Published online May 15, 2014. doi: 10.4251/wjgo.v6.i5.139
Revised: February 19, 2014
Accepted: April 11, 2014
Published online: May 15, 2014
AIM: To evaluate the influence of baseline maximum standardized uptake value (SUVmax) on survival in a cohort of patients, undergoing positron emission tomography-computed tomography (PET-CT) scan for esophageal carcinoma.
METHODS: The pre-treatment SUVmax numeric reading was determined in patients with confirmed esophageal or junctional cancer having PET-CT scan during the time period 1st January 2007 until 31st July 2012. A minimum follow up of 12 mo was required. Patients were subdivided into quartiles according to SUVmax value and the influence of SUVmax on survival was assessed using univariate and multivariate analysis. The following pre-treatment factors were investigated: patient characteristics, tumor characteristics and planned treatment.
RESULTS: The study population was 271 patients (191 male) with esophageal or junctional carcinoma. The median age was 65 years (range 40-85) and histologic subtype was adenocarcinoma in 197 patients and squamous carcinoma in 74 patients. The treatment intent was radical in 182 and palliative in 89 patients. SUVmax was linked to histologic subtype (P = 0.008), tumor site (P = 0.01) and Union for International Cancer Control (UICC) stage (P < 0.001). On univariate analysis, prognosis was significantly associated with SUVmax (P = 0.001), T-stage (P < 0.001) and UICC stage (P < 0.001). On multivariate analysis, only T-stage and UICC stage remained significant.
CONCLUSION: Pretreatment SUVmax was not a useful marker in isolation for determining prognosis of patients with esophageal carcinoma.
Core tip: Positron emission tomography-computed tomography (PET-CT) is integral to the staging of esophageal cancer. It is unclear whether the value of PET-CT extends beyond the identification of metastatic disease. The influence of PET-CT maximum standardized uptake value (SUVmax) on prognosis was determined for 271 patients. Although SUVmax was closely linked to disease stage, it did not exert an independent effect and was not a useful prognostic marker.
- Citation: Al-Taan OS, Eltweri A, Sharpe D, Rodgers PM, Ubhi SS, Bowrey DJ. Prognostic value of baseline FDG uptake on PET-CT in esophageal carcinoma. World J Gastrointest Oncol 2014; 6(5): 139-144
- URL: https://www.wjgnet.com/1948-5204/full/v6/i5/139.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v6.i5.139
Positron emission tomography (PET) is an important component in the staging algorithm for patients with cancers of the esophagus and gastroesophageal junction[1,2]. At some centers, it is employed early in the staging pathway with all patients being assessed by this modality. In other centers, it features later in the staging pathway, only being utilized if computed tomography (CT) and endoscopic ultrasound demonstrate potentially resectable tumor characteristics[1,2].
Its principal application is in the identification of occult metastatic disease, not identified on CT imaging, or in the confirmation of high fluorodeoxyglucose (FDG) uptake in suspicious areas on CT imaging[1].
We have previously shown that PET-CT influences the treatment decision overall for 10% of patients with esophageal cancer, and for 26% of patients free of definite metastatic disease after initial CT imaging[2].
The maximum standardized uptake value (SUVmax) is a measure of the relative metabolic activity of the cancer. A recent meta-analysis confirmed the close link between the SUVmax and both tumor stage and prognosis[3]. Whether the SUVmax exerted an independent effect, unrelated to known clinical prognostic markers was unclear.
The majority of studies have assessed selected patient groups, typically only those receiving one form of treatment such as chemoradiotherapy, palliative chemotherapy or surgery (with or without neoadjuvant chemotherapy)[4-24]. It is likely that this has resulted in clustering of SUVmax values. Only four smaller studies have assessed the influence of SUVmax in unselected patients undergoing PET-CT[8,14,21,24]. Those studies concluded that SUVmax was significantly associated with prognosis but that this was not independent of existing clinical markers such as Union for International Cancer Control (UICC) stage.
The aim of the current study was to assess whether the SUVmax provided additional prognostic information, over and above the UICC stage and known clinical prognostic markers in a large cohort of unselected patients.
The use of anonymized patient information was approved by the Institutional Clinical Audit and Effectiveness Board. Individual patient consent was not required as no change in patient management was effected for the purposes of this audit.
The study was a retrospective review of all patients undergoing PET-CT during the time period 1st January 2007 to 31st July 2012. At our institution, PET-CT became incorporated into the staging algorithm of routine clinical practice in November 2006. Patients undergoing PET-CT after 31st July 2012 were not included, so that a minimum patient follow up time of 12 mo would be obtained.
All patients with a diagnosis of esophageal or gastric cancer are discussed at a weekly multi-disciplinary meeting and treatment intention and schedule determined. The staging algorithm has previously been published[2].
The 7th edition of the UICC stage was determined by consensus decision at the multi-disciplinary meeting based upon pre-treatment imaging.
During the years 2006-2008 coregistered PET-CT was performed using a General Electric Discovery ST PET-CT scanner with eight-slice CT scan, producing fused single image scans. Since 2008, imaging has been performed using a Siemens Biograph TruePoint PET-CT scanner. Half-body PET acquisition was obtained (from eyes to knees). Patients were fasted for 6 h prior to injection with 350-420 MBq of 18F-FDG (4.5 MBq/kg) that was administered to patients lying supine in a quiet and warm environment. Whole-body two-dimensional image acquisition was obtained 60 min after injection of 18F-FDG using a 128 × 128 matrix. Fused PET-CT images were single reported with quality assurance validation of 10% of scans. The diagnostic CT and previous imaging was available at the time of reporting. The threshold for the diagnosis of metastatic disease on PET-CT was a standardized uptake value in excess of 2.5.
The influence of patient characteristics (age and sex), tumor characteristics (tumor location, histologic subtype, T stage, N stage and UICC stage), planned treatment strategy and baseline SUVmax on PET-CT were investigated using univariate analysis. Significant variables were then investigated using Cox regression analysis.
Parametric data were analyzed using the unpaired t-test and non-parametric data were analyzed using the Mann-Whitney and Kruskal-Wallis test. Statistical analysis was performed using SPSS software version 15 (SPSS, Chicago, IL, United States). Significance was assumed at the 5% level.
The study population comprised 271 patients (191 males) of median age 65 years (range 40-85). Primary tumor location was upper esophagus in 13 patients, middle esophagus in 50 patients, lower esophagus in 136 patients and gastroesophageal junction in 72 patients. Histologic subtype was adenocarcinoma in 197 patients and squamous cell carcinoma in 74 patients.
Distribution of UICC stage was as follows: Stage 0 or 1 (45 patients), Stage 2 (50 patients), Stage 3 (99 patients) and Stage 4 (77 patients). Stage 4 disease was defined on the basis of distant metastatic disease in 31 patients and on the basis of celiac axis lymphadenopathy in 46 patients. Lymphadenopathy anterior to the left gastric pedicle was defined as locoregional disease as this would be routinely within the field of surgical resection. Lymphadenopathy posterior to the left gastric pedicle was defined as celiac axis lymphadenopathy and would not be included in the field of surgical resection.
Of note, there was no significant difference in the SUVmax readings obtained during the two time periods, when the two PET-CT scanners were employed. Specifically, with the study population divided into quintiles, there was no significant difference between successive quintiles of SUVmax (P = 0.55).
According to the multi-disciplinary panel, the treatment intention was radical (curative) for 182 patients and palliative for 89 patients. For the 182 patients treated with radical intent, principal treatment modality was surgery with or without neoadjuvant chemotherapy (114 patients), chemoradiotherapy (63 patients) and endoscopic mucosal resection (5 patients). Nineteen of the surgically treated patients underwent exploratory surgery because of the identification of unresectable T4 disease or peritoneal disease (19/114, 17%).
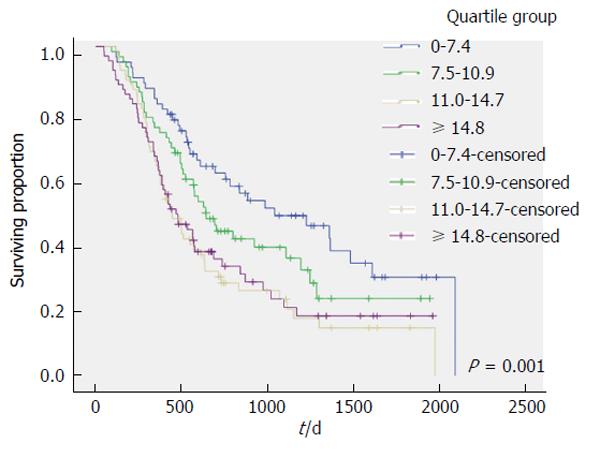
The outcome of univariate analysis comparing associations between patients factors (Table 1), tumor factors (Table 2) and treatment factors (Table 3) and SUVmax is shown. These show that SUVmax increased as disease burden (T stage, N stage and UICC stage) increased. Figure 1 plots survival for patients when stratified into quartiles of SUVmax (1st quartile 0-7.4, 2nd quartile 7.5-10.9, 3rd quartile 11.0-14.7, 4th quartile > 14.7). The strong link between SUVmax and survival is evident. The significance of SUVmax was lost on multivariate analysis. Using Cox regression analysis, the only factors significantly associated with survival were T-stage (P < 0.001) and UICC stage (P < 0.001). The same findings were evident when both the complete cohort was analyzed and when subgroup analysis of individual treatment groups (chemoradiotherapy, surgery, palliative chemotherapy) and histologic subtype (adenocarcinoma, squamous carcinoma) was performed.
| Factor | Mean SUVmax (95%CI) | Median survival in days (95%CI) |
| Sex | ||
| Male (n = 191) | 11.4 (10.5, 12.3) | 566 (491, 641) |
| Female (n = 80) | 12.1 (10.2, 14.0) | 884 (403, 1364) |
| P = 0.950 | P = 0.05 | |
| Age in years | ||
| Age ≤ 65 (n = 136) | 11.5 (10.5, 12.5) | 575 (456, 694) |
| Age > 65 (n = 135) | 11.7 (10.4, 13.1) | 586 (418, 754) |
| P = 0.770 | P = 0.25 | |
| Histology | ||
| Adenocarcinoma (n = 197) | 11.3 (10.2, 12.4) | 570 (483, 657) |
| Squamous carcinoma (n = 74) | 12.4 (11.3, 13.6) | 629 (445, 813) |
| P = 0.008 | P = 0.75 | |
| Tumor location | ||
| Upper esophagus (n = 13) | 15.6 (11.4, 19.8) | 973 (142,1804) |
| Mid esophagus (n = 50) | 12.8 (11.0, 14.6) | 425 (252, 598) |
| Lower esophagus (n = 136) | 10.8 (9.6, 12.0) | 586 (464, 708) |
| Junctional (n = 72) | 11.6 (10.0, 13.1) | 684 (430, 938) |
| P = 0.010 | P = 0.14 |
| Factor | Mean SUVmax (95%CI) | Median survival in days (95%CI) |
| T stage | ||
| T0 or T1 (n = 15) | 3.1 (1.5, 4.7) | Not reached |
| T2 (n = 49) | 8.7 (7.0, 10.4) | 1225 (742, 1708) |
| T3 (n = 183) | 12.7 (11.7, 13.7) | 495 (413, 577) |
| T4 (n = 24) | 14.1 (11.6, 16.7) | 390 (186, 594) |
| P < 0.001 | P < 0.001 | |
| N stage | ||
| N0 (n = 107) | 9.1 (8.1, 10.2) | 1094 (835, 1352) |
| N1 (n = 89) | 12.9 (11.4, 14.5) | 466 (371, 561) |
| N2 (n = 61) | 13.4 (11.6, 15.1) | 477 (307, 646) |
| N3 (n = 14) | 14.4 (8.8, 19.9) | 530 (350, 710) |
| P < 0.001 | P < 0.001 | |
| UICC stage | ||
| Stage 0 or 1 (n = 45) | 5.6 (4.2, 7.0) | 2092 (1060, 3124) |
| Stage 2 (n = 50) | 12.1 (10.6, 13.6) | 780 (195,1365) |
| Stage 3 (n = 99) | 11.9 (10.7, 13.2) | 594 (473, 715) |
| Stage 4 (n = 77) | 14.4 (12.6, 16.1) | 349 (280, 418) |
| P < 0.001 | P < 0.001 |
| Factor | Mean SUVmax (95%CI) | Median survival in days (95%CI) |
| Treatment intention | ||
| Curative (n = 182) | 10.6 (9.7, 11.5) | 984 (699, 1269) |
| Palliative (n = 89) | 13.6 (11.9, 15.2) | 370 (332, 408) |
| P = 0.001 | P < 0.001 | |
| Treatment type | ||
| Endoscopic resection (n = 5) | 1.3 (-1.0, 3.6) | Not reached |
| Surgical resection (n = 95) | 10.7 (9.5, 11.9) | 1285 (962, 1608) |
| Chemoradiotherapy (n = 63) | 11.6 (10.0, 13.1) | 700 (411, 988) |
| Palliative (n = 89) | 13.8 (12.1, 15.5) | 370 (349, 390) |
| Exploratory surgery (n = 19) | 8.8 (6.6, 11.0) | 340 (280, 400) |
| P < 0.001 | P < 0.001 |
Twenty-one studies published to date have assessed the influence of pretreatment SUVmax on the prognosis of cancer of the esophagus in 1960 patients (Table 4)[4-24]. By cancer subtype, the proportion of patients with adenocarcinoma in the studies has ranged from 0% to 100%, with a median of 78%. As was noted in the current study, squamous carcinoma is associated with higher FDG uptake than adenocarcinoma. Sixteen of the studies assessed only patients being treated with radical intent, either surgery (with or without neoadjuvant chemotherapy) or chemoradiotherapy. Only four studies assessed patients treated with both radical and palliative intent. The current study represents the largest unselected study to date.
| Ref. | Patients (n) | Adeno-carcinoma (%) | Treatment intention of studied group | Median (or mean) SUVmax | SUVmax significant on univariate analysis | SUVmax significant on multivariate analysis | Other significant associations on multivariate analysis |
| Fukunaga et al[4], 1998 | 48 | Not stated | Curative | 7 | Yes | Not assessed | Not assessed |
| Choi et al[5], 2004 | 69 | 0% | Curative | 6.3/13.7 (thresholds) | Yes | No | UICC stage |
| Hong et al[6], 2005 | 47 | 87% | Curative | Not stated | No | No | Number of abnormalities on PET-CT |
| Stahl et al[7], 2005 | 40 | 100% | Curative | 10.5 | No | Not assessed | |
| van Westreenen et al[8], 2005 | 40 | 70% | Curative and palliative | 6.7 | Yes | No | Treatment |
| Cerfolio et al[9], 2006 | 89 | 53% | Curative | 6.6 | Yes | Yes | UICC stage |
| Choi et al[10], 2006 | 51 | 0% | Curative | Not stated | Yes | No | UICC stage, N1 status (on PET-CT), immunohistochemical markers |
| Westerterp et al[11], 2008 | 26 | 100% | Curative | 0.26 | Yes | Not assessed | |
| Omloo et al[12], 2008 | 125 | 85% | Curative | 0.27 | Yes | No | UICC stage |
| Cheze-Le Rest et al[13], 2008 | 47 | 77% | Curative | 9 | Yes | Yes | Treatment, number of abnormalities on PET-CT |
| Chatterton et al[14], 2008 | 129 | 19% | Curative and palliative | 8.2 | No | Not assessed | Not assessed |
| Makino et al[15], 2008 | 38 | 100% | Curative | 11.1 | Yes | No | N1 status (on PET-CT) |
| Javeri et al[16], 2009 | 161 | 100% | Curative | 10.1 | No | No | |
| Kato et al[17], 2009 | 184 | 0% | Curative | 4.5 | Yes | Yes | N1 status |
| Rizk et al[18], 2009 | 189 | 100% | Curative | 4.5 (preset threshold) | Yes | Not assessed | Not assessed |
| Sepesi et al[19], 2009 | 72 | 83% | Curative | 6.2 | Yes | Yes | |
| Shenfine et al[20], 2009 | 45 | 100% | Curative | 5.7 | Yes | No | UICC stage |
| Hyun et al[21], 2010 | 151 | 3% | Curative and palliative | 17.2 | Yes | No | UICC stage, metabolic tumor volume |
| Brown et al[22], 2012 | 103 | 80% | Curative | 6.4 (early)/8.8 (later scans) | Yes | No | N1 status, age |
| Gillies et al[23], 2012 | 121 | 100% | Curative | 8.5 | Yes | No | N1 status (on PET-CT) |
| Chan et al[24], 2013 | 185 | 75% | Curative and palliative | 8.9 | Yes | No | N1 status, tumor volume on EUS |
There were wide variations in the median, mean and threshold SUVmax noted in the published studies. The median value of 10.9 identified in the current study was higher than the majority of the studies and likely reflects the unselected population evaluated. Of note, the scans obtained in this study were obtained using two PET-CT machines, although there was no evidence that this had any influence on the measurements.
Pan et al[3], in a meta-analysis of the literature published up to 2009 identified SUVmax to be associated with a hazard ratio of 1.86 for overall survival, with higher values reflecting poorer survival. The authors however assessed the link between uptake and survival using univariate analysis. In the current study, a significant link between SUVmax and prognosis was noted on univariate analysis, but this effect disappeared on multivariate analysis. Table 4 indicates that 17 of the 21 studies (81%) identified a significant association between SUVmax and prognosis on univariate analysis, but only four of 16 studies (25%) found that this effect persisted on multivariate analysis.
The reason for this is likely to be the close relationship between SUVmax and UICC stage, and the over-riding effect of UICC stage on all other prognostic markers. The literature taken en masse report similar themes. Other factors that have been identified as being of prognostic value indirectly relate to cancer stage such as PET-CT N stage, the absolute number of abnormalities on PET-CT and the endoscopic ultrasound derived tumor node metastasis stage or tumor volume.
The current study has assessed the influence of a single pretreatment uptake value on cancer outcome, although other studies have suggested that serial PET-CT scanning may yield additional information by comparing pre- and post-treatment values[25,26]. At our institution, it is not standard practice to perform serial PET-CT. Patients undergo only one pretreatment examination.
We have previously shown that PET-CT alters the cancer stage in 26% of patients and that this translates into a change in management for 18%[2]. The implications of the current study are that the value of the PET-CT remains in the diagnosis of “occult” metastatic disease or confirming suspicious abnormalities on initial CT imaging. Its role is purely in triangulating with other information in order to predict pretreatment stage. The pretreatment SUVmax measurement, while closely linked to prognosis does not provide additional meaningful information that can be used in clinical decision making.
Several studies have noted that FDG uptake in regional lymph nodes may provide additional prognostic information[10,15,22-24]. At our institution, no attempt has been made to stage local peritumoral lymphadenopathy on the basis of PET-CT. We have considered the spatial resolution of the imaging insufficient to allow distinction between primary tumor and local lymphadenopathy. Local nodal staging is assessed by endoscopic ultrasound.
In conclusion, this study did not demonstrate the utility of PET-CT scanning, over and above determination of UICC stage. Pre-treatment SUVmax did not yield additional useful information.
Positron emission tomography-computed tomography (PET-CT) imaging is routinely employed in the staging of esophageal cancer. Its principal role is in the identification of metastatic disease. Some previous reports have suggested that the fluorodeoxyglucose (FDG) uptake [maximum standardized uptake value (SUVmax)] may afford additional prognostic information.
The research hotspot is to determine whether or not the effect of SUVmax on prognosis is independent of known prognostic markers, such as Union for International Cancer Control (UICC) stage
Univariate analysis identified that prognosis was linked to baseline pre-treatment) SUVmax in patients with esophageal cancer. However, this effect did not persist on regression analysis, with conventional prognostic markers (UICC stage and tumor stage) assuming significance.
The principal value of PET-CT in this patient group remains the identification of distant metastatic disease.
The authors are to be congratulated on their effort. Although a retrospective study, it is well written and the authors have experience in this technology. They have addressed the clinical question about the independent effect of the prognostic information that maximum PET-CT FDG uptake could provide.
P- Reviewers: Chen XZ, Raul B S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | National Oesophago-gastric Cancer Audit 2010. 3rd Annual Report. NHS Information Centre. 2010; Available from: https: //catalogue.ic.nhs.uk/publications/clinical/oesophago-gastric/nati-clin-audi-supp-prog-oeso-gast-canc-2010/clin-audi-supp-prog-oeso-gast-2010-rep1.pdf. [Cited in This Article: ] |
| 2. | Williams RN, Ubhi SS, Sutton CD, Thomas AL, Entwisle JJ, Bowrey DJ. The early use of PET-CT alters the management of patients with esophageal cancer. J Gastrointest Surg. 2009;13:868-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Pan L, Gu P, Huang G, Xue H, Wu S. Prognostic significance of SUV on PET/CT in patients with esophageal cancer: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2009;21:1008-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Fukunaga T, Okazumi S, Koide Y, Isono K, Imazeki K. Evaluation of esophageal cancers using fluorine-18-fluorodeoxyglucose PET. J Nucl Med. 1998;39:1002-1007. [PubMed] [Cited in This Article: ] |
| 5. | Choi JY, Jang HJ, Shim YM, Kim K, Lee KS, Lee KH, Choi Y, Choe YS, Kim BT. 18F-FDG PET in patients with esophageal squamous cell carcinoma undergoing curative surgery: prognostic implications. J Nucl Med. 2004;45:1843-1850. [PubMed] [Cited in This Article: ] |
| 6. | Hong D, Lunagomez S, Kim EE, Lee JH, Bresalier RS, Swisher SG, Wu TT, Morris J, Liao Z, Komaki R. Value of baseline positron emission tomography for predicting overall survival in patient with nonmetastatic esophageal or gastroesophageal junction carcinoma. Cancer. 2005;104:1620-1626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Stahl A, Stollfuss J, Ott K, Wieder H, Fink U, Schwaiger M, Weber WA. FDG PET and CT in locally advanced adenocarcinomas of the distal oesophagus. Clinical relevance of a discordant PET finding. Nuklearmedizin. 2005;44:249-255; quiz N55- N56. [PubMed] [Cited in This Article: ] |
| 8. | van Westreenen HL, Heeren PA, van Dullemen HM, van der Jagt EJ, Jager PL, Groen H, Plukker JT. Positron emission tomography with F-18-fluorodeoxyglucose in a combined staging strategy of esophageal cancer prevents unnecessary surgical explorations. J Gastrointest Surg. 2005;9:54-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Cerfolio RJ, Bryant AS. Maximum standardized uptake values on positron emission tomography of esophageal cancer predicts stage, tumor biology, and survival. Ann Thorac Surg. 2006;82:391-394; discussion 391-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Choi JY, Jang KT, Shim YM, Kim K, Ahn G, Lee KH, Choi Y, Choe YS, Kim BT. Prognostic significance of vascular endothelial growth factor expression and microvessel density in esophageal squamous cell carcinoma: comparison with positron emission tomography. Ann Surg Oncol. 2006;13:1054-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Westerterp M, Sloof GW, Hoekstra OS, Ten Kate FJ, Meijer GA, Reitsma JB, Boellaard R, van Lanschot JJ, Molthoff CF. 18FDG uptake in oesophageal adenocarcinoma: linking biology and outcome. J Cancer Res Clin Oncol. 2008;134:227-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Omloo JM, Sloof GW, Boellaard R, Hoekstra OS, Jager PL, van Dullemen HM, Fockens P, Plukker JT, van Lanschot JJ. Importance of fluorodeoxyglucose-positron emission tomography (FDG-PET) and endoscopic ultrasonography parameters in predicting survival following surgery for esophageal cancer. Endoscopy. 2008;40:464-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Cheze-Le Rest C, Metges JP, Teyton P, Jestin-Le Tallec V, Lozac’h P, Volant A, Visvikis D. Prognostic value of initial fluorodeoxyglucose-PET in esophageal cancer: a prospective study. Nucl Med Commun. 2008;29:628-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Chatterton BE, Ho Shon I, Baldey A, Lenzo N, Patrikeos A, Kelley B, Wong D, Ramshaw JE, Scott AM. Positron emission tomography changes management and prognostic stratification in patients with oesophageal cancer: results of a multicentre prospective study. Eur J Nucl Med Mol Imaging. 2009;36:354-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Makino T, Doki Y, Miyata H, Yasuda T, Yamasaki M, Fujiwara Y, Takiguchi S, Higuchi I, Hatazawa J, Monden M. Use of (18)F-fluorodeoxyglucose-positron emission tomography to evaluate responses to neo-adjuvant chemotherapy for primary tumor and lymph node metastasis in esophageal squamous cell carcinoma. Surgery. 2008;144:793-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Javeri H, Xiao L, Rohren E, Lee JH, Liao Z, Hofstetter W, Maru D, Bhutani MS, Swisher SG, Macapinlac H. The higher the decrease in the standardized uptake value of positron emission tomography after chemoradiation, the better the survival of patients with gastroesophageal adenocarcinoma. Cancer. 2009;115:5184-5192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Kato H, Nakajima M, Sohda M, Tanaka N, Inose T, Miyazaki T, Fukuchi M, Oriuchi N, Endo K, Kuwano H. The clinical application of (18)F-fluorodeoxyglucose positron emission tomography to predict survival in patients with operable esophageal cancer. Cancer. 2009;115:3196-3203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Rizk N, Downey RJ, Akhurst T, Gonen M, Bains MS, Larson S, Rusch V. Preoperative 18[F]-fluorodeoxyglucose positron emission tomography standardized uptake values predict survival after esophageal adenocarcinoma resection. Ann Thorac Surg. 2006;81:1076-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Sepesi B, Raymond DP, Polomsky M, Watson TJ, Litle VR, Jones CE, Hu R, Qiu X, Peters JH. Does the value of PET-CT extend beyond pretreatment staging? An analysis of survival in surgical patients with esophageal cancer. J Gastrointest Surg. 2009;13:2121-2127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Shenfine J, Barbour AP, Wong D, Thomas J, Martin I, Gotley DC, Smithers BM. Prognostic value of maximum standardized uptake values from preoperative positron emission tomography in resectable adenocarcinoma of the esophagus treated by surgery alone. Dis Esophagus. 2009;22:668-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, Lee JY, Lee KH, Kim BT. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010;17:115-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 22. | Brown C, Howes B, Jamieson GG, Bartholomeusz D, Zingg U, Sullivan TR, Thompson SK. Accuracy of PET-CT in predicting survival in patients with esophageal cancer. World J Surg. 2012;36:1089-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Gillies RS, Middleton MR, Han C, Marshall RE, Maynard ND, Bradley KM, Gleeson FV. Role of positron emission tomography-computed tomography in predicting survival after neoadjuvant chemotherapy and surgery for oesophageal adenocarcinoma. Br J Surg. 2012;99:239-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Chan DS, Fielding P, Roberts SA, Reid TD, Ellis-Owen R, Lewis WG. Prognostic significance of 18-FDG PET/CT and EUS-defined tumour characteristics in patients with oesophageal cancer. Clin Radiol. 2013;68:352-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Vallböhmer D, Hölscher AH, Dietlein M, Bollschweiler E, Baldus SE, Mönig SP, Metzger R, Schicha H, Schmidt M. [18F]-Fluorodeoxyglucose-positron emission tomography for the assessment of histopathologic response and prognosis after completion of neoadjuvant chemoradiation in esophageal cancer. Ann Surg. 2009;250:888-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Ott K, Weber WA, Lordick F, Becker K, Busch R, Herrmann K, Wieder H, Fink U, Schwaiger M, Siewert JR. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol. 2006;24:4692-4698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 344] [Article Influence: 19.1] [Reference Citation Analysis (0)] |