Published online Sep 15, 2017. doi: 10.4239/wjd.v8.i9.422
Peer-review started: February 12, 2017
First decision: March 28, 2017
Revised: April 11, 2017
Accepted: May 3, 2017
Article in press: May 5, 2017
Published online: September 15, 2017
To investigate matrix metalloproteinase-11 (MMP-11) expression in adipose tissue dysfunction, using in vitro and in vivo models of insulin resistance.
Culture of mouse 3T3-L1 preadipocytes were induced to differentiation into mature 3T3-L1 adipocytes. Cellular insulin resistance was induced by treating differentiated cultured adipocytes with hypoxia and/or tumor necrosis factor (TNF)-α, and transcriptional changes were analyzed in each condition thereafter. For the in vivo studies, MMP-11 expression levels were measured in white adipose tissue (WAT) from C57BL/6J mice that underwent low fat diet or high-fat feeding in order to induce obesity and obesity-related insulin resistance. Statistical analysis was carried out with GraphPad Prism Software.
MMP-11 mRNA expression levels were significantly higher in insulin resistant 3T3-L1 adipocytes compared to control cells (1.46 ± 0.49 vs 0.83 ± 0.21, respectively; P < 0.00036). The increase in MMP-11 expression was observed even in the presence of TNF-α alone (3.79 ± 1.11 vs 1 ± 0.17, P < 0.01) or hypoxia alone (1.79 ± 0.7 vs 0.88 ± 0.1, P < 0.00023). The results obtained in in vitro experiments were confirmed in the in vivo model of insulin resistance. In particular, MMP-11 mRNA was upregulated in WAT from obese mice compared to lean mice (5.5 ± 2.8 vs 1.1 ± 0.7, respectively; P < 3.72E-08). The increase in MMP-11 levels in obese mice was accompanied by the increase in typical markers of fibrosis, such as collagen type VI alpha 3 (Col6α3), and fibroblast-specific protein 1.
Our results indicate that dysregulation of MMP-11 expression is an early process in the adipose tissue dysfunction, which leads to obesity and obesity-related insulin resistance.
Core tip: 3T3-L1 mature adipocytes are widely used as a cellular model of obesity. We treated 3T3-L1 adipocytes with tumor necrosis factor-α and/or hypoxia for 24 h to induce insulin resistance. Matrix metalloproteinase-11 (MMP-11) expression levels were upregulated in insulin resistant adipocytes, as compared to untreated control cells. This observation was confirmed in vivo, in white adipose tissue from insulin-resistant obese mice. Therefore, our results suggest that MMP-11 could play a role in the dysfunction of adipose tissue, which leads to insulin resistance and type 2 diabetes. Further work is necessary to understand better the functional role of MMP-11 in this context.
- Citation: Arcidiacono B, Chiefari E, Laria AE, Messineo S, Bilotta FL, Britti D, Foti DP, Foryst-Ludwig A, Kintscher U, Brunetti A. Expression of matrix metalloproteinase-11 is increased under conditions of insulin resistance. World J Diabetes 2017; 8(9): 422-428
- URL: https://www.wjgnet.com/1948-9358/full/v8/i9/422.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i9.422
Insulin resistance is a pathological condition in which insulin target tissues fail to properly respond to insulin. It is more frequently associated with overweight and obesity, and constitutes a prominent feature of type 2 diabetes (T2D) and the metabolic syndrome[1,2]. In the past decades, research findings have substantially improved our understanding of the pathophysiology of insulin resistance, thanks to the identification of new genetic defects and molecular events that underlie the abnormalities that occur in both peripheral insulin action and insulin secretion[3-7]. Particular interest in this field has been devoted to the investigation of obesity, as it is considered the major risk factor for insulin resistance, which leads to the development of T2D and other obesity-associated insulin resistant states. Therefore, because of the parallel increasing prevalence of obesity and metabolic diseases, much research has been recently focused on the role of adipose tissue, previously considered as a fat storage tissue only. Evidence from the last years has established the involvement of adipose tissue in the production of hormones and numerous other biologically active molecules collectively called “adipokines” that are implicated in metabolic and inflammatory pathways[8]. Based on the new view of adipose tissue as an endocrine organ, new insights have been gained over the last years into the mechanisms linking adipose tissue to insulin resistance, although the entire sequelae of events that initially trigger adipose tissue dysfunction still remain poorly defined.
The matrix metalloproteinase-11 (MMP-11; also known as stromelysin-3) is a proteinase enzyme that belongs to the family of metalloproteinases, and is involved in remodeling and degradation of extracellular matrix (ECM). Unlike other MMPs that are secreted in an inactive form to be then activated extracellularly, MMP-11 is maturated in the Golgi’s apparatus and secreted in an active form[9]. MMP-11 is implicated in tissue remodeling during embryogenesis, tissue involution and metamorphosis, and in biological process of tissue repair after trauma[10]. In addition, as shown in in vivo studies, MMP-11 plays a role in tumor development and progression. In particular, cancer cells, by inducing the adjacent fat cells to express MMP-11, may contribute to modify the ECM, thereby favoring cancer cell migration into the connective tissue, during the initial step of the invasive process[11]. In this regard, the involvement of MMP-11 in certain types of cancers (i.e., breast, colorectal and lung) has been confirmed in clinical studies, in which it has also been established that higher expression of MMP-11 correlates with tumor aggressiveness and lower survival rate among affected patients[12]. However, although the numerous studies carried out up to date, both in vitro and in vivo, the precise molecular target(s) of MMP-11 and their specific role in normal and pathological conditions have not yet been clarified. It has been demonstrated that active MMP-11 is primarily responsible for the digestion of collagen IV and VI, fibronectin, alpha 2-macroglobulin and insulin-like grow factor binding protein 1 (IGFBP1)[13,14]. However, all these substrates are not specific for this enzyme as they can be also cleaved by other MMPs.
In the present study, we investigated the expression of MMP-11 in an in vitro model of insulin resistance, and in a murine diet-induced model of obesity.
3T3-L1 mouse preadipocytes were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplied with 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin and maintained at 37 °C in 5% CO2 humidified atmosphere. As soon as the confluence was reached, cells were induced to differentiate as reported previously[15,16]. In brief, the differentiation process was started through the addition of 500 μmol/L of 3-isobutyl-1-methylxanthine (IBMX), 1 μmol/L of dexamethasone and 1 μg/mL of insulin. The cells were incubated for three days in the differentiation medium, followed by 2 d of treatment with DMEM containing 1 μg/mL insulin. The medium was replaced every two days and experiments were performed using day 8 to day 12 mature adipocytes.
To induce insulin resistance, mature 3T3-L1 adipocytes were treated with 2.5 nmol/L tumor necrosis factor (TNF)-α, and simultaneously incubated in hypoxic conditions for 24 h[17]. Before inducing insulin resistance, 3T3-L1 adipocytes were cultured in DMEM at low glucose concentration (1 g/L) and 0.5% BSA, plus rh-TNF-α, and put in the hypoxic chamber (1% O2, 5% CO2) at 37 °C for 24 h. Control cells were incubated in the same conditions, but in normal atmosphere (21% O2).
Total RNA was extracted from white adipose tissue (WAT) and 3T3-L1 cells, using Trizol reagent (Invitrogen), according to the manufacturer’s instructions[18]. RNA concentration was measured by a NanoDrop spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA, United States), and its quality confirmed on agarose gel. One microgram of RNA sample was used for cDNA synthesis, using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems), in the presence of the following reagents: 10 × RT Buffer, 100 mmol/L dNTP mix, 10 × RT Random Primers and 0.50 U/μL Multiscribe Reverse Transcriptase. The cDNA thermal-profile was 25 °C for 10 min, 37 °C for 120 min and the enzyme was inactivated at 85 °C for 5 min.
Relative quantification was performed to measure MMP-11 expression, using a real-time thermocycler (Eppendorf Mastercycler ep realplex ES). One microliter of cDNA and 0.2 μmol/L of each primers were mixed with SYBR Green RealMasterMix (Eppendorf). S9 and 18S were used as internal reference controls. Primers were designed for mouse MMP-11, S9 and 18S, using the Primer3web version 4.0[19,20], according to sequences from the GeneBank database. Amplification conditions were: 2 min at 95 °C and three step-cycle of 95 °C for 15 s, 58 °C for 20 s and 68 °C for 20 s, for a total of 40 cycles.
Cells were lysed as described previously[21]. Cellular protein (20 μg) was resolved on 10% SDS-PAGE, transferred to PVDF membrane (Immobilon-PSQ 0.2 μm Millipore ISEQ00010), blotted for 2 h with blocking solution (5% non-fat dry milk), then incubated overnight at 4 °C with primary antibody against MMP-11 (Santa Cruz sc8836 dilution 1:1000), followed by incubation for 1 h at room temperature with a secondary antibody linked to horseradish peroxidase. Immune complexes were visualized by enhanced chemiluminescence (ECL, Amersham).
Five week-old male C57BL/6J mice were housed in individual cages and maintained on 12 h light-dark cycle with controlled temperature (25 °C) and humidity (50% ± 5%), and with free access to water. To induce obesity, ten mice were fed ad libitum with HFD containing 60% calories from fat, 20% from carbohydrates, and 20% from protein for 15 wk time period. Six additional mice (control group) were fed for the same time (15 wk) with low fat diet (LFD) containing 10% calories from fat, 70% from carboydrates, and 20% from protein. Intraperitoneal insulin tolerance test (IITT) was performed following previously described procedures[5,22], using human insulin (Human Actrapid, Novo Nordisk), 0.25 U/kg body weight, then measuring blood glucose levels at 0, 15, 30, 45, 60 min after insulin injection. At the end of 15 wk, mice were euthanized by cervical dislocation, epididimal WAT tissue rapidly removed and frozen in liquid nitrogen until analysis.
All animal procedures were performed according to the guidelines of the Charité universitätsmedizin Berlin and were approved by the Landsamt für Gesundheit und Soziales (Berlin, Germany) for the use of laboratory animals and according to the current version of the German Law on protection of animals for scientific purposes.
All calculations were analyzed with GraphPad Prism Software. Mean values were compared with t-test. A P value < 0.05 (two tailed) was considered significant.
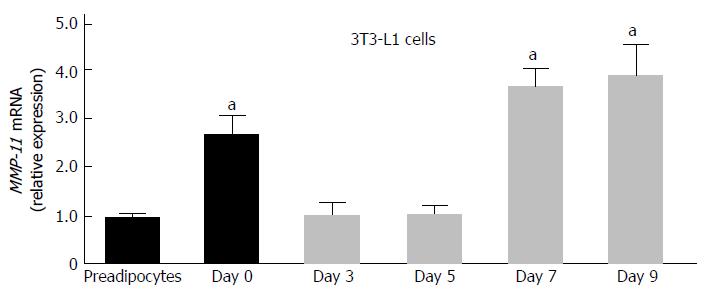
We first examined the expression of MMP-11 during 3T3-L1 adipogenesis. Total RNA was prepared at different stage of adipocyte cell differentiation and MMP-11 mRNA expression levels were measured. As shown in Figure 1, MMP-11 mRNA abundance was low in 3T3-L1 pre-adipocytes, increased in confluent culture cells, reaching maximum expression in mature 3T3-L1 adipocytes (Figure 1).
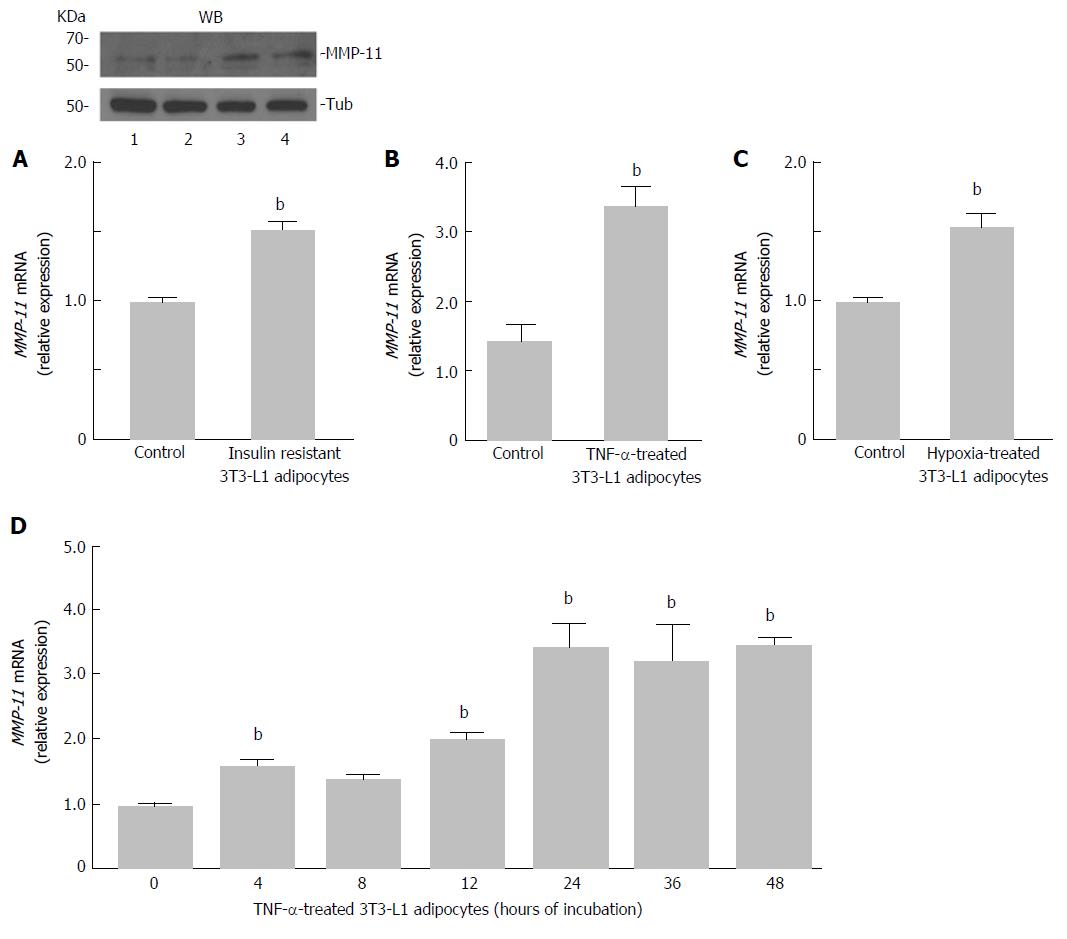
To induce insulin resistance in vitro, fully differentiated 3T3-L1 adipocytes were treated with TNF-α (2.5 nmol/L) and at the same time incubated in hypoxia (1% O2) for 24 h. Then, MMP-11 mRNA and protein expression levels were measured. As shown in Figure 2A, a clear increase in both mRNA and protein expression of the MMP-11 proteinase was observed in insulin resistant 3T3-L1 cells, as compared to normal, non-insulin resistant 3T3-L1 adipocytes. To better understand the effect of each treatment on MMP-11 expression, we carried out separate experiments in which MMP-11 mRNA levels were measured in fully differentiated 3T3-L1 adipocytes treated with either 2.5 nmol/L of TNF-α for 24 h, or subjected to 24 h hypoxia alone. As shown in Figure 2B, MMP-11 mRNA abundance was approximately four-fold higher in TNF-α treated cells compared to untreated 3T3-L1 adipocytes, thereby indicating that upregulation of MMP-11 expression can be at least in part regulated by the pro-inflammatory TNF-α molecule. On the other hand, hypoxia alone induced a slight but significant increase of MMP-11 mRNA expression compared to normoxia (Figure 2C). A time-course study of MMP-11 mRNA expression, using TNF-α alone over a 48 h period, showed that MMP-11 mRNA levels were significantly increased already after 4 h and this increase was maintained thereafter, reaching a plateau level at 24 h of exposure (Figure 2D).
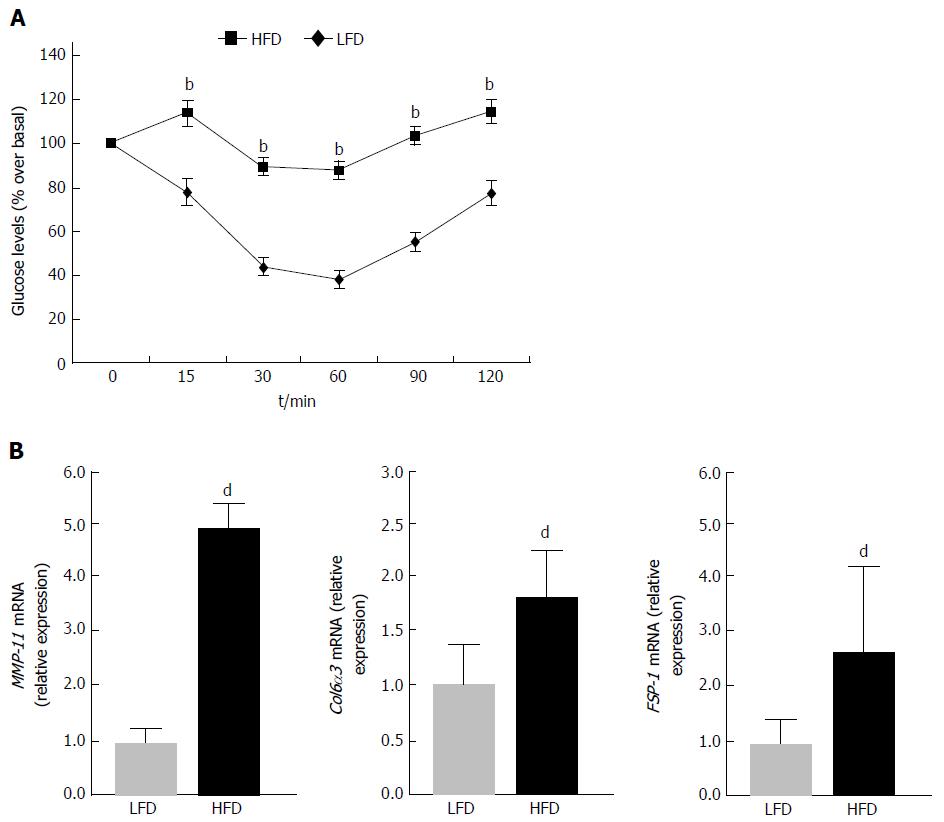
In attempt to validate the results obtained in vitro, in insulin-resistant 3T3-L1 adipocytes, mRNA expression studies were carried out also in vivo, in a mouse model of insulin resistance[23,24]. To this end, ten male mice were fed with HFD for 15 wk, whereas six other mice, which were used as controls, were subjected to normal chow diet, for the same time period. At the end of the diet treatment, mice fed with HFD were obese relative to control mice (43.6 ± 2.1 g vs 27.5 ± 1.7 g, respectively; P < 170829E-06), and developed insulin resistance as assessed by IITT (Figure 3A). Gene expression analysis to evaluate the levels of MMP-11 was then performed in both groups of mice. As shown in Figure 3B, MMP-11 mRNA was significantly higher in WAT from diet-induced obese mice than in WAT from lean mice, indicating that hyperexpression of MMP-11 may also occur in vivo, in the whole animal, after induction of an insulin-resistant state, thereby suggesting that abnormal activation of MMP-11 may have direct consequences on the molecular mechanism(s) related to adipocyte dysfunction. In this regard, the expression profile of two major fibrosis marker genes, collagen type VI alpha 3 (Col6α3) and fibroblast-specific protein 1 (FSP-1), was also measured in parallel experiments. As shown in Figure 3C, both these markers were significantly upregulated in WAT from obese mice compared to lean animals (Figure 3C), highlighting the possibility, in our obese mouse model, for an ECM dysregulation that would support the hypothesis that this ECM remodeling could indeed exert an adverse effect on adipocyte functions.
Adipose tissue is surrounded by ECM elements that provide the right support for adipocyte cell growth and expansion, and maintenance of adipocyte specific functions. Alterations in the organization and flexibility of the ECM as a cause of adipose tissue dysfunction have been reported[25], together with the observation that several MMPs could be involved in these adverse events[26].
In the present work, we focused our attention on the MMP-11 and its activation in conditions of insulin resistance, either in vitro, in 3T3-L1 mouse adipocytes, or in vivo, in obese mice. For the first time, in the present study, we show that MMP-11 was upregulated both in insulin resistant cells treated with TNF-α and/or hypoxia (two elements frequently associated with obesity), and in adipose tissues from insulin-resistant obese mice, suggesting that a direct link may exist between activation of MMP-11 and adipocyte cell dysfunction. Our data are consistent with previous observations that adipokines and hypoxia can alter the expression of MMPs. In this regard, it has been shown that TNF-α upregulated MMP-9 expression in the osteoblast-like MC3T3-E1 cell line[27], while in another study it was found that MMP-2 expression increased in response to hypoxia[28]. Furthermore, an involvement of both MMP-2 and MMP-11 in ECM degradation and collagen accumulation, associated with adipocyte dysfunction, was reported previously[29]. It can be hypothesized that upregulation of MMP-11 in insulin resistance may reflect the increase of nuclear proinflammatory transcription factor(s) whose effective role needs to be investigated.
Fibrosis is considered a new hallmark of the pathological dysfunction of WAT[25]. In our study, it is also interesting to note the alteration in the expression of genes related to fibrosis (Col6α3 and FSP-1) in WAT from nutritionally induced obese mice. A link between Col6α3 and MMP-11 has been reported before[29]. Thus, our data in this context well support previous reports that overexpression of MMPs, via degradation of ECM, could be implicated in adipose tissue remodeling[25], and this can play a role in the pathological dysfunction of adipose tissue, which leads to insulin resistance.
Our results appear to challenge findings obtained by studying the MMP-11 knock-in transgenic mouse[30], in which protection from diet-induced obesity was reported, together with a condition of enhanced glucose tolerance and insulin sensitivity due to increased IGF-I bioactivity[30]. The explanation for these divergent results may reside in the fact that overexpression of active MMP-11 in the skin of the transgenic animal may not necessarily reflect the situation in vitro, in 3T3-L1 adipocytes and in vivo, in WAT from diet-induced obese mice. On the other hand, the existence of compensatory mechanisms/changes that may contribute to counteract genetic manipulation has been proposed[31-35].
Overall, although further studies are still necessary to clarify the role of MMP-11 in insulin resistance, we believe our findings may contribute to shed light on the early process of adipose tissue dysfunction commonly associated with obesity and obesity-related insulin resistance.
Insulin resistance is a common metabolic disorder, in which peripheral target tissues fail to respond adequately to insulin, thereby predisposing to type 2 diabetes and other dysmetabolic conditions. More recent discoveries have now strengthened the hypothesis that adipose tissue dysfunction could be the primum movens in the development of insulin resistance. Therefore, studies have been focused on exploring the molecular mechanism(s) underlying adipocyte dysfunction.
Matrix metalloproteinases (MMPs) are a class of endopeptidases that contribute to the degradation of the extracellular matrix components. It has been discovered that they are involved in adipogenesis and remodelling of adipose tissue. A better understanding of the role and function of MMPs in adipose tissue will open new frontiers of investigations.
For the first time, the authors demonstrate that overexpression of MMP-11 occurs in in vitro and in vivo models of insulin resistance.
This study suggests that MMP-11 could be involved in the early stage of obesity-related insulin resistance. Thus, as a secreted serum protein, MMP-11 could serve as an early biomarker of adipose tissue dysfunction. Research in this area will lead to advancement in understanding the pathophysiology of insulin resistance, as well as advancement in drug development and therapy.
The paper is straight forward, well written, and it adds novel information on the topic.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Beltowski J, Roncucci L S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2168] [Cited by in F6Publishing: 2139] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 2. | Chiefari E, Tanyolaç S, Iiritano S, Sciacqua A, Capula C, Arcidiacono B, Nocera A, Possidente K, Baudi F, Ventura V. A polymorphism of HMGA1 is associated with increased risk of metabolic syndrome and related components. Sci Rep. 2013;3:1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Saltiel AR. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell. 2001;104:517-529. [PubMed] [Cited in This Article: ] |
| 4. | Chiefari E, Iiritano S, Paonessa F, Le Pera I, Arcidiacono B, Filocamo M, Foti D, Liebhaber SA, Brunetti A. Pseudogene-mediated posttranscriptional silencing of HMGA1 can result in insulin resistance and type 2 diabetes. Nat Commun. 2010;1:40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Foti D, Chiefari E, Fedele M, Iuliano R, Brunetti L, Paonessa F, Manfioletti G, Barbetti F, Brunetti A, Croce CM. Lack of the architectural factor HMGA1 causes insulin resistance and diabetes in humans and mice. Nat Med. 2005;11:765-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Chiefari E, Nevolo MT, Arcidiacono B, Maurizio E, Nocera A, Iiritano S, Sgarra R, Possidente K, Palmieri C, Paonessa F. HMGA1 is a novel downstream nuclear target of the insulin receptor signaling pathway. Sci Rep. 2012;2:251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Arcidiacono B, Iiritano S, Chiefari E, Brunetti FS, Gu G, Foti DP, Brunetti A. Cooperation between HMGA1, PDX-1, and MafA is Essential for Glucose-Induced Insulin Transcription in Pancreatic Beta Cells. Front Endocrinol (Lausanne). 2015;5:237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94:206-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 9. | Matziari M, Dive V, Yiotakis A. Matrix metalloproteinase 11 (MMP-11; stromelysin-3) and synthetic inhibitors. Med Res Rev. 2007;27:528-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Rio MC. Stromelysin-3, a particular member of the matrix metalloproteinase family. Kluwer Academic Edition. Vol. 4. Dordrecht: Kluwer Academic Publisher 2002; 81-107. [Cited in This Article: ] |
| 11. | Motrescu ER, Rio MC. Cancer cells, adipocytes and matrix metalloproteinase 11: a vicious tumor progression cycle. Biol Chem. 2008;389:1037-1041. [PubMed] [Cited in This Article: ] |
| 12. | Yan D, Dai H, Liu JW. Serum levels of MMP-11 correlate with clinical outcome in Chinese patients with advanced gastric adenocarcinoma. BMC Cancer. 2011;11:151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Pei D, Majmudar G, Weiss SJ. Hydrolytic inactivation of a breast carcinoma cell-derived serpin by human stromelysin-3. J Biol Chem. 1994;269:25849-25855. [PubMed] [Cited in This Article: ] |
| 14. | Mañes S, Mira E, Barbacid MM, Ciprés A, Fernández-Resa P, Buesa JM, Mérida I, Aracil M, Márquez G, Martínez-A C. Identification of insulin-like growth factor-binding protein-1 as a potential physiological substrate for human stromelysin-3. J Biol Chem. 1997;272:25706-25712. [PubMed] [Cited in This Article: ] |
| 15. | Messineo S, Laria AE, Arcidiacono B, Chiefari E, Luque Huertas RM, Foti DP, Brunetti A. Cooperation between HMGA1 and HIF-1 Contributes to Hypoxia-Induced VEGF and Visfatin Gene Expression in 3T3-L1 Adipocytes. Front Endocrinol (Lausanne). 2016;7:73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Costa V, Foti D, Paonessa F, Chiefari E, Palaia L, Brunetti G, Gulletta E, Fusco A, Brunetti A. The insulin receptor: a new anticancer target for peroxisome proliferator-activated receptor-gamma (PPARgamma) and thiazolidinedione-PPARgamma agonists. Endocr Relat Cancer. 2008;15:325-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Lo KA, Labadorf A, Kennedy NJ, Han MS, Yap YS, Matthews B, Xin X, Sun L, Davis RJ, Lodish HF. Analysis of in vitro insulin-resistance models and their physiological relevance to in vivo diet-induced adipose insulin resistance. Cell Rep. 2013;5:259-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Bianconcini A, Lupo A, Capone S, Quadro L, Monti M, Zurlo D, Fucci A, Sabatino L, Brunetti A, Chiefari E. Transcriptional activity of the murine retinol-binding protein gene is regulated by a multiprotein complex containing HMGA1, p54 nrb/NonO, protein-associated splicing factor (PSF) and steroidogenic factor 1 (SF1)/liver receptor homologue 1 (LRH-1). Int J Biochem Cell Biol. 2009;41:2189-2203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5674] [Cited by in F6Publishing: 5622] [Article Influence: 468.5] [Reference Citation Analysis (0)] |
| 20. | Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics. 2007;23:1289-1291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1799] [Cited by in F6Publishing: 1707] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 21. | Arnoldo L, Sgarra R, Chiefari E, Iiritano S, Arcidiacono B, Pegoraro S, Pellarin I, Brunetti A, Manfioletti G. A novel mechanism of post-translational modulation of HMGA functions by the histone chaperone nucleophosmin. Sci Rep. 2015;5:8552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Foryst-Ludwig A, Hartge M, Clemenz M, Sprang C, Hess K, Marx N, Unger T, Kintscher U. PPARgamma activation attenuates T-lymphocyte-dependent inflammation of adipose tissue and development of insulin resistance in obese mice. Cardiovasc Diabetol. 2010;9:64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Böhm C, Benz V, Clemenz M, Sprang C, Höft B, Kintscher U, Foryst-Ludwig A. Sexual dimorphism in obesity-mediated left ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2013;305:H211-H218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Lombardo GE, Arcidiacono B, De Rose RF, Lepore SM, Costa N, Montalcini T, Brunetti A, Russo D, De Sarro G, Celano M. Normocaloric Diet Restores Weight Gain and Insulin Sensitivity in Obese Mice. Front Endocrinol (Lausanne). 2016;7:49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18:470-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 597] [Cited by in F6Publishing: 612] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 26. | Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:a005058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1129] [Cited by in F6Publishing: 1364] [Article Influence: 104.9] [Reference Citation Analysis (0)] |
| 27. | Tsai CL, Chen WC, Hsieh HL, Chi PL, Hsiao LD, Yang CM. TNF-α induces matrix metalloproteinase-9-dependent soluble intercellular adhesion molecule-1 release via TRAF2-mediated MAPKs and NF-κB activation in osteoblast-like MC3T3-E1 cells. J Biomed Sci. 2014;21:12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Trayhurn P. Hypoxia and adipocyte physiology: implications for adipose tissue dysfunction in obesity. Annu Rev Nutr. 2014;34:207-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 29. | Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 778] [Cited by in F6Publishing: 778] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 30. | Motrescu ER, Blaise S, Etique N, Messaddeq N, Chenard MP, Stoll I, Tomasetto C, Rio MC. Matrix metalloproteinase-11/stromelysin-3 exhibits collagenolytic function against collagen VI under normal and malignant conditions. Oncogene. 2008;27:6347-6355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Dali-Youcef N, Hnia K, Blaise S, Messaddeq N, Blanc S, Postic C, Valet P, Tomasetto C, Rio MC. Matrix metalloproteinase 11 protects from diabesity and promotes metabolic switch. Sci Rep. 2016;6:25140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Speakman J, Hambly C, Mitchell S, Król E. The contribution of animal models to the study of obesity. Lab Anim. 2008;42:413-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Chiefari E, Paonessa F, Iiritano S, Le Pera I, Palmieri D, Brunetti G, Lupo A, Colantuoni V, Foti D, Gulletta E. The cAMP-HMGA1-RBP4 system: a novel biochemical pathway for modulating glucose homeostasis. BMC Biol. 2009;7:24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Iiritano S, Chiefari E, Ventura V, Arcidiacono B, Possidente K, Nocera A, Nevolo MT, Fedele M, Greco A, Greco M. The HMGA1-IGF-I/IGFBP system: a novel pathway for modulating glucose uptake. Mol Endocrinol. 2012;26:1578-1589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Pullinger CR, Goldfine ID, Tanyolaç S, Movsesyan I, Faynboym M, Durlach V, Chiefari E, Foti DP, Frost PH, Malloy MJ. Evidence that an HMGA1 gene variant associates with type 2 diabetes, body mass Index, and high-density lipoprotein cholesterol in a Hispanic-American population. Metab Syndr Relat Disord. 2014;12:25-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |