Published online Feb 15, 2017. doi: 10.4239/wjd.v8.i2.66
Peer-review started: September 19, 2016
First decision: October 21, 2016
Revised: December 6, 2016
Accepted: December 27, 2016
Article in press: December 28, 2016
Published online: February 15, 2017
To investigate the association of NFKB1 gene -94 ATTG insertion/deletion (rs28362491) polymorphism with inflammatory markers and risk of diabetic nephropathy in Asian Indians.
A total of 300 subjects were recruited (100 each), normoglycemic, (NG); type 2 diabetes mellitus (T2DM) without any complications (DM) and T2DM with diabetic nephropathy [DM-chronic renal disease (CRD)]. Analysis was carried out by polymerase chain reaction-restriction fragment length polymorphism and ELISA. Pearson’s correlation, analysis of variance and logistic regression were used for statistical analysis.
The allelic frequencies of -94 ATTG insertion/deletion were 0.655/0.345 (NG), 0.62/0.38 (DM) and 0.775/0.225 (DM-CRD). The -94 ATTG ins allele was associated with significantly increased levels of urinary monocyte chemoattractant protein-1 (uMCP-1); uMCP-1 (P = 0.026) and plasma tumor necrosis factor-alpha (TNF-α); TNF-α (P = 0.030) and almost doubled the risk of diabetic nephropathy (OR = 1.91, 95%CI: 1.080-3.386, P = 0.025).
-94 ATTG ins/ins polymorphism might be associated with increased risk of developing nephropathy in Asian Indian subjects with diabetes mellitus.
Core tip: Type 2 diabetes mellitus (T2DM) is considered as long standing inflammatory disease. Diabetic nephropathy (DN) is the most common micro-vascular complication of T2DM. Pro-inflammatory cytokines like Monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor-alpha (TNF-α) plays a crucial role in the pathogenesis of DN. Therefore we investigated -94 ins/del ATTG polymorphism in NFKB1 gene and its association with the risk of DN in Asian Indians. -94 ins/del ATTG single nucleotide polymorphism was found to increase the urinary MCP-1 and plasma TNF-α levels. Our findings open a new area of research to explore that -94 ins/del ATTG may be considered as genetic markers for early detection of diabetic patients who are at greater risk of development of nephropathy.
- Citation: Gautam A, Gupta S, Mehndiratta M, Sharma M, Singh K, Kalra OP, Agarwal S, Gambhir JK. Association of NFKB1 gene polymorphism (rs28362491) with levels of inflammatory biomarkers and susceptibility to diabetic nephropathy in Asian Indians. World J Diabetes 2017; 8(2): 66-73
- URL: https://www.wjgnet.com/1948-9358/full/v8/i2/66.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i2.66
Chronic renal disease (CRD) is an intricate pathological process, often leading to end stage renal disease. The causes of CRD are quite multi-factorial ranging from infections to heredity, but type 2 diabetes mellitus (T2DM) is the major culprit amongst them[1]. In spite of the improvement in our knowledge about the etiopathogenesis of diabetic nephropathy (DN), the intricate mechanisms leading to the development of renal injury from chronic hyperglycemia are not yet fully understood. DN has been considered a micro-vascular complication of hyperglycemia, but various clinical and experimental studies have observed that there is a close link between hyperglycemia, inflammation and oxidative stress (OS)[2]. OS may also be involved in promoting a low grade systemic inflammation in patients with T2DM and vice versa[3]. Nuclear factor-kappa B (NF-κB) activation through hyperglycemia induced OS may lead to increased concentration of inflammatory cytokines[4].
NF-κB was identified as a transcription factor which controls the expression of numerous genes affecting immune response, inflammation, cell-growth control, apoptosis and therefore, is an emerging candidate for studies on the pathogenesis of inflammatory diseases including DN. There are five members of the NF-κB family in mammals: NF-κB1: p105/p50, NF-κB2: p52/p100, RelA: p65, RelB, and c-Rel. The chief form of NF-κB is a hetero-dimer of the p50 and p65/RelA subunits, encoded by the NFKB1 and RelA gene. Normally, inactive NF-κB is found in the cytoplasm bound to IkBs, which are specific inhibitor proteins in cytoplasm. Cell when exposed to a variety of proinflammatory stimuli leads to the quick phosphorylation followed by ubiquitinylation, and finally proteolytic breakdown of I-κB. This causes transfer of NF-κB in nucleus and thus leading to increased transcription of gene[5]. NF-κB transcriptionally regulates many downstream proinflammatory genes, mainly including monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-alpha (TNF-α)[6].
MCP-1 is an important proinflammatory chemokine which affects the recruitment and function of monocyte[7]. MCP-1 is synthesized in response to a various proinflammatory stimuli by kidney cell[8]. A study done by Wada et al[9] in 2000 has shown that expression of MCP-1 increases in inflammation induced kidney diseases including DN. Urinary MCP-1 (uMCP-1) is a potential biomarker for renal damage[10]. Hyperglycemia induced secretion of abundant MCP-1 from renal parenchymal cells, attract monocytes into the kidney stimulating myofibroblast-like properties in mesangial cells. Kidney macrophages when exposed to MCP-1 in diabetic milieu promotes activation of macrophage. Thus, leading to release of reactive oxygen species (ROS), various pro-inflammatory cytokines and profibrotic growth factors[11,12]. Thus, resulting in exaggerated inflammation that leads to renal injury through proliferation of myofibroblast, augmented production of extracellular matrix by mesangial cells and fibroblasts.
TNF-α is a well known proinflammatory cytokine associated with systemic inflammation[13,14]. It is produced predominantly by macrophages and monocytes[13,14]. TNF-α acts via NF-κB signaling and mediates the transcription of various cytokines performing roles in cell survival, proliferation, inflammatory responses, cell adhesion and inflammation[15]. A study has shown that there is upregulation of TNF-α expression in glomeruli of diabetic rats[16]. TNF-α is well acknowledged to cause damage to renal cells by enhancing renal hypertrophy, hemodynamic imbalance, albumin permeability[17]. The harmful effects of these responses lead to the development of renal disease in patients with T2DM, hence resulting in the progression of renal failure.
In addition to poor glycemic control, OS and inflammation; genetic factors seem to be main determinants of DN in terms of both occurrence and severity[18]; however the genetic mechanism causing DN is still unexplored. In our knowledge, there is no study available regarding the polymorphisms of NFKB1 and their correlation with levels of uMCP-1 and plasma TNF-α. We have reported[19] increased uMCP-1, plasma TNF-α levels in subjects with DN when compared to subjects with T2DM without nephropathy and observed a positive correlation between uMCP-1 and plasma TNF-α[20]. We have also highlighted that DN is associated with TNFA gene single nucleotide polymorphism (SNP)[20]. In recent times, a new functional NFKB1 promoter SNP consisting of a insertion/deletion (-94ins/del ATTG) (rs28362491) has been identified which can elicit a regulatory effect on the NFKB1 gene[21]. Since above mentioned polymorphism has been associated with various inflammatory diseases, autoimmune diseases and cancers[22], therefore, it is worthwhile to further investigate the association of -94 ins/del ATTG NFKB1 gene SNP with levels of uMCP-1, plasma TNF-α and nephropathy risk in subjects with T2DM.
The present study comprises of total 300 subjects visiting Nephrology Outpatient Clinic and Medicine OPD at University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi. Subjects were divided into three groups of 100 each namely; Group 1: Normoglycemic (NG), Group 2: Subjects with T2DM for ≥ 10 years without nephropathy (DM), Group 3: Subjects with T2DM for ≥ 5 years with nephropathy (DM-CRD). T2DM was diagnosed according to revised ADA criteria[23]. Detailed clinical history and physical examination were recorded. Blood pressure (BP) of subjects was estimated using sphygmomanometer in the sitting position after a resting period of 10 min. The estimated glomerular filtration rate (eGFR) was measured by Modification of Diet in Renal Disease Abbreviated Equation (MDRD)[24].
The presence of micro-albuminuria in T2DM subjects was detected by Urine Test 11 MAU dipstick (Piramal Diagnostic, sensitivity: 10-15 mg/dL), and all participants having proteinuria and micro-albuminuria were clubbed in Group 3. All participants with nephropathy were in pre-dialysis stage. Normoglycemic (Group 1) subjects were recruited from employees of UCMS and GTB Hospital with the following criteria: (1) they did not have of diabetes mellitus (fasting plasma glucose < 100 mg% or postprandial glucose < 140 mg% or HbA1c < 5.7%) according to ADA criteria; (2) there was no presence of diabetes in their first or second degree relatives; and (3) they had normal BP, with systolic and diastolic BP not > 120 mmHg and 80 mmHg[25].
To circumvent any possible confounding factors, patients having renal disorders (hypertensive nephropathy, chronic glomerular nephritis, chronic interstitial disease, ischemic nephropathy, obstructive nephropathy), acute and chronic infections, congestive heart failure, malignancy and liver disorder were not included into the study. All subjects in Group 3 had retinopathy; but participants with macro-vascular complications like coronary artery disease and stroke were not included into the study. Patients taking renin-angiotension aldosterone system inhibitors, aspirin and vitamin D analogues were advised to discontinue these drugs for a period of a week before inclusion in the study since they have been found to influence the synthesis of uMCP-1 and TNF-α. However, patients were prescribed beta-blockers to control BP in that duration of one week. The Institutional Ethics Committee for Human Research approved the protocol of this study (approval number-UCMS/IEC-HR/2010/10). Prior to the inclusion into the present study, informed written consent was taken from all participants.
Under aseptic conditions fasting venous blood samples were withdrawn and collected into EDTA and fluoride vials. For glycosylated hemoglobin (HbA1c) 200 µL whole blood was preserved at 4 °C-8 °C and processed within one week of collection. Blood samples collected in EDTA vial was subjected to centrifugation at 3000 rpm for 10 min in order to separate the plasma. Early morning first mid-stream urine sample was collected and stored in aliquots at -20 °C for estimation of MCP-1, albumin and creatinine.
Routine investigations such as fasting and post-prandial plasma glucose, urea, creatinine and uric acid were carried out using commercially available kits on autoanalyser (Olympus AU-400). HbA1c was estimated by ion-exchange resin chromatography using commercially available kits (Fortress, United Kingdom). Urinary protein excretion was expressed as albumin to creatinine ratio.
uMCP-1 (Weldon, California; sensitivity less than 7.8 pg/mL) and plasma TNF-α (Diaclone, France; sensitivity less than 8 pg/mL) were estimated by commercially available ELISA kit.
Cellular DNA of every individual was extracted from 200 μL EDTA-anticoagulated peripheral blood sample by means of DNA isolation kit (Zymo research, United States). The polymerase chain reaction was carried out in Thermocycler (Eppendorf Mastercycler Gradient-5331). In brief, 0.1 μg of DNA was amplified in a reaction mixture of 20 μL containing 0.5 μmol/L each of the following primer pairs (Forward 5’-TGGGCACAAGTCGTTTATGA-3’ and Reverse 5’CTGGAGCCGGTAGGGAAG-3’). The reaction mixture also contained 0.5 mmol/L (dNTP mix), 2 μL (10 × PCR buffer) and 2.0 units Taq DNA polymerase, 2 mmol/L MgCl2. The PCR protocol consist an initial temperature of 94 °C (5 min) followed by 35 cycles of amplification (30 s at 94 °C, 45 s at 59 °C, and extension for 1 min at 72 °C). Final extension step was carried out for 2-min at 72 °C[22].
For the study of the -94 insertion/deletion ATTG SNP in NFKB1, PCR product (281/285 bp) was subjected to fast digestion with restriction enzyme PfIMI. PCR products was treated with enzyme PfIMI in at 37 °C for 1 h and inactivated at 65 °C for 20 min. The insertion allele (ins) was cut down into two fragments of 45 bp and 240 bp by PfIMI restriction enzyme. But, there was no cleavage at the deletion allele (del) that has only one ATTG at its promoter[22]. The bands of digested products were visualized in 2% agarose gel electrophoresis stained with ethidium bromide.
Demographic profiles and routine investigation was compared by χ2 and Student’s t test and one-way ANOVA was used. To associate all the study groups with genotype two-way ANOVA followed by post-hoc Tukey’s test was used. For association of genotypes with uMCP-1 and plasma TNF-α levels, analysis of variance was used. Logistic regressions was used to evaluate the risk of development of DN at the single SNP level. Power of sample size keeping 5% significance level and 80% power was calculated by genetic power calculator. A P value < 0.05 was considered statistically significant (two-tailed). All statistical tests were performed using SPSS version 20.
Biochemical and demographic parameters of the various study groups are shown in Table 1. There was no difference in sex distribution and BMI within all the three study groups. The subjects of Group 2 (DM) and Group 3 (DM-CRD) were older than Group 1 (NG) subjects; however the period of diabetes was more in Group 2 (DM) than Group 3 (DM-CRD) which was as per our selection criteria. Incidence of hypertension was significantly higher in Group 2 (DM) and Group 3 (DM-CRD) participants as suggested by raised SBP and DBP (P < 0.001) when compared to NG. Poor glucose control was observed in DM-CRD as compared to DM as suggested by significantly higher (P < 0.001) fasting, postprandial plasma glucose and HbA1c. Renal function tests suggested that blood urea, plasma creatinine, and uric acid were significantly higher (P < 0.001) and eGFR was decreased (P < 0.001) in Group 3 (DM-CRD) as compared to Group 2 (DM).
| Variables | NG (n = 100) | DM (n = 100) | DM-CRD (n = 100) |
| Age (yr) | 46.0 ± 4.0 | 56.40 ± 3.5 | 55.7 ± 4.2 |
| Sex ratio (male/female) | 52/48 | 54/46 | 52/48 |
| Duration of DM (yr) | - | 12.7 ± 1.5 | 8.1 ± 2.3d |
| BMI (kg/m2) | 20.1 ± 1.7 | 21.1 ± 2.1 | 21.6 ± 3.4 |
| SBP (mmHg) | 118.1 ± 0.5 | 138.0 ± 2.1b | 137.7 ± 2.8b |
| DBP (mmHg) | 75.2 ± 1.0 | 81.6 ± 1.9b | 82.8 ± 0.0b |
| Fasting glucose (mg/dL) | 82.4 ± 3.1 | 153.5 ± 3.5b | 184.3 ± 9.2bd |
| Postprandial glucose (mg/dL) | 118.2 ± 2.4 | 201.7 ± 10.1b | 261.1 ± 12.2bd |
| HbA1c (%) | 5.11 ± 0.46 | 7.10 ± 0.25b | 9.16 ± 0.16bd |
| Urea (mg/dL) | 31.5 ± 5.5 | 30.7 ± 5.8 | 93.2 ± 4.8bd |
| Creatinine (mg/dL) | 0.83 ± 0.23 | 0.90 ± 0.20 | 3.7 ± 1.5bd |
| Uric acid (mg/dL) | 4.2 ± 0.8 | 4.9 ± 0.6 | 9.1 ± 0.8bd |
| eGFR (mL/min per 1.73 m2) | 99.1 ± 0.7 | 96.4 ± 0.6 | 51.2 ± 0.9d |
| Urinary albumin/creatinine | - | - | 0.42 ± 0.35 |
The allele frequencies and genotype of the NFKB1 gene for -94 insertion/deletion ATTG SNP in various study groups are shown in Table 2. The distribution percentage of ins/ins, ins/del, del/del genotypes in Group 1 (NG), Group 2 (DM) and Group 3 (DM-CRD) (expressed in percentage) were 41%, 49% and 10%; 38%, 48% and 14%; and 61%, 33% and 6% respectively. The frequency of del/del genotype was significantly lower (P < 0.001) in Group 3 (DM-CRD) as compared to Group 2 (DM). However, allele frequencies of -94 insertion/deletion ATTG were 65.5%/34.5% in Group 1 (NG), 62%/38% in Group 2 (DM) and 77.5%/22.5% in Group 3 (DM-CRD).
| NG (n = 100) n (%) | DM (n = 100) n (%) | DM-CRD (n = 100) n (%) | |
| ins/ins | 41 (41) | 38 (38) | 61 (61) |
| ins/del | 49 ( 49) | 48 (48) | 33 (33) |
| del/del | 10 (10) | 14 (14) | 06b (06) |
| ins allele | 131 (65.5) | 124 (62) | 155 (77.5) |
| del allele | 69 (34.5) | 76 (38) | 45 (22.5) |
Correlation of -94 ins/del AGGT SNP with levels of uMCP-1 and plasma TNF-α have been studied and the results are shown in Table 3. The -94 ins allele were associated with increased levels of uMCP-1 (P = 0.026) and plasma TNF-α (P = 0.030) in the disease study groups, i.e., Group 2 (DM), Group 3 (DM-CRD).
| Inflammatory marker | Groups | NG (n = 100) | DM (n = 100) | DM-CRD (n = 100) | P value |
| uMCP-1 (pg/mg creatinine) | Total | 130.00 ± 42.22 | 271.00 ± 120.01 | 5632.70 ± 1007.20ab | |
| del/del | 85.1 ± 9.2 | 200.6 ± 66.5 | 4609.9 ± 900.6 | P = 0.026 | |
| ins/del | 110.9 ± 15.6 | 278.9 ± 105.9 | 5879.9 ± 1016.3 | ||
| ins/ins | 166.8 ± 26.8 | 302.2 ± 100.1 | 6405.1 ± 1550.6 | ||
| Plasma TNF-α (pg/mL) | Total | 15.55 ± 2.22 | 16.51 ± 3.75 | 21.38 ± 3.67ab | |
| del/del | 8.27 ± 1.06 | 10.21 ± 1.32 | 17.31 ± 1.17 | P = 0.030 | |
| ins/del | 11.55 ± 0.05 | 14.05 ± 0.18 | 19.31 ± 0.44 | ||
| ins/ins | 15.08 ± 1.15 | 16.36 ± 1.20 | 23.12 ± 0.70 |
The associations at the level of genotype is shown in Table 4. Highly significant association was observed for -94 ins/del AGGT polymorphism in subjects with Group 3 (DM-CRD) in comparison to Group 1 (NG); P = 0.022. In our present study, -94 ins SNP was found to increase risk for the development of DN by 1.91-fold in subjects with diabetes (OR = 1.91, 95%CI: 1.080-3.386, P = 0.025).
| Genotype | OR | 95%CI | P value |
| DM vs NG ref | 1.04 | 0.607-4.987 | 0.887 |
| DM-CRD vs NG ref | 1.95 | 1.101-3.467 | 0.022 |
| DM-CRD vs DM ref | 1.91 | 1.080-3.386 | 0.025 |
Polymorphism in the NFKB1 promoter region at position -94 ins/del AGGT has been correlated with many long standing inflammatory diseases like autoimmune diseases such as rheumatoid arthritis, asthma, AIDS, cancers and various diabetic complications[26,27]. Our study is the first to report the association of above mentioned polymorphism with DN in North Indian population. In the current study, we observed that the frequency distribution of ins/del is maximum in NG and DM subjects followed by ins/ins, with least distribution of del/del in the same. However the trend was different in DM-CRD subjects with respect to ins/del genotype which was less as compared to ins/ins this group. The frequency of different genotypes observed in the present study were in accordance with studies on NFKB1 polymorphism in healthy volunteer in different ethnic population like Turkish[22], Caucasians[28], English[29], Polish[30]. But our results were not in agreement with healthy Chinese population[28]. When our findings were compared with studies on inflammatory diseases like cancer, they are in accordance with a studies conducted in Asian by Huo et al[31] and Zhou et al[32]. However our results were in contrast with a genomic study on cancer conducted by Yang et al[28] in 2014. The dissimilarity of results could be due to diverse geographical distribution and ethnicity between our study and theirs was different, which could result in diverse genetic background.
Latest evidence has shown that the production of MCP-1 by kidney affected by diabetes along with TNF-α is a major cause of inflammation, renal injury and fibrosis in DN[10,17]. The present study is the foremost one to document the correlation of -94 ins/del AGGT SNP with levels of inflammatory markers namely uMCP-1 and plasma TNF-α in DN from North Indian patients. In our previous study, we have observed that plasma TNF-α and uMCP-1levels were significantly raised in patients with T2DM and so more in patients with DN[19]. To explicate the role of NFKB1 gene SNP in the development of DN, -94 ins/del AGGT SNP were analyzed in various study, i.e., Group 2 (DM) and Group 3 (DM-CRD) and further correlated with measured inflammatory markers like uMCP-1 and plasma TNF-αlevels. Interestingly, this study has also shown that ins allele was significantly associated with increased urinary MCP-1 and plasma TNF-α levels in NG as well as patient groups. However, there is no report in literature to compare our results.
A recent study has shown that TNF-α stimulates the MCP-1 production via NF-κB signalling pathway in rat astrocyte cultures[33]. TNF-α was found to increase p65 and phosphorylated p65 levels in nuclear extracts of rat astrocytes, hence augmenting MCP-1 levels[33]. This supports our finding that increased levels of TNF-α are associated with increased levels of uMCP-1.
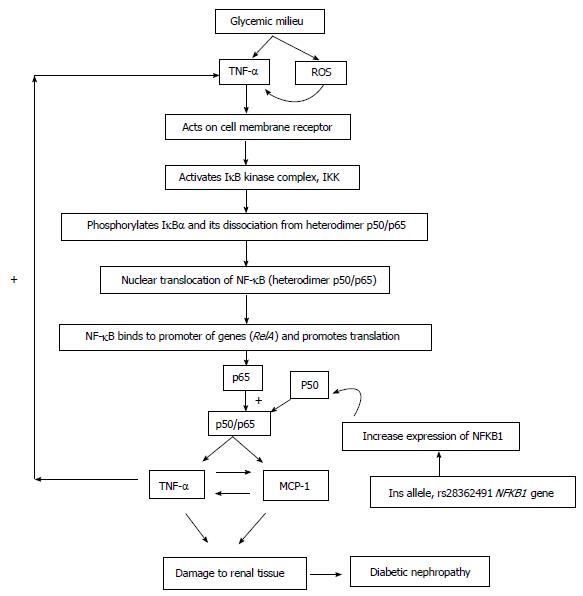
Genetic variations are known to play a vital role in determining risk of DN. A number of studies have investigated the relationship of ins allele of -94 ins/del AGGT polymorphism with various inflammatory diseases. Till date not a single study has tried to evaluate the association between this polymorphism and DN risk. Our study is first to document that patients with T2DM having ins/ins genotype were found to have increased risk of developing nephropathy. Latest studies have reported that p50 null mice have a significantly reduced inflammatory response in various models of inflammation such as asthma[34], arthritis[35], and autoimmune encephalomyelitis[36]. A similar study conducted in sporadic colorectal cancer (CRC)[37] and epithelial ovarian cancer (EOC)[31] has supported our findings which suggested that ins/ins genotype contribute to significantly increased risk of CRC and EOC. The probable mechanism of -94 ins/del AGGT polymorphism leading to increased risk of developing DN is explained in Figure 1. In almost all cell types, NF-κB complexes are typically localized in the cytoplasm where they bind to IKB inhibitory proteins. However, stimulation with hyperglycemia induced ROS and TNF-α leads to rapid phosphorylation of IKB via I-κB kinases complex which is then degraded by ubiquitin-proteosome pathway. On the other hand, simultaneously -94 ins/del AGGT polymorphism might lead to increased synthesis of p50 mRNA. Hence there will be increased production of p50/p65 hetrodimer complex which is a well known proinflammatory molecule, since p50/p65 hetrodimer acts on its downstream proinflammatory targets viz: MCP-1 and TNF-α, leading to over production of MCP-1 and TNF-α. Thus, there occurs a viscous cycle, i.e., MCP-1 is a positive regulator of TNF-α and vice versa. The above mentioned probable hypothesis might lead to increased risk of developing renal damage in T2DM. However results of a recent study from China[38] in bladder cancer is in contradiction to our findings which could be due to ethnic and geographical differences. Furthermore, the sample size of our study was fairly small than aforementioned bladder cancer study.
The results of the current study suggest that the NFKB1 promoter -94 ins/del AGGT SNP is associated with increased possibility of developing nephropathy in patients with diabetes. This SNP may be considered as genetic markers for susceptibility to develop nephropathy in patients with T2DM. The limitation of the study is the small sample size. Therefore, further evaluation is necessary in big sample size to look for the possibility of this polymorphisms as potential genetic markers in the near future. This would help to identify patients with type 2 diabetics who may be at higher risk of developing nephropathy.
This study was financially supported by Indian Council of Medical Research and Postgraduate Research Grant, University College of Medical Sciences, New Delhi. The authors are thankful to the Department of Biostatistics and Medical Informatics, University College of Medical Sciences, Delhi for statistical analysis.
Type 2 diabetes mellitus (T2DM) is considered as a long standing inflammatory disease. Nuclear factor-kappa B (NF-κB) controls the expression of numerous genes affecting inflammation, immune response. Immunogenic and inflammatory cytokines like monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor-alpha (TNF-α) plays a crucial role in the pathogenesis of micro-vascular complication of T2DM, i.e., diabetic nephropathy (DN) and clinical outcome.
In spite of the present advances in our knowledge about the etiopathogenesis of DN, the intricate mechanisms leading to the development of renal injury from chronic hyperglycemia are not yet fully understood. NFKB1 promoter polymorphism -94 ins/del ATTG has been associated with inflammatory diseases, autoimmune diseases and cancers. However, its role in the development of T2DM and DN has not been explored till date. The authors hypothesized that the -94 ins/del ATTG polymorphism would affect the levels of urinary MCP-1 and plasma TNF-α and therefore might be culprit in developing DN.
The authors have recently reported that -94 ATTG ins allele was associated with significantly increased levels of urinary MCP-1, plasma TNF-α and was found to increase risk for the development of DN by 1.91-fold in subjects with diabetes.
-94 ins/del AGGT polymorphisms can be considered as genetic marker for identifying those more susceptible and provide suitable interventions to delay the progression of DN. This study provides a ground for the development of newer anti-inflammatory therapeutic agents that may have potential to affect primary mechanisms contributing to the pathogenesis of DN.
DN: Diabetic nephropathy; NF-κB: Nuclear factor-kappa B; NFKB1: Nuclear factor-kappa B1 gene; T2DM: Type 2 diabetes mellitus; TNF-α: Tumor necrosis factor-alpha; uMCP-1: Urinary Monocyte chemoattractant protein-1.
The manuscript is well informative.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lehtonen SH, Sameer AS, Wada J S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089-2100. [PubMed] [Cited in This Article: ] |
| 2. | Pan HZ, Zhang L, Guo MY, Sui H, Li H, Wu WH, Qu NQ, Liang MH, Chang D. The oxidative stress status in diabetes mellitus and diabetic nephropathy. Acta Diabetol. 2010;47 Suppl 1:71-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Arnalich F, Hernanz A, López-Maderuelo D, Peña JM, Camacho J, Madero R, Vázquez JJ, Montiel C. Enhanced acute-phase response and oxidative stress in older adults with type II diabetes. Horm Metab Res. 2000;32:407-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067-2072. [PubMed] [Cited in This Article: ] |
| 5. | Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3652] [Cited by in F6Publishing: 3650] [Article Influence: 152.1] [Reference Citation Analysis (0)] |
| 6. | Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066-1071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3573] [Cited by in F6Publishing: 3522] [Article Influence: 130.4] [Reference Citation Analysis (0)] |
| 7. | Gu L, Tseng SC, Rollins BJ. Monocyte chemoattractant protein-1. Chem Immunol. 1999;72:7-29. [PubMed] [Cited in This Article: ] |
| 8. | Rovin BH, Yoshiumura T, Tan L. Cytokine-induced production of monocyte chemoattractant protein-1by cultured human mesangial cell. J Immunol. 1992;148:2148-2153. [Cited in This Article: ] |
| 9. | Wada T, Furuichi K, Sakai N, Iwata Y, Yoshimoto K, Shimizu M, Takeda SI, Takasawa K, Yoshimura M, Kida H. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 2000;58:1492-1499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 262] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 10. | Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:F697-F701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 11. | Ihm CG, Park JK, Hong SP, Lee TW, Cho BS, Kim MJ, Cha DR, Ha H. A high glucose concentration stimulates the expression of monocyte chemotactic peptide 1 in human mesangial cells. Nephron. 1998;79:33-37. [PubMed] [Cited in This Article: ] |
| 12. | Banba N, Nakamura T, Matsumura M, Kuroda H, Hattori Y, Kasai K. Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int. 2000;58:684-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 204] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Sugimoto H, Shikata K, Wada J, Horiuchi S, Makino H. Advanced glycation end products-cytokine-nitric oxide sequence pathway in the development of diabetic nephropathy: aminoguanidine ameliorates the overexpression of tumour necrosis factor-alpha and inducible nitric oxide synthase in diabetic rat glomeruli. Diabetologia. 1999;42:878-886. [PubMed] [Cited in This Article: ] |
| 14. | Pamir N, McMillen TS, Kaiyala KJ, Schwartz MW, LeBoeuf RC. Receptors for tumor necrosis factor-alpha play a protective role against obesity and alter adipose tissue macrophage status. Endocrinology. 2009;150:4124-4134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Luo SF, Fang RY, Hsieh HL, Chi PL, Lin CC, Hsiao LD, Wu CC, Wang JS, Yang CM. Involvement of MAPKs and NF-kappaB in tumor necrosis factor alpha-induced vascular cell adhesion molecule 1 expression in human rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2010;62:105-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Nakamura T, Fukui M, Ebihara I, Osada S, Nagaoka I, Tomino Y, Koide H. mRNA expression of growth factors in glomeruli from diabetic rats. Diabetes. 1993;42:450-456. [PubMed] [Cited in This Article: ] |
| 17. | Rivero A, Mora C, Muros M, García J, Herrera H, Navarro-González JF. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin Sci (Lond). 2009;116:479-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Makuc J, Petrovič D. A review of oxidative stress related genes and new antioxidant therapy in diabetic nephropathy. Cardiovasc Hematol Agents Med Chem. 2011;9:253-261. [PubMed] [Cited in This Article: ] |
| 19. | Gupta S, Gambhir JK, Kalra O, Gautam A, Shukla K, Mehndiratta M, Agarwal S, Shukla R. Association of biomarkers of inflammation and oxidative stress with the risk of chronic kidney disease in Type 2 diabetes mellitus in North Indian population. J Diabetes Complications. 2013;27:548-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Gupta S, Mehndiratta M, Kalra S, Kalra OP, Shukla R, Gambhir JK. Association of tumor necrosis factor (TNF) promoter polymorphisms with plasma TNF-α levels and susceptibility to diabetic nephropathy in North Indian population. J Diabetes Complications. 2015;29:338-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Karban AS, Okazaki T, Panhuysen CI, Gallegos T, Potter JJ, Bailey-Wilson JE, Silverberg MS, Duerr RH, Cho JH, Gregersen PK. Functional annotation of a novel NFKB1 promoter polymorphism that increases risk for ulcerative colitis. Hum Mol Genet. 2004;13:35-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 271] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 22. | Senol Tuncay S, Okyay P, Bardakci F. Identification of NF-kappaB1 and NF-kappaBIAlpha polymorphisms using PCR-RFLP assay in a Turkish population. Biochem Genet. 2010;48:104-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | American Diabetic Association. Standards of medical care in diabetes-2015. Diabetes Care. 2015;38:S1-S9. [Cited in This Article: ] |
| 24. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [PubMed] [Cited in This Article: ] |
| 25. | James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5429] [Cited by in F6Publishing: 5261] [Article Influence: 526.1] [Reference Citation Analysis (0)] |
| 26. | Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem. 1999;45:7-17. [PubMed] [Cited in This Article: ] |
| 27. | Romzova M, Hohenadel D, Kolostova K, Pinterova D, Fojtikova M, Ruzickova S, Dostal C, Bosak V, Rychlik I, Cerna M. NFkappaB and its inhibitor IkappaB in relation to type 2 diabetes and its microvascular and atherosclerotic complications. Hum Immunol. 2006;67:706-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Yang X, Li P, Tao J, Qin C, Cao Q, Gu J, Deng X, Wang J, Liu X, Wang Z. Association between NFKB1 -94ins/del ATTG Promoter Polymorphism and Cancer Susceptibility: An Updated Meta-Analysis. Int J Genomics. 2014;2014:612972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Mirza MM, Fisher SA, Onnie C, Lewis CM, Mathew CG, Sanderson J, Forbes A. No association of the NFKB1 promoter polymorphism with ulcerative colitis in a British case control cohort. Gut. 2005;54:1205-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Kurylowicz A, Hiromatsu Y, Jurecka-Lubieniecka B, Kula D, Kowalska M, Ichimura M, Koga H, Kaku H, Bar-Andziak E, Nauman J. Association of NFKB1 -94ins/del ATTG promoter polymorphism with susceptibility to and phenotype of Graves' disease. Genes Immun. 2007;8:532-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Huo ZH, Zhong HJ, Zhu YS, Xing B, Tang H. Roles of functional NFKB1 and β-TrCP insertion/deletion polymorphisms in mRNA expression and epithelial ovarian cancer susceptibility. Genet Mol Res. 2013;12:3435-3443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Zhou B, Qie M, Wang Y, Yan L, Zhang Z, Liang A, Wang T, Wang X, Song Y, Zhang L. Relationship between NFKB1 -94 insertion/deletion ATTG polymorphism and susceptibility of cervical squamous cell carcinoma risk. Ann Oncol. 2010;21:506-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Thompson WL, Van Eldik LJ. Inflammatory cytokines stimulate the chemokines CCL2/MCP-1 and CCL7/MCP-3 through NFkB and MAPK dependent pathways in rat astrocytes [corrected]. Brain Res. 2009;1287:47-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 34. | Yang L, Cohn L, Zhang DH, Homer R, Ray A, Ray P. Essential role of nuclear factor kappaB in the induction of eosinophilia in allergic airway inflammation. J Exp Med. 1998;188:1739-1750. [PubMed] [Cited in This Article: ] |
| 35. | Campbell IK, Gerondakis S, O'Donnell K, Wicks IP. Distinct roles for the NF-kappaB1 (p50) and c-Rel transcription factors in inflammatory arthritis. J Clin Invest. 2000;105:1799-1806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 132] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Hilliard B, Samoilova EB, Liu TS, Rostami A, Chen Y. Experimental autoimmune encephalomyelitis in NF-kappa B-deficient mice: roles of NF-kappa B in the activation and differentiation of autoreactive T cells. J Immunol. 1999;163:2937-2943. [PubMed] [Cited in This Article: ] |
| 37. | Mohd Suzairi MS, Tan SC, Ahmad Aizat AA, Mohd Aminudin M, Siti Nurfatimah MS, Andee ZD, Ankathil R. The functional -94 insertion/deletion ATTG polymorphism in the promoter region of NFKB1 gene increases the risk of sporadic colorectal cancer. Cancer Epidemiol. 2013;37:634-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Li P, Gu J, Yang X, Cai H, Tao J, Yang X, Lu Q, Wang Z, Yin C, Gu M. Functional promoter -94 ins/del ATTG polymorphism in NFKB1 gene is associated with bladder cancer risk in a Chinese population. PLoS One. 2013;8:e71604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |