Published online Aug 15, 2017. doi: 10.4251/wjgo.v9.i8.314
Peer-review started: December 14, 2016
First decision: March 6, 2017
Revised: March 20, 2017
Accepted: June 12, 2017
Article in press: June 16, 2017
Published online: August 15, 2017
To determine whether S-1 induces hepatic steatosis in patients being treated for pancreatic cancer.
This retrospective study evaluated 22 patients who received oral S-1 as a first-line treatment for pancreatic cancer between January 2008 and July 2015 at the Ishikawa Prefectural Central Hospital. Patients underwent abdominal computed tomography (CT) scans before chemotherapy and within 3 mo from the start of treatment. CT numbers of the liver and spleen were measured before and after S-1 administration. Steatosis was diagnosed when the ratio of the CT number of the liver to that of the spleen (liver/spleen ratio) was < 0.9.
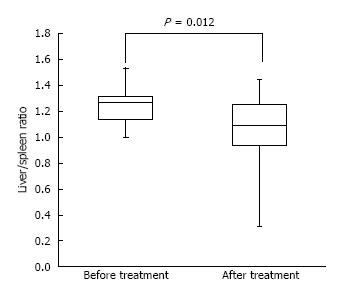
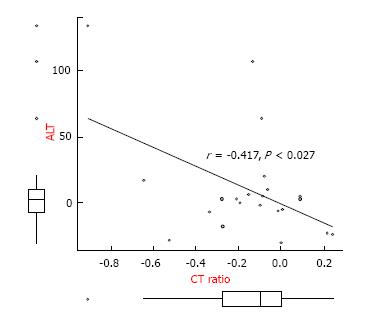
Median patient age was 68 years (range, 48-85 years), and median body mass index was 21 kg/m2 (range, 18-27 kg/m2). Of the 22 patients, six (27%) regularly consumed alcohol, and five (23%) had liver metastases. The mean ratio of CT number of the liver to the spleen was significantly higher before administration of S-1 (1.27 vs 1.09, P = 0.012) compared with after. Of the 22 patients, five (23%) had hepatic steatosis and 17 (77%) did not. The pretreatment demographic and clinical characteristics of these two groups showed no significant differences. The relationship between liver/spleen ratio and alanine transaminase activity in these patients. A statistically significant inverse correlation was observed (r = -0.417, P < 0.027).
Of the 22 patients with pancreatic cancer, five (23%) experienced S-1 induced hepatic steatosis. Care should be taken during S-1 treatment of patients with pancreatic cancer.
Core tip: Drug induced hepatic steatosis is a rare form of liver injury. Although hepatic steatosis has been observed in some patients with pancreatic cancer who were administered S-1, the ability of 5-fluorouracil alone to induce hepatic steatosis has not been evaluated systematically. The purpose of our study was to determine whether S-1 induces hepatic steatosis in patients being treated for pancreatic cancer. After analyzing a total of 22 patients, we found that S-1 chemotherapy induced hepatic steatosis in some patients with pancreatic cancer within three months and the correlation between the development of hepatic steatosis and liver function was weak in these patients.
- Citation: Tsuji K, Doyama H. S-1 induced hepatic steatosis in patients with pancreatic cancer: Retrospective analysis. World J Gastrointest Oncol 2017; 9(8): 314-318
- URL: https://www.wjgnet.com/1948-5204/full/v9/i8/314.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i8.314
S-1 is an oral antitumor drug that combines tegafur, a prodrug of 5-fluorouracil (5-FU), with 5-chloro-2,4-dihydroxypyridine and potassium oxonate[1]. Following S-1 administration, fluorouracil concentrations in blood remain high for long periods of time[1]. Several phase III trials have shown the efficacy and safety of S-1 in cancer patients[2-5], and S-1 has been approved in Japan for the treatment of various cancers, including gastric, head and neck, colorectal, lung, breast, pancreatic, and biliary tract cancers.
Nonalcoholic fatty liver disease is the most common form of chronic liver disease in Western countries, and its incidence is increasing, partly owing to the increasing prevalence of diabetes and obesity[6]. In Japan also, nonalcoholic fatty liver diseases has become one of the most frequent types of liver disease[7].
In contrast to nonalcoholic fatty liver diseases, drug induced hepatic steatosis is a rare form of liver injury. Drugs found to induce hepatic steatosis include amiodarone, tamoxifen, irinotecan, and valproic acid, with their toxicities due to their effects on hepatocyte mitochondria[8,9]. 5-FU, when combined with interferon (IFN) and folinic acid, has also been reported to induce hepatic steatosis[10,11], but the ability of 5-FU alone to induce hepatic steatosis has not been evaluated systematically.
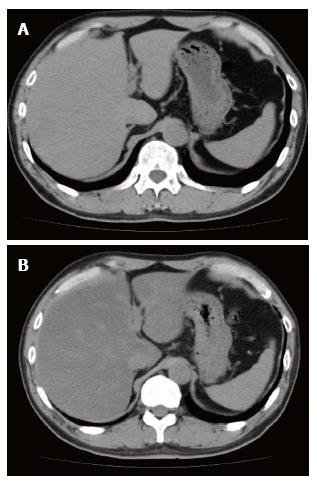
Although hepatic steatosis has been observed in some patients with pancreatic cancer who were administered S-1 (Figure 1), it is not clear whether S-1 was responsible for drug induced hepatic steatosis in these patients. This study therefore evaluated whether S-1 induced hepatic steatosis in patients with pancreatic cancer.
This retrospective study analyzed patients with pancreatic cancer who underwent chemotherapy for pancreatic cancer at Ishikawa Prefectural Central Hospital between January 2008 and July 2015. Of the 107 pancreatic cancer patients who received chemotherapy during the study period, 37 received oral S-1 as first-line treatment. Patients were included if they: (1) were aged > 20 years; (2) had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-2; (3) underwent regular computed tomography (CT) examinations; and (4) had no other cancer or serious complications, such as active infectious disease or serious heart disease. Twenty-two patients were deemed eligible. This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Ishikawa Prefectural Central Hospital.
All patients underwent abdominal CT scans before the start of chemotherapy and within three months from the start of treatment. CT numbers of the liver and spleen were evaluated before and after administration of S-1. Steatosis was diagnosed when the ratio of the CT number of the liver to that of the spleen (liver/spleen ratio) was < 0.9[12]. Blood was collected and blood tests performed at the time of each CT examination.
Continuous variables were reported as median (range) and analyzed using non-parametric Mann-Whitney U tests. Categorical variables were reported as number (percentage) and analyzed using Fisher’s exact tests or Wilcoxon signed rank tests. A P-value < 0.05 was considered statistically significant. All statistical analyses were performed with EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Australia). More precisely, EZR is a modified version of R commander designed to add statistical functions frequently used in biostatistics[13].
The clinical characteristics of the 22 included patients are shown in Table 1. Their median age was 68 years (range, 48-85 years), and their median body mass index was 21 kg/m2 (range, 18-27 kg/m2). Of the 22 patients, six (27%) regularly consumed alcohol, and five (23%) had liver metastases.
| Number of patients (n = 22) | |
| Age (yr), median (range) | 68 (48-85) |
| Sex | |
| Male | 9 |
| Female | 13 |
| Body mass index (kg/m2) median (range) | 21 (18-27) |
| ECOG PS | |
| 0 | 15 |
| 1 | 5 |
| 2 | 2 |
| Drinking habit | |
| Yes | 6 |
| No | 16 |
| Diabetes mellitus | |
| Yes | 6 |
| No | 16 |
| Disease status | |
| Locally advanced | 14 |
| Metastatic | 8 |
| Liver metastasis | |
| Yes | 5 |
| No | 17 |
| Biliary drainage | |
| Yes | 7 |
| No | 15 |
| Combination with radiotherapy | |
| Yes | 12 |
| No | 10 |
| Alanine transaminase concentration (U/L), median (range) | 21 (8-73) |
Figure 2 shows the median liver/spleen ratios in these patients before and after administration of S-1. This ratio was significantly higher before than after administration of S-1 (1.27 vs 1.09, P = 0.012).
Of the 22 patients, five (23%) had hepatic steatosis and 17 (77%) did not. The pretreatment demographic and clinical characteristics of these two groups showed no significant differences (Table 2).
| Without hepatic steatosis (n = 11) | With hepatic steatosis (n = 5) | P value | |
| Age (yr), median (range) | 70 (57-85) | 67 (48-78) | 0.39 |
| Sex | |||
| Male | 7 | 2 | 1 |
| Female | 10 | 3 | |
| Body mass index (kg/m2), median (range) | 21.5 (17.9-26.9) | 22.6 (19.0-25.3) | 0.75 |
| ECOG PS | |||
| 0 | 11 | 4 | 1 |
| 1 | 4 | 1 | |
| 2 | 2 | 0 | |
| Drinking habit | |||
| Yes | 5 | 1 | 1 |
| No | 12 | 4 | |
| Diabetes mellitus | |||
| Yes | 5 | 1 | 1 |
| No | 12 | 4 | |
| Disease status | |||
| Locally advanced | 10 | 4 | 0.61 |
| Metastatic | 7 | 1 | |
| Liver metastasis | |||
| Yes | 5 | 0 | 0.29 |
| No | 12 | 5 | |
| Biliary drainage | |||
| Yes | 4 | 3 | 0.27 |
| No | 13 | 2 | |
| Combination with radiotherapy | |||
| Yes | 9 | 3 | 1 |
| No | 8 | 2 | |
| Alanine transaminase concentration (U/L), median (range) | 24 (8-57) | 20.5 (11-73) | 0.97 |
Figure 3 shows the relationship between liver/spleen ratio and alanine transaminase activity in these patients. A statistically significant inverse correlation was observed (r = -0.417, P < 0.027).
This study showed that S-1 chemotherapy induced hepatic steatosis in some patients with pancreatic cancer within three months. This study also found that the correlation between the development of hepatic steatosis and liver function was weak in these patients.
Recognition of steatosis in patients receiving chemotherapy is important. The liver is of higher attenuation than the hepatic metastases, but it is difficult to delineate making assessment of size of the hepatic metastases as the liver becomes steatosis[10]. Obesity, insulin resistance, and the metabolic syndrome have been found to induce nonalcoholic fatty liver disease, a fairly common entity. In contrast, drug induced steatosis is a rare form of liver injury. Agents found to induce steatosis include amiodarone, tamoxifen, irinotecan, and valproic acid[8,9]. Drug induced steatosis is largely due to mitochondrial damage. In addition, mitochondrial damage can be induced by the inhibition of fatty acid beta oxidation, oxidative phosphorylation, and mitochondrial respiration[14].
In this study, 23% of patients with pancreatic cancer developed hepatic steatosis within three months of starting the oral chemotherapeutic agent S-1. S-1 is a combination of tegafur, a prodrug of 5-FU; 5-chloro-2,4-dihydroxypyridine, an inhibitor of dihydropyrimidine dehydrogenase, the enzyme responsible for generating 5-FU from tegafur; and potassium oxonate[1]. 5-FU has been linked to the development of steatosis[8]. For example, one study reported that 47% of patients receiving 5-FU-based therapy for advanced colorectal cancers developed steatosis during treatment[10], and a second study found that 30% of patients treated with interferon-alfa 2a and 5-FU developed steatosis[11]. To date, however, hepatic steatosis had not been found to be induced by 5-FU alone.
Although a statistically significant inverse correlation was observed between the development of hepatic steatosis and the loss of liver function, this correlation was weak (r = -0.417, P < 0.027). A previous study reported no correlation between liver function test results and the degree of steatosis in patients receiving 5-FU-based therapy[10]. Our findings are not strong enough to conclude that liver damage and the degree of steatosis are related.
In general, S-1 is safe for the liver, with few reports of S-1 induced fatal liver dysfunction. Indeed, the GEST trial, which compared S-1 and gemcitabine treatment in patients with mild to moderate pancreatic cancer, reported that the percentages of patients with grade ≥ 3 elevated AST and ALT levels were significantly lower in the S-1 than in the gemcitabine group[3]. Steatosis and liver damage may be underdiagnosed because the laboratory abnormalities are slight. Further investigations are needed to assess the relationship between liver damage and the degree of steatosis in patients receiving S-1.
This study had at least four limitations. First, it was performed at a single institution. Second, this study had a retrospective design. Prospective studies are needed to show the rate of S-1 induced hepatic steatosis in patients with pancreatic and other cancers. Third, none of our patients was histologically evaluated by liver biopsy because all had mild liver damage. CT, however, is reliable in diagnosing hepatic steatosis, with significantly superior diagnostic sensitivity and accuracy than other modalities in diagnosing hepatic steatosis. Fourth, this study was limited to patients with unresectable pancreatic cancer. We experienced the S-1 induced steatohepatitis to patients with gastric cancer in adjuvant therapy. However, Even gastrectomy alone has been found to induce hepatic steatosis[15]. Further study is needed to assess the effects of S-1 on steatosis in other types of cancer.
In conclusion, this study found that 23% of patients with pancreatic cancer who were treated with first-line S-1 chemotherapy developed hepatic steatosis. Because adjuvant S-1 chemotherapy has become a standard treatment in patients resected for pancreatic cancer, clinical use of S-1 and the risk of S-1-induced steatosis are expected to increase. The liver function of patients treated with S-1 should therefore be closely monitored during follow-up.
S-1 is an oral chemotherapeutic agent, consisting of tegafur, a prodrug of 5-fluorouracil, 5-chloro-2,4-dihydroxypyridine, and potassium oxonate. Recent randomized controlled trials in Japan have shown the clinical utility of S-1 treatment in patients with pancreatic cancer. Liver dysfunction, primarily steatohepatitis, has been observed in some S-1-treated patients, but it is not clear whether S-1 induced steatohepatitis.
It is not clear whether S-1 was responsible for drug induced steatohepatitis.
The literature suggests 5-FU has been linked to the development of steatosis, but the ability of 5-FU alone to induce hepatic steatosis has not been evaluated systematically. The current study adds that S-1 chemotherapy induced hepatic steatosis in some patients.
This study serves as additional evidence supporting the closely investigation of liver function in patients treated with S-1.
S-1 is a combination of tegafur, a prodrug of 5-FU; 5-chloro-2,4-dihydroxypyridine, an inhibitor of dihydropyrimidine dehydrogenase, the enzyme responsible for generating 5-FU from tegafur; and potassium oxonate.
The paper is well-written and contributes important information.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Tang Y S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
| 1. | Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, Fukushima M. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 586] [Cited by in F6Publishing: 610] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 2. | Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1320] [Cited by in F6Publishing: 1365] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 3. | Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640-1648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 435] [Cited by in F6Publishing: 442] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 4. | Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 635] [Cited by in F6Publishing: 645] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 5. | Takashima T, Mukai H, Hara F, Matsubara N, Saito T, Takano T, Park Y, Toyama T, Hozumi Y, Tsurutani J. Taxanes versus S-1 as the first-line chemotherapy for metastatic breast cancer (SELECT BC): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2016;17:90-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Wang YC, Colditz GA, Kuntz KM. Forecasting the obesity epidemic in the aging U.S. population. Obesity (Silver Spring). 2007;15:2855-2865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Okanoue T, Umemura A, Yasui K, Itoh Y. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in Japan. J Gastroenterol Hepatol. 2011;26 Suppl 1:153-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Maor Y, Malnick S. Liver injury induced by anticancer chemotherapy and radiation therapy. Int J Hepatol. 2013;2013:815105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Choti MA. Chemotherapy-associated hepatotoxicity: do we need to be concerned? Ann Surg Oncol. 2009;16:2391-2394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Peppercorn PD, Reznek RH, Wilson P, Slevin ML, Gupta RK. Demonstration of hepatic steatosis by computerized tomography in patients receiving 5-fluorouracil-based therapy for advanced colorectal cancer. Br J Cancer. 1998;77:2008-2011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 140] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Sørensen P, Edal AL, Madsen EL, Fenger C, Poulsen MR, Petersen OF. Reversible hepatic steatosis in patients treated with interferon alfa-2a and 5-fluorouracil. Cancer. 1995;75:2592-2596. [PubMed] [Cited in This Article: ] |
| 12. | Kato K, Takayama T, Katada N. Evaluation of computed tomography in the diagnosis of liver diseases. Kanzo. 1980;21:1340-1351. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9275] [Cited by in F6Publishing: 10632] [Article Influence: 966.5] [Reference Citation Analysis (0)] |
| 14. | Schumacher JD, Guo GL. Mechanistic review of drug-induced steatohepatitis. Toxicol Appl Pharmacol. 2015;289:40-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Yamasaki H, Tanemura M, Morimoto Y, Hashimoto J, Sakamoto T, Yamasaki Y, Kuwata K. [Study of postgastrectomy fatty liver]. Nihon Geka Gakkai Zasshi. 1992;93:1384-1389. [PubMed] [Cited in This Article: ] |