Published online Nov 27, 2011. doi: 10.4240/wjgs.v3.i11.159
Revised: November 3, 2011
Accepted: November 10, 2011
Published online: November 27, 2011
CDX2 is a nuclear homeobox transcription factor that belongs to the caudal-related family of CDX homeobox genes. The gene encoding CDX2 is a nonclustered hexapeptide located on chromosome 13q12-13. Homeobox genes play an essential role in the control of normal embryonic development. CDX2 is crucial for axial patterning of the alimentary tract during embryonic development and is involved in the processes of intestinal cell proliferation, differentiation, adhesion, and apoptosis. It is considered specific for enterocytes and has been used for the diagnosis of primary and metastatic colorectal adenocarcinoma. CDX2 expression has been reported to be organ specific and is normally expressed throughout embryonic and postnatal life within the nuclei of epithelial cells of the alimentary tract from the proximal duodenum to the distal rectum. In this review, the authors elaborate on the diagnostic utility of CDX2 in gastrointestinal tumors and other neoplasms with intestinal differentiation. Limitations with its use as the sole predictor of a gastrointestinal origin of metastatic carcinomas are also discussed.
- Citation: Saad RS, Ghorab Z, Khalifa MA, Xu M. CDX2 as a marker for intestinal differentiation: Its utility and limitations. World J Gastrointest Surg 2011; 3(11): 159-166
- URL: https://www.wjgnet.com/1948-9366/full/v3/i11/159.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v3.i11.159
CDX2 is a nuclear homeobox transcription factor that belongs to the caudal-related family of CDX homeobox genes[1-3]. The gene encoding CDX2 is a nonclustered hexapeptide located on chromosome 13q12-13[1,2]. Homeobox genes play an essential role in the control of normal embryonic development[1,2]. CDX2 is crucial for axial patterning of the alimentary tract during embryonic development[4,5] and is involved in the processes of intestinal cell proliferation, differentiation, adhesion, and apoptosis[4-6]. CDX2 functions within the cell to induce differentiation and inhibit proliferation at the level of gene transcription[4]. It stimulates intestinal epithelium differentiation through activating the transcription of proteins specific to the intestine, such as MUC2, sucrase, isomaltase, and carbonic anhydrase I[4,5]. CDX2 inhibits epithelial proliferation through upregulating WAF1/p21, a cdk inhibitor that arrests the cell cycle upon DNA damage[6]. CDX2 expression has been reported to be organ specific and is normally expressed throughout embryonic and postnatal life within the nuclei of epithelial cells of the alimentary tract from the proximal duodenum to the distal rectum[4-7].
The majority of homeobox genes are considered as proto-oncogenes, with few exceptions[8]. Expression of CDX2 decreases in human colorectal cancers in proportion to the tumor grade and it is lost in minimally differentiated colon carcinomas[9]. In addition, CDX2 is downregulated by oncogenic pathways in colon cancer cells. These observations have suggested that CDX2 has a tumor suppressor function. In addition, Bonhomme et al[8] have provided experimental evidence that CDX2 is a colon tumor suppressor gene. Unlike other colon tumor suppressor genes such as APC and p53[10], which act also outside the gut, CDX2 is the first intestine-specific tumor suppressor[8].
Since CDX2 is a transcription factor, it shows a nuclear immunostaining pattern. In practice, nuclear expression of transcription factors has several distinct advantages over cytoplasmic “differentiation” markers. Firstly, transcription factors generally yield an “all or none” signal, with the vast majority of positive cases containing positive signal in > 90% of the target cell population. Secondly, the nuclear localization of the signal is much less likely to be confused with biotin or other sources of false-positive cytoplasmic signals. Third, there is no association between the levels of expression of nuclear transcription factors and the state of differentiation of the tumor.
CDX2 is expressed in normal small and large intestinal epithelial cells, including absorptive, endocrine and Paneth cells[4]. Recent immunohistochemical studies have reported that CDX2 is a specific and sensitive marker for adenocarcinoma of the gastrointestinal tract, particularly colorectal adenocarcinoma[7,11-14]. Moskaluk et al[12], examined CDX2 expression in tissue microarrays containing 745 cancers from many anatomic sites and observed strong positive staining in 90% of colonic adenocarcinomas, 20%-30% of carcinomas of the stomach, esophagus and ovary (limited to endometrioid and mucinous types) and in less than 1% of all other carcinoma types. Another study conducted by De Lott et al[13], investigated CDX2 expression in tissue microarrays from 71 colorectal adenocarcinomas, 47 lung adenocarcinomas, 31 hepatocellular carcinomas, 55 squamous cell carcinomas of the lung, 69 neuroendocrine carcinomas of the lung, 43 neuroendocrine carcinomas of the pancreas, 57 pancreatic adenocarcinomas, and 256 endometrial adenocarcinomas. Positive results were found in about 72% of colorectal cancers and in only 6% of endometrial carcinomas[13]. Tumors from other sites were either negative or rarely positive. Similarly, Werling et al[14] found CDX2 expression in the majority of colorectal carcinomas, with only few exceptions. A heterogeneous focal staining pattern was found in pancreatic, gastric and gastroesophageal adenocarcinomas and cholangiocarcinomas. CDX2 was rarely expressed in carcinomas of the breast, genitourinary tracts, gynecologic tracts, lung, head and neck[14]. Bakaris et al[15] observed that CDX2 expression was seen in all cases of colonic adenoma, and the majority of colorectal adenocarcinomas. These previous studies have illustrated the value of CDX2 expression in determining tumor origin in the diagnostic settings[12].
Previous studies have reported a wide variation in the proportion of colorectal adenocarcinomas that express CDX2. Some studies have reported its expression in 98% to 100% of cases, while others have observed loss of CDX2 expression in 14% to 37% of cases[7,12,16]. Loss of CDX2 expression in colorectal cancer has been found to correlate with high tumor grade, microsatellite instability or advanced tumor stage[7,15,17]. Considering the role of CDX2 in promoting cellular differentiation and inhibiting proliferation[2,3], loss of CDX2 expression could conceivably contribute to aggressive tumor behavior and increase the likelihood of metastatic disease[17]. Choi et al[18] analyzed the expression of CDX2 in 123 cases of sporadic colorectal cancers and found loss of its expression in 29/123 (23.6%) specimens. Again, this loss of expression was found to correlate with higher tumor stage and positive lymph node metastasis[18]. Loss of CDX2 is also more frequently encountered in mismatch repair-deficient colorectal cancer[9]. Utilizing a database of 621 colorectal cancers, Baba et al[9] found that CDX2 loss was correleted directly with female gender, high tumor grade, stage IV disease, and inversely with LINE-1 hypomethylation, p53 expression, and β-catenin activation. CDX2 loss was associated with high overall mortality among patients with a family history of colorectal cancer[9]. This implies the importance of CDX2 in the suppression of tumorgenesis in a subset of colorectal cancers and its potential for use as a prognostic marker in identifying high risk patients.
Rectal adenocarcinomas are commonly lumped together with colonic tumors, making their proper immunoprofiling difficult[19]. We recently investigated the expression of CDX2, along with CK7 and CK20, in rectal adenocarcinoma. In our experience, CDX2 was expressed in the majority of cases of rectal adenocarcinoma, and staining was predominantly nuclear with occasional faint cytoplasmic staining[19]. In our study, CDX2 expression did not appear to correlate with tumor grade (tumor differentiation).
CDX2 is not expressed in normal esophageal and gastric epithelial cells but is expressed in intestinal metaplasia of the esophagus[20-22]. In some patients, Barrett’s esophagus is complicated by the development of esophageal adenocarcinoma[20,21]. Lord et al[22] investigated the expression of CDX2 and PITX1 in Barrett’s esophagus and associated adenocarcinoma. Negative CDX2 staining was observed in normal squamous esophageal lining, while strong (3+) nuclear staining was seen in all cases of Barrett’s intestinal metaplasia, dysplasia, and associated adenocarcinoma[22]. The level of CDX2 mRNA expression was found to coincide with immunohistochemical CDX2 expression as both were upregulated in Barrett’s intestinal metaplasia tissues and remained elevated in dysplastic and adenocarcinoma cells[22]. In contrast, a recent study has reported CDX2 expression which was significantly weaker or absent in esophageal dysplasia and adenocarcinoma in comparison to metaplastic cells[23].
Gastric carcinoma is frequently found in association with intestinal metaplasia[24]. Studies have reported CDX2 expression in both intestinal metaplasia of the stomach and intestinal-type gastric carcinoma[25-31]. Furthermore, incomplete intestinal metaplasia, which expresses both gastric and intestinal mucins, shows lower CDX2 expression compared with complete intestinal metaplasia[32]. Although incomplete intestinal metaplasia morphologically resembles colon, its CDX2 expression was apparently lower than that seen in the normal colon. Similar to esophageal dysplasia, previous studies showed decreasing CDX2 expression from metaplasia to dysplasia to adenocarcinoma[32]. Intestinal metaplasia or dysplasia with low expression of CDX2 may potentially serve as predictive markers for gastric cancer[32].
Song et al[33] reported a significantly better outcome for CDX2-positive gastric tumors over CDX2-negative tumors. Other studies have similarly demonstrated that positive CDX2 expression in gastric cancer significantly correlated with better differentiation and prognosis[34,35]. CDX2 expression has been evaluated in 69 cases of gastric epithelial dysplasia, 88 early gastric cancers and 56 advanced gastric cancers. Increased CDX2 expression was more frequently associated with adenomatous-type gastric epithelial dysplasia (87%), compared with the foveolar (47%) or hybrid (44%) types. CDX2 expression levels also gradually decreased from gastric dysplasia, to early and advanced gastric cancers. Moreover, a negative correlation was observed between CDX2 expression and the depth of tumor invasion[26]. A recent study showed that absence of nuclear CDX2 expression may serve as a powerful predictor of lymph node metastasis in gastric cancer[36]. Overexpression of CDX2 has recently been shown to inhibit cell growth and proliferation in vitro and can effectively inhibit gastric cancer progression, making this a potential therapeutic target[37].
Despite the large surface area, malignancies of the small intestine are quite rare and account for only 2% of primary gastrointestinal tumors[38]. Small intestinal adenocarcinoma shows similarities in morphology and risk factors with its colorectal counterpart[38]. However, it has been found to be immunophenotypically distinct from colorectal adenocarcinoma. Zhang et al[38] examined the expression of CDX2 in small intestinal adenocarcinoma and found that CDX2 was expressed in 60% of cases of small intestinal adenocarcinoma in comparison to 98% of colorectal adenocarcinoma.
Gallbladder adenocarcinoma is a highly malignant neoplasm with variable incidence depending on gender and geographic distribution[39]. Sakamoto et al[40] investigated the expression of CDX2 in human gallbladders with cholelithiasis and reported CDX2 expression in 92% of gallbladder intestinal metaplasias. CDX2 expression has been found in dysplasia, carcinoma and intestinal metaplasia of the gallbladder and carcinogenesis may proceed through intestinal metaplasia as seen in esophageal metaplasia[39,40].
Wu et al[39] examined the expression of CDX2 in 68 primary gallbladder carcinomas and compared its expression with various clinicopathologic factors. Positive staining was observed in 25/68 (36.8%) cases with no significant correlation with clinicopathologic prognostic parameters. Well-differentiated carcinomas had high CDX2 expression (54.8%) compared to moderately differentiated (7.1%) and poorly differentiated carcinomas (0%)[39]. In contrast, Chang et al[41] reported CDX2 positivity in 29% of their cases and that expression was an independent prognostic factor in patients with biliary tract carcinoma.
Hong et al[42] found CDX2 expression in 37% of their extrahepatic bile duct carcinoma cases. They observed more frequent CDX2 expression in tumors with papillary growth (60%) than in those with a nodular (25%) or infiltrative (34.9%) pattern. CDX2 expression was also more frequent in cases without vascular invasion (41.3%) than in those with vascular invasion (23%). In univariant analysis, CDX2/MUC2 positive patients had a significantly higher survival rate than negative patients[42].
CDX2 expression is focal and patchy in normal pancreatic epithelium[20] and CDX2 is infrequently expressed in pancreatic adenocarcinoma. In our experience, CDX2 is focally expressed in less than 10% of pancreatic duct adenocarcinomas[19]. Another report found CDX2 expression in only 3 of the 57 (5%) pancreatic adenocarcinoma cases studied[13]. In general, the staining pattern is usually focal and less intense than that found in colorectal adenocarcinoma.
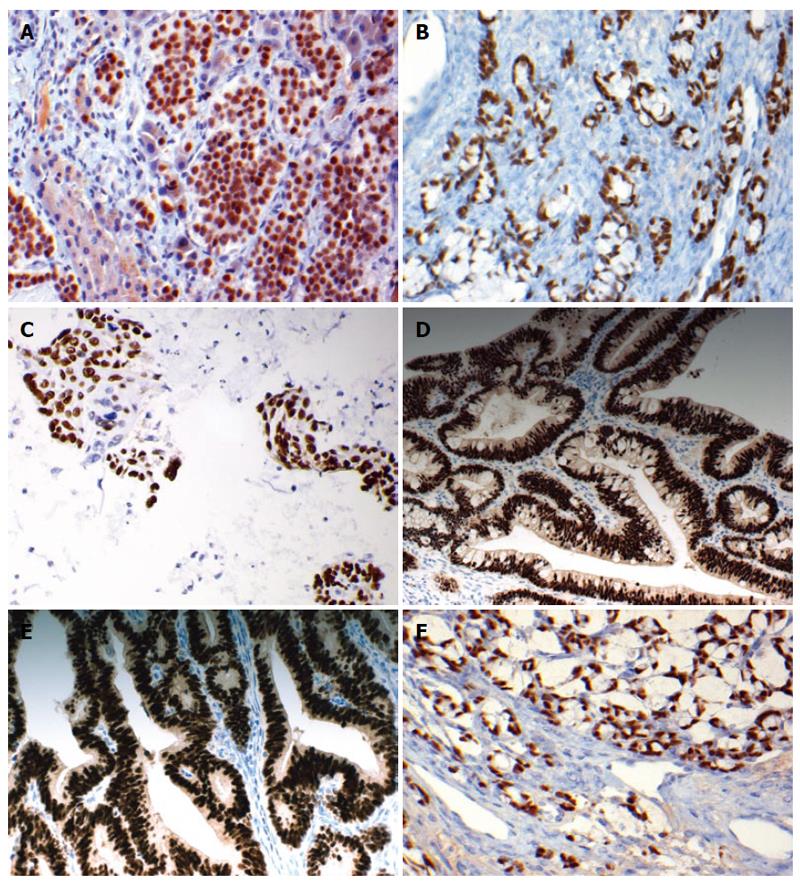
We have also examined the use of CDX2 and TTF1 in differentiating metastatic neuroendocrine neoplasms of unknown origin[43]. Expression of CDX2 was found in 28/60 (47%) gastrointestinal neuroendocrine tumors with high prevalence in ileal, appendiceal and colonic origin[43] (Figure 1). Similarly, previous studies documented exclusive positive staining for CDX2 in ileal and appendiceal neuroendocrine tumors, while all rectal, gastric and duodenal neuroendocrine tumors were negative[44,45]. No CDX2 expression was observed in neuroendocrine tumors of other origins, including skin, ovary or thymus[43]. Pancreatic endocrine tumors show focal and heterogeneous staining for CDX2[43].
Rabban et al[46] have evaluated CDX2 expression in metastatic and primary ovarian carcinoids. They reported diffuse nuclear CDX-2 expression in majority of primary ovarian and metastatic intestinal carcinoids involving the ovary, particularly insular and mucinous types. They concluded that CDX2 is not specific and cannot be used to determine the site of ovarian carcinoids. All primary ovarian carcinoids were negative for TTF-1, CK7 and CK20[46].
CDX2 immunohistochemical staining for diagnosis of metastatic adenocarcinoma has recently come into practice[47-49]. Due to its limited expression in the spectrum of human tissues and neoplasia, CDX2 has been investigated for its usefulness in diagnosing a metastatic adenocarcinoma as being of a gastrointestinal origin[47-49].
The diagnostic utility of CDX2 as a marker to identify the gastrointestinal origin of a metastatic tumor was addressed a study where we used CDX2 to distinguish bronchioloalveolar adenocarcinoma of the lung from metastatic mucinous colorectal adenocarcinoma[42]. Using surgical material, Saad et al[47] and Barbareschi et al[49] both found CDX2 expression in metastatic colorectal adenocarcinoma to the lung compared but absent in primary lung adenocarcinoma. Similarly, CDX2 was useful in cytology specimens as a marker of metastatic gastrointestinal adenocarcinoma when compared to other metastatic tumors (Figure 1C). CDX2 expression was found in 19/22 (86%) confirmed metastatic gastrointestinal specimens[50]. All other metastatic adenocarcinomas, from lung, breast, ovaries, pancreas, and prostate sites, were negative for CDX2[50]. Similarly, Lora and Kanitakis investigated CDX2 expression in 68 cutaneous metastatic tumors of various origin and found that CDX2 was a specific immunohistochemical marker for cutaneous metastases from intestinal and urothelial carcinomas[48].
Expression of CDX2 tumors outside the colorectum has been reported[12,14]. Tot[51] reported that the CK20+/CK7- pattern is more specific than CDX2 expression in predicting the colorectal origin of metastatic adenocarcinoma. It is usually recommended to use CDX2 as a part of immunostaining panel including CK7, CK20, mCEA and villin to prove the intestinal origin of a metastatic tumor[50].
Despite the relatively restricted CDX2 expression profile, expression of CDX2 in tumors outside the colorectum has been previously reported. Moskaluk et al[12] and Werling et al[14] reported that a significant fraction of ovarian mucinous carcinomas and primary bladder adenocarcinomas were CDX2-positive and there have been other studies reporting CDX2 expression in adenocarcinomas of various anatomic sites.
Intestinal differentiation of cervical adenocarcinoma, in the form of goblet cells and/or Paneth cells, is uncommon but may generate diagnostic dilemmas. Invasive cervical adenocarcinomas with intestinal differentiation could mimic the histology of colorectal adenocarcinoma, raising the possibility of metastasis or direct spread. Also, a distant metastasis from an intestinal type cervical adenocarcinoma could be easily mistaken for a metastatic adenocarcinoma of an intestinal origin, based on morphology alone.
CDX2 immunostaining has been studied in a few large series of cervical adenocarcinomas of various histologic subtypes. McCluggage et al[52] have recently reported CDX2 positivity in the majority of intestinal-type endocervical adenocarcinomas in situ (20/21 cases) and in all the three invasive intestinal-type adenocarcinomas (ITAC) studied. We compared the expression of CDX2 in 119 cases of different types of cervical adenocarcinoma with that in rectal adenocarcinoma[53]. Our study is the largest reported to date and confirms that the majority of invasive and in situ endocervical adenocarcinomas of intestinal-type show CDX2 immunoreactivity[53], in agreement with the results of McCluggage et al[52] (Figure 1D).
Of all ovarian epithelial tumors, mucinous tumors pose the greatest difficulty with regard to differentiation between primary and metastatic tumors. Previous studies have demonstrated conflicting results regarding the value of CDX2 in distinguishing primary tumors from metastatic carcinomas of the ovary with mucinous morphology. CDX2 expression has been reported in from 0 to 100% of ovarian mucinous tumors and in 0% to 30% of ovarian endometrioid carcinomas[54-64]. In contrast, a recent study showed that almost all primary ovarian carcinomas lacked immunoreactivity for CDX2, while the majority of metastatic colorectal carcinomas of the ovary were CDX2-positive[57]. In an attempt to reconcile the wide gap in data from different studies, the authors claimed that previous studies may have misclassified ovarian metastases as primary tumors. Taken together, the results available to date suggest that the differential diagnosis of primary and metastatic mucinous carcinoma still poses a great problem because these tumors can share their immunophenotype, gross and microscopic features.
Wani et al[65] investigated CDX2 expression in 225 cases of endometrial biopsies including 101 endometrioid carcinomas. Normal and non-proliferative endometrium showed negative CDX2 staining. Endometrioid carcinoma with squamous differentiation was positive for CDX2 in 73% of cases, whereas only 14% of tumors without squamous differentiation were positive (Figure 1E). In addition, the authors found that the larger the number of squamous foci the greater the number of CDX2 positive cells which correlated strongly with nuclear β-catenin expression. This may suggest an important role of CDX2 in squamous morular formation[65].
Herawi et al[66] have investigated CDX2 expression in prostatic adenocarcinoma, including 708 tissue microarrays containing either benign or malignant prostate tissue as well normal tissues from various anatomic sites. Out of 185 prostatic adenocarcinomas, only four cases (6%) showed focal positive staining while benign prostatic tissue was positive in 12% of cases. No cases of metastatic prostatic carcinoma expressed CDX2[66]. Another study found CDX2 expression in 31% of prostatic adenocarcinoma with mucinous or signet cell differentiation[67]. However, in routine pathology practice, positive PSA immunostaining and clinical findings should prove more helpful when a prostatic origin is suspected for a metastatic adenocarcinoma[66,67].
The majority of urachal epithelial neoplasms are adenocarcinomas with enteric or nonenteric histologies. Urachal adenocarcinoma may mimic metastatic adenocarcinoma of different origins. Paner et al[68] studied CDX2 expression in 32 urachal adenocarcinomas and reported CDX2 expression in 85% of their cases. CDX2 expression can be diffuse in urachal adenocarcinomas, even without the classic enteric morphology. In urachal adenocarcinoma subtypes, CDX2 expression was see in 8/8 (100%) of mucinous, 10/11 (91%) of enteric type, 5/7 (71%) of not otherwise specified, and in 4/6 (67%) of signet ring cell type. In addition, CDX2 was expressed by urachal remnants of glandular type, and noninvasive urachal mucinous cystic tumors[68].
ITAC of the nasal cavity and paranasal sinuses are uncommon[69]. They are clinically aggressive and generally present at an advanced stage. Franchi et al[69] demonstrated nuclear expression of CDX2 in all their cases of ITAC, with strong nuclear staining identified in the majority. CDX2 staining was not present in normal respiratory mucosa or seromucous glands. A similar study detected strong and diffuse nuclear expression of CDX2 in all cases of ITAC[70] (Figure 1F). Choi et al[71] suggested that the development of ITAC is preceded by intestinal metaplasia, with conversion from a normal CK7+/CK20-/CDX2-/villin- phenotype to an abnormal CK7-/CK20+/CDX2+/villin- intestinal phenotype.
CDX2 is expressed in 90% of acute myeloid leukemia (AML) but not in hematopoietic stem and progenitor cells derived from normal individuals[72,73]. Frequent expression of CDX2 in the adult hematopoietic compartment suggests a role for CDX2 as part of a common effector pathway that promotes the proliferative capacity and self-renewal potential of myeloid progenitor cells[73].
CDX2 is a useful immunohistochemical marker for a colorectal origin of metastatic carcinoma. However, CDX2 can be expressed in other neoplasms, especially those with intestinal differentiation, irrespective of their origin. Therefore, CDX2 should not be used as the sole basis for the conclusion that the gastrointestinal tract is the primary origin of metastatic carcinomas. We recommend that CDX2 should always be used as a part of a broader immunohistochemical panel.
We thank Pauline Henry, MD for her critical review of this article.
Peer reviewer: Yong-Song Guan, MD, PhD, Professor, Department of Oncology and Radiology, State Key Laboratory of Biotherapy, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
| 1. | Beck F. The role of Cdx genes in the mammalian gut. Gut. 2004;53:1394-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Chawengsaksophak K, James R, Hammond VE, Köntgen F, Beck F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature. 1997;386:84-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 517] [Cited by in F6Publishing: 491] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 3. | Eda A, Osawa H, Yanaka I, Satoh K, Mutoh H, Kihira K, Sugano K. Expression of homeobox gene CDX2 precedes that of CDX1 during the progression of intestinal metaplasia. J Gastroenterol. 2002;37:94-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Mizoshita T, Inada K, Tsukamoto T, Kodera Y, Yamamura Y, Hirai T, Kato T, Joh T, Itoh M, Tatematsu M. Expression of Cdx1 and Cdx2 mRNAs and relevance of this expression to differentiation in human gastrointestinal mucosa--with special emphasis on participation in intestinal metaplasia of the human stomach. Gastric Cancer. 2001;4:185-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Suh E, Chen L, Taylor J, Traber PG. A homeodomain protein related to caudal regulates intestine-specific gene transcription. Mol Cell Biol. 1994;14:7340-7351. [PubMed] [Cited in This Article: ] |
| 6. | Bai YQ, Miyake S, Iwai T, Yuasa Y. CDX2, a homeobox transcription factor, upregulates transcription of the p21/WAF1/CIP1 gene. Oncogene. 2003;22:7942-7949. [PubMed] [Cited in This Article: ] |
| 7. | Kaimaktchiev V, Terracciano L, Tornillo L, Spichtin H, Stoios D, Bundi M, Korcheva V, Mirlacher M, Loda M, Sauter G. The homeobox intestinal differentiation factor CDX2 is selectively expressed in gastrointestinal adenocarcinomas. Mod Pathol. 2004;17:1392-1399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Bonhomme C, Duluc I, Martin E, Chawengsaksophak K, Chenard MP, Kedinger M, Beck F, Freund JN, Domon-Dell C. The Cdx2 homeobox gene has a tumour suppressor function in the distal colon in addition to a homeotic role during gut development. Gut. 2003;52:1465-1471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Baba Y, Nosho K, Shima K, Freed E, Irahara N, Philips J, Meyerhardt JA, Hornick JL, Shivdasani RA, Fuchs CS. Relationship of CDX2 loss with molecular features and prognosis in colorectal cancer. Clin Cancer Res. 2009;15:4665-4673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | Chung DC. The genetic basis of colorectal cancer: insights into critical pathways of tumorigenesis. Gastroenterology. 2000;119:854-865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 257] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 11. | Witek ME, Nielsen K, Walters R, Hyslop T, Palazzo J, Schulz S, Waldman SA. The putative tumor suppressor Cdx2 is overexpressed by human colorectal adenocarcinomas. Clin Cancer Res. 2005;11:8549-8556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Moskaluk CA, Zhang H, Powell SM, Cerilli LA, Hampton GM, Frierson HF. Cdx2 protein expression in normal and malignant human tissues: an immunohistochemical survey using tissue microarrays. Mod Pathol. 2003;16:913-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 208] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | De Lott LB, Morrison C, Suster S, Cohn DE, Frankel WL. CDX2 is a useful marker of intestinal-type differentiation: a tissue microarray-based study of 629 tumors from various sites. Arch Pathol Lab Med. 2005;129:1100-1105. [PubMed] [Cited in This Article: ] |
| 14. | Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 539] [Cited by in F6Publishing: 475] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 15. | Bakaris S, Cetinkaya A, Ezberci F, Ekerbicer H. Expression of homeodomain protein CDX2 in colorectal adenoma and adenocarcinoma. Histol Histopathol. 2008;23:1043-1047. [PubMed] [Cited in This Article: ] |
| 16. | Lugli A, Tzankov A, Zlobec I, Terracciano LM. Differential diagnostic and functional role of the multi-marker phenotype CDX2/CK20/CK7 in colorectal cancer stratified by mismatch repair status. Mod Pathol. 2008;21:1403-1412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Hinoi T, Tani M, Lucas PC, Caca K, Dunn RL, Macri E, Loda M, Appelman HD, Cho KR, Fearon ER. Loss of CDX2 expression and microsatellite instability are prominent features of large cell minimally differentiated carcinomas of the colon. Am J Pathol. 2001;159:2239-2248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 176] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Choi BJ, Kim CJ, Cho YG, Song JH, Kim SY, Nam SW, Lee SH, Yoo NJ, Lee JY, Park WS. Altered expression of CDX2 in colorectal cancers. APMIS. 2006;114:50-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Saad RS, Silverman JF, Khalifa MA, Rowsell C. CDX2, cytokeratins 7 and 20 immunoreactivity in rectal adenocarcinoma. Appl Immunohistochem Mol Morphol. 2009;17:196-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Groisman GM, Amar M, Meir A. Expression of the intestinal marker Cdx2 in the columnar-lined esophagus with and without intestinal (Barrett's) metaplasia. Mod Pathol. 2004;17:1282-1288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 99] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 21. | Kazumori H, Ishihara S, Rumi MA, Kadowaki Y, Kinoshita Y. Bile acids directly augment caudal related homeobox gene Cdx2 expression in oesophageal keratinocytes in Barrett's epithelium. Gut. 2006;55:16-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Lord RV, Brabender J, Wickramasinghe K, DeMeester SR, Holscher A, Schneider PM, Danenberg PV, DeMeester TR. Increased CDX2 and decreased PITX1 homeobox gene expression in Barrett's esophagus and Barrett's-associated adenocarcinoma. Surgery. 2005;138:924-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Weimann A, Zimmermann M, Gross M, Slevogt H, Rieger A, Morawietz L. CDX2 and LI-cadherin expression in esophageal mucosa: use of both markers can facilitate the histologic diagnosis of Barrett's esophagus and carcinoma. Int J Surg Pathol. 2010;18:330-337. [PubMed] [Cited in This Article: ] |
| 24. | Bai YQ, Yamamoto H, Akiyama Y, Tanaka H, Takizawa T, Koike M, Kenji Yagi O, Saitoh K, Takeshita K, Iwai T. Ectopic expression of homeodomain protein CDX2 in intestinal metaplasia and carcinomas of the stomach. Cancer Lett. 2002;176:47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Park do Y, Srivastava A, Kim GH, Mino-Kenudson M, Deshpande V, Zukerberg LR, Song GA, Lauwers GY. CDX2 expression in the intestinal-type gastric epithelial neoplasia: frequency and significance. Mod Pathol. 2010;23:54-61. [PubMed] [Cited in This Article: ] |
| 26. | Mizoshita T, Tsukamoto T, Nakanishi H, Inada K, Ogasawara N, Joh T, Itoh M, Yamamura Y, Tatematsu M. Expression of Cdx2 and the phenotype of advanced gastric cancers: relationship with prognosis. J Cancer Res Clin Oncol. 2003;129:727-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Almeida R, Silva E, Santos-Silva F, Silberg DG, Wang J, De Bolós C, David L. Expression of intestine-specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. J Pathol. 2003;199:36-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 212] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 28. | Ge J, Chen Z, Wu S, Yuan W, Hu B, Chen Z. A clinicopathological study on the expression of cadherin-17 and caudal-related homeobox transcription factor (CDX2) in human gastric carcinoma. Clin Oncol (R Coll Radiol). 2008;20:275-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Shiotani A, Kamada T, Yamanaka Y, Manabe N, Kusunoki H, Hata J, Haruma K. Sonic hedgehog and CDX2 expression in the stomach. J Gastroenterol Hepatol. 2008;23 Suppl 2:S161-S166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Xin S, Huixin C, Benchang S, Aiping B, Jinhui W, Xiaoyan L, Yu WB, Minhu C. Expression of Cdx2 and claudin-2 in the multistage tissue of gastric carcinogenesis. Oncology. 2007;73:357-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Kim HS, Lee JS, Freund JN, Min KW, Lee JS, Kim W, Juhng SW, Park CS. CDX-2 homeobox gene expression in human gastric carcinoma and precursor lesions. J Gastroenterol Hepatol. 2006;21:438-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Liu Q, Teh M, Ito K, Shah N, Ito Y, Yeoh KG. CDX2 expression is progressively decreased in human gastric intestinal metaplasia, dysplasia and cancer. Mod Pathol. 2007;20:1286-1297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Song JH, Kim CJ, Cho YG, Chae JS, Cao Z, Nam SW, Lee JY, Park WS. Genetic alterations of the Cdx2 gene in gastric cancer. APMIS. 2008;116:74-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Fan Z, Li J, Dong B, Huang X. Expression of Cdx2 and hepatocyte antigen in gastric carcinoma: correlation with histologic type and implications for prognosis. Clin Cancer Res. 2005;11:6162-6170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Seno H, Oshima M, Taniguchi MA, Usami K, Ishikawa TO, Chiba T, Taketo MM. CDX2 expression in the stomach with intestinal metaplasia and intestinal-type cancer: Prognostic implications. Int J Oncol. 2002;21:769-774. [PubMed] [Cited in This Article: ] |
| 36. | Okayama H, Kumamoto K, Saitou K, Hayase S, Kofunato Y, Sato Y, Miyamoto K, Nakamura I, Ohki S, Sekikawa K. CD44v6, MMP-7 and nuclear Cdx2 are significant biomarkers for prediction of lymph node metastasis in primary gastric cancer. Oncol Rep. 2009;22:745-755. [PubMed] [Cited in This Article: ] |
| 37. | Xie Y, Li L, Wang X, Qin Y, Qian Q, Yuan X, Xiao Q. Overexpression of Cdx2 inhibits progression of gastric cancer in vitro. Int J Oncol. 2010;36:509-516. [PubMed] [Cited in This Article: ] |
| 38. | Zhang MQ, Lin F, Hui P, Chen ZM, Ritter JH, Wang HL. Expression of mucins, SIMA, villin, and CDX2 in small-intestinal adenocarcinoma. Am J Clin Pathol. 2007;128:808-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Wu XS, Akiyama Y, Igari T, Kawamura T, Hiranuma S, Shibata T, Tsuruta K, Koike M, Arii S, Yuasa Y. Expression of homeodomain protein CDX2 in gallbladder carcinomas. J Cancer Res Clin Oncol. 2005;131:271-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Sakamoto H, Mutoh H, Ido K, Satoh K, Hayakawa H, Sugano K. A close relationship between intestinal metaplasia and Cdx2 expression in human gallbladders with cholelithiasis. Hum Pathol. 2007;38:66-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Chang YT, Hsu C, Jeng YM, Chang MC, Wei SC, Wong JM. Expression of the caudal-type homeodomain transcription factor CDX2 is related to clinical outcome in biliary tract carcinoma. J Gastroenterol Hepatol. 2007;22:389-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Hong SM, Cho H, Moskaluk CA, Frierson HF, Yu E, Ro JY. CDX2 and MUC2 protein expression in extrahepatic bile duct carcinoma. Am J Clin Pathol. 2005;124:361-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Lin X, Saad RS, Luckasevic TM, Silverman JF, Liu Y. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15:407-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Srivastava A, Hornick JL. Immunohistochemical staining for CDX-2, PDX-1, NESP-55, and TTF-1 can help distinguish gastrointestinal carcinoid tumors from pancreatic endocrine and pulmonary carcinoid tumors. Am J Surg Pathol. 2009;33:626-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 45. | Saqi A, Alexis D, Remotti F, Bhagat G. Usefulness of CDX2 and TTF-1 in differentiating gastrointestinal from pulmonary carcinoids. Am J Clin Pathol. 2005;123:394-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Rabban JT, Lerwill MF, McCluggage WG, Grenert JP, Zaloudek CJ. Primary ovarian carcinoid tumors may express CDX-2: a potential pitfall in distinction from metastatic intestinal carcinoid tumors involving the ovary. Int J Gynecol Pathol. 2009;28:41-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Saad RS, Cho P, Silverman JF, Liu Y. Usefulness of Cdx2 in separating mucinous bronchioloalveolar adenocarcinoma of the lung from metastatic mucinous colorectal adenocarcinoma. Am J Clin Pathol. 2004;122:421-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Lora V, Kanitakis J. CDX2 expression in cutaneous metastatic carcinomas and extramammary Paget's Disease. Anticancer Res. 2009;29:5033-5037. [PubMed] [Cited in This Article: ] |
| 49. | Barbareschi M, Murer B, Colby TV, Chilosi M, Macri E, Loda M, Doglioni C. CDX-2 homeobox gene expression is a reliable marker of colorectal adenocarcinoma metastases to the lungs. Am J Surg Pathol. 2003;27:141-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Saad RS, Essig DL, Silverman JF, Liu Y. Diagnostic utility of CDX-2 expression in separating metastatic gastrointestinal adenocarcinoma from other metastatic adenocarcinoma in fine-needle aspiration cytology using cell blocks. Cancer. 2004;102:168-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Tot T. Identifying colorectal metastases in liver biopsies: the novel CDX2 antibody is less specific than the cytokeratin 20+/7- phenotype. Med Sci Monit. 2004;10:BR139-BR143. [PubMed] [Cited in This Article: ] |
| 52. | McCluggage WG, Shah R, Connolly LE, McBride HA. Intestinal-type cervical adenocarcinoma in situ and adenocarcinoma exhibit a partial enteric immunophenotype with consistent expression of CDX2. Int J Gynecol Pathol. 2008;27:92-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 53. | Saad RS, Ismiil N, Dubé V, Nofech-Mozes S, Khalifa MA. CDX-2 expression is a common event in primary intestinal-type endocervical adenocarcinoma. Am J Clin Pathol. 2009;132:531-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Fraggetta F, Pelosi G, Cafici A, Scollo P, Nuciforo P, Viale G. CDX2 immunoreactivity in primary and metastatic ovarian mucinous tumours. Virchows Arch. 2003;443:782-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Vang R, Gown AM, Barry TS, Wheeler DT, Yemelyanova A, Seidman JD, Ronnett BM. Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases. Am J Surg Pathol. 2006;30:1130-1139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 56. | Vang R, Gown AM, Wu LS, Barry TS, Wheeler DT, Yemelyanova A, Seidman JD, Ronnett BM. Immunohistochemical expression of CDX2 in primary ovarian mucinous tumors and metastatic mucinous carcinomas involving the ovary: comparison with CK20 and correlation with coordinate expression of CK7. Mod Pathol. 2006;19:1421-1428. [PubMed] [Cited in This Article: ] |
| 57. | Tornillo L, Moch H, Diener PA, Lugli A, Singer G. CDX-2 immunostaining in primary and secondary ovarian carcinomas. J Clin Pathol. 2004;57:641-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Kim MJ. The usefulness of CDX-2 for differentiating primary and metastatic ovarian carcinoma: an immunohistochemical study using a tissue microarray. J Korean Med Sci. 2005;20:643-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Logani S, Oliva E, Arnell PM, Amin MB, Young RH. Use of novel immunohistochemical markers expressed in colonic adenocarcinoma to distinguish primary ovarian tumors from metastatic colorectal carcinoma. Mod Pathol. 2005;18:19-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 60. | Lagendijk JH, Mullink H, Van Diest PJ, Meijer GA, Meijer CJ. Tracing the origin of adenocarcinomas with unknown primary using immunohistochemistry: differential diagnosis between colonic and ovarian carcinomas as primary sites. Hum Pathol. 1998;29:491-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 150] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 61. | McCluggage WG. Recent advances in immunohistochemistry in the diagnosis of ovarian neoplasms. J Clin Pathol. 2000;53:327-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Groisman GM, Meir A, Sabo E. The value of Cdx2 immunostaining in differentiating primary ovarian carcinomas from colonic carcinomas metastatic to the ovaries. Int J Gynecol Pathol. 2004;23:52-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Lagendijk JH, Mullink H, van Diest PJ, Meijer GA, Meijer CJ. Immunohistochemical differentiation between primary adenocarcinomas of the ovary and ovarian metastases of colonic and breast origin. Comparison between a statistical and an intuitive approach. J Clin Pathol. 1999;52:283-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 64. | Raspollini MR, Amunni G, Villanucci A, Baroni G, Taddei A, Taddei GL. Utility of CDX-2 in distinguishing between primary and secondary (intestinal) mucinous ovarian carcinoma: an immunohistochemical comparison of 43 cases. Appl Immunohistochem Mol Morphol. 2004;12:127-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Wani Y, Notohara K, Saegusa M, Tsukayama C. Aberrant Cdx2 expression in endometrial lesions with squamous differentiation: important role of Cdx2 in squamous morula formation. Hum Pathol. 2008;39:1072-1079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Herawi M, De Marzo AM, Kristiansen G, Epstein JI. Expression of CDX2 in benign tissue and adenocarcinoma of the prostate. Hum Pathol. 2007;38:72-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Leite KR, Mitteldorf CA, Srougi M, Dall'oglio MF, Antunes AA, Pontes J, Camara-Lopes LH. Cdx2, cytokeratin 20, thyroid transcription factor 1, and prostate-specific antigen expression in unusual subtypes of prostate cancer. Ann Diagn Pathol. 2008;12:260-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Paner GP, McKenney JK, Barkan GA, Yao JL, Frankel WL, Sebo TJ, Shen SS, Jimenez RE. Immunohistochemical analysis in a morphologic spectrum of urachal epithelial neoplasms: diagnostic implications and pitfalls. Am J Surg Pathol. 2011;35:787-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 69. | Franchi A, Massi D, Palomba A, Biancalani M, Santucci M. CDX-2, cytokeratin 7 and cytokeratin 20 immunohistochemical expression in the differential diagnosis of primary adenocarcinomas of the sinonasal tract. Virchows Arch. 2004;445:63-67. [PubMed] [Cited in This Article: ] |
| 70. | Kennedy MT, Jordan RC, Berean KW, Perez-Ordoñez B. Expression pattern of CK7, CK20, CDX-2, and villin in intestinal-type sinonasal adenocarcinoma. J Clin Pathol. 2004;57:932-937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 71. | Choi HR, Sturgis EM, Rashid A, DeMonte F, Luna MA, Batsakis JG, El-Naggar AK. Sinonasal adenocarcinoma: evidence for histogenetic divergence of the enteric and nonenteric phenotypes. Hum Pathol. 2003;34:1101-1107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Riedt T, Ebinger M, Salih HR, Tomiuk J, Handgretinger R, Kanz L, Grünebach F, Lengerke C. Aberrant expression of the homeobox gene CDX2 in pediatric acute lymphoblastic leukemia. Blood. 2009;113:4049-4051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Thoene S, Rawat VP, Heilmeier B, Hoster E, Metzeler KH, Herold T, Hiddemann W, Gökbuget N, Hoelzer D, Bohlander SK. The homeobox gene CDX2 is aberrantly expressed and associated with an inferior prognosis in patients with acute lymphoblastic leukemia. Leukemia. 2009;23:649-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |