Published online Oct 27, 2010. doi: 10.4240/wjgs.v2.i10.306
Revised: September 12, 2010
Accepted: September 19, 2010
Published online: October 27, 2010
Intraductal papillary-mucinous neoplasm (IPMN) of the pancreas is a clinically and morphologically distinctive precursor lesion of pancreatic cancer, characterized by gradual progression through a sequence of neoplastic changes. Based on the nature of the constituting neoplastic epithelium, degree of dysplasia and location within the pancreatic duct system, IPMNs are divided in several types which differ in their biological properties and clinical outcome. Molecular analysis and recent animal studies suggest that IPMNs develop in the context of a field-defect and reveal their possible relationship with other neoplastic precursor lesions of pancreatic cancer.
- Citation: Verbeke CS. Intraductal papillary-mucinous neoplasia of the pancreas: Histopathology and molecular biology. World J Gastrointest Surg 2010; 2(10): 306-313
- URL: https://www.wjgnet.com/1948-9366/full/v2/i10/306.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v2.i10.306
Since the first report on intraductal papillary-mucinous neoplasm (IPMN) of the pancreas in 1982[1] and the recognition of this entity by the World Health Organisation in 1996, it has become increasingly clear that in fact IPMNs constitute a heterogeneous group with a wide range of gross and microscopic features. In this review, the panoply of morphological and molecular characteristics of IPMNs will be briefly discussed along with recent developments that provide new insight into the development of IPMNs and their relationship with other neoplastic precursor lesions in the pancreas.
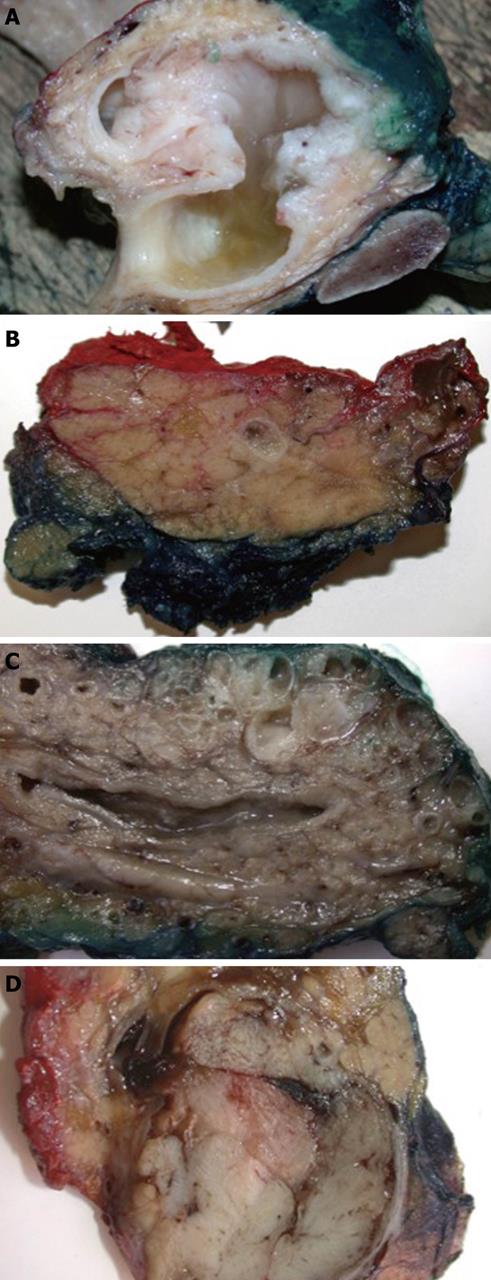
IPMN is defined as an intraductal proliferation of mucin-producing neoplastic cells arranged in papillary formations[2]. Duct dilatation is the key macroscopic feature of IPMN; however, this can vary significantly, depending on the degree of mucin production and papillary tumor formation. The latter can range from a mere granularity of the duct lining to bulky, several centimetres large protrusions within the dilated duct lumen. Similarly, intraductal mucin can be hardly detectable in some cases, whereas in others, copious amounts of mucus cause marked duct distension and occasionally extrude through the papilla of Vater. Gross appearance further depends on which part of the pancreatic duct system is involved and on the extent of the lesion (Figure 1). Any solid or gelatinous nodular areas suggest the presence of associated invasive adenocarcinoma. Seventy percent of IPMNs arise in the pancreatic head and up to 10% involve diffusely the entire gland[3].
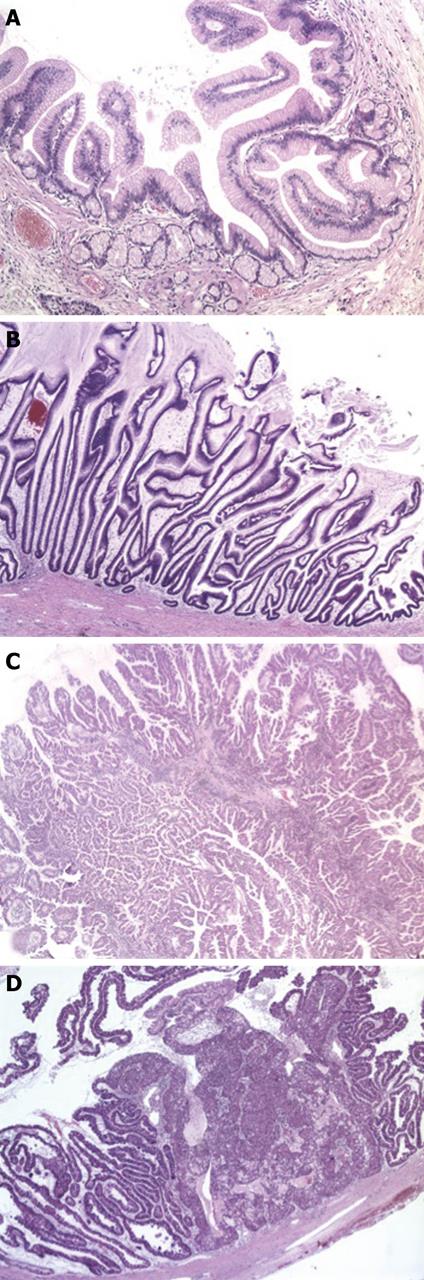
IPMNs are divided into 3 groups according to the degree of cyto-architectural atypia: adenoma or low-grade dysplasia, borderline or moderate dysplasia and in-situ carcinoma or high-grade dysplasia[4]. Similar to the adenoma-carcinoma sequence in colonic cancer, these three groups are thought to reflect neoplastic progression. A further classification is based on the morphology of the neoplastic epithelium (Figure 2)[5]. In gastric-type IPMNs, neoplastic epithelium resembling gastric foveolae forms short finger-like papillae or can be flat, and small pyloric-type glands are often present at the base of these lesions. Long villous projections lined with mucin-rich columnar cells, reminiscent of colonic villous adenoma, are characteristic of intestinal-type IPMNs. Pancreatobiliary-type IPMNs consist of complex arborizing papillae which are lined with cuboidal cells containing little mucin. In oncocytic-type IPMNs, the neoplastic epithelia have abundant eosinophilic cytoplasm but usually little mucin and line the papillae in several layers which are complex and merge into solid aggregates. This rare type of IPMN is regarded by some as a separate lesion (“intraductal oncocytic papillary neoplasm”)[6,7], mainly because of the lack of KRAS mutations which are frequent in IPMNs[8,9]. The direction of differentiation in the different types of IPMN is reflected in the expression of mucins. MUC1, a membrane-bound mucin detected in adult pancreas, is expressed in pancreatobiliary-type IPMN while the intestinal type secretory mucin MUC2 is found in intestinal-type IPMN. MUC5AC and MUC6 (gastric mucins) are expressed in gastric-type IPMN. MUC5AC in combination with MUC1 or MUC2 can also be found in pancreatobiliary or intestinal type IPMN respectively[4,5,10].
IPMNs are further subdivided depending on whether they involve the main duct, branch ducts or both. It is common for IPMNs to extend microscopically several centimetres beyond the grossly visible lesions[11].
Invasive adenocarcinoma is present in approximately 35% of IPMN-bearing pancreata and can be of colloid (65%) or intestinal type (15%)[12-15]. The former, also known as mucinous noncystic carcinoma, consists of mucin pools with free-floating clusters of cancer cells, expresses MUC2 but not MUC1 and is usually associated with intestinal-type IPMN[16]. It has a more favourable outcome than tubular adenocarcinoma which is identical to conventional pancreatic ductal adenocarcinoma (PDAC) in terms of histomorphology, mucin profile (MUC1+, MUC2-) and prognosis and is often, but not exclusively, associated with pancreatobiliary-type IPMN[17].
Interestingly, there is significant association between the epithelial type, grade of dysplasia, localisation in the pancreatic duct system and risk and type of associated invasive carcinoma. Gastric-type IPMNs usually present as small lesions in branch ducts, with mild dysplasia and a low risk of associated invasive cancer. In contrast, intestinal and pancreatobiliary type IPMNs are larger lesions that involve the main duct and/or connecting branch ducts, exhibit higher-grade dysplasia and bear a higher risk of being invasive[14,18]. These associations suggest that location of IPMNs in the duct system is not a random event but rather reflects intrinsic biological difference[19]. The associations also concur with the observation that invasive carcinoma is more frequently found in main duct than branch duct IPMNs (42% vs 12%)[20-23] which has important clinical implications and shaped the current guidelines for the management of IPMN patients[24].
With the growing awareness of IPMNs, two morphologically similar mass-forming intraductal neoplastic lesions have been recently described. Intraductal tubular neoplasia shares with IPMN the intraductal localisation and associated duct dilatation but differs by its predominantly tubular growth pattern and overall more favourable outcome[25-27]. Intraductal tubulopapillary neoplasia forms solid nodular tumors that obstruct dilated pancreatic ducts, is devoid of any visible mucin and exhibits a tubulopapillary growth pattern with high-grade dysplasia[28]. While both entities are supposedly unrelated to IPMN, a possible link between intraductal tubular adenoma and gastric-type IPMN has been suggested[29].
Multiple studies have investigated whether the difference in behavior between IPMN and PDAC is reflected in distinctive genetic aberrations. While activating mutation of KRAS is an early event in IPMN development, a significant proportion (14%-69%) of these lesions harbor the wild-type gene[30-33], suggesting that alternative ways of stimulating the Ras-Raf-MEK-MAP kinase pathway are used[34]. The reported frequency of inactivation of P53, P16 and SMAD4/DPC4 varies greatly between reports and depends on the degree of dysplasia of the lesion[35,36]. Overall, however, inactivation of SMAD4/DPC4 appears to be significantly less common in IPMN compared to PDAC[37,38]. IPMNs of patients with Peutz-Jeghers syndrome harbor germline mutations of the STK11/LKB1 gene and somatic mutation is an uncommon finding in sporadic IPMN[39]. PIK3CA is the only gene so far that is mutated in some IPMNs but not in PDAC[40].
Recent global genomic analyses confirm the gradual accumulation of chromosomal imbalances (losses more than gains) in IPMNs in parallel with increasing grades of dysplasia while the average fractional allelic loss appears to be lower compared to PDAC[41,42]. Whereas some chromosomal losses (5q, 6q, 11q) are more frequent in high-grade dysplastic or invasive IPMNs than PDAC, others (8p, 15q, 18q, 22q) occur with similar frequency in both[41-44].
Large-scale gene expression profiling studies of IPMNs reveal up- or down-regulation of numerous genes that are also differentially expressed in PDAC and therefore either relate to early events in carcinogenesis or functions that are common to most cancers[45,46].
Aberrant methylation is common in IPMNs and may contribute more to tumor suppressor gene inactivation than mutational events. It increases in prevalence with grades of dysplasia and is largely completed prior to the transition into invasive carcinoma[47,48]. Invasive IPMNs have multiple methylated genes which are related to cell cycle control (p16, p73, APC), DNA repair (MGMT, hMLH1) and cell adhesion (E-cadherin, claudin 5, TSLC1/IGSF1)[47,49].
Most (non-)invasive IPMNs are microsatellite stable and normally express mismatch repair genes[50,51]. Only a single case of high-level microsatellite instability has been reported in a patient with proven Lynch syndrome[52].
Recently, a large number of other pathways and molecular markers have been investigated. Wnt signalling and DNA damage checkpoint pathways, sonic hedgehog and telomere shortening appear to be aberrant in a proportion of IPMNs, however, further studies are awaited to clarify the significance of these findings[53-56].
Meticulous examination of pancreatic specimens with IPMN demonstrated that up to 32% of cases contain multiple apparently discontinuous lesions which often harbor different KRAS mutations[57-59]. In addition, KRAS mutation and X-chromosomal inactivation studies revealed that up to 80% of IPMNs are poly- or oligoclonal in origin[59-61]. This indicates that the majority of IPMNs can be considered as the result of fusion of two or more independent monoclonal precursor lesions. Multicentric or “field” cancerisation as the basis of IPMN development is further supported by the detection of genetic abnormalities (e.g. monosomy of chromosomes 6 & 17) in morphologically normal duct epithelium lining unremarkable or slightly dilated ducts and in adjacent unequivocal IPMNs[43,61]. FISH analysis demonstrated that within these morphologically normal duct epithelia, cells harboring monosomy 6 or 17 were admixed with cells of a normal karyotype[43]. Hence, IPMNs are not sharply delineated but rather surrounded by a grey zone, an area of as yet unknown extent, containing a mixed population of epithelial cells with or without genetic aberrations. Meanwhile, these findings have been corroborated by the increased prevalence of low-level aberrant methylation in morphologically normal duct epithelium of pancreata from IPMN patients[47].
These observations have important implications. Firstly, they indicate that morphology does not allow accurate identification of epithelial cells with early genomic aberrations. Secondly, the morphologically unremarkable cell populations that harbor genomic alterations could be responsible for local tumor recurrence after partial pancreatectomy with clear margins. Recent reports on concomitant but topographically separate PDAC in 9% of patients followed-up for branch duct IPMN, also point at a field-defect[12,62-64]. IPMNs are therefore not only precursor lesions of invasive carcinoma but also markers of unstable duct epithelium that is at higher risk of carcinogenesis. The underlying molecular mechanisms are, however, as yet unknown and whether these concomitant cancers develop from (small) IPMNs or PanINs is currently not clear[63,65].
Systematic study of IPMN is hampered by the practical issues related to the establishment of large series that adequately represent the inter- and intratumor heterogeneity of IPMNs in terms of dysplasia, epithelial type and main or branch duct involvement. Because of the significant association between these different features, it is particularly difficult to assess the significance of each feature individually. Moreover, gastric-type IPMNs involving branch ducts are often underrepresented because of the limited availability of tissue samples from these generally small lesions. Hence, large-scale studies with extensive sampling from different, well-characterized areas are needed to clarify the clinical and biological significance of these features and their mutual relationships.
Recent data indicate that morphologically normal duct epithelium adjacent to or away from IPMNs can harbor genomic aberrations[43,47,61]. Systematic analysis of “normal” duct epithelium is therefore required to characterize the molecular nature and extent of the putative field-defect. This will provide information regarding the development and natural history of IPMNs and is likely to have important implications for patient management. For instance, the current practice of guiding the extent of surgery by intra-operative microscopic examination of the resection margin may need reconsideration[4]. In addition, the presence of molecular abnormality in morphologically unremarkable background duct epithelium could possibly allow risk stratification of individual patients in terms of future development of IPMN recurrence or concomitant PDAC.
One key unanswered question remains that of the relationship between IPMN and pancreatic intraepithelial neoplasia (PanIN). Both are intraductal precursor lesions of invasive adenocarcinoma, progress through a sequence of increasingly severe dysplastic features and share certain molecular aberrations[66-68]. PanINs are usually incidental microscopic findings that involve small branch ducts whereas IPMNs generally produce gross lesions that are manifest clinically or on imaging. However, there is considerable histological overlap, making microscopic distinction often impossible and resulting in a low interobserver agreement, even when using the consensus definitions[69]. According to the latter, distinction is based on size, with lesions < 5 mm regarded as PanINs and those > 10 mm deemed to be IPMNs[70]. This definition has two main disadvantages. Firstly, it obviously leaves a grey area for lesions measuring between 5 and 10 mm in size. Secondly, as it seems reasonable to presume that IPMNs do not ab initio reach a size of 10 mm, adherence to the consensus definition effectively precludes the study of IPMNs at an early stage of development. Interestingly, Shi et al[71] recently introduced the notion of “incipient IPMNs” which they defined as morphologically typical IPMNs measuring 5 to 10 mm in size. Systematic reporting of the putative early IPMNs as PanINs bears the risk of obfuscating the true relationship between both lesions.
The closest relationship seems to exist between gastric-type IPMN and lower-grade PanIN which share morphological features, the mucin profile and location within branch ducts. PanINs have been reported to frequently occur next to gastric-type IPMNs[14,72] and both lesions frequently co-exist in patients with a family history of PDAC[71,73,74]. These similarities and co-existence of both lesions suggest they may be aspects of the same disease, whereby low-grade PanINs would represent “small gastric-type IPMNs” and the latter a focal accentuation of an essentially diffuse disease[72].
Several genetically engineered mouse (GEM) models currently exist in which PanIN and PDAC are faithfully reproduced[75,76]. A model for mucinous cystic neoplasia of the pancreas, a third known precursor of pancreatic cancer, has been described recently in a GEM model characterized by concomitant expression of KRASG12D and haploinsufficiency of SMAD4/DPC4[77]. Furthermore, selective biallelic deletion of the latter in combination with the activated KRASG12D allele has been reported to produce IPMN-like neoplastic lesions[78]. From the work with various GEM models, a complex picture emerges in which the sequence as well as the context in which the same overall spectrum of critical mutations occurs, determine the ensuing pathology[77]. Common to the pathways of different precursor lesions of pancreatic cancer is the initiating event of KRAS mutation with formation of early PanIN-lesions. Depending on the subsequent events, e.g. mutations of P53, P16 or SMAD4/DPC4, progression occurs along the same pathway and higher-grade PanINs develop, or, diversion into a different pathway leads to cystic neoplasia such as the mucinous cystic neoplasm or, possibly, IPMN. In particular, the timing of SMAD4/DPC4 mutation seems to determine which of the pleiotropic effects of this event will be exerted on the evolving precursor neoplasm[77,78]. These observations underscore the limitations of our largely static view of IPMN so far and the need for further development of a GEM model that recapitulates both the clinicopathological features of IPMN and the particular kinetics of this route of carcinogenesis. Through careful comparison with human IPMN, it will allow preclinical testing of novel risk stratification markers and treatment strategies and may provide the rationale for refined follow-up protocols.
IPMN is a clinically and morphologically distinct precursor lesion which offers a unique opportunity to study pancreatic carcinogenesis. Further molecular characterization and animal models of IPMN will further clarify the development and progression of this lesion and may provide clinically useful markers for early detection and risk stratification of patients affected by IPMN.
Peer reviewer: Jorg H Kleeff, MD, Department of General Surgery, Klinikum rechts der Isar, Technical University of Munich, Ismaningerstr 22, Munich 81675, Germany
S- Editor Wang JL L- Editor Roemmele A E- Editor Yang C
| 1. | Ohhashi K, Murakami F, Maruyama M. Four cases of mucous secreting pancreatic cancer. Prog Dig Endosc. 1982;20:348–351. [Cited in This Article: ] |
| 2. | Longnecker DS, Adler G, Hruban RH, Kloppel G. Intraductal papillary mucinous neoplasms. World Health Organization Classification of Tumors. Pathology and Genetics of Tumors of the Digestive System. Lyon: IARC Press 2000; 237-241. [Cited in This Article: ] |
| 3. | Cellier C, Cuillerier E, Palazzo L, Rickaert F, Flejou JF, Napoleon B, Van Gansbeke D, Bely N, Ponsot P, Partensky C. Intraductal papillary and mucinous tumors of the pancreas: accuracy of preoperative computed tomography, endoscopic retrograde pancreatography and endoscopic ultrasonography, and long-term outcome in a large surgical series. Gastrointest Endosc. 1998;47:42-49. [Cited in This Article: ] |
| 4. | Hruban RH, Pitman MB, Klimstra DS. Tumors of the pancreas. Atlas of Tumor Pathology.4th series, fascicle 6. Washington, DC: Armed Forces Institute of Pathology 2007; . [Cited in This Article: ] |
| 5. | Furukawa T, Klöppel G, Volkan Adsay N, Albores-Saavedra J, Fukushima N, Horii A, Hruban RH, Kato Y, Klimstra DS, Longnecker DS. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794-799. [Cited in This Article: ] |
| 6. | Adsay NV, Adair CF, Heffess CS, Klimstra DS. Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol. 1996;20:980-994. [Cited in This Article: ] |
| 7. | Jyotheeswaran S, Zotalis G, Penmetsa P, Levea CM, Schoeniger LO, Shah AN. A newly recognized entity: intraductal "oncocytic" papillary neoplasm of the pancreas. Am J Gastroenterol. 1998;93:2539-2543. [Cited in This Article: ] |
| 8. | Chung SM, Hruban RH, Iacobuzio-Donahue , Adsay NV, Zee SY, Klimstra DS. Analysis of molecular alterations and differentiation pathways in intraductal oncocytic papillary neoplasm of the pancreas. Mod Pathol. 2005;18:277A. [Cited in This Article: ] |
| 9. | Patel SA, Adams R, Goldstein M, Moskaluk CA. Genetic analysis of invasive carcinoma arising in intraductal oncocytic papillary neoplasm of the pancreas. Am J Surg Pathol. 2002;26:1071-1077. [Cited in This Article: ] |
| 10. | Terris B, Dubois S, Buisine MP, Sauvanet A, Ruszniewski P, Aubert JP, Porchet N, Couvelard A, Degott C, Fléjou JF. Mucin gene expression in intraductal papillary-mucinous pancreatic tumours and related lesions. J Pathol. 2002;197:632-637. [Cited in This Article: ] |
| 11. | Furukawa T, Takahashi T, Kobari M, Matsuno S. The mucus-hypersecreting tumor of the pancreas. Development and extension visualized by three-dimensional computerized mapping. Cancer. 1992;70:1505-1513. [Cited in This Article: ] |
| 12. | Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M, Clain JE, Norton IA, Pearson RK, Petersen BT. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500-1507. [Cited in This Article: ] |
| 13. | Bernard P, Scoazec JY, Joubert M, Kahn X, Le Borgne J, Berger F, Partensky C. Intraductal papillary-mucinous tumors of the pancreas: predictive criteria of malignancy according to pathological examination of 53 cases. Arch Surg. 2002;137:1274-1278. [Cited in This Article: ] |
| 14. | Ban S, Naitoh Y, Mino-Kenudson M, Sakurai T, Kuroda M, Koyama I, Lauwers GY, Shimizu M. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: its histopathologic difference between 2 major types. Am J Surg Pathol. 2006;30:1561-1569. [Cited in This Article: ] |
| 15. | Adsay NV, Conlon KC, Zee SY, Brennan MF, Klimstra DS. Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94:62-77. [Cited in This Article: ] |
| 16. | Adsay NV, Pierson C, Sarkar F, Abrams J, Weaver D, Conlon KC, Brennan MF, Klimstra DS. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25:26-42. [Cited in This Article: ] |
| 17. | Sadakari Y, Ohuchida K, Nakata K, Ohtsuka T, Aishima S, Takahata S, Nakamura M, Mizumoto K, Tanaka M. Invasive carcinoma derived from the nonintestinal type intraductal papillary mucinous neoplasm of the pancreas has a poorer prognosis than that derived from the intestinal type. Surgery. 2010;147:812-817. [Cited in This Article: ] |
| 18. | Nakamura A, Horinouchi M, Goto M, Nagata K, Sakoda K, Takao S, Imai K, Kim YS, Sato E, Yonezawa S. New classification of pancreatic intraductal papillary-mucinous tumour by mucin expression: its relationship with potential for malignancy. J Pathol. 2002;197:201-210. [Cited in This Article: ] |
| 19. | Hingorani SR. Location, location, location: precursors and prognoses for pancreatic cancer. Gastroenterology. 2007;133:345-350. [Cited in This Article: ] |
| 20. | Kobari M, Egawa S, Shibuya K, Shimamura H, Sunamura M, Takeda K, Matsuno S, Furukawa T. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg. 1999;134:1131-1136. [Cited in This Article: ] |
| 21. | Terris B, Ponsot P, Paye F, Hammel P, Sauvanet A, Molas G, Bernades P, Belghiti J, Ruszniewski P, Fléjou JF. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372-1377. [Cited in This Article: ] |
| 22. | Salvia R, Fernández-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, Pederzoli P, Warshaw AL. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678-685; discussion 685-687. [Cited in This Article: ] |
| 23. | Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M, Thayer SP, Lauwers GY, Capelli P, Mino-Kenudson M. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72-79; quiz 309-310. [Cited in This Article: ] |
| 24. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [Cited in This Article: ] |
| 25. | Bakotic BW, Robinson MJ, Sturm PD, Hruban RH, Offerhaus GJ, Albores-Saavedra J. Pyloric gland adenoma of the main pancreatic duct. Am J Surg Pathol. 1999;23:227-231. [Cited in This Article: ] |
| 26. | Albores-Saavedra J, Sheahan K, O'Riain C, Shukla D. Intraductal tubular adenoma, pyloric type, of the pancreas: additional observations on a new type of pancreatic neoplasm. Am J Surg Pathol. 2004;28:233-238. [Cited in This Article: ] |
| 27. | Nakayama Y, Inoue H, Hamada Y, Takeshita M, Iwasaki H, Maeshiro K, Iwanaga S, Tani H, Ryu S, Yasunami Y. Intraductal tubular adenoma of the pancreas, pyloric gland type: a clinicopathologic and immunohistochemical study of 6 cases. Am J Surg Pathol. 2005;29:607-616. [Cited in This Article: ] |
| 28. | Yamaguchi H, Shimizu M, Ban S, Koyama I, Hatori T, Fujita I, Yamamoto M, Kawamura S, Kobayashi M, Ishida K. Intraductal tubulopapillary neoplasms of the pancreas distinct from pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2009;33:1164-1172. [Cited in This Article: ] |
| 29. | Chetty R, Serra S. Intraductal tubular adenoma (pyloric gland-type) of the pancreas: a reappraisal and possible relationship with gastric-type intraductal papillary mucinous neoplasm. Histopathology. 2009;55:270-276. [Cited in This Article: ] |
| 30. | Z'graggen K, Rivera JA, Compton CC, Pins M, Werner J, Fernández-del Castillo C, Rattner DW, Lewandrowski KB, Rustgi AK, Warshaw AL. Prevalence of activating K-ras mutations in the evolutionary stages of neoplasia in intraductal papillary mucinous tumors of the pancreas. Ann Surg. 1997;226:491-498; discussion 498-500. [Cited in This Article: ] |
| 31. | Satoh K, Shimosegawa T, Moriizumi S, Koizumi M, Toyota T. K-ras mutation and p53 protein accumulation in intraductal mucin-hypersecreting neoplasms of the pancreas. Pancreas. 1996;12:362-368. [Cited in This Article: ] |
| 32. | Sessa F, Solcia E, Capella C, Bonato M, Scarpa A, Zamboni G, Pellegata NS, Ranzani GN, Rickaert F, Klöppel G. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: an investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch. 1994;425:357-367. [Cited in This Article: ] |
| 33. | Yanagisawa A, Kato Y, Ohtake K, Kitagawa T, Ohashi K, Hori M, Takagi K, Sugano H. c-Ki-ras point mutations in ductectatic-type mucinous cystic neoplasms of the pancreas. Jpn J Cancer Res. 1991;82:1057-1060. [Cited in This Article: ] |
| 34. | Schönleben F, Qiu W, Bruckman KC, Ciau NT, Li X, Lauerman MH, Frucht H, Chabot JA, Allendorf JD, Remotti HE. BRAF and KRAS gene mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/IPMC) of the pancreas. Cancer Lett. 2007;249:242-248. [Cited in This Article: ] |
| 35. | Sasaki S, Yamamoto H, Kaneto H, Ozeki I, Adachi Y, Takagi H, Matsumoto T, Itoh H, Nagakawa T, Miyakawa H. Differential roles of alterations of p53, p16, and SMAD4 expression in the progression of intraductal papillary-mucinous tumors of the pancreas. Oncol Rep. 2003;10:21-25. [Cited in This Article: ] |
| 36. | Kawahira H, Kobayashi S, Kaneko K, Asano T, Ochiai T. p53 protein expression in intraductal papillary mucinous tumors (IPMT) of the pancreas as an indicator of tumor malignancy. Hepatogastroenterology. 2000;47:973-977. [Cited in This Article: ] |
| 37. | Biankin AV, Biankin SA, Kench JG, Morey AL, Lee CS, Head DR, Eckstein RP, Hugh TB, Henshall SM, Sutherland RL. Aberrant p16(INK4A) and DPC4/Smad4 expression in intraductal papillary mucinous tumours of the pancreas is associated with invasive ductal adenocarcinoma. Gut. 2002;50:861-868. [Cited in This Article: ] |
| 38. | Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, Wilentz RE, Argani P, Sohn TA, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: comparison with conventional ductal adenocarcinomas. Am J Pathol. 2000;157:755-761. [Cited in This Article: ] |
| 39. | Sato N, Rosty C, Jansen M, Fukushima N, Ueki T, Yeo CJ, Cameron JL, Iacobuzio-Donahue CA, Hruban RH, Goggins M. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 2001;159:2017-2122. [Cited in This Article: ] |
| 40. | Schönleben F, Qiu W, Ciau NT, Ho DJ, Li X, Allendorf JD, Remotti HE, Su GH. PIK3CA mutations in intraductal papillary mucinous neoplasm/carcinoma of the pancreas. Clin Cancer Res. 2006;12:3851-3855. [Cited in This Article: ] |
| 41. | Abe T, Fukushima N, Brune K, Boehm C, Sato N, Matsubayashi H, Canto M, Petersen GM, Hruban RH, Goggins M. Genome-wide allelotypes of familial pancreatic adenocarcinomas and familial and sporadic intraductal papillary mucinous neoplasms. Clin Cancer Res. 2007;13:6019-6025. [Cited in This Article: ] |
| 42. | Fritz S, Fernandez-del Castillo C, Mino-Kenudson M, Crippa S, Deshpande V, Lauwers GY, Warshaw AL, Thayer SP, Iafrate AJ. Global genomic analysis of intraductal papillary mucinous neoplasms of the pancreas reveals significant molecular differences compared to ductal adenocarcinoma. Ann Surg. 2009;249:440-447. [Cited in This Article: ] |
| 43. | Soldini D, Gugger M, Burckhardt E, Kappeler A, Laissue JA, Mazzucchelli L. Progressive genomic alterations in intraductal papillary mucinous tumours of the pancreas and morphologically similar lesions of the pancreatic ducts. J Pathol. 2003;199:453-461. [Cited in This Article: ] |
| 44. | Schleger C, Arens N, Zentgraf H, Bleyl U, Verbeke C. Identification of frequent chromosomal aberrations in ductal adenocarcinoma of the pancreas by comparative genomic hybridization (CGH). J Pathol. 2000;191:27-32. [Cited in This Article: ] |
| 45. | Terris B, Blaveri E, Crnogorac-Jurcevic T, Jones M, Missiaglia E, Ruszniewski P, Sauvanet A, Lemoine NR. Characterization of gene expression profiles in intraductal papillary-mucinous tumors of the pancreas. Am J Pathol. 2002;160:1745-1754. [Cited in This Article: ] |
| 46. | Sato N, Fukushima N, Maitra A, Iacobuzio-Donahue CA, van Heek NT, Cameron JL, Yeo CJ, Hruban RH, Goggins M. Gene expression profiling identifies genes associated with invasive intraductal papillary mucinous neoplasms of the pancreas. Am J Pathol. 2004;164:903-914. [Cited in This Article: ] |
| 47. | Hong SM, Kelly D, Griffith M, Omura N, Li A, Li CP, Hruban RH, Goggins M. Multiple genes are hypermethylated in intraductal papillary mucinous neoplasms of the pancreas. Mod Pathol. 2008;21:1499-1507. [Cited in This Article: ] |
| 48. | Nakayama S, Semba S, Maeda N, Matsushita M, Kuroda Y, Yokozaki H. Hypermethylation-mediated reduction of WWOX expression in intraductal papillary mucinous neoplasms of the pancreas. Br J Cancer. 2009;100:1438-1443. [Cited in This Article: ] |
| 49. | House MG, Guo M, Iacobuzio-Donahue C, Herman JG. Molecular progression of promoter methylation in intraductal papillary mucinous neoplasms (IPMN) of the pancreas. Carcinogenesis. 2003;24:193-198. [Cited in This Article: ] |
| 50. | Lüttges J, Beyser K, Pust S, Paulus A, Rüschoff J, Klöppel G. Pancreatic mucinous noncystic (colloid) carcinomas and intraductal papillary mucinous carcinomas are usually microsatellite stable. Mod Pathol. 2003;16:537-542. [Cited in This Article: ] |
| 51. | Handra-Luca A, Couvelard A, Degott C, Fléjou JF. Correlation between patterns of DNA mismatch repair hmlh1 and hmsh2 protein expression and progression of dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Virchows Arch. 2004;444:235-238. [Cited in This Article: ] |
| 52. | Sparr JA, Bandipalliam P, Redston MS, Syngal S. Intraductal papillary mucinous neoplasm of the pancreas with loss of mismatch repair in a patient with Lynch syndrome. Am J Surg Pathol. 2009;33:309-312. [Cited in This Article: ] |
| 53. | Chetty R, Serra S, Salahshor S, Alsaad K, Shih W, Blaszyk H, Woodgett JR, Tsao MS. Expression of Wnt-signaling pathway proteins in intraductal papillary mucinous neoplasms of the pancreas: a tissue microarray analysis. Hum Pathol. 2006;37:212-217. [Cited in This Article: ] |
| 54. | Miyasaka Y, Nagai E, Yamaguchi H, Fujii K, Inoue T, Ohuchida K, Yamada T, Mizumoto K, Tanaka M, Tsuneyoshi M. The role of the DNA damage checkpoint pathway in intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res. 2007;13:4371-4377. [Cited in This Article: ] |
| 55. | Ohuchida K, Mizumoto K, Fujita H, Yamaguchi H, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M, Tanaka M. Sonic hedgehog is an early developmental marker of intraductal papillary mucinous neoplasms: clinical implications of mRNA levels in pancreatic juice. J Pathol. 2006;210:42-48. [Cited in This Article: ] |
| 56. | Hashimoto Y, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Ohge H, Fukuda E, Shimamoto F, Sueda T, Hiyama E. Telomere shortening and telomerase expression during multistage carcinogenesis of intraductal papillary mucinous neoplasms of the pancreas. J Gastrointest Surg. 2008;12:17-28; discussion 28-29. [Cited in This Article: ] |
| 57. | Bendix Holme J, Jacobsen NO, Rokkjaer M, Kruse A. Total pancreatectomy in six patients with intraductal papillary mucinous tumour of the pancreas: the treatment of choice. HPB (Oxford). 2001;3:257-262. [Cited in This Article: ] |
| 58. | Obara T, Saitoh Y, Maguchi H, Ura H, Kitazawa S, Koike Y, Okamura K, Namiki M. Multicentric development of pancreatic intraductal carcinoma through atypical papillary hyperplasia. Hum Pathol. 1992;23:82-85. [Cited in This Article: ] |
| 59. | Kitago M, Ueda M, Aiura K, Suzuki K, Hoshimoto S, Takahashi S, Mukai M, Kitajima M. Comparison of K-ras point mutation distributions in intraductal papillary-mucinous tumors and ductal adenocarcinoma of the pancreas. Int J Cancer. 2004;110:177-182. [Cited in This Article: ] |
| 60. | Izawa T, Obara T, Tanno S, Mizukami Y, Yanagawa N, Kohgo Y. Clonality and field cancerization in intraductal papillary-mucinous tumors of the pancreas. Cancer. 2001;92:1807-1817. [Cited in This Article: ] |
| 61. | Yoshizawa K, Nagai H, Sakurai S, Hironaka M, Morinaga S, Saitoh K, Fukayama M. Clonality and K-ras mutation analyses of epithelia in intraductal papillary mucinous tumor and mucinous cystic tumor of the pancreas. Virchows Arch. 2002;441:437-443. [Cited in This Article: ] |
| 62. | Ingkakul T, Sadakari Y, Ienaga J, Satoh N, Takahata S, Tanaka M. Predictors of the presence of concomitant invasive ductal carcinoma in intraductal papillary mucinous neoplasm of the pancreas. Ann Surg. 2010;251:70-75. [Cited in This Article: ] |
| 63. | Uehara H, Nakaizumi A, Ishikawa O, Iishi H, Tatsumi K, Takakura R, Ishida T, Takano Y, Tanaka S, Takenaka A. Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut. 2008;57:1561-1565. [Cited in This Article: ] |
| 64. | Yamaguchi K, Ohuchida J, Ohtsuka T, Nakano K, Tanaka M. Intraductal papillary-mucinous tumor of the pancreas concomitant with ductal carcinoma of the pancreas. Pancreatology. 2002;2:484-490. [Cited in This Article: ] |
| 65. | Biankin AV, Kench JG, Biankin SA, Lee CS, Morey AL, Dijkman FP, Coleman MJ, Sutherland RL, Henshall SM. Pancreatic intraepithelial neoplasia in association with intraductal papillary mucinous neoplasms of the pancreas: implications for disease progression and recurrence. Am J Surg Pathol. 2004;28:1184-1192. [Cited in This Article: ] |
| 66. | Takaori K, Kobashi Y, Matsusue S, Matsui K, Yamamoto T. Clinicopathological features of pancreatic intraepithelial neoplasias and their relationship to intraductal papillary-mucinous tumors. J Hepatobiliary Pancreat Surg. 2003;10:125-136. [Cited in This Article: ] |
| 67. | Furukawa T, Fujisaki R, Yoshida Y, Kanai N, Sunamura M, Abe T, Takeda K, Matsuno S, Horii A. Distinct progression pathways involving the dysfunction of DUSP6/MKP-3 in pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms of the pancreas. Mod Pathol. 2005;18:1034-1042. [Cited in This Article: ] |
| 68. | Moriya T, Kimura W, Semba S, Sakurai F, Hirai I, Ma J, Fuse A, Maeda K, Yamakawa M. Biological similarities and differences between pancreatic intraepithelial neoplasias and intraductal papillary mucinous neoplasms. Int J Gastrointest Cancer. 2005;35:111-119. [Cited in This Article: ] |
| 69. | Longnecker DS, Adsay NV, Fernandez-del Castillo C, Hruban RH, Kasugai T, Klimstra DS, Klöppel G, Lüttges J, Memoli VA, Tosteson TD. Histopathological diagnosis of pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms: interobserver agreement. Pancreas. 2005;31:344-349. [Cited in This Article: ] |
| 70. | Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, Biankin SA, Compton C, Fukushima N, Furukawa T. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977-987. [Cited in This Article: ] |
| 71. | Shi C, Klein AP, Goggins M, Maitra A, Canto M, Ali S, Schulick R, Palmisano E, Hruban RH. Increased Prevalence of Precursor Lesions in Familial Pancreatic Cancer Patients. Clin Cancer Res. 2009;15:7737-7743. [Cited in This Article: ] |
| 72. | Andrejevic-Blant S, Kosmahl M, Sipos B, Klöppel G. Pancreatic intraductal papillary-mucinous neoplasms: a new and evolving entity. Virchows Arch. 2007;451:863-869. [Cited in This Article: ] |
| 73. | Brune K, Abe T, Canto M, O'Malley L, Klein AP, Maitra A, Volkan Adsay N, Fishman EK, Cameron JL, Yeo CJ. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol. 2006;30:1067-1076. [Cited in This Article: ] |
| 74. | Meckler KA, Brentnall TA, Haggitt RC, Crispin D, Byrd DR, Kimmey MB, Bronner MP. Familial fibrocystic pancreatic atrophy with endocrine cell hyperplasia and pancreatic carcinoma. Am J Surg Pathol. 2001;25:1047-1053. [Cited in This Article: ] |
| 75. | Leach SD. Mouse models of pancreatic cancer: the fur is finally flying! Cancer Cell. 2004;5:7-11. [Cited in This Article: ] |
| 76. | Ottenhof NA, Milne AN, Morsink FH, Drillenburg P, Ten Kate FJ, Maitra A, Offerhaus GJ. Pancreatic intraepithelial neoplasia and pancreatic tumorigenesis: of mice and men. Arch Pathol Lab Med. 2009;133:375-381. [Cited in This Article: ] |
| 77. | Izeradjene K, Combs C, Best M, Gopinathan A, Wagner A, Grady WM, Deng CX, Hruban RH, Adsay NV, Tuveson DA. KrasG12D and Smad4/Dpc4 Haploinsufficiency Cooperate to Induce Mucinous Cystic Neoplasms and Invasive Adenocarcinoma of the Pancreas. Cancer Cell. 2007;11:229-243. [Cited in This Article: ] |