fistula height with 2DEAUS as can be seen by the lower specificity for high transsphincteric fistulas and lower sensitivity for low transsphincteric fistulas shown in Table 4.
| S | SP | PPV | NPV | ||
| IO identified (%) | PE | 76 | 33 | 96 | 6 |
| 2D-EAUS | 98 | 66 | 98 | 66 | |
| 3D-EAUS | 98 | 66 | 98 | 66 | |
| Primary tract (%) | |||||
| Intersphincteric | PE | 69 | 82 | 47 | 92 |
| 2D-EAUS | 77 | 93 | 71 | 95 | |
| 3D-EAUS | 22 | 98 | 90 | 93 | |
| Transsphincteric | |||||
| Low | PE | 45 | 81 | 68 | 67 |
| 2D-EAUS | 67 | 92 | 88 | 75 | |
| 3D-EAUS | 85 | 84 | 82 | 86 | |
| High | PE | 38 | 87 | 68 | 78 |
| 2D-EAUS | 64 | 76 | 70 | 70 | |
| 3D-EAUS | 88 | 91 | 84 | 93 | |
| Secondary tract (%) | PE | 40 | 97 | 67 | 91 |
| 2D-EAUS | 90 | 90 | 60 | 98 | |
| 3D-EAUS | 90 | 88 | 56 | 98 | |
Secondary fistulous tracts: One or more secondary fistula tracts were diagnosed by 2D-EAUS and 3D-EAUS in 15 and 16 patients respectively with a good concordance with surgical findings (91.4%, k = 0.66; 92.8%, k = 0.60) (Table 3).
Abscesses and adjacent cavities: 2D-EAUS diagnosed abscesses in 17 (24.3%) patients and 3D-EAUS in 19 (27.1%) patients. 12 cases (17.1%) were diagnosed by PE. 8 patients (11.4%) presented with an abscess at the time of surgery. There was a moderate concordance between EAUS and surgery (k=0.57, k = 0.54, respectively). There was a low concordance between PE and intraoperative findings (k = 0.30) (Table 3).
The sensitivity and specificity (efficacy indexes) of the different examinations with respect to intraoperative findings are shown in Table 4.
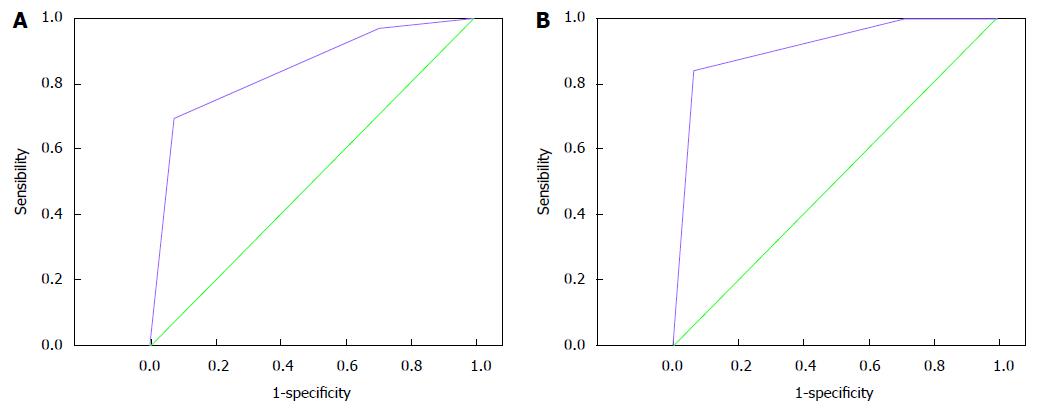
ROC curves (Receiver Operating Characteristic) for the diagnosis of transsphincteric fistulas by PE and 2D/3D-EAUS are adequate for the diagnosis of low transsphincteric fistulas. 3D-EAUS is superior for the diagnosis of high transsphincteric fistulas (Figure 2). PE is clearly deficient for the classification of transsphincteric fistulas (Table 5).
| Area under curve | 95%CI | P value | ||
| Low transsphincteric fistula | PE | 0.608 | 0.474-0.742 | 0.120 |
| 2D-EAUS | 0.819 | 0.714-0.924 | 0.0001 | |
| 3D-EAUS | 0.829 | 0.724-0.934 | 0.0001 | |
| High transsphincteric fistula | PE | 0.672 | 0.541-0.803 | 0.019 |
| 2D-EAUS | 0.842 | 0.745-0.939 | 0.0001 | |
| 3D-EAUS | 0.910 | 0.835-0.985 | 0.0001 |
3D-EAUS is a novel technique for the diagnosis of perianal fistulas and multiple studies such as ours demonstrate its’ superiority with respect to 2D-EAUS. 3D-EAUS is a useful tool that gives a more reliable preoperative diagnosis of perianal fistulas with accurate diagnosis of the IO, primary tracts, secondary tracts and adjacent abscesses or cavities. Ratto et al[10] published a rate of exact diagnosis with 3D-EAUS of primary and secondary tracts of 98.5% and 96.4% for the IO compared with 89.9%, 83.3% and 87.9% respectively with 2D-EAUS. Santoro et al[11,12] in their study in 57 patients confirm that 3D-EAUS improves diagnosis accuracy of the IO when compared to 2D-EAUS (2D-EAUS: 66.7% vs 3D-EAUS: 89.5%; P = 0.0033). However, both techniques were similar for diagnosis of primary and secondary tracts and abscesses[11,12]. Our study showed a 97.1% concordance for the diagnosis of the IO (for both types of EAUS), 78.6% for primary tracts, 91.4% for secondary tracts and 87.1% for cavities and abscesses with 2D-EAUS as opposed to 82.8%, 92.8% and 85.7% respectively when using 3D-EAUS. A preliminary study in 29 patients carried out by our group showed a concordance rate between intraoperative findings (gold standard) and 3D-EAUS of 79% for primary fistula tracts validating the latter as a useful technique in the evaluation of perianal fistulas[13].
There are various classifications for perianal fistulas. As a practical method, various authors have modified the Parks classification[14]. The subdivision of transsphincteric fistulas with regards to the level at which they cross the anal canal tends to be arbitrary dividing these in equal thirds. We propose a new division of transsphincteric fistulas dividing them into low (less than 66% of the total length of the EAS involved) and high (over 66% of the EAS involved). This way we can simplify the classification and guide the indication for surgery.
This study shows a good correlation between 3D-EAUS and surgical findings, with superior results to PE and 2D-EAUS, in particular with regards to high transsphincteric fistulas which are the ones raising more doubts in diagnosis and choice of treatment. According to our results, 2D-EAUS in particular for high transsphincteric fistulas tends to overestimate the amount of anal sphincter involved thus classifying them as higher than they really are, as can be seen by the lower specificity for high transsphincteric fistulas and lower sensitivity for low transsphincteric fistulas. These errors are minimized with 3D-EAUS with a notable improvement in sensitivity and specificity. According to the ROC curves, the best technique for diagnosing high transsphincteric fistulas is 3D-EAUS. Although both types of EAUS are adequate for the diagnosis of low transsphincteric fistulas, 3D-EAUS seems to be slightly superior.
The large variability between examinations have not allowed for the calculation of the IO Kappa coefficient, there were no significant differences between examination techniques and surgical findings with regards to diagnosis of the IO. These results are similar to the study in 21 patients published by Poen et al[15]. The three examinations show high sensitivity and specificity when diagnosing the location and distance from the anal margin of the IO.
Even though both types of EAUS have a good concordance 3D-EAUS has shown a higher concordance and accuracy than 2D-EAUS when compared to intraoperative findings (k = 0.75 vs k = 0.67). Various studies have shown a very good concordance between 2D and 3D-EAUS and surgery for diagnosis of the primary tract using the instillation of hydrogen peroxide[8,13]. We did not use hydrogen peroxide in all our patients and included patients with a closed EO. This could explain the difference in results.
Similar to the results published by Poen et al[15] (k = 0.61), 3D-EAUS shows a good concordance with surgery for the diagnosis of secondary tracts (k = 0.60). The concordance coefficient for 2D-EAUS is slightly higher than 3D-EAUS (k = 0.66 vs k = 0.60). In addition, EAUS diagnosed more secondary tracts than surgery. These complex or high fistulous tracts could go unnoticed during surgery. As a result of these findings we should possibly reconsider, as have done other authors, which of these examinations is truly the gold standard for the diagnosis of perianal fistulas. Surgery may not be the best diagnostic tool and we should consider MRI with an endoanal coil or 3D-EAUS[16].
The diagnosis of adjacent abscesses and cavities shows a moderate concordance with surgery (2D-EAUS, k = 0.57; 3D-EAUS, k = 0.54) and insignificant concordance with PE. This is probably due to the fact that these cavities may not be obvious on PE but as patients had to wait sometime between examination and surgery there were probably changes (improvement or deterioration) in these parameters.
There are various studies that defend the routine use of preoperative 2D-EAUS for the diagnosis of both simple and complex perianal fistulas[14,17]. Some simple perianal fistulas can be diagnosed on PE and we believe a routine EAUS is unnecessary. 3D-EAUS has clearly overtaken 2D-EAUS however, and is more efficient offering more detailed information[10,18]. Due to the common problem that these fistulas represent and the difficulty in obtaining a definitive treatment, there are various groups that as we do, use 3D-EAUS for the preoperative diagnosis of perianal fistulas[19]. Murad-Regadas et al[20] published a study in 33 patients confirming that preoperative 3D-EAUS was useful for the diagnosis of anterior transsphincteric fistulas, assisting in choosing the most appropriate treatment and reducing the incontinence rates.
Our work shows the value of 3D-EAUS in predicting the amount of sphincter involved by the fistula in an objective and quantitative manner, and allowing a more accurate classification of the fistula.
Despite the results obtained in this study there were some limitations. These include the low number of patients included even though this was similar or superior to other published studies, the exclusion of suprasphincteric and extrasphincteric fistulas, whose prevalence is very low and where the role of IRM vs 3D-EAUS is debatable[7,16]; and that all measurements and scans in this study were performed by the same surgeon. This last point may be beneficial on the one hand as it reduces interobserver variability, but at the same time may offer some bias. We believe it would be more correct to perform the measurements by two independent examiners and then analyse the differences between them.
According to our results we can conclude that 3D-EAUS is more accurate than 2D-EAUS for estimating the height of the primary tract in transsphincteric fistulas. Both 2D and 3D-EAUS techniques show a good concordance with examination under anesthesia for the diagnosis of primary tracts with slightly superior results for 3D-EAUS. Therefore, we agree with other authors that EAUS is a fundamental tool in the evaluation of perianal fistulas allowing for a better classification. 3D-EAUS provides new advantages with respect to 2D-EAUS and is a superior technique allowing for objective and quantitative, and not only subjective, information.
Perianal fistulas are a common problem in the general population and affect around 10 per 100000 population per year. The relationship between fistulous tract, the sphincters and adequate management is still a challenge today. Imaging techniques play an important role in diagnosis. Various authors including us prefer endoanal ultrasound (EAUS). It is cheaper, easy to use with training, fast, non-invasive and can be used in the operating room if necessary.
Controversy has been raised over the last few years over which technique [magnetic resonance imaging (MRI), ultrasound or examination under anesthesia] is the gold standard for diagnosis of perianal fistulas. The choice between EAUS and MRI mainly depends on their availability. MRI may seem to offer better results for the diagnosis of perianal fistulas but is outweighed by its’ expense and lower availability. In addition, three-dimensional (3D)-EAUS has considerably improved when compared with MRI.
This study compares the results of PE, 2D-EAUS and 3D-EAUS with examination under anesthesia for perianal fistulas providing concordance data for the different techniques. This allows the authors to determine which technique is best in each case, the need to use them as diagnostic tools and for providing optimal management of perianal fistulas.
The results of this study suggest 3D-EAUS is superior to 2D-EAUS for the diagnosis of high transsphincteric fistulas and could now be considered the gold standard for diagnosis of this pathology. EAUS is a fundamental tool for the evaluation of perianal fistulas offers an accurate classification and therefore betters treatment.
Perianal fistulas are a chronic phase of a suppurated anal disease. The currently available imaging techniques for classifying anal fistulas are: Fistulography (no longer used), MRI and EAUS. EAUS can be 2D EAUS (distance, area and volume measurements cannot be taken, with poorer imaging of spatial relations and loss of relevant information) or 3D-EAUS (offers a view of all planes and distances, angles, areas and volumes can be accurately measured).
The paper offers an interesting comparison of the diagnostic yield of 2D-EAUS vs 3D-EAUS for perianal fistulas. The gold standard in the present study are intraoperative findings, although other groups think that MRI can demonstrate perianal fistulas missed by the surgeon.
P- Reviewer: Armellini E, Guadalajara H S- Editor: Gong ZM L- Editor: A E- Editor: Jiao XK
| 1. | Voyvodic F, Rieger NA, Skinner S, Schloithe AC, Saccone GT, Sage MR, Wattchow DA. Endosonographic imaging of anal sphincter injury: does the size of the tear correlate with the degree of dysfunction? Dis Colon Rectum. 2003;46:735-741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Law PJ, Talbot RW, Bartram CI, Northover JM. Anal endosonography in the evaluation of perianal sepsis and fistula in ano. Br J Surg. 1989;76:752-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 123] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Cataldo PA, Senagore A, Luchtefeld MA. Intrarectal ultrasound in the evaluation of perirectal abscesses. Dis Colon Rectum. 1993;36:554-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Lindsey I, Humphreys MM, George BD, Mortensen NJ. The role of anal ultrasound in the management of anal fistulas. Colorectal Dis. 2002;4:118-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Bartram CI, Frundiger A. Handbook of anal endosonography. Petersfield, Wrightson Biomedical, 1997: 15-20. . [Cited in This Article: ] |
| 6. | Hildebrandt U, Feifel G, Schwarz HP, Scherr O. Endorectal ultrasound: instrumentation and clinical aspects. Int J Colorectal Dis. 1986;1:203-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 109] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Law PJ, Bartram CI. Anal endosonography: technique and normal anatomy. Gastrointest Radiol. 1989;14:349-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 229] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | West RL, Dwarkasing S, Felt-Bersma RJ, Schouten WR, Hop WC, Hussain SM, Kuipers EJ. Hydrogen peroxide-enhanced three-dimensional endoanal ultrasonography and endoanal magnetic resonance imaging in evaluating perianal fistulas: agreement and patient preference. Eur J Gastroenterol Hepatol. 2004;16:1319-1324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Cho DY. Endosonographic criteria for an internal opening of fistula-in-ano. Dis Colon Rectum. 1999;42:515-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Ratto C, Grillo E, Parello A, Costamagna G, Doglietto GB. Endoanal ultrasound-guided surgery for anal fistula. Endoscopy. 2005;37:722-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Santoro GA, Fortling B. The advantages of volume rendering in three-dimensional endosonography of the anorectum. Dis Colon Rectum. 2007;50:359-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Santoro GA, Ratto C, Di Falco G. Three-dimensional reconstructions improve the accuracy of endoanal ultrasonography in the identification of internal openings of anal fistulas. Colorectal Dis. 2004;6 Suppl 2:214. [Cited in This Article: ] |
| 13. | Garcés Albir M, García Botello S, Esclápez Valero P, Sanahuja Santafé A, Espí Macías A, Flor Lorente B, García-Granero E. [Evaluation of three-dimensional endoanal endosonography of perianal fistulas and correlation with surgical findings]. Cir Esp. 2010;87:299-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Navarro-Luna A, García-Domingo MI, Rius-Macías J, Marco-Molina C. Ultrasound study of anal fistulas with hydrogen peroxide enhancement. Dis Colon Rectum. 2004;47:108-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Poen AC, Felt-Bersma RJ, Eijsbouts QA, Cuesta MA, Meuwissen SG. Hydrogen peroxide-enhanced transanal ultrasound in the assessment of fistula-in-ano. Dis Colon Rectum. 1998;41:1147-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 131] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | West RL, Zimmerman DD, Dwarkasing S, Hussain SM, Hop WC, Schouten WR, Kuipers EJ, Felt-Bersma RJ. Prospective comparison of hydrogen peroxide-enhanced three-dimensional endoanal ultrasonography and endoanal magnetic resonance imaging of perianal fistulas. Dis Colon Rectum. 2003;46:1407-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Pascual Migueláñez I, García-Olmo D, Martínez-Puente MC, Pascual Montero JA. Is routine endoanal ultrasound useful in anal fistulas? Rev Esp Enferm Dig. 2005;97:323-327. [PubMed] [Cited in This Article: ] |
| 18. | Kruskal JB, Kane RA, Morrin MM. Peroxide-enhanced anal endosonography: technique, image interpretation, and clinical applications. Radiographics. 2001;21 Spec No:S173-S189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Brillantino A, Iacobellis F, Di Sarno G, D’Aniello F, Izzo D, Paladino F, De Palma M, Castriconi M, Grassi R, Di Martino N. Role of tridimensional endoanal ultrasound (3D-EAUS) in the preoperative assessment of perianal sepsis. Int J Colorectal Dis. 2015;30:535-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Murad-Regadas SM, Regadas FS, Rodrigues LV, Holanda Ede C, Barreto RG, Oliveira L. The role of 3-dimensional anorectal ultrasonography in the assessment of anterior transsphincteric fistula. Dis Colon Rectum. 2010;53:1035-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |