Published online Oct 15, 2014. doi: 10.4239/wjd.v5.i5.586
Revised: April 10, 2014
Accepted: July 12, 2014
Published online: October 15, 2014
Hepatitis C virus (HCV) infection and diabetes mellitus are two major public health problems that cause devastating health and financial burdens worldwide. Diabetes can be classified into two major types: type 1 diabetes mellitus (T1DM) and T2DM. T2DM is a common endocrine disorder that encompasses multifactorial mechanisms, and T1DM is an immunologically mediated disease. Many epidemiological studies have shown an association between T2DM and chronic hepatitis C (CHC) infection. The processes through which CHC is associated with T2DM seem to involve direct viral effects, insulin resistance, proinflammatory cytokines, chemokines, and other immune-mediated mechanisms. Few data have been reported on the association of CHC and T1DM and reports on the potential association between T1DM and acute HCV infection are even rarer. A small number of studies indicate that interferon-α therapy can stimulate pancreatic autoimmunity and in certain cases lead to the development of T1DM. Diabetes and CHC have important interactions. Diabetic CHC patients have an increased risk of developing cirrhosis and hepatocellular carcinoma compared with non-diabetic CHC subjects. However, clinical trials on HCV-positive patients have reported improvements in glucose metabolism after antiviral treatment. Further studies are needed to improve prevention policies and to foster adequate and cost-effective programmes for the surveillance and treatment of diabetic CHC patients.
Core tip: Many studies have shown an association between type 2 diabetes mellitus (T2DM) and chronic hepatitis C (CHC) infection. The processes through which CHC is associated with T2DM seem to involve direct viral effects, insulin resistance, proinflammatory cytokines, and chemokines. Few data have been reported on the association of CHC and T1DM. A small number of studies indicate that interferon-α therapy can induce T1DM. Diabetic CHC patients have an increased risk of developing cirrhosis and hepatocellular carcinoma compared with non-diabetics. Clinical trials on hepatitis C virus-positive patients have reported improvements in glucose metabolism after antiviral treatment.
- Citation: Antonelli A, Ferrari SM, Giuggioli D, Di Domenicantonio A, Ruffilli I, Corrado A, Fabiani S, Marchi S, Ferri C, Ferrannini E, Fallahi P. Hepatitis C virus infection and type 1 and type 2 diabetes mellitus. World J Diabetes 2014; 5(5): 586-600
- URL: https://www.wjgnet.com/1948-9358/full/v5/i5/586.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i5.586
Hepatitis C virus (HCV) infection and diabetes mellitus (DM) are two major public health problems that cause devastating health and financial burdens worldwide[1,2]. Diabetes can be classified into two major types: type 1 (T1DM) and T2DM[3,4]. T2DM is a common endocrine disorder that encompasses multifactorial mechanisms. These mechanisms include resistance to the action of insulin, increased hepatic glucose production, and a defect in insulin secretion, all of which contribute to the development of overt hyperglycaemia[5]. T1DM is an immunologically mediated disease. Prevention and treatment of T1DM are hampered by the fact that the key immunological mechanisms of the pathogenesis of the disease are still under debate[6,7]. However, a Th1 immune response is involved in β-cell destruction[8] and the importance of islet autoantibodies has been highlighted[9-11].
Chronic hepatitis C (CHC) infection has a global prevalence of 2%-3%. Approximately 170 million people are thought to be currently infected (approximately 3% of the world’s population), and an additional 3-4 million are infected each year[12,13]. HCV is the main reason for liver transplantation in the developed world and the main cause of liver-related morbidity and mortality in a number of countries, including Italy. This virus is not only a frequent cause of chronic liver diseases, including hepatitis, cirrhosis, and hepatocellular carcinoma (HCC), but it is also involved in the pathogenesis of various autoimmune and rheumatic disorders (e.g., arthritis, vasculitis, sicca syndrome, porphyria cutanea tarda, lichen planus, nephropathies, and lung fibrosis) and in the development of B-cell lymphoproliferative diseases[14,15].
CHC is a multifaceted disorder that is associated with extrahepatic manifestations, including endocrinological disorders, thyroid disorders and diabetes[16,17].
In this paper, we review the increasing evidence linking HCV infection and DM in multiple fields (epidemiology, pathogenesis, clinical aspects, prevention, and treatment).
The liver plays an important role in carbohydrate metabolism, and liver diseases such as chronic hepatitis and cirrhosis are associated with a higher prevalence of disturbed glucose homeostasis, impaired glucose tolerance, and insulin resistance (IR)[18,19], which can eventually lead to DM[20-23]. Asymptomatic, moderate serum aminotransferase elevation has frequently been found in patients with DM, particularly in those with T2DM[24,25]. This phenomenon has often been related to fatty infiltration of the liver without further investigation[26,27]. In particular, steatosis has been related to IR and T2DM, beyond intracellular fat accumulation[28].
Liver fibrosis progression has also long been considered to be responsible for the development of IR and T2DM in patients with chronic liver diseases[29]. However, diabetes often occurs in the early stages of liver disease[30].
The aetiological factors that underlie the development of glucose homeostasis alterations were initially thought to be exclusively related to general long-term hepatocyte damage. However, later studies showed that patients with hepatitis B virus infection have a lower prevalence of T2DM compared with HCV-infected patients[31,32]. Thus, the question is as follows: “Does HCV infection itself have diabetogenic action?”
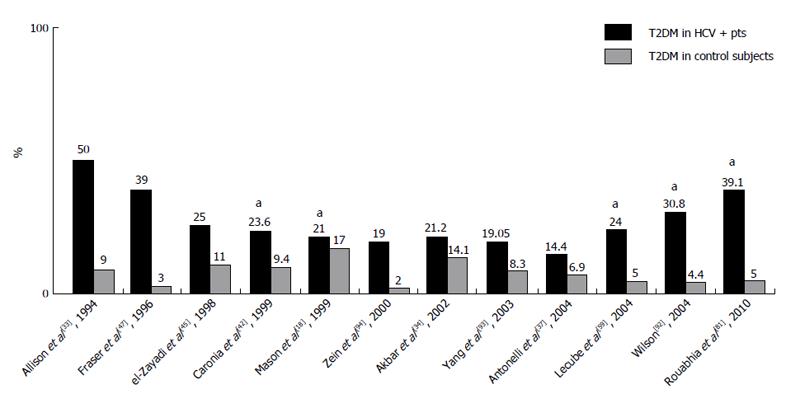
Since the discovery of HCV in 1989, attention has been paid to the association of CHC with the development of DM. Additionally from 1994[33] until now, several epidemiological studies on the seroprevalence of HCV have shown higher prevalences in diabetic patients than in controls (Figure 1). Moreover, analyses have shown a higher prevalence of DM in patients who are seropositive for HCV than in controls without HCV infection.
To analyse the epidemiological data, we searched for published studies in the PubMed database, covering the period from 1994 to December 2012. The literature search was performed using combinations of the terms “diabetes”, “diabetes mellitus”, “type 2 diabetes mellitus”, “T2DM”, “type 2 DM”, “non-insulin dependent diabetes”, or “NIDDM”; “hepatitis”, “hepatitis C”, “hepatitis C virus”, “HCV”, “HVC”, or “chronic hepatitis”; and “risk”, “risk factor”, “case-control”, “cohort”, “clinical trial”, “cross sectional”, “epidemiology”, “observational”, “meta-analysis”, “systematic review”, or “review”. For epidemiological studies, we only searched human studies and publications in English and Italian, the languages understood by the authors.
The data represent a very heterogeneous population regarding gender, age, and ethnic group. Globally, approximately seventy studies are in agreement with an association[18,26,30-96], although not all of them have shown significant data. However, some of the non-significant data may be attributed to small sample sizes and other methodological factors (Figure 1).
Certain negative data that are not in agreement with an association between HCV infection and T2DM have also been reported[97-104]. However, the number of published epidemiological studies that are in agreement with the association between HCV infection and T2DM is higher than the number of studies in disagreement with this hypothesis.
HCV is hepatotropic and noncytopathic; nevertheless, its genome has been identified in a number of tissues beyond the liver, including pancreatic acinar cells and epithelial cells of the pancreatic duct[105,106]. Although post-mortem studies have revealed that HCV replicates in the pancreas[107] and animal models have suggested a direct effect of HCV infection on IR in the liver[108], the evidence is scanty.
Of interest are the roles of structural and non-structural HCV proteins. HCV has an RNA genome of 9.6 kb that encodes approximately 3010 amino acids and is translated into structural (core, E1, and E2) and non-structural (NS3-NS5B) proteins. These proteins play a role in the development of IR and oxidative stress via reactive oxygen species at the cellular level[109-113]. The HCV core protein, alone or in combination with other viral proteins, increases phosphorylation of insulin receptor substrate-1 (IRS-1), which is the basis of IR[114-116]. Phosphorylated IRS-1 activates phosphatidylinositol 3-kinase (PI3K)[117,118], and the activation of PI3K and one of its downstream targets, Akt, is essential for most of the metabolic effects of insulin[119-126]. Therefore, defects at the level of the association of PI3K with IRS-1 and a lack of PI3K activation may contribute to IR and the increased prevalence of diabetes in HCV-infected patients. Indeed, this mechanism ultimately promotes glucose transporter-4 translocation to the plasma membrane to enhance glucose uptake[127,128]. Within the IR mechanism impairment of the activation of Akt/PKB is the key step that can inhibit glucose uptake[30,129,130].
The detailed molecular events leading to IR in HCV-infected patients are, however, unclear. Recent evidence supports the existence of a significant extrahepatic component of HCV-induced IR. Thus, the molecular pathogenesis of the glucose metabolism disturbances observed in hepatitis C is much more complex than expected[131].
Recently, Eslam et al[132] showed that polymorphisms in the IFNL3 (IL28B) region are associated with spontaneous and treatment-induced recovery from HCV infection. Furthermore, circumstantial evidence suggests a link between single-nucleotide polymorphisms in IFNL3 and lipid metabolism, steatosis, and IR in CHC. The emerging picture suggests that the responder genotypes of IFNL3 polymorphisms are associated with higher serum lipid levels and less frequent steatosis and IR[132].
Viral innate immune evasion strategies and human genetic determinants underlie the transition of acute HCV infection into viral persistence and chronic infection. Host genetic factors can influence both the outcome of the infection and the response to antiviral therapy. Recent insights into how HCV regulates immune signalling within the liver reveal a complex interaction of the patient’s genetic background with viral and host factors related to the innate immune triggering and control that dictate the outcome of HCV infection and immunity[133].
Beyond the direct effects of HCV on IRS-1/PI3K, the HCV core protein may induce IR indirectly via stimulation of the secretion of proinflammatory cytokines[115]. In patients with CHC, most likely due to HCV-induced inflammation, there is hypersecretion of insulin-resistant proinflammatory cytokines such as interleukin (IL)-6 and tumour necrosis factor (TNF)-α[134-138]. Proinflammatory cytokines also upregulate suppressors of cytokine signalling proteins as part of a negative feedback loop to attenuate cytokine signalling[139,140]. This phenomenon may contribute to increased gluconeogenesis due to a lack of Akt-mediated inhibition of phosphoenolpyruvate carboxykinase gene expression. In this context, it is interesting to note that leptin can modulate the action of insulin in liver cells by antagonising insulin-stimulated IRS-1 tyrosine phosphorylation, increasing phosphoenolpyruvate carboxykinase gene expression, and decreasing glucokinase expression, which results in increased gluconeogenesis[141]. Together with the increase in gluconeogenesis, the enhanced production and accumulation of lipids mediated by inhibition of the AMP-activated protein kinase occur after HCV infection[142]. Additionally TNF-α plays a role in lipid metabolism. Indeed, the lipolysis-stimulating effect of TNF-α leads to increased serum levels of free fatty acids, which reduces insulin sensitivity[143,144].
Cytokines are intercellular mediators involved in viral control and in the liver damage induced by infection with HCV. The complex cytokine network that operates during the initial infection allows the coordinated, effective development of both the innate and the adaptive immune responses. However, HCV interferes with cytokines at various levels and escapes the immune response by inducing a Th2/T cytotoxic 2 cytokine profile. The inability to control infection leads to the recruitment of inflammatory infiltrates into the liver parenchyma by interferon (IFN)-γ-inducible CXC chemokine ligand (CXCL)9, CXCL10, and CXCL11, which result in sustained liver damage and eventually liver cirrhosis. The most important systemic HCV-related extrahepatic diseases (mixed cryoglobulinemia, lymphoproliferative disorders, thyroid autoimmune disorders, and T2DM) are associated with complex dysregulation of the cytokine/chemokine network, involving proinflammatory and Th1 chemokines[145,146].
Few data on this association have been reported, and published studies have shown only small proportions of CHC patients positive for one or more markers of pancreatic autoimmunity[18,147-150].
Even rarer are reports on the potential association between autoimmune diabetes and acute HCV infection. Only two cases have been described in the literature[151,152]. Several mechanisms have been postulated to initiate the process. Even if HCV can infect extrahepatic tissue in patients with hepatitis C[16,107,153], no direct involvement of HCV in the onset of T1DM has been clarified yet. Nevertheless, the direct destruction of β-cells by viral infection could be a good explanation. Beyond the undemonstrated direct mechanisms, HCV infection surely initiates an immune reaction against β-cells or causes an acceleration of diabetes onset when an immune reaction against β-cells is already present. Some authors have also suggested the involvement of a process of molecular mimicry as a trigger of HCV-related autoimmunity[154,155]. Indeed, glutamic acid decarboxylase (GAD) 65 shares amino acid sequence similarities with antigenic regions of the HCV polyprotein[156]. Of interest, HCV/self-homologous autoantigenic regions are also mimicked by other microbial agents. Such mimics may give rise to β-cell autoimmunity through a multiple-hit mechanism of molecular mimicry[154,155,157]. Cross-reactive immunity does not exclude the possible involvement of additional factors, such as proinflammatory cytokines, which may act in concert, leading to the development and/or maintenance of pancreatic autoimmunity during acute HCV infection[156]. Another possibility is the induction of antibody reactivity against GAD and the development of full-blown diabetes, mediated by IL-18 and other proinflammatory cytokines. In particular, IL-18 is presumed to play a pathogenetic role in T1DM, specifically because this cytokine appears to be involved in acceleration of the development of overt disease[152,158-160]. IL-18 can induce both Th1 and Th2 responses, depending on the surrounding cytokines[161], and this cytokine plays a pathogenic role in several diseases[161], including acute hepatic injury[162]. Other proinflammatory cytokines, such as TNF-α and IL-1β, which are elevated in patients with acute hepatitis[163], can also induce autoimmune diabetes[164-167].
Immune aspects have been reported in both T1DM and T2DM, and based on the immunology, it is clear that the lines separating T1DM from latent autoimmune diabetes in adults (LADA) and T2DM are not well delineated[10,11,16,37,145,168-170].
The type of diabetes manifested by patients with CHC is not classical T2DM, and the labelling of HCV patients as having T2DM is purely conventional and possibly inaccurate. The lines separating T1DM from LADA and T2DM are fading away as new pathogenetic information is obtained[170].
Three studies have reported[37,38,171] that HCV patients with T2DM are leaner than T2DM controls and show significantly lower low-density lipoprotein-cholesterol levels and systolic and diastolic blood pressures. Furthermore, patients with HCV-associated mixed cryoglobulinaemia (MC + HCV) and T2DM had non-organ-specific autoantibodies more frequently (34% vs 18%, respectively) than did non-diabetic MC + HCV patients[37]. An immune-mediated mechanism for MC + HCV-associated diabetes has been postulated[37], and a similar pathogenesis might be involved in diabetes in HCV patients. This hypothesis is strengthened by the finding that autoimmune phenomena are more common in T2DM patients than previously thought[10]. However, as the prevalence of classic β-cell autoimmune markers is not increased in HCV patients[70], other immune phenomena might be involved[168]. Chemokines could be important in this context. In fact, in children with newly diagnosed T1DM, raised serum CXCL10 and normal chemokine (C-C motif) ligand 2 concentrations signal a predominantly Th1-driven autoimmune process, which shifts toward Th2 immunity 2 years after diagnosis[172].
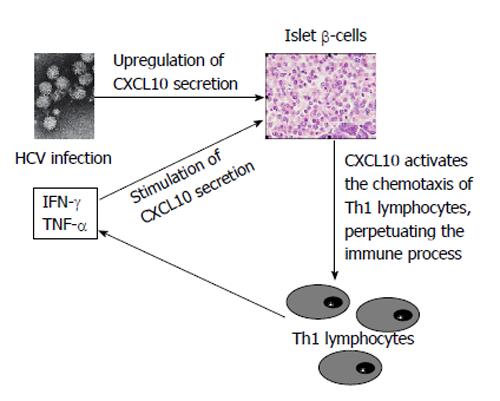
Based on the abovementioned concepts, HCV infection of β-cells[106] may act by upregulating CXCL10 gene expression and secretion (as previously shown in human hepatocytes[173]) and recruiting Th1 lymphocytes that secrete IFN-γ and TNF-α, which induce CXCL10 secretion by β-cells and thus perpetuate the immune cascade. This cascade may lead to the appearance of β-cell dysfunction in genetically predisposed subjects (Figure 2). Recently, certain studies have confirmed this hypothesis, demonstrating higher serum levels of CXCL10 in HCV patients with T2DM than in those without[16,169].
An important research area concerns the relationship between diabetes and IFN-α therapy in HCV-infected patients. In particular, studies have shown a high prevalence of markers of pancreatic autoimmunity in HCV-positive patients after or during IFN-α therapy, most likely due to the immunostimulatory effects of this cytokine. Indeed, IFN-α has antiviral, antiproliferative, and immunomodulatory activities[174]. Thus, in predisposed individuals, IFN-α can either induce a diabetogenic process or accelerate a diabetogenic process that is already underway[18,175,176]. For this reason, islet cell autoantibodies and GADAb should be investigated before and during IFN treatment to identify subjects who are at high risk of developing T1DM[177-180]. A small number of patients can develop de novo pancreatic autoimmunity and fall into a group of patients at risk of developing DM. In general, patients who are initially positive for organ-specific autoantibodies (in particular, thyroid- and pancreas-specific autoantibodies) and those who seroconvert seem to be at high risk of developing clinical autoimmune disease after treatment with IFN-α[181]. Timely suspension of IFN-α therapy is rarely accompanied by regression of clinical DM. No correlation has been documented between the response to antiviral therapy and the development of DM.
IFN-α increases HLA class I antigen expression and natural killer cell and T cell activities, and this cytokine may be an important cofactor in the development of a Th1 immune reaction. This reaction can contribute to the development of autoimmune disease by the activation of CD4+ lymphocytes that secrete IL-2, IFN-γ and TNF-β. These cytokines help in the generation of CD8+ cytotoxic T cells[182]. In addition to its immunomodulatory properties, IFN-α can also increase IR and induce hyperglycaemia[183-188]. Fabris et al[189] documented the first case of T1DM development during IFN-α therapy. Other studies suggest that IFN-α therapy can stimulate pancreatic autoimmunity and, in certain cases, lead to the development of T1DM[150,175,177,180,181,190-223].
The relationship with T1DM does not account for all of the effects of IFN-α therapy on diabetes. Indeed, from a completely different perspective, antiviral therapy with IFN should also be considered in HCV-positive patients because of its potential role in limiting the progression of this metabolic disturbance (see later discussion).
CHC is an insidiously progressive form of liver disease that leads to cirrhosis[224-226] and HCC[227-231]. Diabetic HCV-positive patients have increased risk compared with non-diabetic subjects, and DM itself seems to have a selective impact on HCC development[232-251].
The main characteristic of diabetic patients is IR, which plays a crucial role in fibrosis progression and has a negative impact on treatment responses to antiviral therapy in patients with CHC[52,252,253]. Reduced insulin sensitivity is at the basis of compensatory hyperinsulinemia and elevated levels of insulin-like growth factor 1 (IGF-1), which stimulates cell proliferation and inhibits apoptosis. Additionally, this phenomenon has strong mitogenic effects on a wide variety of cancer cell lines[254-256]. At the same time, insulin activates the IGF-1 receptor, which has a growth-promoting effect that includes modulating cell cycle progression. Excess insulin may also indirectly affect the development of cancer by downregulating the level of IGF-binding protein 1, which increases the level and bioavailability of total circulating IGF-1. Additional factors, such as obesity and physical inactivity, also cause hyperinsulinemia and are thus also ultimately associated with accelerated cancer progression[255-258].
Genotype differences in terms of liver disturbance progression have been described as well. Genotype 3a is more strongly correlated with steatosis than other genotypes[259,260], and the HCV genotype 3 may have a cytopathic effect[261]. Steatosis in genotype 1 infection is instead thought to be an expression of metabolic syndrome caused by the activation of proinflammatory mechanisms as well as underlying obesity and IR[262]. The degree of steatosis in this genotype is independent of the HCV viral load, and antiviral therapy does not improve steatosis in these patients. Similar data have been obtained for genotype 4 infection, whereas few data are available for genotype 2[263].
The presence of HCV infection in patients with DM may also increase the proportion of DM-related chronic nephrologic complications[86,264].
CHC is a complex disease with systemic effects that require a multidisciplinary treatment approach[265].
The potential relationship between HCV infection and the development of DM increases the need for the implementation of prevention measures. Prevention must be directed toward lifestyle changes that can reduce the risk of HCV infection and/or diabetes development[266]; regular diabetes screening for anti-HCV-positive people; and the analysis of other risk factors that can accelerate the progression of both CHC and DM, such as obesity, dyslipidaemia, and alcohol consumption. In these high-risk patients, comprehensive treatment, including lifestyle modifications, must be recommended. Animal models also provide clues regarding the prevention and clinical management of diabetes in the setting of HCV infection[108]. Indeed, identifying patients who are at risk of developing diabetes, and have CHC, reduces liver disturbance progression[267,268], the incidence of HCC and transplant-related morbidity and mortality. Additionally, this identification improves the response to antiviral therapy[269-271], even reducing the side effects of the treatment[270] by encouraging the pretreatment of IR and DM[265].
Moreover, clinical trials on HCV-positive patients have reported improvement in glucose metabolism after antiviral treatment[187]. As discussed earlier, many factors may surely affect the antiviral response that modulates the IFN signalling pathway. Among these factors, the HCV genotype, genetic host factors, and comorbidities have been taken into account. In particular, recent studies have reported obesity[272] and hypercholesterolaemia[273] as potential factors that interfere with a sustained viral response. These observations suggest additional therapeutic options for HCV infection, including dietary changes, anti-diabetic drugs, and statins. Concerning anti-diabetic drugs, it is not currently clear whether the best approach is to use a peroxisome proliferator-activated receptor agonist or a biguanide, such as metformin[274-276]. Concerning statins, these drugs are capable of inhibiting HCV replication in vitro[277-279] but not in vivo[280].
Further studies are needed to improve prevention policies and to foster adequate and cost-effective programmes for the surveillance and treatment of diabetic CHC patients. The final goal must be to cure two diseases, diabetes and CHC, with one multifaceted treatment.
Many epidemiological studies have shown an association between T2DM and CHC. The processes through which HCV is associated with DM seem to involve direct viral effects, IR, proinflammatory cytokines, chemokines, suppressors of cytokine signalling, and other immune-mediated mechanisms. Other factors, such as metabolic syndrome and a family history of diabetes, also seem to be important risk factors for the development of diabetes. Few data on the association of CHC and T1DM have been reported, and reports on the potential association between T1DM and acute HCV infection are even rarer. A small number of studies have indicated that IFN-α therapy can stimulate pancreatic autoimmunity and, in certain cases, lead to the development of T1DM. Diabetes and CHC have important interactions. Diabetic CHC patients have an increased risk of developing cirrhosis and HCC compared with non-diabetic CHC subjects. Additionally, clinical trials on HCV-positive patients have reported improvement in glucose metabolism after antiviral treatment. Further studies are needed to improve prevention policies and to foster adequate and cost-effective programmes for the surveillance and treatment of diabetic CHC patients.
P- Reviewer: Dashora U S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41-52. [PubMed] [Cited in This Article: ] |
| 2. | Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3804] [Cited by in F6Publishing: 3581] [Article Influence: 155.7] [Reference Citation Analysis (0)] |
| 3. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [PubMed] [Cited in This Article: ] |
| 4. | Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197. [PubMed] [Cited in This Article: ] |
| 5. | Ferrannini E. Physiology of glucose homeostasis and insulin therapy in type 1 and type 2 diabetes. Endocrinol Metab Clin North Am. 2012;41:25-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Barbeau WE. What is the key environmental trigger in type 1 diabetes--is it viruses, or wheat gluten, or both? Autoimmun Rev. 2012;12:295-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Askenasy EM, Askenasy N. Is autoimmune diabetes caused by aberrant immune activity or defective suppression of physiological self-reactivity? Autoimmun Rev. 2013;12:633-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Lo J, Clare-Salzler MJ. Dendritic cell subsets and type I diabetes: focus upon DC-based therapy. Autoimmun Rev. 2006;5:419-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Lernmark A, Larsson HE. Immune therapy in type 1 diabetes mellitus. Nat Rev Endocrinol. 2013;9:92-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Antonelli A, Tuomi T, Nannipieri M, Fallahi P, Nesti C, Okamoto H, Groop L, Ferrannini E. Autoimmunity to CD38 and GAD in Type I and Type II diabetes: CD38 and HLA genotypes and clinical phenotypes. Diabetologia. 2002;45:1298-1306. [PubMed] [Cited in This Article: ] |
| 11. | Antonelli A, Baj G, Marchetti P, Fallahi P, Surico N, Pupilli C, Malavasi F, Ferrannini E. Human anti-CD38 autoantibodies raise intracellular calcium and stimulate insulin release in human pancreatic islets. Diabetes. 2001;50:985-991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436-2441. [PubMed] [Cited in This Article: ] |
| 13. | Hepatitis C--global prevalence (update). Wkly Epidemiol Rec. 1999;74:425-427. [PubMed] [Cited in This Article: ] |
| 14. | Antonelli A, Ferri C, Galeazzi M, Giannitti C, Manno D, Mieli-Vergani G, Menegatti E, Olivieri I, Puoti M, Palazzi C. HCV infection: pathogenesis, clinical manifestations and therapy. Clin Exp Rheumatol. 2008;26:S39-S47. [PubMed] [Cited in This Article: ] |
| 15. | Ferri C, Antonelli A, Mascia MT, Sebastiani M, Fallahi P, Ferrari D, Pileri SA, Zignego AL. HCV-related autoimmune and neoplastic disorders: the HCV syndrome. Dig Liver Dis. 2007;39 Suppl 1:S13-S21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Antonelli A, Ferri C, Ferrari SM, Colaci M, Fallahi P. Immunopathogenesis of HCV-related endocrine manifestations in chronic hepatitis and mixed cryoglobulinemia. Autoimmun Rev. 2008;8:18-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Ferri C, Antonelli A, Mascia MT, Sebastiani M, Fallahi P, Ferrari D, Giunti M, Pileri SA, Zignego AL. B-cells and mixed cryoglobulinemia. Autoimmun Rev. 2007;7:114-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, Guo L, Jacob S, Regenstein FG, Zimmerman R. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 467] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 19. | Weinman SA, Belalcazar LM. Hepatitis C: a metabolic liver disease. Gastroenterology. 2004;126:917-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 574] [Cited by in F6Publishing: 565] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 21. | Cruezfeldt W, Frerichs H, Sickinger K. Liver diseases and diabetes mellitus. Progress in Liver Disease. New York: Grune and Stratton 1970; 371-407. [Cited in This Article: ] |
| 22. | Felig P, Sherin R. Carbohydrate homeostasis, liver and diabetes. Progress in Liver Disease. New York: Grune and Stratton 1976; 149-171. [Cited in This Article: ] |
| 23. | Kruszynska YT, McIntyre N. Carbohydrate metabolism. Offord Textbook of Clinical Hepatology. Oxford: Oxford University Press 1991; 129-143. [Cited in This Article: ] |
| 24. | Nagore N, Scheuer PJ. The pathology of diabetic hepatitis. J Pathol. 1988;156:155-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Morgan C, Hyland C, Young IF. Hepatitis C antibody and transaminase activities in blood donors. Lancet. 1990;335:921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Gray H, Wreghitt T, Stratton IM, Alexander GJ, Turner RC, O’Rahilly S. High prevalence of hepatitis C infection in Afro-Caribbean patients with type 2 diabetes and abnormal liver function tests. Diabet Med. 1995;12:244-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 87] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Salmela PI, Sotaniemi EA, Niemi M, Mäentausta O. Liver function tests in diabetic patients. Diabetes Care. 1984;7:248-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C, Tataranni PA. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:1889-1895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 482] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 29. | Romero-Gómez M. Insulin resistance and hepatitis C. World J Gastroenterol. 2006;12:7075-7080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Petit JM, Bour JB, Galland-Jos C, Minello A, Verges B, Guiguet M, Brun JM, Hillon P. Risk factors for diabetes mellitus and early insulin resistance in chronic hepatitis C. J Hepatol. 2001;35:279-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 193] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Mehta SH, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, Szklo M, Thomas DL. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 288] [Cited by in F6Publishing: 301] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 32. | Huang JF, Dai CY, Hwang SJ, Ho CK, Hsiao PJ, Hsieh MY, Lee LP, Lin ZY, Chen SC, Hsieh MY. Hepatitis C viremia increases the association with type 2 diabetes mellitus in a hepatitis B and C endemic area: an epidemiological link with virological implication. Am J Gastroenterol. 2007;102:1237-1243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135-1139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 332] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 34. | Akbar DH, Siddique AM, Ahmed MM. Prevalence of Type-2 diabetes in patients with hepatitis C and B virus infection in Jeddah, Saudi Arabia. Med Princ Pract. 2002;11:82-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Alexander GJ. An association between hepatitis C virus infection and type 2 diabetes mellitus: what is the connection? Ann Intern Med. 2000;133:650-652. [PubMed] [Cited in This Article: ] |
| 36. | AlDosary AA, Ramji AS, Elliott TG, Sirrs SM, Thompson DM, Erb SR, Steinbrecher UP, Yoshida EM. Post-liver transplantation diabetes mellitus: an association with hepatitis C. Liver Transpl. 2002;8:356-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Antonelli A, Ferri C, Fallahi P, Sebastiani M, Nesti C, Barani L, Barale R, Ferrannini E. Type 2 diabetes in hepatitis C-related mixed cryoglobulinaemia patients. Rheumatology (Oxford). 2004;43:238-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Antonelli A, Ferri C, Fallahi P, Pampana A, Ferrari SM, Goglia F, Ferrannini E. Hepatitis C virus infection: evidence for an association with type 2 diabetes. Diabetes Care. 2005;28:2548-2550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Arao M, Murase K, Kusakabe A, Yoshioka K, Fukuzawa Y, Ishikawa T, Tagaya T, Yamanouchi K, Ichimiya H, Sameshima Y. Prevalence of diabetes mellitus in Japanese patients infected chronically with hepatitis C virus. J Gastroenterol. 2003;38:355-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Bernsmeier C, Heim MH. Insulin resistance in chronic hepatitis C: mechanisms and clinical relevance. Swiss Med Wkly. 2009;139:678-684. [PubMed] [Cited in This Article: ] |
| 41. | Boschi-Pinto C, Stuver S, Okayama A, Trichopoulos D, Orav EJ, Tsubouchi H, Mueller N. A follow-up study of morbidity and mortality associated with hepatitis C virus infection and its interaction with human T lymphotropic virus type I in Miyazaki, Japan. J Infect Dis. 2000;181:35-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Caronia S, Taylor K, Pagliaro L, Carr C, Palazzo U, Petrik J, O’Rahilly S, Shore S, Tom BD, Alexander GJ. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059-1063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 281] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 43. | Chehadeh W, Abdella N, Ben-Nakhi A, Al-Arouj M, Al-Nakib W. Risk factors for the development of diabetes mellitus in chronic hepatitis C virus genotype 4 infection. J Gastroenterol Hepatol. 2009;24:42-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Chen HF, Li CY, Chen P, See TT, Lee HY. Seroprevalence of hepatitis B and C in type 2 diabetic patients. J Chin Med Assoc. 2006;69:146-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | el-Zayadi AR, Selim OE, Hamdy H, Dabbous H, Ahdy A, Moniem SA. Association of chronic hepatitis C infection and diabetes mellitus. Trop Gastroenterol. 1998;19:141-144. [PubMed] [Cited in This Article: ] |
| 46. | Everhart J. A confluence of epidemics: does hepatitis C cause type 2 diabetes? Hepatology. 2001;33:762-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Fraser GM, Harman I, Meller N, Niv Y, Porath A. Diabetes mellitus is associated with chronic hepatitis C but not chronic hepatitis B infection. Isr J Med Sci. 1996;32:526-530. [PubMed] [Cited in This Article: ] |
| 48. | Fukui M, Kitagawa Y, Nakamura N, Yoshikawa T. Hepatitis C virus and atherosclerosis in patients with type 2 diabetes. JAMA. 2003;289:1245-1246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Gulcan A, Gulcan E, Toker A, Bulut I, Akcan Y. Evaluation of risk factors and seroprevalence of hepatitis B and C in diabetic patients in Kutahya, Turkey. J Investig Med. 2008;56:858-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 50. | Grimbert S, Valensi P, Lévy-Marchal C, Perret G, Richardet JP, Raffoux C, Trinchet JC, Beaugrand M. High prevalence of diabetes mellitus in patients with chronic hepatitis C. A case-control study. Gastroenterol Clin Biol. 1996;20:544-548. [PubMed] [Cited in This Article: ] |
| 51. | Howard AA, Klein RS, Schoenbaum EE. Association of hepatitis C infection and antiretroviral use with diabetes mellitus in drug users. Clin Infect Dis. 2003;36:1318-1323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, McCaughan GW, George J. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected]. Gastroenterology. 2003;125:1695-1704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 519] [Cited by in F6Publishing: 550] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 53. | Imazeki F, Yokosuka O, Fukai K, Kanda T, Kojima H, Saisho H. Prevalence of diabetes mellitus and insulin resistance in patients with chronic hepatitis C: comparison with hepatitis B virus-infected and hepatitis C virus-cleared patients. Liver Int. 2008;28:355-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Jadoon NA, Shahzad MA, Yaqoob R, Hussain M, Ali N. Seroprevalence of hepatitis C in type 2 diabetes: evidence for a positive association. Virol J. 2010;7:304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Kaabia N, Ben Jazia E, Slim I, Fodha I, Hachfi W, Gaha R, Khalifa M, Hadj Kilani A, Trabelsi H, Abdelaziz A. Association of hepatitis C virus infection and diabetes in central Tunisia. World J Gastroenterol. 2009;15:2778-2781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Knobler H, Schattner A. Association of hepatitis C and diabetes mellitus. Ann Intern Med. 2001;135:141; author reply 142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Knobler H, Schihmanter R, Zifroni A, Fenakel G, Schattner A. Increased risk of type 2 diabetes in noncirrhotic patients with chronic hepatitis C virus infection. Mayo Clin Proc. 2000;75:355-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 58. | Labropoulou-Karatza C, Goritsas C, Fragopanagou H, Repandi M, Matsouka P, Alexandrides T. High prevalence of diabetes mellitus among adult beta-thalassaemic patients with chronic hepatitis C. Eur J Gastroenterol Hepatol. 1999;11:1033-1036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Lecube A, Hernández C, Genescà J, Esteban JI, Jardí R, Simó R. High prevalence of glucose abnormalities in patients with hepatitis C virus infection: a multivariate analysis considering the liver injury. Diabetes Care. 2004;27:1171-1175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 60. | Lonardo A, Adinolfi LE, Petta S, Craxì A, Loria P. Hepatitis C and diabetes: the inevitable coincidence? Expert Rev Anti Infect Ther. 2009;7:293-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Marzouk D, Sass J, Bakr I, El Hosseiny M, Abdel-Hamid M, Rekacewicz C, Chaturvedi N, Mohamed MK, Fontanet A. Metabolic and cardiovascular risk profiles and hepatitis C virus infection in rural Egypt. Gut. 2007;56:1105-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Mason A, Nair S. Is type II diabetes another extrahepatic manifestation of HCV infection? Am J Gastroenterol. 2003;98:243-246. [PubMed] [Cited in This Article: ] |
| 63. | Mayo MJ. Extrahepatic manifestations of hepatitis C infection. Am J Med Sci. 2003;325:135-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 64. | Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592-599. [PubMed] [Cited in This Article: ] |
| 65. | Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Hepatology. 2001;33:1554. [PubMed] [Cited in This Article: ] |
| 66. | Mehta SH, Strathdee SA, Thomas DL. Association between hepatitis C virus infection and diabetes mellitus. Epidemiol Rev. 2001;23:302-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Negro F, Alaei M. Hepatitis C virus and type 2 diabetes. World J Gastroenterol. 2009;15:1537-1547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 102] [Cited by in F6Publishing: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 68. | Ndako JA, Echeonwu GO, Shidali NN, Bichi IA, Paul GA, Onovoh E, Okeke LA. Occurrence of hepatitis C virus infection in type 2 diabetic patients attending Plateau state specialist hospital Jos Nigeria. Virol J. 2009;6:98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 69. | Zein NN. Hepatitis C and diabetes mellitus: an ongoing controversy. Am J Gastroenterol. 1998;93:2320-2322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 70. | Noto H, Raskin P. Hepatitis C infection and diabetes. J Diabetes Complications. 2006;20:113-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 71. | Nwokediuko SC, Oli JM. Hepatitis C virus infection in Nigerians with diabetes mellitus. Niger J Clin Pract. 2008;11:94-99. [PubMed] [Cited in This Article: ] |
| 72. | Olokoba AB, Badung LH, Abdulrahman MB, Salawu FK, Danburam A, Aderibigbe S, Midala J, Tidi SK. Hepatitis C virus infection in Nigerians with diabetes mellitus. Am J Sci Ind Res. 2010;1:135-138. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 73. | Okan V, Araz M, Aktaran S, Karsligil T, Meram I, Bayraktaroglu Z, Demirci F. Increased frequency of HCV but not HBV infection in type 2 diabetic patients in Turkey. Int J Clin Pract. 2002;56:175-177. [PubMed] [Cited in This Article: ] |
| 74. | Ozyilkan E, Erbaş T, Simşek H, Telatar F, Kayhan B, Telatar H. Increased prevalence of hepatitis C virus antibodies in patients with diabetes mellitus. J Intern Med. 1994;235:283-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 75. | Papatheodoridis GV, Chrysanthos N, Savvas S, Sevastianos V, Kafiri G, Petraki K, Manesis EK. Diabetes mellitus in chronic hepatitis B and C: prevalence and potential association with the extent of liver fibrosis. J Viral Hepat. 2006;13:303-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 76. | Parolin MB, Réa R, Vargas RM, de Almeida AC, Baldanzi GR, Lopes RW. [Prevalence of hepatitis C infection in patients with type 2 diabetes mellitus]. Arq Gastroenterol. 2006;43:77-80. [PubMed] [Cited in This Article: ] |
| 77. | Picerno I, Di Pietro A, Spataro P, Di Benedetto A, Romano G, Scoglio ME. Is diabetes mellitus a risk factor for HCV infection? Ann Ig. 2002;14:473-477. [PubMed] [Cited in This Article: ] |
| 78. | Qureshi H, Ahsan T, Mujeeb SA, Jawad F, Mehdi I, Ahmed W, Alam SE. Diabetes mellitus is equally frequent in chronic HCV and HBV infection. J Pak Med Assoc. 2002;52:280-283. [PubMed] [Cited in This Article: ] |
| 79. | Roaeid RBM, Ciasuddin ASM, Shakmak AA. Hepatitis C virus: seropositivity and diabetes in Benghazi, Libya. Diabetes Int. 2002;12:28-29. [Cited in This Article: ] |
| 80. | Ratziu V, Heurtier A, Bonyhay L, Poynard T, Giral P. Review article: an unexpected virus-host interaction--the hepatitis C virus-diabetes link. Aliment Pharmacol Ther. 2005;22 Suppl 2:56-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Rouabhia S, Malek R, Bounecer H, Dekaken A, Bendali Amor F, Sadelaoud M, Benouar A. Prevalence of type 2 diabetes in Algerian patients with hepatitis C virus infection. World J Gastroenterol. 2010;16:3427-3431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 36] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 82. | Ryu JK, Lee SB, Hong SJ, Lee S. Association of chronic hepatitis C virus infection and diabetes mellitus in Korean patients. Korean J Intern Med. 2001;16:18-23. [PubMed] [Cited in This Article: ] |
| 83. | Sangiorgio L, Attardo T, Gangemi R, Rubino C, Barone M, Lunetta M. Increased frequency of HCV and HBV infection in type 2 diabetic patients. Diabetes Res Clin Pract. 2000;48:147-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Simó R, Hernández C, Genescà J, Jardí R, Mesa J. High prevalence of hepatitis C virus infection in diabetic patients. Diabetes Care. 1996;19:998-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 189] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 85. | Singal AK, Ayoola AE. Prevalence and factors affecting occurrence of type 2 diabetes mellitus in Saudi patients with chronic liver disease. Saudi J Gastroenterol. 2008;14:118-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 86. | Soma J, Saito T, Taguma Y, Chiba S, Sato H, Sugimura K, Ogawa S, Ito S. High prevalence and adverse effect of hepatitis C virus infection in type II diabetic-related nephropathy. J Am Soc Nephrol. 2000;11:690-699. [PubMed] [Cited in This Article: ] |
| 87. | Thuluvath PJ, John PR. Association between hepatitis C, diabetes mellitus, and race. a case-control study. Am J Gastroenterol. 2003;98:438-441. [PubMed] [Cited in This Article: ] |
| 88. | Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Community-based study of hepatitis C virus infection and type 2 diabetes: an association affected by age and hepatitis severity status. Am J Epidemiol. 2003;158:1154-1160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 89. | Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Hepatitis C virus infection and the development of type 2 diabetes in a community-based longitudinal study. Am J Epidemiol. 2007;166:196-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 90. | Wang LF, Wu CH, Shan Y, Fan XH, Huo N, Lu HY, Xu XY. Prevalence of abnormal glycometabolism in patients with chronic hepatitis C and related risk factors in China. Chin Med J (Engl). 2011;124:183-188. [PubMed] [Cited in This Article: ] |
| 91. | White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 317] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 92. | Wilson C. Hepatitis C infection and type 2 diabetes in American-Indian women. Diabetes Care. 2004;27:2116-2119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Yang SQ, Chen HS, Jiang D, Wei L, Ji LN, Wang Y. [Relationship between chronic hepatitis C and type II diabetes mellitus]. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 2003;17:46-49. [PubMed] [Cited in This Article: ] |
| 94. | Zein NN, Abdulkarim AS, Wiesner RH, Egan KS, Persing DH. Prevalence of diabetes mellitus in patients with end-stage liver cirrhosis due to hepatitis C, alcohol, or cholestatic disease. J Hepatol. 2000;32:209-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 164] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 95. | Zein CO, Levy C, Basu A, Zein NN. Chronic hepatitis C and type II diabetes mellitus: a prospective cross-sectional study. Am J Gastroenterol. 2005;100:48-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 96. | Zhao P, Wang JB, Jiao J. [Investigation on the incidence of diabetes in chronic hepatitis C patients and their HCV genotypes]. Zhonghua Ganzangbing Zazhi. 2006;14:86-88. [PubMed] [Cited in This Article: ] |
| 97. | Adegoke OA, Kolawole BA, Ikem RT, Adediran A, Aboderin AO, Salawu A. Seroprevalence of hepatitis C virus infection in Nigerians with type 2 diabetes mellitus. Niger J Clin Pract. 2008;11:199-201. [PubMed] [Cited in This Article: ] |
| 98. | Balogun WO, Adeleye JO, Akinlade KS, Kuti M, Otegbayo JA. Low prevalence of hepatitis-C viral seropositivity among patients with type-2 diabetes mellitus in a tertiary hospital. J Natl Med Assoc. 2006;98:1805-1808. [PubMed] [Cited in This Article: ] |
| 99. | Costa LM, Mussi AD, Brianeze MR, Souto FJ. Hepatitis C as a risk factor for diabetes type 2: lack of evidence in a hospital in central-west Brazil. Braz J Infect Dis. 2008;12:24-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 100. | Mangia A, Schiavone G, Lezzi G, Marmo R, Bruno F, Villani MR, Cascavilla I, Fantasia L, Andriulli A. HCV and diabetes mellitus: evidence for a negative association. Am J Gastroenterol. 1998;93:2363-2367. [PubMed] [Cited in This Article: ] |
| 101. | Sotiropoulos A, Peppas TA, Skliros E, Apostolou O, Kotsini V, Pappas SI. Low prevalence of hepatitis C virus infection in Greek diabetic patients. Diabet Med. 1999;16:250-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Perret JL, Moussavou-Kombila JB, Delaporte E, Pemba LF, Larouze B. [Lack of association between hepatitis C virus infection and diabetes mellitus in Gabon]. Gastroenterol Clin Biol. 2000;24:135-136. [PubMed] [Cited in This Article: ] |
| 103. | Vírseda I, Jaqueti J, Nicolás MD, Prieto RI, Somoza MA, Navarro F. [Hepatitis C virus and type-2 diabetes mellitus. Is there a connection?]. Enferm Infecc Microbiol Clin. 2002;20:96-97. [PubMed] [Cited in This Article: ] |
| 104. | Wolff C, Moñoz S, Raddatz V. [Is there is association between hepatitis C virus and diabetes?]. Medicina (B Aires). 1999;59:315-316. [PubMed] [Cited in This Article: ] |
| 105. | Gowans EJ, Jones KL, Bharadwaj M, Jackson DC. Prospects for dendritic cell vaccination in persistent infection with hepatitis C virus. J Clin Virol. 2004;30:283-290. [PubMed] [Cited in This Article: ] |
| 106. | Masini M, Campani D, Boggi U, Menicagli M, Funel N, Pollera M, Lupi R, Del Guerra S, Bugliani M, Torri S. Hepatitis C virus infection and human pancreatic beta-cell dysfunction. Diabetes Care. 2005;28:940-941. [PubMed] [Cited in This Article: ] |
| 107. | Laskus T, Radkowski M, Wang LF, Vargas H, Rakela J. Search for hepatitis C virus extrahepatic replication sites in patients with acquired immunodeficiency syndrome: specific detection of negative-strand viral RNA in various tissues. Hepatology. 1998;28:1398-1401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 108. | Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 559] [Cited by in F6Publishing: 590] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 109. | Bureau C, Bernad J, Chaouche N, Orfila C, Béraud M, Gonindard C, Alric L, Vinel JP, Pipy B. Nonstructural 3 protein of hepatitis C virus triggers an oxidative burst in human monocytes via activation of NADPH oxidase. J Biol Chem. 2001;276:23077-23083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 110. | Gale M, Blakely CM, Kwieciszewski B, Tan SL, Dossett M, Tang NM, Korth MJ, Polyak SJ, Gretch DR, Katze MG. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol Cell Biol. 1998;18:5208-5218. [PubMed] [Cited in This Article: ] |
| 111. | Mitsuyoshi H, Itoh Y, Sumida Y, Minami M, Yasui K, Nakashima T, Okanoue T. Evidence of oxidative stress as a cofactor in the development of insulin resistance in patients with chronic hepatitis C. Hepatol Res. 2008;38:348-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 112. | Miyamoto H, Moriishi K, Moriya K, Murata S, Tanaka K, Suzuki T, Miyamura T, Koike K, Matsuura Y. Involvement of the PA28gamma-dependent pathway in insulin resistance induced by hepatitis C virus core protein. J Virol. 2007;81:1727-1735. [PubMed] [Cited in This Article: ] |
| 113. | Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology. 2008;47:2127-2133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 114. | Banerjee S, Saito K, Ait-Goughoulte M, Meyer K, Ray RB, Ray R. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream akt/protein kinase B signaling pathway for insulin resistance. J Virol. 2008;82:2606-2612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 115. | Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499-1508. [PubMed] [Cited in This Article: ] |
| 116. | Pazienza V, Clément S, Pugnale P, Conzelman S, Foti M, Mangia A, Negro F. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 117. | Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541-6551. [PubMed] [Cited in This Article: ] |
| 118. | Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261-1274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4237] [Cited by in F6Publishing: 4568] [Article Influence: 268.7] [Reference Citation Analysis (0)] |
| 119. | Burén J, Liu HX, Jensen J, Eriksson JW. Dexamethasone impairs insulin signalling and glucose transport by depletion of insulin receptor substrate-1, phosphatidylinositol 3-kinase and protein kinase B in primary cultured rat adipocytes. Eur J Endocrinol. 2002;146:419-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 120. | Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655-1657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4101] [Cited by in F6Publishing: 4147] [Article Influence: 188.5] [Reference Citation Analysis (0)] |
| 121. | Dominici FP, Hauck S, Argentino DP, Bartke A, Turyn D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate (IRS)-1 and IRS-2 in liver of Ames dwarf mice. J Endocrinol. 2002;173:81-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 155] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 122. | Jiang G, Zhang BB. Pi 3-kinase and its up- and down-stream modulators as potential targets for the treatment of type II diabetes. Front Biosci. 2002;7:d903-d907. [PubMed] [Cited in This Article: ] |
| 123. | Kaburagi Y, Yamauchi T, Yamamoto-Honda R, Ueki K, Tobe K, Akanuma Y, Yazaki Y, Kadowaki T. The mechanism of insulin-induced signal transduction mediated by the insulin receptor substrate family. Endocr J. 1999;46 Suppl:S25-S34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 124. | Matsumoto M, Ogawa W, Teshigawara K, Inoue H, Miyake K, Sakaue H, Kasuga M. Role of the insulin receptor substrate 1 and phosphatidylinositol 3-kinase signaling pathway in insulin-induced expression of sterol regulatory element binding protein 1c and glucokinase genes in rat hepatocytes. Diabetes. 2002;51:1672-1680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 125. | Sun Y, Liu S, Ferguson S, Wang L, Klepcyk P, Yun JS, Friedman JE. Phosphoenolpyruvate carboxykinase overexpression selectively attenuates insulin signaling and hepatic insulin sensitivity in transgenic mice. J Biol Chem. 2002;277:23301-23307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 126. | Ueki K, Fruman DA, Brachmann SM, Tseng YH, Cantley LC, Kahn CR. Molecular balance between the regulatory and catalytic subunits of phosphoinositide 3-kinase regulates cell signaling and survival. Mol Cell Biol. 2002;22:965-977. [PubMed] [Cited in This Article: ] |
| 127. | Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1943] [Cited by in F6Publishing: 1923] [Article Influence: 106.8] [Reference Citation Analysis (3)] |
| 128. | Thirone AC, Huang C, Klip A. Tissue-specific roles of IRS proteins in insulin signaling and glucose transport. Trends Endocrinol Metab. 2006;17:72-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 129. | Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003;38:1384-1392. [PubMed] [Cited in This Article: ] |
| 130. | Bernsmeier C, Duong FH, Christen V, Pugnale P, Negro F, Terracciano L, Heim MH. Virus-induced over-expression of protein phosphatase 2A inhibits insulin signalling in chronic hepatitis C. J Hepatol. 2008;49:429-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 131. | Negro F. Mechanisms of hepatitis C virus-related insulin resistance. Clin Res Hepatol Gastroenterol. 2011;35:358-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 132. | Eslam M, Booth DR, George J, Ahlenstiel G. Interaction of IFNL3 with insulin resistance, steatosis and lipid metabolism in chronic hepatitis C virus infection. World J Gastroenterol. 2013;19:7055-7061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 133. | Horner SM, Gale M. Regulation of hepatic innate immunity by hepatitis C virus. Nat Med. 2013;19:879-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 134. | Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert JJ, Capeau J, Hainque B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87:2084-2089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 135. | Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245:621-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 586] [Cited by in F6Publishing: 550] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 136. | Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745-E751. [PubMed] [Cited in This Article: ] |
| 137. | Malaguarnera M, Di Fazio I, Romeo MA, Restuccia S, Laurino A, Trovato BA. Elevation of interleukin 6 levels in patients with chronic hepatitis due to hepatitis C virus. J Gastroenterol. 1997;32:211-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 138. | Nelson DR, Lim HL, Marousis CG, Fang JW, Davis GL, Shen L, Urdea MS, Kolberg JA, Lau JY. Activation of tumor necrosis factor-alpha system in chronic hepatitis C virus infection. Dig Dis Sci. 1997;42:2487-2494. [PubMed] [Cited in This Article: ] |
| 139. | Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells. 2001;19:378-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 590] [Cited by in F6Publishing: 621] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 140. | Naka T, Tsutsui H, Fujimoto M, Kawazoe Y, Kohzaki H, Morita Y, Nakagawa R, Narazaki M, Adachi K, Yoshimoto T. SOCS-1/SSI-1-deficient NKT cells participate in severe hepatitis through dysregulated cross-talk inhibition of IFN-gamma and IL-4 signaling in vivo. Immunity. 2001;14:535-545. [PubMed] [Cited in This Article: ] |
| 141. | Oncül O, Top C, Cavuplu T. Correlation of serum leptin levels with insulin sensitivity in patients with chronic hepatitis-C infection. Diabetes Care. 2002;25:937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 142. | Mankouri J, Tedbury PR, Gretton S, Hughes ME, Griffin SD, Dallas ML, Green KA, Hardie DG, Peers C, Harris M. Enhanced hepatitis C virus genome replication and lipid accumulation mediated by inhibition of AMP-activated protein kinase. Proc Natl Acad Sci USA. 2010;107:11549-11554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 143. | Cheung AT, Wang J, Ree D, Kolls JK, Bryer-Ash M. Tumor necrosis factor-alpha induces hepatic insulin resistance in obese Zucker (fa/fa) rats via interaction of leukocyte antigen-related tyrosine phosphatase with focal adhesion kinase. Diabetes. 2000;49:810-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 144. | Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14:447-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 342] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 145. | Fallahi P, Ferri C, Ferrari SM, Corrado A, Sansonno D, Antonelli A. Cytokines and HCV-related disorders. Clin Dev Immunol. 2012;2012:468107. [PubMed] [Cited in This Article: ] |
| 146. | Antonelli A, Ferri C, Fallahi P, Ferrari SM, Sebastiani M, Ferrari D, Giunti M, Frascerra S, Tolari S, Franzoni F. High values of CXCL10 serum levels in mixed cryoglobulinemia associated with hepatitis C infection. Am J Gastroenterol. 2008;103:2488-2494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 147. | Ando H, Nagai Y, Yokoyama M, Takamura T, Kobayashi K. Antibodies to GAD in diabetic patients with chronic hepatitis C. Diabet Med. 1998;15:797. [PubMed] [Cited in This Article: ] |
| 148. | Betterle C, Zanette F, Pedini B, Presotto F, Rapp LB, Monciotti CM, Rigon F. Clinical and subclinical organ-specific autoimmune manifestations in type 1 (insulin-dependent) diabetic patients and their first-degree relatives. Diabetologia. 1984;26:431-436. [PubMed] [Cited in This Article: ] |
| 149. | Hiéronimus S, Fredenrich A, Tran A, Benzaken S, Fénichel P. Antibodies to GAD in chronic hepatitis C patients. Diabetes Care. 1997;20:1044. [PubMed] [Cited in This Article: ] |
| 150. | Piquer S, Hernández C, Enriquez J, Ross A, Esteban JI, Genescà J, Bonifacio E, Puig-Domingo M, Simó R. Islet cell and thyroid antibody prevalence in patients with hepatitis C virus infection: effect of treatment with interferon. J Lab Clin Med. 2001;137:38-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 151. | Chen LK, Chou YC, Tsai ST, Hwang SJ, Lee SD. Hepatitis C virus infection-related Type 1 diabetes mellitus. Diabet Med. 2005;22:340-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 152. | Masuda H, Atsumi T, Fujisaku A, Shimizu C, Yoshioka N, Koike T. Acute onset of type 1 diabetes accompanied by acute hepatitis C: the potential role of proinflammatory cytokine in the pathogenesis of autoimmune diabetes. Diabetes Res Clin Pract. 2007;75:357-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 153. | Yan FM, Chen AS, Hao F, Zhao XP, Gu CH, Zhao LB, Yang DL, Hao LJ. Hepatitis C virus may infect extrahepatic tissues in patients with hepatitis C. World J Gastroenterol. 2000;6:805-811. [PubMed] [Cited in This Article: ] |
| 154. | Bogdanos DP, Mieli-Vergani G, Vergani D. Virus, liver and autoimmunity. Dig Liver Dis. 2002;32:440-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 155. | Bogdanos DP, Choudhuri K, Vergani D. Molecular mimicry and autoimmune liver disease: virtuous intentions, malign consequences. Liver. 2001;21:225-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 156. | Bogdanos DP, Rigopoulou EI. Viral/self-mimicry and immunological cross-reactivity as a trigger of hepatic C virus associated autoimmune diabetes. Diabetes Res Clin Pract. 2007;77:155-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 157. | Bogdanos DP, Lenzi M, Okamoto M, Rigopoulou EI, Muratori P, Ma Y, Muratori L, Tsantoulas D, Mieli- Vergani G, Bianchi FB. Multiple viral/self immunological cross-reactivity in liver kidney microsomal antibody positive hepatitis C virus infected patients is associated with the possession of HLA B51. Int J Immunopathol Pharmacol. 2004;17:83-92. [PubMed] [Cited in This Article: ] |
| 158. | Hanifi-Moghaddam P, Schloot NC, Kappler S, Seissler J, Kolb H. An association of autoantibody status and serum cytokine levels in type 1 diabetes. Diabetes. 2003;52:1137-1142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 159. | Nicoletti F, Conget I, Di Marco R, Speciale AM, Morìnigo R, Bendtzen K, Gomis R. Serum levels of the interferon-gamma-inducing cytokine interleukin-18 are increased in individuals at high risk of developing type I diabetes. Diabetologia. 2001;44:309-311. [PubMed] [Cited in This Article: ] |
| 160. | Oikawa Y, Shimada A, Kasuga A, Morimoto J, Osaki T, Tahara H, Miyazaki T, Tashiro F, Yamato E, Miyazaki J. Systemic administration of IL-18 promotes diabetes development in young nonobese diabetic mice. J Immunol. 2003;171:5865-5875. [PubMed] [Cited in This Article: ] |
| 161. | Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53-72. [PubMed] [Cited in This Article: ] |
| 162. | Yumoto E, Higashi T, Nouso K, Nakatsukasa H, Fujiwara K, Hanafusa T, Yumoto Y, Tanimoto T, Kurimoto M, Tanaka N. Serum gamma-interferon-inducing factor (IL-18) and IL-10 levels in patients with acute hepatitis and fulminant hepatic failure. J Gastroenterol Hepatol. 2002;17:285-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 163. | Torre D, Zeroli C, Giola M, Ferrario G, Fiori GP, Bonetta G, Tambini R. Serum levels of interleukin-1 alpha, interleukin-1 beta, interleukin-6, and tumor necrosis factor in patients with acute viral hepatitis. Clin Infect Dis. 1994;18:194-198. [PubMed] [Cited in This Article: ] |
| 164. | Yang XD, Tisch R, Singer SM, Cao ZA, Liblau RS, Schreiber RD, McDevitt HO. Effect of tumor necrosis factor alpha on insulin-dependent diabetes mellitus in NOD mice. I. The early development of autoimmunity and the diabetogenic process. J Exp Med. 1994;180:995-1004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 238] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 165. | Eizirik DL, Mandrup-Poulsen T. A choice of death--the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44:2115-2133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 653] [Cited by in F6Publishing: 652] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 166. | Thomas HE, Irawaty W, Darwiche R, Brodnicki TC, Santamaria P, Allison J, Kay TW. IL-1 receptor deficiency slows progression to diabetes in the NOD mouse. Diabetes. 2004;53:113-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 167. | Lee LF, Xu B, Michie SA, Beilhack GF, Warganich T, Turley S, McDevitt HO. The role of TNF-alpha in the pathogenesis of type 1 diabetes in the nonobese diabetic mouse: analysis of dendritic cell maturation. Proc Natl Acad Sci USA. 2005;102:15995-16000. [PubMed] [Cited in This Article: ] |
| 168. | Antonelli A, Ferri C, Fallahi P, Ferrari SM, Goglia F, Ferrannini E. Hepatitis C virus infection: evidence for an association with type 2 diabetes (Response to Skowronski et al). Diabetes Care. 2006;29:751. [DOI] [Cited in This Article: ] |
| 169. | Antonelli A, Ferri C, Ferrari SM, Colaci M, Sansonno D, Fallahi P. Endocrine manifestations of hepatitis C virus infection. Nat Clin Pract Endocrinol Metab. 2009;5:26-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 170. | Gale EA. Latent autoimmune diabetes in adults: a guide for the perplexed. Diabetologia. 2005;48:2195-2199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 171. | Skowroński M, Zozulińska D, Juszczyk J, Wierusz-Wysocka B. Hepatitis C virus infection: evidence for an association with type 2 diabetes. Diabetes Care. 2006;29:750; author reply 751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 172. | Antonelli A, Fallahi P, Ferrari SM, Pupilli C, d’Annunzio G, Lorini R, Vanelli M, Ferrannini E. Serum Th1 (CXCL10) and Th2 (CCL2) chemokine levels in children with newly diagnosed Type 1 diabetes: a longitudinal study. Diabet Med. 2008;25:1349-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 173. | Apolinario A, Majano PL, Lorente R, Núñez O, Clemente G, García-Monzón C. Gene expression profile of T-cell-specific chemokines in human hepatocyte-derived cells: evidence for a synergistic inducer effect of cytokines and hepatitis C virus proteins. J Viral Hepat. 2005;12:27-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 174. | Vial T, Descotes J. Clinical toxicity of the interferons. Drug Saf. 1994;10:115-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 187] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 175. | Fabris P, Betterle C, Greggio NA, Zanchetta R, Bosi E, Biasin MR, de Lalla F. Insulin-dependent diabetes mellitus during alpha-interferon therapy for chronic viral hepatitis. J Hepatol. 1998;28:514-517. [PubMed] [Cited in This Article: ] |
| 176. | Hadziyannis SJ. The spectrum of extrahepatic manifestations in hepatitis C virus infection. J Viral Hepat. 1997;4:9-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 103] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 177. | di Cesare E, Previti M, Russo F, Brancatelli S, Ingemi MC, Scoglio R, Mazzù N, Cucinotta D, Raimondo G. Interferon-alpha therapy may induce insulin autoantibody development in patients with chronic viral hepatitis. Dig Dis Sci. 1996;41:1672-1677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 178. | Jasonek J, Kacprzak-Bergman I, Zaleska I. [The treatment of HCV infection with interferon alpha and ribavirin in a child with diabetes I type]. Przegl Epidemiol. 2006;60:259-263. [PubMed] [Cited in This Article: ] |
| 179. | Schories M, Peters T, Rasenack J, Reincke M. [Autoantibodies against islet cell antigens and type 1 diabetes after treatment with interferon-alpha]. Dtsch Med Wochenschr. 2004;129:1120-1124. [PubMed] [Cited in This Article: ] |
| 180. | Schreuder TC, Gelderblom HC, Weegink CJ, Hamann D, Reesink HW, Devries JH, Hoekstra JB, Jansen PL. High incidence of type 1 diabetes mellitus during or shortly after treatment with pegylated interferon alpha for chronic hepatitis C virus infection. Liver Int. 2008;28:39-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 181. | Betterle C, Fabris P, Zanchetta R, Pedini B, Tositti G, Bosi E, de Lalla F. Autoimmunity against pancreatic islets and other tissues before and after interferon-alpha therapy in patients with hepatitis C virus chronic infection. Diabetes Care. 2000;23:1177-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 182. | Chakrabarti D, Hultgren B, Stewart TA. IFN-alpha induces autoimmune T cells through the induction of intracellular adhesion molecule-1 and B7.2. J Immunol. 1996;157:522-528. [PubMed] [Cited in This Article: ] |
| 183. | Frankart L, Lejeune D, Donckier J. Diabetes mellitus and interferon therapy. Diabet Med. 1997;14:405. [PubMed] [Cited in This Article: ] |
| 184. | Hayakawa M, Gando S, Morimoto Y, Kemmotsu O. Development of severe diabetic keto-acidosis with shock after changing interferon-beta into interferon-alpha for chronic hepatitis C. Intensive Care Med. 2000;26:1008. [PubMed] [Cited in This Article: ] |
| 185. | Imano E, Kanda T, Ishigami Y, Kubota M, Ikeda M, Matsuhisa M, Kawamori R, Yamasaki Y. Interferon induces insulin resistance in patients with chronic active hepatitis C. J Hepatol. 1998;28:189-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 186. | Koivisto VA, Pelkonen R, Cantell K. Effect of interferon on glucose tolerance and insulin sensitivity. Diabetes. 1989;38:641-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 187. | Konrad T, Zeuzem S, Vicini P, Toffolo G, Briem D, Lormann J, Herrmann G, Berger A, Kusterer K, Teuber G. Evaluation of factors controlling glucose tolerance in patients with HCV infection before and after 4 months therapy with interferon-alpha. Eur J Clin Invest. 2000;30:111-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 188. | Nemesánszky E, Pusztay M, Csepregi A. Effects of interferon treatment on the glucose metabolism of patients with chronic hepatitis C. Eur J Intern Med. 2000;11:151-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 189. | Fabris P, Betterle C, Floreani A, Greggio NA, de Lazzari F, Naccarato R, Chiaramonte M. Development of type 1 diabetes mellitus during interferon alfa therapy for chronic HCV hepatitis. Lancet. 1992;340:548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 145] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 190. | Bhatti A, McGarrity TJ, Gabbay R. Diabetic ketoacidosis induced by alpha interferon and ribavirin treatment in a patient with hepatitis C. Am J Gastroenterol. 2001;96:604-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 191. | Bosi E, Minelli R, Bazzigaluppi E, Salvi M. Fulminant autoimmune Type 1 diabetes during interferon-alpha therapy: a case of Th1-mediated disease? Diabet Med. 2001;18:329-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 192. | Cozzolongo R, Betterle C, Fabris P, Paola Albergoni M, Lanzilotta E, Manghisi OG. Onset of type 1 diabetes mellitus during peginterferon alpha-2b plus ribavirin treatment for chronic hepatitis C. Eur J Gastroenterol Hepatol. 2006;18:689-692. [PubMed] [Cited in This Article: ] |
| 193. | Eibl N, Gschwantler M, Ferenci P, Eibl MM, Weiss W, Schernthaner G. Development of insulin-dependent diabetes mellitus in a patient with chronic hepatitis C during therapy with interferon-alpha. Eur J Gastroenterol Hepatol. 2001;13:295-298. [PubMed] [Cited in This Article: ] |
| 194. | Fattovich G, Giustina G, Favarato S, Ruol A. A survey of adverse events in 11,241 patients with chronic viral hepatitis treated with alfa interferon. J Hepatol. 1996;24:38-47. [PubMed] [Cited in This Article: ] |
| 195. | Figge E, Reiser M, Schmiegel W, Nauck MA. Manifestation eines Typ-1-Diabetes bei einem patienten mit hepatitis C wahrend einer therapie mit interferon-a and ribavirin. Diabetes Stoffwechsel. 2001;10:133-138. [Cited in This Article: ] |
| 196. | Floreani A, Chiaramonte M, Greggio NA, Fabris P, De Lazzari F, Naccarato R, Betterle C. Organ-specific autoimmunity and genetic predisposition in interferon-treated HCV-related chronic hepatitis patients. Ital J Gastroenterol Hepatol. 1998;30:71-76. [PubMed] [Cited in This Article: ] |
| 197. | Fujioka T, Honda M, Yoshizaki T, Ogawa M, Matsuno H, Shimokawa K, Koyama K. A case of type 1 diabetes onset and recurrence of Graves’ disease during pegylated interferon-α plus ribavirin treatment for chronic hepatitis C. Intern Med. 2010;49:1987-1990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 198. | Giuntoli P, Mariani S, Avoli D, Giammarco V. Diabete mellito insulino-dipendente indotto da interferon-alfa. Minerva Endocrinol. 1995;20:243-245. [Cited in This Article: ] |
| 199. | Hayashi M, Kataoka Y, Tachikawa K, Koguchi H, Tanaka H. Dual onset of type 1 diabetes mellitus and Graves’ disease during treatment with pegylated interferon alpha-2b and ribavirin for chronic hepatitis C. Diabetes Res Clin Pract. 2009;86:e19-e21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 200. | Huang X, Yuang J, Goddard A, Foulis A, James RF, Lernmark A, Pujol-Borrell R, Rabinovitch A, Somoza N, Stewart TA. Interferon expression in the pancreases of patients with type I diabetes. Diabetes. 1995;44:658-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 201. | Imagawa A, Itoh N, Hanafusa T, Oda Y, Waguri M, Miyagawa J, Kono N, Kuwajima M, Matsuzawa Y. Autoimmune endocrine disease induced by recombinant interferon-alpha therapy for chronic active type C hepatitis. J Clin Endocrinol Metab. 1995;80:922-926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 202. | Kado S, Miyamoto J, Komatsu N, Iwaki Y, Ozaki H, Taguchi H, Kure M, Sarashina G, Watanabe T, Katsura Y. Type 1 diabetes mellitus caused by treatment with interferon-beta. Intern Med. 2000;39:146-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 203. | Mathieu E, Fain O, Sitbon M, Thomas M. [Autoimmune diabetes after treatment with interferon-alpha]. Presse Med. 1995;24:238. [PubMed] [Cited in This Article: ] |
| 204. | Mofredj A, Howaizi M, Grasset D, Licht H, Loison S, Devergie B, Demontis R, Cadranel JF. Diabetes mellitus during interferon therapy for chronic viral hepatitis. Dig Dis Sci. 2002;47:1649-1654. [PubMed] [Cited in This Article: ] |
| 205. | Nakamura K, Kawasaki E, Abiru N, Jo O, Fukushima K, Satoh T, Kuriya G, Kobayashi M, Kuwahara H, Yamasaki H. Trajectories of anti-islet autoantibodies before development of type 1 diabetes in interferon-treated hepatitis C patients. Case reports and a literature review. Endocr J. 2010;57:947-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 206. | Ogihara T, Katagiri H, Yamada T, Kudo H, Imai J, Ishigaki Y, Hinokio Y, Yamagiwa Y, Ueno Y, Shimosegawa T. Peginterferon (PEG-IFN) plus ribavirin combination therapy, but neither interferon nor PGE-IFN alone, induced type 1 diabetes in a patient with chronic hepatitis C. Intern Med. 2009;48:1387-1390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 207. | Oka R, Hiroi N, Shigemitsu R, Sue M, Oshima Y, Yoshida-Hiroi M. Type 1 Diabetes Mellitus Associated with Pegylated Interferon-α Plus Ribavirin Treatment for Chronic Hepatitis C: Case Report and Literature Review. Clin Med Insights Endocrinol Diabetes. 2011;4:39-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 208. | Okada K, Kikuoka H, Kokawa M, Ihozaki M, Takeuchi K, Okamura T, Nakao T, Hoso T, Kondo M. A case of insulin-dependent diabetes mellitus (IDDM) following interferon alpha therapy for type C chronic hepatitis. J Japan Diab Soc. 1995;38:625-630. [Cited in This Article: ] |
| 209. | Okanoue T, Sakamoto S, Itoh Y, Minami M, Yasui K, Sakamoto M, Nishioji K, Katagishi T, Nakagawa Y, Tada H. Side effects of high-dose interferon therapy for chronic hepatitis C. J Hepatol. 1996;25:283-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 341] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 210. | Recasens M, Aguilera E, Ampurdanés S, Sánchez Tapias JM, Simó O, Casamitjana R, Conget I. Abrupt onset of diabetes during interferon-alpha therapy in patients with chronic hepatitis C. Diabet Med. 2001;18:764-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 211. | Rostaing L, Oksman F, Izopet J, Baron E, Cisterne JM, Hoff M, Abbal M, Durand D. Serological markers of autoimmunity in renal transplant patients before and after alpha-interferon therapy for chronic hepatitis C. Am J Nephrol. 1996;16:478-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 212. | Seifarth C, Benninger J, Böhm BO, Wiest-Ladenburger U, Hahn EG, Hensen J. [Augmentation of the immune response to islet cell antigens with development of diabetes mellitus caused by interferon-alpha therapy in chronic hepatitis C]. Z Gastroenterol. 1999;37:235-239. [PubMed] [Cited in This Article: ] |
| 213. | Shiba T, Morino Y, Tagawa K, Fujino H, Unuma T. Onset of diabetes with high titer anti-GAD antibody after IFN therapy for chronic hepatitis. Diabetes Res Clin Pract. 1995;30:237-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 214. | Hiltunen Y, Ala-Korpela M, Jokisaari J, Eskelinen S, Kiviniitty K, Savolainen M, Kesäniemi YA. A lineshape fitting model for 1H NMR spectra of human blood plasma. Magn Reson Med. 1991;21:222-232. [PubMed] [Cited in This Article: ] |
| 215. | Tanaka J, Sugimoto K, Shiraki K, Beppu T, Yoneda K, Fuke H, Yamamoto N, Ito K, Takei Y. Type 1 diabetes mellitus provoked by peginterferon alpha-2b plus ribavirin treatment for chronic hepatitis C. Intern Med. 2008;47:747-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 216. | Tohda G, Oida K, Higashi S, Hayashi T, Miyamori I. Interferon-alpha and development of type 1 diabetes: a case without insulin resistance. Diabetes Care. 1998;21:1774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 217. | Tosone G, Borgia G, Gentile I, Cerini R, Conte MC, Orlando R, Piazza M. A case of pegylated interferon alpha-related diabetic ketoacidosis: can this complication be avoided? Acta Diabetol. 2007;44:167-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 218. | Uto H, Matsuoka H, Murata M, Okamoto T, Miyata Y, Hori T, Ido A, Hirono S, Hayashi K, Tsubouchi H. A case of chronic hepatitis C developing insulin-dependent diabetes mellitus associated with various autoantibodies during interferon therapy. Diabetes Res Clin Pract. 2000;49:101-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 219. | Wasmuth HE, Stolte C, Geier A, Gartung C, Matern S. Induction of multiple autoantibodies to islet cell antigens during treatment with interferon alpha for chronic hepatitis C. Gut. 2001;49:596-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 220. | Waguri M, Hanafusa T, Itoh N, Imagawa A, Miyagawa J, Kawata S, Kono N, Kuwajima M, Matsuzawa Y. Occurrence of IDDM during interferon therapy for chronic viral hepatitis. Diabetes Res Clin Pract. 1994;23:33-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 221. | Wesche B, Jaeckel E, Trautwein C, Wedemeyer H, Falorni A, Frank H, von zur Mühlen A, Manns MP, Brabant G. Induction of autoantibodies to the adrenal cortex and pancreatic islet cells by interferon alpha therapy for chronic hepatitis C. Gut. 2001;48:378-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 222. | Yamazaki M, Sato A, Takeda T, Komatsu M. Distinct clinical courses in type 1 diabetes mellitus induced by peg-interferon-alpha treatment for chronic hepatitis C. Intern Med. 2010;49:403-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 223. | Yanagisawa K, Amemiya T, Morita Y, Kuroki H, Sanaka M, Uchigata Y, Omori Y, Hashimoto E, Hayashi N. A case of insulindependent diabetes mellitus developing during interferon therapy for chronic hepatitis type C. J Japan Diab Soc. 1995;38:283-288. [Cited in This Article: ] |
| 224. | Alter MJ, Margolis HS, Krawczynski K, Judson FN, Mares A, Alexander WJ, Hu PY, Miller JK, Gerber MA, Sampliner RE. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med. 1992;327:1899-1905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1137] [Cited by in F6Publishing: 1157] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 225. | Kiyosawa K, Furuta S. Review of hepatitis C in Japan. J Gastroenterol Hepatol. 1991;6:383-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 226. | van Rossum TG, Vulto AG, de Man RA, Brouwer JT, Schalm SW. Review article: glycyrrhizin as a potential treatment for chronic hepatitis C. Aliment Pharmacol Ther. 1998;12:199-205. [PubMed] [Cited in This Article: ] |
| 227. | Colombo M, Kuo G, Choo QL, Donato MF, Del Ninno E, Tommasini MA, Dioguardi N, Houghton M. Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet. 1989;2:1006-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 492] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 228. | Hasan F, Jeffers LJ, De Medina M, Reddy KR, Parker T, Schiff ER, Houghton M, Choo QL, Kuo G. Hepatitis C-associated hepatocellular carcinoma. Hepatology. 1990;12:589-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 187] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 229. | Ikeda K, Saitoh S, Koida I, Arase Y, Tsubota A, Chayama K, Kumada H, Kawanishi M. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology. 1993;18:47-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 230. | Kew MC, Houghton M, Choo QL, Kuo G. Hepatitis C virus antibodies in southern African blacks with hepatocellular carcinoma. Lancet. 1990;335:873-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 186] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 231. | Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, Nakanishi K, Fujimoto I, Inoue A, Yamazaki H. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797-1801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 818] [Cited by in F6Publishing: 778] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 232. | Adami HO, Chow WH, Nyrén O, Berne C, Linet MS, Ekbom A, Wolk A, McLaughlin JK, Fraumeni JF. Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst. 1996;88:1472-1477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 274] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 233. | Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 406] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 234. | Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 482] [Cited by in F6Publishing: 480] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 235. | El-Serag HB, Richardson PA, Everhart JE. The role of diabetes in hepatocellular carcinoma: a case-control study among United States Veterans. Am J Gastroenterol. 2001;96:2462-2467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 236. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3846] [Cited by in F6Publishing: 4102] [Article Influence: 241.3] [Reference Citation Analysis (2)] |
| 237. | Gao C, Yao SK. Diabetes mellitus: a “true” independent risk factor for hepatocellular carcinoma? Hepatobiliary Pancreat Dis Int. 2009;8:465-473. [PubMed] [Cited in This Article: ] |
| 238. | Hung CH, Lee CM, Wang JH, Hu TH, Chen CH, Lin CY, Lu SN. Impact of diabetes mellitus on incidence of hepatocellular carcinoma in chronic hepatitis C patients treated with interferon-based antiviral therapy. Int J Cancer. 2011;128:2344-2352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 239. | Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med. 2006;166:1871-1877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 392] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 240. | Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 682] [Cited by in F6Publishing: 686] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 241. | Lagiou P, Kuper H, Stuver SO, Tzonou A, Trichopoulos D, Adami HO. Role of diabetes mellitus in the etiology of hepatocellular carcinoma. J Natl Cancer Inst. 2000;92:1096-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 242. | Lai MS, Hsieh MS, Chiu YH, Chen TH. Type 2 diabetes and hepatocellular carcinoma: A cohort study in high prevalence area of hepatitis virus infection. Hepatology. 2006;43:1295-1302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 243. | La Vecchia C, Negri E, Franceschi S, D’Avanzo B, Boyle P. A case-control study of diabetes mellitus and cancer risk. Br J Cancer. 1994;70:950-953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 235] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 244. | Lawson DH, Gray JM, McKillop C, Clarke J, Lee FD, Patrick RS. Diabetes mellitus and primary hepatocellular carcinoma. Q J Med. 1986;61:945-955. [PubMed] [Cited in This Article: ] |
| 245. | N’Kontchou G, Paries J, Htar MT, Ganne-Carrie N, Costentin L, Grando-Lemaire V, Trinchet JC, Beaugrand M. Risk factors for hepatocellular carcinoma in patients with alcoholic or viral C cirrhosis. Clin Gastroenterol Hepatol. 2006;4:1062-1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 246. | Taura N, Ichikawa T, Miyaaki H, Yatsuhashi H, Ishibashi H, Nakao K. Prevalence of type 2 diabetes mellitus in Japanese patients with hepatocellular carcinoma. Exp Ther Med. 2011;2:81-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 247. | Tazawa J, Maeda M, Nakagawa M, Ohbayashi H, Kusano F, Yamane M, Sakai Y, Suzuki K. Diabetes mellitus may be associated with hepatocarcinogenesis in patients with chronic hepatitis C. Dig Dis Sci. 2002;47:710-715. [PubMed] [Cited in This Article: ] |
| 248. | Veldt BJ, Chen W, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, de Knegt RJ, Zeuzem S, Manns MP, Hansen BE. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47:1856-1862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 206] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 249. | Yu MC, Tong MJ, Govindarajan S, Henderson BE. Nonviral risk factors for hepatocellular carcinoma in a low-risk population, the non-Asians of Los Angeles County, California. J Natl Cancer Inst. 1991;83:1820-1826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 132] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 250. | Yuan JM, Govindarajan S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer. 2004;101:1009-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 230] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 251. | Wideroff L, Gridley G, Mellemkjaer L, Chow WH, Linet M, Keehn S, Borch-Johnsen K, Olsen JH. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89:1360-1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 484] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 252. | D’Souza R, Sabin CA, Foster GR. Insulin resistance plays a significant role in liver fibrosis in chronic hepatitis C and in the response to antiviral therapy. Am J Gastroenterol. 2005;100:1509-1515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 253. | Romero-Gómez M, Del Mar Viloria M, Andrade RJ, Salmerón J, Diago M, Fernández-Rodríguez CM, Corpas R, Cruz M, Grande L, Vázquez L. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 553] [Cited by in F6Publishing: 567] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 254. | Alexia C, Fallot G, Lasfer M, Schweizer-Groyer G, Groyer A. An evaluation of the role of insulin-like growth factors (IGF) and of type-I IGF receptor signalling in hepatocarcinogenesis and in the resistance of hepatocarcinoma cells against drug-induced apoptosis. Biochem Pharmacol. 2004;68:1003-1015. [PubMed] [Cited in This Article: ] |
| 255. | Stuver SO, Kuper H, Tzonou A, Lagiou P, Spanos E, Hsieh CC, Mantzoros C, Trichopoulos D. Insulin-like growth factor 1 in hepatocellular carcinoma and metastatic liver cancer in men. Int J Cancer. 2000;87:118-121. [PubMed] [Cited in This Article: ] |
| 256. | Su TS, Liu WY, Han SH, Jansen M, Yang-Fen TL, P’eng FK, Chou CK. Transcripts of the insulin-like growth factors I and II in human hepatoma. Cancer Res. 1989;49:1773-1777. [PubMed] [Cited in This Article: ] |
| 257. | Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med. 1997;336:633-640. [PubMed] [Cited in This Article: ] |
| 258. | Macaulay VM. Insulin-like growth factors and cancer. Br J Cancer. 1992;65:311-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 371] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 259. | Ellidokuz E, Cömlekçi A, Ellidokuz H, Akpinar H, Gökçe C, Tankurt E, Sagol O, Simsek I, Gönen O. The role of serum leptin levels in chronic hepatitis C with steatosis. Hepatogastroenterology. 2003;50 Suppl 2:cclxix-cclxxii. [PubMed] [Cited in This Article: ] |
| 260. | Rubbia-Brandt L, Quadri R, Abid K, Giostra E, Malé PJ, Mentha G, Spahr L, Zarski JP, Borisch B, Hadengue A. Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J Hepatol. 2000;33:106-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 437] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 261. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 778] [Cited by in F6Publishing: 813] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 262. | Ratziu V, Trabut JB, Poynard T. Fat, diabetes, and liver injury in chronic hepatitis C. Curr Gastroenterol Rep. 2004;6:22-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 263. | Tsochatzis E, Papatheodoridis GV, Manesis EK, Chrysanthos N, Kafiri G, Petraki K, Hadziyannis E, Pandelidaki H, Zafiropoulou R, Savvas S. Hepatic steatosis in genotype 4 chronic hepatitis C is mainly because of metabolic factors. Am J Gastroenterol. 2007;102:634-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 264. | Crook ED, Penumalee S, Gavini B, Filippova K. Hepatitis C is a predictor of poorer renal survival in diabetic patients. Diabetes Care. 2005;28:2187-2191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 265. | Pattullo V, Heathcote J. Hepatitis C and diabetes: one treatment for two diseases? Liver Int. 2010;30:356-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 266. | Wang CS, Yao WJ, Chang TT, Wang ST, Chou P. The impact of type 2 diabetes on the development of hepatocellular carcinoma in different viral hepatitis statuses. Cancer Epidemiol Biomarkers Prev. 2009;18:2054-2060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 267. | Kita Y, Mizukoshi E, Takamura T, Sakurai M, Takata Y, Arai K, Yamashita T, Nakamoto Y, Kaneko S. Impact of diabetes mellitus on prognosis of patients infected with hepatitis C virus. Metabolism. 2007;56:1682-1688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 268. | Petta S, Cammà C, Di Marco V, Alessi N, Cabibi D, Caldarella R, Licata A, Massenti F, Tarantino G, Marchesini G. Insulin resistance and diabetes increase fibrosis in the liver of patients with genotype 1 HCV infection. Am J Gastroenterol. 2008;103:1136-1144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 269. | Bressler BL, Guindi M, Tomlinson G, Heathcote J. High body mass index is an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Hepatology. 2003;38:639-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 266] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 270. | Elgouhari HM, Zein CO, Hanouneh I, Feldstein AE, Zein NN. Diabetes mellitus is associated with impaired response to antiviral therapy in chronic hepatitis C infection. Dig Dis Sci. 2009;54:2699-2705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 271. | Konishi I, Horiike N, Hiasa Y, Tokumoto Y, Mashiba T, Michitaka K, Miyake Y, Nonaka S, Joukou K, Matsuura B. Diabetes mellitus reduces the therapeutic effectiveness of interferon-alpha2b plus ribavirin therapy in patients with chronic hepatitis C. Hepatol Res. 2007;37:331-336. [PubMed] [Cited in This Article: ] |
| 272. | Walsh MJ, Jonsson JR, Richardson MM, Lipka GM, Purdie DM, Clouston AD, Powell EE. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut. 2006;55:529-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 218] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 273. | Sanyal AJ. Role of insulin resistance and hepatic steatosis in the progression of fibrosis and response to treatment in hepatitis C. Liver Int. 2011;31 Suppl 1:23-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 274. | Gunton JE, Delhanty PJ, Takahashi S, Baxter RC. Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J Clin Endocrinol Metab. 2003;88:1323-1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 275. | Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. [PubMed] [Cited in This Article: ] |
| 276. | Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642-1646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1494] [Cited by in F6Publishing: 1438] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 277. | Ikeda M, Abe K, Yamada M, Dansako H, Naka K, Kato N. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology. 2006;44:117-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 278. | Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci USA. 2005;102:2561-2566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 399] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 279. | Ye J, Wang C, Sumpter R, Brown MS, Goldstein JL, Gale M. Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc Natl Acad Sci USA. 2003;100:15865-15870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 301] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 280. | O’Leary JG, Chan JL, McMahon CM, Chung RT. Atorvastatin does not exhibit antiviral activity against HCV at conventional doses: a pilot clinical trial. Hepatology. 2007;45:895-898. [PubMed] [Cited in This Article: ] |