Published online Apr 27, 2013. doi: 10.4240/wjgs.v5.i4.73
Revised: January 17, 2013
Accepted: February 5, 2013
Published online: April 27, 2013
AIM: To evaluate acute cholecystitis, complicated by peritonitis, acute phase response and immunological status in patients treated by laparoscopic or open approach.
METHODS: From January 2002 to May 2012, we conducted a prospective randomized study on 45 consecutive patients (27 women, 18 men; mean age 58 years). These subjects were taken from a total of 681 patients who were hospitalised presenting similar preoperative findings: acute upper abdominal pain with tenderness, involuntary guarding under the right hypochondrium and/or in the flank; fever higher than 38 °C, leukocytosis greater than 10 × 109/L or both, and ultrasonographic evidence of calculous cholecystitis possibly complicated by peritonitis. These patients had undergone cholecystectomy for acute calculous cholecystitis, complicated by bile peritonitis. Randomly, 23 patients were assigned to laparoscopic cholecystectomy (LC), and 22 patients to open cholecystectomy (OC). Blood samples were collected from all patients before operation and at days 1, 3 and 6 after surgery. Serum bacteraemia, endotoxaemia, white blood cells (WBCs), WBC subpopulations, human leukocyte antigen-DR (HLA-DR), neutrophil elastase, interleukin-1 (IL-1) and IL-6, and C-reactive protein (CRP) were measured at 0, 30, 60, 90, 120 and 180 min, at 4, 6, 12, 24 h, and then daily (8 A.M.) until post-op day 6.
RESULTS: The two groups were comparable in the severity of peritoneal contamination as indicated by the viable bacterial count (open group = 90% of positive cultures vs laparoscopic group = 87%) and endotoxin level (open group = 33.21 ± 6.32 pg/mL vs laparoscopic group = 35.02 ± 7.23 pg/mL). Four subjects in the OC group (18.1%) and 1 subject (4.3%) in the LC group (P < 0.05) developed intra-abdominal abscess. Severe leukocytosis (range 15.8-19.6/mL) was observed only after OC but not after LC, mostly due to an increase in neutrophils (days 1 and 3, P < 0.05). This value returned to the normal range within 3-4 d after LC and 5-7 d after OC. Other WBC types and lymphocyte subpopulations showed no significant variation. On the first day after surgery, a statistically significant difference was observed in HLA-DR expression between LC (13.0 ± 5.2) and OC (6.0 ± 4.2) (P < 0.05). A statistically significant change in plasma elastase concentration was recorded post-operatively at days 1, 3, and 6 in patients from the OC group when compared to the LC group (P < 0.05). In the OC group, the serum levels of IL-1 and IL-6 began to increase considerably from the first to the sixth hour after surgery. In the LC group, the increase of serum IL-1 and IL-6 levels was delayed and the peak values were notably lower than those in the OC group. Significant differences between the groups, for these two cytokines, were observed from the second to the twenty-fourth hour (P < 0.05) after surgery. The mean values of serum CRP in the LC group on post-operative days (1 and 3) were also lower than those in the OC group (P < 0.05). Systemic concentration of endotoxin was higher in the OC group at all intra-operative sampling times, but reached significance only when the gallbladder was removed (OC group = 36.81 ± 6.4 ρg/mL vs LC group = 16.74 ± 4.1 ρg/mL, P < 0.05). One hour after surgery, microbiological analysis of blood cultures detected 7 different bacterial species after laparotomy, and 4 species after laparoscopy (P < 0.05).
CONCLUSION: OC increased the incidence of bacteraemia, endotoxaemia and systemic inflammation compared with LC and caused lower transient immunological defense, leading to enhanced sepsis in the patients examined.
Core Tip: Laparoscopic techniques are being increasingly used in diffuse or localised peritonitis. However, a possible concern is that increased intra-abdominal pressure may promote bacteraemia and the systemic inflammatory response during laparoscopic surgery. The majority of reports in the literature are on experimental studies made using animal models. This study, instead, is a prospective randomized study conducted on human subjects. Experimental studies on peritonitis showed that the inflammatory response was significantly higher in the open cholecystectomy (OC) group than in the laparoscopic cholecystectomy (LC) group in the animal models, suggesting that carbon dioxide pneumoperitoneum has a protective effect against bacterial peritonitis. This study, in contrast to the previous ones, is the first work demonstrating that OC after biliary peritonitis increases the incidence of bacteraemia, endotoxaemia and systemic inflammation, compared with the LC group. The authors also demonstrated that early enhanced post-operative systemic inflammation may cause lower transient immunologic defense after laparotomy (decrease of human leukocyte antigen-DR), leading to increased sepsis in these patients.
-
Citation: Sista F, Schietroma M, Santis GD, Mattei A, Cecilia EM, Piccione F, Leardi S, Carlei F, Amicucci G. Systemic inflammation and immune response after laparotomy
vs laparoscopy in patients with acute cholecystitis, complicated by peritonitis. World J Gastrointest Surg 2013; 5(4): 73-82 - URL: https://www.wjgnet.com/1948-9366/full/v5/i4/73.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v5.i4.73
Laparoscopic techniques are increasingly used in surgical conditions complicated by diffuse or localised peritonitis. Successful treatment of acute appendicitis, acute cholecystitis, perforated peptic ulcers and acute diverticulitis has been reported with low morbidity[1-3]. However, a possible concern is that the increased intra-abdominal pressure during laparoscopic surgery may promote bacteraemia and systemic inflammatory response. Data regarding the effects of pneumoperitoneum on physiological changes and systemic inflammation during sepsis are contradictory[4,5]. Furthermore, only the early effects of pneumoperitoneum during peritonitis have been assessed, and the differences between conventional and laparoscopic surgery have been compared only in animal models[6,7].
Therefore, the influence of laparotomy and laparoscopy on bacteraemia and endotoxaemia, peripheral leukocytic subpopulations (neutrophils, total lymphocytes, lymphocyte subpopulations), human leukocyte antigen-DR (HLA-DR), neutrophil elastase, interleukin-1 (IL-1), IL-6, and C-reactive protein (CRP) were investigated in a prospective, randomized study in subjects with acute calculous cholecystitis, complicated by bile peritonitis, who randomly underwent open or laparoscopic cholecystectomy.
From January 2002 to May 2012, we conducted a prospective randomized study on 45 consecutive patients (27 women, 18 men; mean age 58 years), who showed intra-operatively bile peritonitis with acute calculous cholecystitis. Bile peritonitis is an inflammatory, irritative response to the abnormal presence of bile and bacteria in the peritoneal cavity[8]. These patients were taken from a total of 681 subjects who were admitted presenting similar preoperative findings: acute upper abdominal pain with tenderness, involuntary guarding under the right hypochondrium and/or in the flank; fever higher than 38 °C, leukocytosis greater than 10 × 109/L or both, and ultrasonographic symptoms (thickened gallbladder wall, edematous gallbladder wall, presence of gallstones, ultrasonographic Murphy’s sign and pericholecystitis and/or Douglas space fluid collection). These diagnostic criteria suggested acute calculous cholecystitis possibly complicated by peritonitis. All the subjects were randomly assigned to be treated by laparoscopic or open approach, according to a computer-generated table of random numbers. Randomization was performed by an independent computer consultant. The patient and the surgeon were informed of the type of approach just before the intervention.
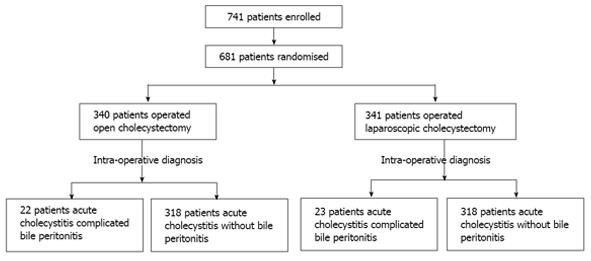
The diagnosis of bile peritonitis was confirmed in 45 patients (6.6%), intraoperatively. Thirty-one patients had rupture of the gallbladder, 11 gangrenous gallbladder and three bile peritonitis without perforation. In Figure 1 the randomization is described in detail.
Exclusion criteria were as follows: acute cholangitis; other acute inflammation; current or recent (6 mo) acute pancreatitis; hematological disorder; anticoagulant treatment; current or recent (6 mo) thromboembolic disorders; renal, hepatic, rheumatic or vascular disease; pregnancy; recent (6 mo) surgery; current or recent (3 years) malignancy; immunosuppressive therapy. Five of the selected patients with clinical suspicion of common bile duct stones were subjected to pre-operative endoscopic retrograde cholangiopancreatography. When common bile duct stones were discovered (3 cases), endoscopic sphincterotomy was performed and ductal clearance achieved before operation. All five patients were excluded by the study. Therefore, there were no indications for intra-operative cholangiography in either group.
Twenty-two patients (14 women, 8 men; mean age 58 years, Table 1) underwent open cholecystectomy (OC) using a right subcostal incision. The remaining 23 patients (13 women, 10 men; mean age 57 years, Table 1) were subjected to laparoscopic cholecystectomy (LC) using the standard technique with four trocar incisions and 14 mmHg CO2 pneumoperitoneum. All procedures were performed by surgeons experienced in hepatobiliary surgery and advanced laparoscopic surgery (Carlei F, Amicucci G).
| Parameter | Open chol-ecystectomyn = 22 | LaparoscopiccholecystectomYn = 231 | P value |
| Age (yr) | 58.3 (36-84) | 56.9 (32-83) | NS |
| Sex (F/M) | 14/8 | 13/10 | NS |
| ASA grade | |||
| I | 3 | 3 | NS |
| II | 12 | 13 | NS |
| III | 5 | 6 | NS |
| IV | 2 | 1 | NS |
| APACHE | |||
| score < 15 | 12 | 13 | NS |
| score > 15 | 10 | 10 | NS |
| MPI | |||
| score < 20 | 13 | 14 | NS |
| score > 20 | 10 | 9 | NS |
| Anaesthesia (min) | 51.6 (46-72) | 61.8 (51-81) | 0.01 |
| Operative time (min) | 46.7 (40-62) | 51.2 (42-64) | NS |
| Post-operative complications2 | 4 (18.1%) | 1 (4.3%) | 0.01 |
| Mortality | 5 (22.7%) | 1 (4.3%) | 0.02 |
| Post-operative hospitalization (d) | 10.2 (6-18) | 5.4 (2-13) | 0.02 |
| Adjusted length of stay (d) | 6.8 (6-9) | 4.6 (2-6) | 0.004 |
The severity of sepsis was evaluated by Acute Physiologic and Chronic Health Evaluation (APACHE) II Score and by message passing interface (MPI) Score (Mannheim Peritonitis Index) (Table 1)[9,10]. Peritoneal lavage was performed with at least 4 washes with warm normal saline solution or until the recovered fluid was clear.
This trial was conducted according to the principles of good clinical practice and received ethics committee approval. Informed consent was obtained from every subject. The patients were classified as grade I II III or IV according to the American Society of Anesthesiologists (ASA) grading system[11].
The initial supportive care during the acute phase was the same for both groups of patients. All subjects received intravenous fluid infusion, intravenous antibiotics (cefotaxime: 2 g per 8 h, tobramicine: 100 mg per 12 h), a proton pump inhibitor (omeprazole: 40 mg iv per 24 h) and pain relief (ketorolac trometamine: 30 mg im per 6 h). There were no indications for blood transfusions.
Anaesthesia was achieved in both groups using the same procedure. Preanaesthesia was accomplished using atropine (0.01 mg/kg), plus promethazine (0.5 mg/kg); induction was conducted using sodium thiopental (5 mg/kg) and atracurium (0.5 mg/kg); tracheal intubation and assisted ventilation were performed using NO2/O2 2:1. After intubation, anaesthesia was maintained with oxygen in air, sevoflurane and remifentanil (0.25 μg/kg per minute). LC and OC were performed as soon as possible, within 12 h of admission.
Wound infections were graded using a classification described elsewhere[12]. Infections were considered grade I in the case of erythema, indurations, and pain; grade II as grade I but with serous fluid; grade III, in the presence of contaminated fluid in less than half the wound; grade IV as grade III but contaminated fluid was in more than half the wound. Wound dehiscence was considered to be present when surgical closure of the cutaneous or subcutaneous tissue (superficial) or the fascia and muscular plane (deep) was necessary in the early post-operative period.
Blood samples were collected from all patients before operation and at days 1, 3 and 6 after surgery. Serum concentration of interleukin-1 (IL-1), IL-6 and endotoxin were measured at 0, 30, 60, 90, 120 and 180 min, at 4, 6, 12, 24 h, and then daily (8 A.M.) until post-op day 6.
Bacterial assay was performed immediately before (within 60 min) and then after operation (within 60 min and within 7 d). Blood was collected in pyrogen-free tubes and centrifuged at 4 °C at 2000 rpm for 10 min, aliquoted into sterile cryotubes (NUNC 36341, Intermed, Denmark) and stored at -80 °C until analysis for the subsequent determination of endotoxin. To determine the severity of peritoneal contamination, undiluted peritoneal fluid was aspirated from the Douglas space during surgery for quantitative bacterial and endotoxin assays. All samples were tested for total white blood cell (WBC) count, and WBC populations, T-helper lymphocytes (CD4), T-suppressor lymphocytes (CD8), natural killer lymphocytes (CD16 and CD56), pan-B cell antigen (CD20), TCR gamma/delta and the T-helper/T-suppressor ratio (CD4/CD8).
Human leukocyte antigen-DR (HLA-DR) in peripheral monocytes was measured by a cytofluorimetric method. All blood samples (10 mL) were collected with ethylenediaminetetraacetic acid (EDTA) (0.5 mL). A FITC (Fluorescein Isothiocyanate)-conjugated (10 μL) monoclonal antibody for the HLA-DR antigen was added. Whole blood (100 μL) from each patient was then added, and the tubes were vortex mixed and stored at 4 °C for 30 min. Two mL of lysis solution were added to each sample. All samples were stirred and then incubated for 15 min at room temperature. Finally, an Ortho cytofluorimeter was used for the assay.
Elastase concentration was photometrically determined, using an immune-activation immunoassay (Merck, Damstadt, Germany), as a complex with 1-proteinase inhibitor, according to the method described by Hafner et al[13].
The plasma concentration of CRP was measured using a competitive CRP Elisa Kit. Serum IL-1 and IL-6 concentrations were measured using a quantitative “sandwich” enzyme-linked immunosorbent assay (ELISA) kit (R and D Systems, Minneapolis, United States), according to the manufacturer’s description (range IL-β 3.9-250 pg/mL; IL-6 3.13-300 pg/mL). Samples of serum (100 μL) were dispensed into wells of 96-well microlitre plates which had been coated with the relevant monoclonal cytokine antibody. After a 2-h incubation at room temperature, unbound proteins were washed away from the wells. An enzyme-linked antibody directed against the relevant cytokine was then added and plates were incubated for 2 more hours at room temperature. After further rinsing to remove unbound antibody, a substrate solution was added to each well and the mixture was incubated for 20 min at 37 °C. The reaction was terminated with the addition of a stop solution. Adsorbance was determined by using an ELISA plate reader at 450 nm. Serial dilution of the relevant recombinant cytokine provided the standard curve. Assays were performed on duplicate samples. Samples were appropriately diluted with the diluent provided in the kit if the levels of neat samples were beyond the linear measuring range.
The microorganisms were grown on chocolate agar (tryptic soy agar supplemented with 10% defibrinated sheep blood, heated for 10 min to 80 °C), blood agar (Columbia agar supplemented with 5% defribrinated sheep blood), Endo agar, and Sabouraud agar in both an aerobic and anaerobic atmosphere. The phenotypic identification of all strains was carried out by testing the carbohydrate fermentation reactions or by using commercially available enzyme activity and fermentation test (API, Bio Mérieux, Nürtingen, Germany).
Microbiological analysis of blood was performed immediately before the operation, 1 h after and 3 d after surgery. The initial blood cultures were drawn prior to antibiotic administration. Endotoxin was quantified in duplicates using a modified chromogenic Limulus amoebocyte lysate assay (Quadratech, Epsom, United Kingdom). Test plasma samples and standards were diluted 1:10 in pyrogen-free water and heated to 75 °C for 10 min to remove plasma inhibitors. The concentration of endotoxin in the sample was taken as the average of the duplicates calculated from a standard curve. The assay had a sensitivity of 8 g/mL and was linear in the range 8-100 pg/mL. Each aliquot was assayed for endotoxin only once. If the assay gave poor duplicates or very high values, indicating possible contamination, a fresh aliquot of the same sample was retested.
The sample size of the study was calculated a priori on the assumption that it would have been clinically relevant to have a 15% reduction in the parameter (hypothesis of IL-1 and IL-6 reduction between laparoscopy and laparotomy) with a 10% standard deviation. Furthermore, a power (1-β) of 80% was computed for the two-sided null hypothesis. A sample size of at least 20 patients was needed in each group to have a type I error of less than 5% and a type II error of less than 20%, using a two-tailed test. Comparisons between groups were on an intention-to treat basis.
The primary efficacy variable was the proportion of patients with immunological status improvement at the evaluation performed 1 d, 3 d and 6 d after the end of either of the treatments. The normality distribution of the data was checked with the Shapiro-Wilk test. Data were analyzed using non-parametric statistics, which are more powerful when the data show a skewed distribution. Since the data were not normally distributed, an analysis of variance (non-parametric Friedman’s repeated measures comparisons) was performed in both groups to determine differences between post-operative values and baseline. In the presence of significant difference, post-hoc analysis were made using the Mann-Whitney U test, to compare the values between the two groups Thus, all continuous variables were expressed as mean and standard deviation and compared using the Mann-Whitney U test. χ2 test and Fisher’s exact test were used to compare nominal data. Statistical calculations were performed with the help of Stata/MP 12.1, and a P value of less than 0.05 was considered to indicate statistical significance.
There was no difference between the two groups of patients in terms of co-morbidity, pre-operative clinical features (severity and duration of symptoms, involuntary guarding), biochemical (WBCs count) and radiological (ultrasounds, computed tomography) features of acute calculous cholecystitis possibly complicated by peritonitis. Three subjects (8%) in the laparoscopic group were converted to open surgery and were excluded from the study. As shown in Table 1, no significant differences were observed between the two groups with respect to age, sex, ASA grades, APACHE II Score, MPI score and operation time (P > 0.05), while considerably shorter hospitalization and time of anaesthesia were observed in the LC group (P < 0.05).
LC required almost the same operative time as OC, but it required shorter hospitalization (P < 0.05; Table 1). The two groups were comparable with respect to the severity of peritoneal contamination, as indicated by the viable bacterial count (OC group = 90% of positive cultures vs LC group = 87%) and endotoxin level (open group = 33.21 ± 6.32 pg/mL vs laparoscopic group = 35.02 ± 7.23 pg/mL). Four patients who had undergone OC (18.1%) developed intra-abdominal abscess (Table 1). Clinical and ultrasonographic findings demonstrated a subphrenic abscess in all cases. The characteristics of these subjects are reported in Table 2. Monocyte expression antigen (HLA-DR), which was low one day after operation, remained low even 7 d after surgery and normalised 8-10 d after operation. In patients number 2 and 4, HLA-DR normalised 13 d after surgery (Table 2). The patients were discharged from hospital as a mean 13.8 d after their admission (range 9-18 d). The patient of the LC group with a subphrenic abscess was discharged from after hospital 13 d.
Largely because of the five patients who developed post-operative abscess, the unadjusted median length of stay was rather long for both groups. In fact, the OC group’s unadjusted length of stay (10.2 d) was significantly longer than that of the LC group (5.4 d, P < 0.05). However, if these 5 patients are counted out the calculation, the median length of stay for the OC group falls to 6.8 d and to 4.6 d (P = 0.004) for the LC group (Table 1).
The rate of overall wound infections was 23.8% (10 out of 42). Seven patients (31.8%) in the OC group and 3 (15%) in the LC group had a wound infection (P < 0.05). Two infections of the laparotomy wound appeared at between 1 and 6 mo after surgery. Wound infection was consistently lower in the LC group than in the OC group (P < 0.05). No wound dehiscence was observed in any patient. No subject required surgical revision or re-operation for this complication and all wound infections were successfully managed with secondary closure.
The overall mortality rate was 13.3% (6/45); five in the OC group (22.7%) and one (4.3%) in the LC group (P < 0.05). The mortality rate associated with intra-abdominal abscess was 50% (3/6), all in patients who underwent OC. The remaining 3 subjects died from myocardial infarction (1 patient in the OC group and 1 patient in the LC group) and pulmonary embolism (1 patient in the OC group).
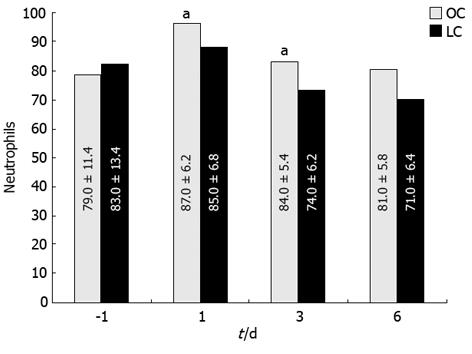
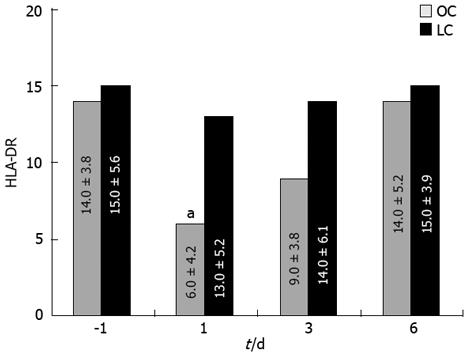
Severe leukocytosis was observed after OC (range 15.8-19.6/mL) but not after LC (range 12.1-14.4/mL), mostly due to an increment of neutrophils (Figure 2) on days 1 and 3 (P < 0.05). Values returned to the normal range within 3-4 d after LC and 5-7 d after OC. Other WBC types showed no significant variation. There were no differences between the two groups of patients before and after operation in relation to lymphocyte populations. A statistically significant change in HLA-DR expression was recorded post-operatively at day 1 as a reduction of this antigen expressed on the monocyte surface in patients from the OC group; no changes were noted in LC patients (Figure 3; P < 0.05). In this case HLA-DR expression returned to normal levels within 7 d after surgery.
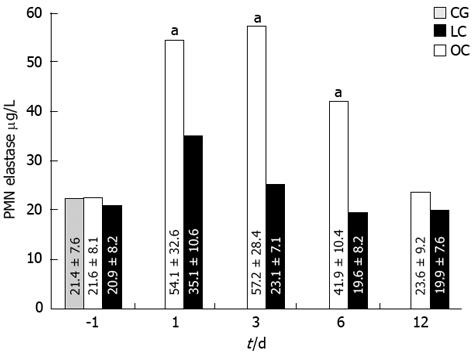
A statistically significant change in plasma elastase concentration was recorded post-operatively at days 1, 3, and 6: the increase of plasma elastase in the OC group patients was higher than in the LC group patients (Figure 4; P < 0.05). In the OC group, plasma elastase concentration returned to normal values within 10 d after operation.
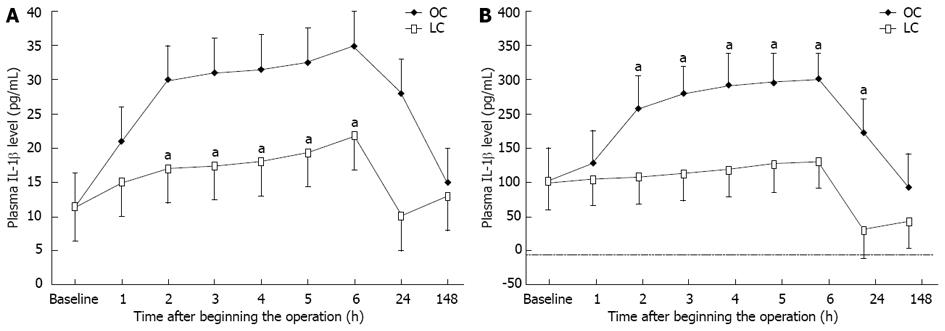
Before the operation, the serum levels of neither IL-1 nor IL-6 were significantly different between the two groups. Figure 5 shows the chronological change in the serum level of IL-1β and IL-6β after surgery. In the OC group, the serum levels of IL-1β and IL-6β began to increase consistently 1 h from the beginning of the operation, reaching a peak at the sixth hour (approximately 4 h after surgery) and, thereafter, declining to pre-operative levels by 7 d. On the other hand, in LC group patients, the increase in the serum levels of IL-1β and IL-6β was delayed and the peak values were significantly lower than those in the OC group. The differences between baseline values and post-operative levels of IL-1β and IL-6β values were significant in both groups (using the Friedman test, P < 0.05). In addition , the Mann-Whitney U test indicated significant differences between values for the groups at 2, 3, 4, 5, 6 and 24 h after the operation (Figure 5; P < 0.05).
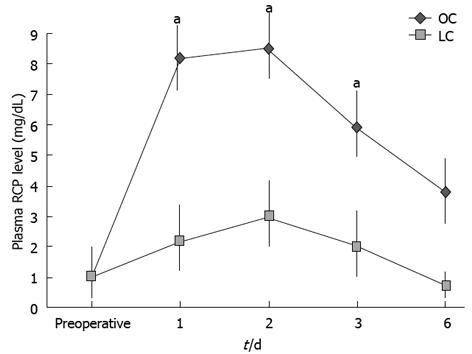
Moreover, the mean values of the serum CRP on post-operative days (1 and 3) were lower in the LC group than in the OC group. The differences between baseline values and postoperative values for CRP were significant in both groups (using the Friedman test, P < 0.05). Besides, significance was obtained by comparing values between the groups at days 1, 2 and 3 after surgery, using the Mann-Whitney U test (Figure 6; P < 0.05). In this case CRP concentration returned to normal values within 7 d after operation.
The number of blood cultures positive for organisms was higher in the OC group than in the LC group (Table 3; P < 0.05). There was no difference in bacteraemia between the groups one week after intervention.
| Time intervention | Opencholecystectomy(n = 22) | Laparoscopic cholecystectomy(n = 20) | P value |
| Within 60 min before | 10 (45.4) | 8 (40) | NS |
| Within 60 min after1 | 10 (45.4) | 4 (20) | 0.001 |
| Within 7 d after | 1 (4.5) | 0 | NS |
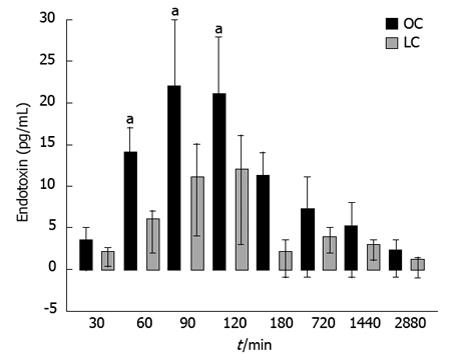
One hour after surgery, microbiological analysis of blood cultures detected 7 different bacterial species after laparotomy and 4 species after laparoscopy. Aerobic bacteria were not found in the LC group. The systemic endotoxin concentration significantly increased during the course of surgery but returned to near baseline by day 2 (Figure 7). Systemic concentrations of endotoxin were higher in the OC group at all intra-operative sampling times, but reached significance only when the gallbladder was removed (OC group = 36.81 ± 6.4 pg/mL vs LC group = 16.74 ± 4.1 pg/mL, P < 0.05) (Figure 7).
Laparoscopic surgery is increasingly used treatment of for intra-abdominal disease complicated by inflammatory processes and peritonitis. In literature there are no studies on stress response after LC or OC for acute calculous cholecystitis complicated by biliary peritonitis. In a recent review[3] it was demonstrated that laparoscopy is superior to conventional open appendectomy in terms of post-operative complications and recovery. Furthermore, laparoscopic management of perforated peptic ulcers has been reported to be simple and followed by a short recovery time, albeit with some limitations[2,14].
Experimental studies on rats showed that the inflammatory response in peritonitis models was significantly higher in the OC group than in the LC group[7,15]. On the other hand, other studies failed to find relevant differences[4,5]. Finally, other authors showed that carbon dioxide pneumoperitoneum has a protective effect against bacterial peritonitis induced in rats[16].
Studying subjects with peritonitis from perforated peptic ulcers, Lau et al[17] compared acute stress responses, endotoxaemia and bacteraemia between laparoscopy and open surgery. They concluded that endotoxaemia and bacteraemia are insignificant in most patients with perforated peptic ulcers. In these cases, laparoscopic patch repair does not reduce acute stress response if compared with open surgery.
Our study shows no difference in the pre-operative clinical parameters (age, ASA grade, Apache II and MPI scores, etc.) between the studied groups. Our results prove that colecystectomy in patients with acute cholecystitis complicated by peritonitis, whether performed by laparoscopic or open approach, is associated with significant stress response and with an increase in the biochemical markers measured. Nonetheless, the LC group was superior to the OC group in terms of post-operative systemic inflammation as well as intra-peritoneal abscess formation. In fact, the immunological status was better preserved and systemic inflammatory response was lower in the laparoscopic group than in the open group.
The number of positive blood cultures for organisms was significantly higher after laparotomy than in the laparoscopic group 1 h after surgery. Moreover, aerobic bacteria were not found after 1 h in the laparoscopic group. Since carbon dioxide is bacteriostatic on aerobic bacteria[16,18], this may explain why aerobic bacteria were only detected in blood cultures after laparotomy at this time.
Unlike laparoscopy, laparotomy caused a significant increase of systemic inflammation in the early post-operative course[19,20]. This difference was found in cytokine and cell-mediated immune responses not only in animal experiments but also in clinical trials[21,22].
In our study, while leukocyte counts recovered on day 2 in the laparoscopic group, they did not recover after laparotomy. Moreover, in the OC group we observed a post-operative decrease in HLA-DR of peripheral monocytes. Patients who underwent LC showed normal levels of HLA-DR expression[23]. Previous studies had demonstrated the crucial role of this antigen in assessing the activity of the immune system[24]. The HLA-DR antigen expression on monocytes plays an important role in antigen presentation to lymphocytes, particularly T-helper lymphocytes[24]. In fact, these cells require both HLA-DR and exogenic antigens on the macrophage surface to initiate proliferation. Moreover, studies have shown that HLA-DR is related to the surgical trauma and the occurrence of post-operative sepsis is strongly correlated with a minor expression of the human leukocyte antigen-DR of peripheral monocytes[22]. Given that HLA-DR expression is not significantly affected by age, sex, or race, this antigen can be considered meaningfull in the post-operative monitoring of surgical patients[24].
Neutrophil function has been examined by measuring neutrophil elastase (PMN-elastase). Neutrophil elastase is one of the major enzymes in neutrophils and is upregulated during activation[25]. During surgical procedures there is a massive release from the neutrophils of elastase [25,26], along with other proteinases. Therefore, the measurement of the elastase-α1-protease inhibitor complex might be a useful indicator of the degree of surgical trauma[26,27]. Varga et al[28] noted an elevation of PMN-elastase on the first postoperative day in both groups (OC and LC). However, in the laparoscopic patients it considerably decreased on the post-operative day 3 while in the OC group it remained high. The same discrepancy was present between the two groups on the 5th day. In the OC group a post-operative increase of plasma elastase concentration has been observed. Patients who underwent LC showed normal activity of leukocyte elastase. Therefore, it is conceivable that they maintain an adequate immune response even during the early post-operative phase, when the risk of infection is higher.
In relation to other serologic parameters (B and T lymphocytes, lymphocyte subpopulations), we observed no significant differences in pre- and postoperative values between the two groups of patients or between patients within each group.
IL-1, IL-6 and CRP showed significantly higher values in the OC group one hour after intervention. It may be that an abdominal incision causes a greater tissue trauma, leading to an increase of inflammatory cytokines and enhancing post-operative systemic inflammation[29,30]. In patients undergoing colectomy, systemic inflammation was significantly higher after an open approach than after a laparoscopic procedure, which is due to less trauma in the LC group[31-34]. Endotoxin is a potent stimulator of the release of cytokines such as IL-6 and tumor necrosis factor (TNF)[33,35,36]. These inflammatory mediators play an important role in the pathogenesis of systemic inflammatory response syndrome and multiple organ dysfunction syndrome[37]. In our study, a systemic concentration of endotoxin was higher in the open group, supporting the clinical findings. Intra-peritoneal abscess formation was detected in 5 patients after laparotomy and was significantly higher in this group than in the laparoscopic group. Therefore, it is conceivable that the patients who undergo LC maintain an adequate immune response even during the early post-operative phase, when the risk of infection is higher.
In conclusion, OC after biliary peritonitis increases the incidence of bacteraemia, endotoxaemia and systemic inflammation, compared with LC. Early enhanced post-operative systemic inflammation may cause lower transient immunological defense after laparotomy (decrease of HLA-DR), leading to enhanced sepsis in these patients.
Laparoscopic techniques are being increasingly used in diffuse or localised peritonitis. However, a possible concern is that increased intra-abdominal pressure may promote bacteraemia and the systemic inflammatory response during laparoscopic surgery. Data regarding the effects of pneumoperitoneum on physiologic changes and systemic inflammation during sepsis are controversial. Furthermore, only early effects of pneumoperitoneum during peritonitis have been evaluated, and the differences between conventional and laparoscopic surgery have been compared only in animal models. The influence of laparotomy and laparoscopy on bacteraemia and endotoxaemia, peripheral leukocytic subpopulations (neutrophils, total lymphocytes, lymphocyte subpopulation), human leukocyte antigen-DR (HLA-DR), neutrophil elastase, interleukin-1 (IL-1), IL-6, C-reactive protein and skin Multitest was investigated in a prospective, randomized study in patients with acute calculous cholecystitis, complicated by biliary peritonitis, undergoing a random open or laparoscopic cholecystectomy. The results showed that open cholecystectomy (OC) increased the incidence of bacteraemia, endotoxaemia and systemic inflammation compared with the laparoscopic cholecystectomy (LC). It also caused lower transient immunologic defense, leading to enhanced sepsis in these patients. Few studies in the literature show a detailed evaluation of these parameters by prospective randomized study and there are no studies on stress response after LC or OC for acute calculous cholecystitis, complicated by biliary peritonitis.
The study of inflammation biomarkers such as IL-1 and IL-6 allows us to directly quantify the response of the immune system since these interleukins are the basis of the activation of the cell-mediated immune system. The evidence of a faster normalization of the leukocyte count at post-op day 2 in the LC group compared to the OC group is a further demonstration of this. Another substantial piece of evidence, not showed in any related study in the literature, can be inferred from the study of HLA-DR in both groups, given the crucial role of this antigen in the activation of the T-helper cell-mediated response of the immune-system. In the OC group we observed a post-operative decrease in the HLA-DR of peripheral monocyte. Patients who undergone LC showed a normal level of HLA-DR expression. The study of serum concentrations of neutrophils and of the enzymes produced by them (PNM-elastase) in the two groups allowed us to optimize the study with the evaluation of the polymorphic-nucleated component of the immune system.By these means, the study has demonstrated how the immune status has been better preserved and the inflammation response has been less in the LC group than in the OC group.
The majority of studies presented in the literature are experimental studies using animal models. This study is, instead, a prospective randomized study conducted on human subjects. Experimental studies on peritonitis showed that the inflammatory response was significantly higher in the OC group than in the LC group in the animal models, suggesting that carbon dioxide pneumoperitoneum has a protective effect against bacterial peritonitis. This study, in contrast to the previous ones, is the first demonstrating that OC after biliary peritonitis increases the incidence of bacteraemia, endotoxaemia and systemic inflammation more than LC The authors also demonstrated that early enhanced post-operative systemic inflammation may cause lower transient immunological defense after laparotomy (decrease of HLA-DR), leading to enhanced sepsis in these patients.
This study lays the foundations for future applications of laparoscopy in emergency surgery. The immunologic implications of laparoscopic surgery shown in this study on acute cholecystitis, complicated by bile peritonitis indicate a new management for peritoneal sepsis. However, this study has been limited to the observance of bile peritonitis. This means that further random prospective studies on immunologic responses in other forms of peritonitis (chemical and stercoraceous) treated by laparoscopy may be useful to detect new guidelines on the laparoscopic or open approach to colic perforations, such as diverticular perforations generating diffuse peritonitis (Hincey > 2). Moreover, further studies comparing the two methods when applied to the variations of peritoneal bacterial charges during stercoraceous or chemical peritonitis may give useful indications on the surgical approach to prefer. New studies of this type may foster the replacement of explorative laparotomy with the laparoscopic approach in case of peritonitis, as it presents the undeniable advantages of lower invasiveness and shorter recovery in patients.
Bile peritonitis is an inflammatory, irritative response to the abnormal presence of bile and bacteria in the peritoneum. It may occur in up to 15% of patients with acute cholecystitis, even in the absence of gallbladder perforation. During bile peritonitis some chemical mediators of inflammation playing specific roles are studied: IL-1 is a cytokine excreted by various types of immune system cells including macrophages and monocytes in response to bacterial infections. The role of this molecule is to recruit macrophages and lymphocytes to the site of infection promoting the maturation and clonal expansion of T-helper lymphocytes and B lymphocytes. IL-6 acts as a cytokine with a pro- and anti-inflammatory effect. It is secreted by T-lymphocytes and macrophages to stimulate the immune response to specific microbial molecules. IL-6 is one of the main mediators of fever and responses in the acute phase. It can cross the hemato-cephalic barrier and start prostaglandin E2 synthesis in the hypothalamus, thus provoking the increase of body temperature. Another mechanism through which IL-6 causes the increase of body temperature is the stimulation of the catabolism of energetic substrates in muscles and adipose tissue. HLA-DR is a major histocompatibility complex class II cell surface receptor. The HLA-DR molecules are upregulated in response to signaling. During an infection, the peptide is bound into a DR molecule and presented to a few of the many great T-cell receptors found on T-helper cells. These cells then bind to antigens on the surface of B-cells, stimulating B-cell proliferation. The HLA-DR antigen expression on monocytes plays an important role in antigen presentation to lymphocytes, particularly T-helper lymphocytes. In fact, these cells require both HLA-DR and exogenous antigens on the macrophage surface to initiate proliferation. Neutrophil elastase is an enzyme of the serine protease family, released by neutrophils during the inflammatory process. It coordinates the destruction of bacteria and extraneous cells.
The authors evaluated the systemic inflammation and immune response after laparotomy vs laparoscopy in patients with bile peritonitis caused by acute cholecystitis. The study design is unclear making interpretation of the data difficult.
P- Reviewer Linke GR S- Editor Song XX L- Editor A E- Editor Lu YJ
| 1. | Karamanakos SN, Sdralis E, Panagiotopoulos S, Kehagias I. Laparoscopy in the emergency setting: a retrospective review of 540 patients with acute abdominal pain. Surg Laparosc Endosc Percutan Tech. 2010;20:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Sneider EB, Cahan MA, Litwin DE. Laparoscopic repair of acute surgical diseases in the 21st century. Minerva Chir. 2010;65:275-296. [PubMed] [Cited in This Article: ] |
| 3. | Sauerland S, Jaschinski T, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2010;CD001546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Chatzimavroudis G, Pavlidis TE, Koutelidakis I, Giamarrelos-Bourboulis EJ, Atmatzidis S, Kontopoulou K, Marakis G, Atmatzidis K. CO(2) pneumoperitoneum prolongs survival in an animal model of peritonitis compared to laparotomy. J Surg Res. 2009;152:69-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Gurtner GC, Robertson CS, Chung SC, Ling TK, Ip SM, Li AK. Effect of carbon dioxide pneumoperitoneum on bacteraemia and endotoxaemia in an animal model of peritonitis. Br J Surg. 1995;82:844-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Clary EM, Bruch SM, Lau CL, Ali A, Chekan EG, Garcia-Oria MJ, Eubanks S. Effects of pneumoperitoneum on hemodynamic and systemic immunologic responses to peritonitis in pigs. J Surg Res. 2002;108:32-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Jacobi CA, Ordemann J, Zieren HU, Volk HD, Bauhofer A, Halle E, Müller JM. Increased systemic inflammation after laparotomy vs laparoscopy in an animal model of peritonitis. Arch Surg. 1998;133:258-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Cohn Ijr. Bile peritonitis in: Bockus HL, Berk JE, Haubrich WS, Kalser MH, JLA Roth, Vilardel F. Gastro-enterology. Philadelphia, London, Toronto: W.B. Saunders Company 1976; 894-899. [Cited in This Article: ] |
| 9. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 197] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Billing A, Fröhlich D, Schildberg FW. Prediction of outcome using the Mannheim peritonitis index in 2003 patients. Peritonitis Study Group. Br J Surg. 1994;81:209-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 129] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Wolters U, Wolf T, Stützer H, Schröder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77:217-222. [PubMed] [Cited in This Article: ] |
| 12. | Franchi M, Ghezzi F, Zanaboni F, Scarabelli C, Beretta P, Donadello N. Nonclosure of peritoneum at radical abdominal hysterectomy and pelvic node dissection: a randomized study. Obstet Gynecol. 1997;90:622-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Hafner G, Dreher M, Lütgehaus M, Ehrenthal W, Heubner A, Swars H, Prellwitz W. Determination of human granulocyte elastase by the immunoactivation method on the Hitachi 717 automated analyser. Eur J Clin Chem Clin Biochem. 1991;29:179-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Bertleff MJ, Lange JF. Laparoscopic correction of perforated peptic ulcer: first choice? A review of literature. Surg Endosc. 2010;24:1231-1239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Bloechle C, Emmermann A, Treu H, Achilles E, Mack D, Zornig C, Broelsch CE. Effect of a pneumoperitoneum on the extent and severity of peritonitis induced by gastric ulcer perforation in the rat. Surg Endosc. 1995;9:898-901. [PubMed] [Cited in This Article: ] |
| 16. | Sorbello AA, Azevedo JL, Osaka JT, Damy S, França LM, Tolosa EC. Protective effect of carbon dioxide against bacterial peritonitis induced in rats. Surg Endosc. 2010;24:1849-1853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Lau JY, Lo SY, Ng EK, Lee DW, Lam YH, Chung SC. A randomized comparison of acute phase response and endotoxemia in patients with perforated peptic ulcers receiving laparoscopic or open patch repair. Am J Surg. 1998;175:325-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Gill CO, DeLacy KM. Growth of Escherichia coli and Salmonella typhimurium on high-pH beef packed under vacuum or carbon dioxide. Int J Food Microbiol. 1991;13:21-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Schietroma M, Carlei F, Franchi L, Mazzotta C, Sozio A, Lygidakis NJ, Amicucci G. A comparison of serum interleukin-6 concentrations in patients treated by cholecystectomy via laparotomy or laparoscopy. Hepatogastroenterology. 2004;51:1595-1599. [PubMed] [Cited in This Article: ] |
| 20. | Schietroma M, Carlei F, Lezoche E, Agnifili A, Enang GN, Mattucci S, Minervini S, Lygidakis NJ. Evaluation of immune response in patients after open or laparoscopic cholecystectomy. Hepatogastroenterology. 2001;48:642-646. [PubMed] [Cited in This Article: ] |
| 21. | Buunen M, Gholghesaei M, Veldkamp R, Meijer DW, Bonjer HJ, Bouvy ND. Stress response to laparoscopic surgery: a review. Surg Endosc. 2004;18:1022-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Veenhof AA, Sietses C, von Blomberg BM, van Hoogstraten IM, vd Pas MH, Meijerink WJ, vd Peet DL, vd Tol MP, Bonjer HJ, Cuesta MA. The surgical stress response and postoperative immune function after laparoscopic or conventional total mesorectal excision in rectal cancer: a randomized trial. Int J Colorectal Dis. 2011;26:53-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Carlei F, Schietroma M, Cianca G, Risetti A, Mattucci S, Ngome Enang G, Simi M. Effects of laparoscopic and conventional (open) cholecystectomy on human leukocyte antigen-DR expression in peripheral blood monocytes: correlations with immunologic status. World J Surg. 1999;23:18-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Neefjes JJ, Ploegh HL. Intracellular transport of MHC class II molecules. Immunol Today. 1992;13:179-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 171] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Borregaard N. The human neutrophil. Function and dysfunction. Eur J Haematol. 1988;41:401-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 95] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Schietroma M, Carlei F, Rossi M, Mattucci S, Gullà N, Lezoche E. Neutrophil-elastase in patients undergoing open versus laparoscopic cholecystectomy. Surgery. 2001;130:898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Schietroma M, Carlei F, Cappelli S, Pescosolido A, Lygidakis NJ, Amicucci G. Effects of cholecystectomy (laparoscopic versus open) on PMN-elastase. Hepatogastroenterology. 2007;54:342-345. [PubMed] [Cited in This Article: ] |
| 28. | Varga G, Gál I, Róth E, Lantos J, Jaberansari MT. Inflammatory mediators and surgical trauma regarding laparoscopic access: neutrophil function. Acta Chir Hung. 1997;36:368-369. [PubMed] [Cited in This Article: ] |
| 29. | Schietroma M, Carlei F, Mownah A, Franchi L, Mazzotta C, Sozio A, Amicucci G. Changes in the blood coagulation, fibrinolysis, and cytokine profile during laparoscopic and open cholecystectomy. Surg Endosc. 2004;18:1090-1096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Li J, Wang S, Xu J, Dai Q, Xu S, Sun H, Peng L. [Regulation trend of resveratrol on TNFα-,IL-1β, IL-6 expressions in bronchoalveolar lavage fluid of RSV-infected BALB/c mice]. Zhongguo Zhongyao Zazhi. 2012;37:1451-1454. [PubMed] [Cited in This Article: ] |
| 31. | Huang C, Huang R, Jiang T, Huang K, Cao J, Qiu Z. Laparoscopic and open resection for colorectal cancer: an evaluation of cellular immunity. BMC Gastroenterol. 2010;10:127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Schietroma M, Piccione F, Carlei F, Clementi M, Bianchi Z, de Vita F, Amicucci G. Peritonitis from perforated appendicitis: stress response after laparoscopic or open treatment. Am Surg. 2012;78:582-590. [PubMed] [Cited in This Article: ] |
| 33. | Ypsilantis P, Didilis V, Tsigalou C, Pitiakoudis M, Karakatsanis A, Margioulas A, Simopoulos C. Systemic inflammatory response after single-incision laparoscopic surgery versus standard laparoscopic approach. Surg Laparosc Endosc Percutan Tech. 2012;22:21-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Tsamis D, Theodoropoulos G, Stamopoulos P, Siakavellas S, Delistathi T, Michalopoulos NV, Zografos GC. Systemic inflammatory response after laparoscopic and conventional colectomy for cancer: a matched case-control study. Surg Endosc. 2012;26:1436-1443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Schietroma M, Carlei F, Cappelli S, Amicucci G. Intestinal permeability and systemic endotoxemia after laparotomic or laparoscopic cholecystectomy. Ann Surg. 2006;243:359-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Welc SS, Clanton TL. The regulation of interleukin-6 implicates skeletal muscle as an integrative stress sensor and endocrine organ. Exp Physiol. 2013;98:359-371. [PubMed] [Cited in This Article: ] |
| 37. | Emura I, Usuda H. Histopathological and cytological examination of autopsy cases with multiple organ dysfunction syndromes. Pathol Int. 2010;60:443-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |