Published online Jan 15, 2018. doi: 10.4251/wjgo.v10.i1.31
Peer-review started: September 7, 2017
First decision: October 9, 2017
Revised: November 24, 2017
Accepted: December 13, 2017
Article in press: December 13, 2017
Published online: January 15, 2018
To provide evidence regarding the postoperative treatment of patients with T4bN1-3M0/TxN3bM0 gastric cancer, for which guidelines have not been established.
Patients who had undergone curative resection between 1996 and 2014 with a pathological stage of T4bN1-3M0/TxN3bM0 for gastric cancer were retrospectively analyzed; staging was based on the 7th edition of the American Joint Committee on Cancer staging system. The clinicopathological characteristics, administration of adjuvant chemotherapy, and patterns of recurrence were studied. Univariate and multivariate analyses of prognostic factors were conducted. The chemotherapeutic agents mainly included fluorouropyrimidine, platinum and taxanes, used as monotherapy, doublet, or triplet regimens. Patterns of first recurrence were categorized as locoregional recurrence, peritoneal dissemination, or distant metastasis.
The 5-year overall survival (OS) of the whole group (n = 176) was 16.8%, and the median OS was 25.7 mo (95%CI: 20.9-30.5). Lymphovascular invasion and a node positive rate (NPR) ≥ 0.8 were associated with a poor prognosis (P = 0.01 and P = 0.048, respectively). One hundred forty-seven (83.5%) of the 176 patients eventually experienced recurrence; the most common pattern of the first recurrence was distant metastasis. The prognosis was best for patients with locoregional recurrence and worst for those with peritoneal dissemination. Twelve (6.8%) of the 176 patients did not receive adjuvant chemotherapy, while 164 (93.2%) patients received adjuvant chemotherapy. Combined chemotherapy, including doublet and triplet regimens, was associated with a better prognosis than monotherapy, with no significant difference in 5-year OS (17.5% vs 0%, P = 0.613). The triplet regimen showed no significant survival benefit compared with the doublet regimen for 5-year OS (18.5% vs 17.4%, P = 0.661). Thirty-nine (22.1%) patients received adjuvant chemotherapy for longer than six months; the median OS in patients who received adjuvant chemotherapy for longer than six months was 40.2 mo (95%CI: 30.6-48.2), significantly longer than the 21.6 mo (95%CI: 19.1-24.0) in patients who received adjuvant chemotherapy for less than six months (P = 0.001).
Patients with T4bN1-3M0/TxN3bM0 gastric cancer showed a poor prognosis and a high risk of distant metastasis. Adjuvant chemotherapy for longer than six months improved outcomes for them.
Core tip: Patients with T4bN1-3M0/TxN3bM0 gastric cancer have a poor prognosis after curative resection. Due to limited evidence and a lack of guidelines for clinical practice, T4bN1-3M0/TxN3bM0 gastric cancer remains a challenging clinical problem. Our retrospective study is complementary to large-scale phase III prospective trials and showed that the most common pattern of first recurrence for this population is distant metastasis and that prolonged adjuvant chemotherapy may improve patient outcomes. This finding will need to be confirmed by future prospective randomized controlled studies to improve the outcomes for patients with T4bN1-3M0/TxN3bM0 gastric cancer.
- Citation: Wang QW, Zhang XT, Lu M, Shen L. Impact of duration of adjuvant chemotherapy in radically resected patients with T4bN1-3M0/TxN3bM0 gastric cancer. World J Gastrointest Oncol 2018; 10(1): 31-39
- URL: https://www.wjgnet.com/1948-5204/full/v10/i1/31.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i1.31
Nearly one million new cases of gastric cancer (GC) were diagnosed in 2012, making it the fifth most common malignancy worldwide[1]. Geographically, GC is most common in East Asian countries including China, Japan and Korea (45% in China). In contrast to the situation in Japan and Korea, GC in China is often detected at a locally advanced or advanced stage. Complete resection with a D2 lymphadenectomy remains the cornerstone of curative treatment; however, more than half of resectable GC patients develop recurrence despite achieving an R0 resection[2].
Efforts to reduce the risk of recurrence and improve survival have focused on perioperative treatment. Postoperative adjuvant chemotherapy in GC is primarily supported by two large randomized phase III studies: The Japanese ACTS-GC[3] (Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer) and the Asian CLASSIC[4] (Capecitabine and Oxaliplatin Adjuvant Study in Stomach Cancer) trials. Both of these trials showed a survival benefit after D2 gastrectomy compared with surgery alone. A recent study, SAMIT[5] (Japanese Stomach Cancer Adjuvant Multi-Institutional Trial), compared additional chemotherapy with single-agent fluoropyrimidine but failed to show a survival benefit. However, GC patients who were resectable at the most advanced stage (T4bN1-3M0/TxN3bM0, mostly IIIC) were not included in the CLASSIC trial; moreover, this patient population made up only 5% of the sample in the ACTS-GC study and 10% in the SAMIT study. Considering that R0 resection of the primary cancer had barely been achieved due to the locally advanced stage, these patients were at the highest risk for disease recurrence and were more likely to benefit from adjuvant chemotherapy. Due to the limited evidence as well as the difficulties in therapeutic management, T4bN1-3M0/TxN3bM0 gastric cancer remains a challenging problem in clinical practice.
A Korean retrospective study[6] that focused on stage IV [T4N1-3M0/T1-4N3M0, American Joint Committee on Cancer (AJCC) 6th edition[7]] GC patients, who were equivalent to the T4bN1-3M0/TxN3bM0 (AJCC 7th edition[8]) patients in the current study, showed that patients who received adjuvant chemotherapy exhibited a survival benefit compared with patients who received surgery alone. However, the Korean study did not discuss the appropriate adjuvant therapy modality, which remains undefined for T4bN1-3M0/TxN3bM0 GC patients.
In view of the limited evidence regarding T4bN1-3M0/TxN3bM0 GC, the difficulty of R0 resection, and the high risk of disease recurrence in this population, the aim of this retrospective study was to discuss the appropriate adjuvant therapy modality for patients with the most locally advanced GC.
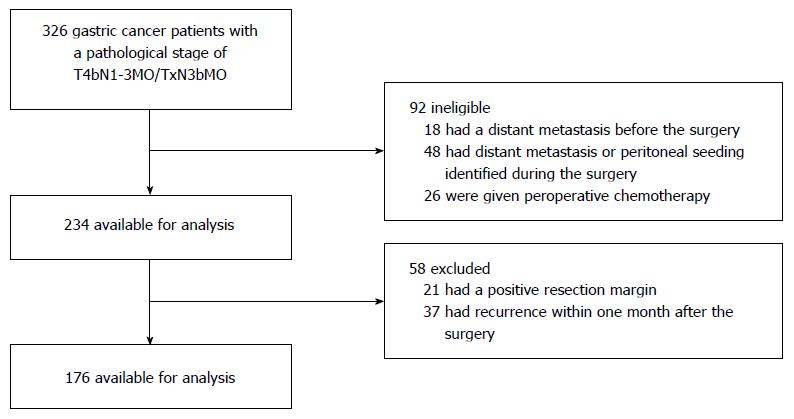
A total of 326 consecutive patients with primary GC with a pathological stage of T4bN1-3M0/TxN3bM0 based on the AJCC (7th edition) staging system who underwent potentially curative resection (R0) between October 1996 and December 2014 were identified in the database of Peking University Cancer Hospital. Of these patients, 18 had a distant metastasis that was detected before surgery, 48 had distant metastasis or peritoneal seeding (including positive peritoneal cytology) identified during the operation, 26 were given preoperative chemotherapy, 21 had a positive resection margin, 37 had recurrence within one month after surgery, and 176 with T4bN1-3M0/TxN3bM0 disease were available for analysis (Figure 1). All patients had histologically confirmed gastric or gastroesophageal junction adenocarcinoma.
A total of 145 (82.4%) patients had metastasis in sixteen or more regional lymph nodes with a median number of 20 metastatic lymph nodes (range: 0-70) and a median node positive rate (NPR) of 0.60 (range: 0.0-1.0). D2 lymph node dissection, according to the NCCN Clinical Practice Guidelines in Oncology-Gastric Cancer (Version 1.2017), was performed in 136 (77.3%) patients, and the median number of dissected lymph nodes was 33 (range: 2-108); 49 (27.8%) patients showed invasion of the adjacent structures and underwent a gastrectomy with bloc resection of the involved structures. A total of 132 (75%) patients underwent resection at a single institution in the Peking University Cancer Hospital.
Adjuvant chemotherapy was administered to 164 (93.2%) patients after curative resection. The chemotherapy regimens included monotherapy (capecitabine/S1/5-FU, n = 10), doublet chemotherapy (FOLFOX, n = 33; XELOX, n = 34; SOX, n = 39; capecitabine/S1+cisplatin, n = 9; paclitaxel+capecitabine, n = 15; paclitaxel+ cisplatin/oxaliplatin, n = 4) and triplet chemotherapy (based on 5-FU including cisplatin, oxaliplatin, epirubicin, paclitaxel, docetaxel, etoposide, and mitomycin, n = 20); 12 patients did not receive adjuvant chemotherapy. Fourteen patients received intra- or postoperative intraperitoneal perfusion of cisplatin/paclitaxel/5-FU, and four patients received postoperative chemoradiotherapy. All adverse events were assessed using the National Cancer Institute Common Toxicity Criteria (NCI-CTC), version 2.0. Dose modifications were made for patients who experienced hematologic or non-hematologic toxicity.
Disease recurrence was determined by radiologic or histological examination; the sites of recurrence were documented separately and included anastomotic sites, regional lymph nodes, peritoneum, ovary, adrenal gland, liver, lung, bone, extra-abdominal lymph nodes, and Virchow’s lymph nodes. Based on these sites, the patterns of the first recurrence were categorized as locoregional recurrence (anastomotic sites and regional lymph nodes), peritoneal dissemination (ovary and the peritoneum), or distant metastasis (the liver, lung, bone, Virchow’s lymph nodes, extra-abdominal lymph nodes, and adrenal gland).
Patients were followed every 3 mo for the first 2 years and then at 6-mo intervals until the fifth year. Regular follow-up evaluations consisted of a physical examination, routine laboratory tests, abdominal computed tomography (CT) scan, endoscopy, and chest X-ray.
The statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) software, version 21.0. Disease-free survival (DFS) was defined as the time from surgery until the recurrence of GC or death from any cause. Overall survival (OS) was defined as the time from surgery until death from any cause. Continuous variables were transformed to dichotomous variables in the survival analysis. χ2 tests were used to compare clinicopathological characteristics between groups. Variables known to have prognostic value were selected in the final multivariable Cox proportional hazards model. Kaplan-Meier curves for disease-free survival and OS were compared using a log-rank test. A P-value of < 0.05 was considered statistically significant.
Our study included a group of 176 patients with metastasis in sixteen or more regional lymph nodes (TxN3bM0) or invasion of adjacent structures (T4bN1-3M0) in whom achieving R0 resection was difficult and who were assumed to be at high risk for recurrence. All patients, including 131 females and 45 males aged 25-81 years (56.4 ± 11.1 years), had histologically confirmed gastric or gastroesophageal junction adenocarcinoma; most had poorly differentiated adenocarcinoma. Of the 176 patients, 156 (88.6%) were classified as stage IIIC based on the AJCC TNM Staging Classification for Carcinoma of the Stomach (7th ed, 2010). The clinicopathological characteristics of the patients are listed in Table 1.
| Clinicopathological characteristics | All patients (n = 176) | 5-yr OS (%) | P value | |
| n | % | |||
| Sex | ||||
| Male | 131 | 74.4% | 17.4% | 0.702 |
| Female | 45 | 25.6% | 15.8% | |
| Age (yr) | ||||
| ≥ 60 | 68 | 38.6% | 21.8% | 0.799 |
| < 60 | 108 | 61.4% | 13.5% | |
| Tumor location | ||||
| Upper third | 43 | 24.4% | 17.7% | 0.614 |
| Middle third | 56 | 31.8% | 19.6% | |
| Lower third | 62 | 35.2% | 19.6% | |
| Total | 15 | 8.5% | 0.0% | |
| Tumor grade (differentiation) | ||||
| Moderate | 15 | 8.5% | 19.3% | 0.241 |
| Poor | 161 | 91.5% | 16.5% | |
| Lymphovascular invasion | ||||
| Yes | 139 | 79.0% | 10.3% | 0.010 |
| No | 37 | 21.0% | 30.6% | |
| No. of positive LNs | ||||
| 0 | 4 | 2.3% | 37.5% | 0.174 |
| 1-6 | 17 | 9.7% | 31.2% | |
| 7-15 | 10 | 5.7% | 0.0% | |
| ≥ 16 | 145 | 82.4% | 15.8% | |
| No. of dissected LNs | ||||
| ≥ 30 | 106 | 60.2% | 20.6% | 0.326 |
| < 30 | 70 | 39.8% | 11.6% | |
| Positive LN ratio | ||||
| ≥ 0.8 | 34 | 19.3% | 6.2% | 0.048 |
| < 0.8 | 142 | 80.7% | 20.5% | |
| Pathologic T stage1 | ||||
| T2 | 5 | 2.8% | 40.0% | 0.420 |
| T3 | 20 | 11.4% | 30.6% | |
| T4a | 102 | 58.0% | 12.6% | |
| T4b | 49 | 27.8% | 21.2% | |
| Stage1 | ||||
| IIIA | 5 | 2.8% | 40.0% | 0.237 |
| IIIB | 15 | 8.5% | 35.9% | |
| IIIC | 156 | 88.6% | 14.0% | |
Based on the follow-up data updated on July 31, 2015, the median follow-up time for the 176 patients was 47.4 mo (range: 2-202 mo). By the end of the follow-up period, 123 patients had died, 37 patients were alive, and 16 patients (9.1%) had been lost to follow-up.
The 5-year OS of the group was 16.8%; the median OS was 25.7 mo (95%CI: 20.9-30.5). The 3-year DFS of the whole group was 9.8%, while the median DFS was 11.7 mo (95%CI: 10.0-13.4). The univariate analysis showed that lymphovascular invasion and NPR ≥ 0.8 were associated with a poor prognosis (P = 0.01 and P = 0.048, respectively), while stage IIIC was not significantly associated with a poor prognosis according to the Kaplan-Meier method (P = 0.237, Table 1).
In the multivariate analysis, lymphovascular invasion was an independent prognostic factor (P = 0.01, HR: 1.8, 95%CI: 1.15-2.8) for OS in T4bN1-3M0/TxN3bM0 GC patients (Table 2).
| Clinicopathological characteristics | P value | Odds ratio | 95%CI | |
| Lower | Upper | |||
| Lymphovascular invasion | 0.01 | 1.80 | 1.15 | 2.8 |
| Node positive rate | 0.14 | 1.36 | 0.90 | 2.1 |
| Stage | 0.49 | 0.71 | 0.34 | 1.5 |
During the follow-up period, 147 (83.5%) of the 176 patients with T4bN1-3M0/TxN3bM0 GC experienced recurrence; the first recurrence was localized to a single site in 78.9% of patients, two sites in 13.6% of patients, and three or more sites in 6.8% of patients. As shown in Table 3, the most common pattern of first recurrence was distant metastasis (45.6%), followed by peritoneal dissemination (25.9%) and locoregional recurrence (22.5%). Nine patients (6.1%) who experienced combined patterns of recurrence were excluded from the survival analysis. The prognosis was best for patients with locoregional recurrence and worst for those who had peritoneal dissemination. Figure 2 presents the OS for each group. The 5-year OS rates were 28.0%, 0% and 14.7% for locoregional recurrence, peritoneal dissemination and distant metastasis, respectively, which showed statistically significant differences (P = 0.001).
| Recurrent sites | Recurrent patients (n = 147) | Median OS (mo) | 5-yr OS(%) | P value | |
| n | % | ||||
| Locoregional | 33 | 22.5% | 33.9 | 28.0% | 0.001 |
| Peritoneal | 38 | 25.9% | 16.0 | 0.0% | |
| Distant | 67 | 45.6% | 21.3 | 14.7% | |
We further analyzed OS according to the most distant metastatic sites; the most frequent site of distant metastasis was the liver, followed by the lung (including malignant pleural effusion), bone, and other distant sites. Eight of ten patients had bone metastases as the first recurrence site without liver or lung metastases. The median OS for patients with bone metastasis from GC was 30.7 mo, while that for patients with other metastatic sites was 21.9 mo (P = 0.35). The median OS for patients with lung metastasis was significantly shorter than that for patients with other metastatic sites (16.8 mo vs 22.4 mo, P = 0.04) (Table 4). The results showed that patients with bone metastasis had a better prognosis, whereas patients with lung and pleura metastasis had a worse prognosis than those with other metastatic sites.
| Distant metastasis site | Recurrent patients (n = 147) | Median OS (mo) | 5-yr OS(%) | |
| n | % | |||
| Liver | 26 | 17.7% | 18.3 | 15.5% |
| Lung and pleura | 12 | 8.2% | 16.8 | 0.0% |
| Bone | 10 | 6.8% | 30.7 | 29.2% |
During the follow-up period after curative resection, 12 patients did not receive adjuvant chemotherapy because of their poor condition or rejection of chemotherapy; 164 (93.2%) of the 176 patients received at least one cycle of adjuvant chemotherapy. Combined chemotherapy, including doublet and triple regimens, was associated with a better prognosis than monotherapy but with no significant difference in 5-year OS (0% in the monotherapy group and 17.5% in the combined chemotherapy group, P = 0.613). Triple adjuvant chemotherapy showed no significant survival benefit over the doublet regimen (P = 0.449). The 5-year OS rates were 0%, 17.4%, and 18
| Treatment | n | Median DFS (mo) | 3-yr DFS (%) | P value | MedianOS (mo) | 5-yr OS (%) | P value | |
| Adjuvant chemotherapy | Yes | 164 | 12.3 | 10.4% | 0.000 | 25.7 | 16.1% | 0.532 |
| No | 12 | 2.8 | 0.0% | 18.7 | 22.2% | |||
| Chemotherapy | Mono- therapy | 10 | 6.7 | 0.0% | 0.583 | 20.3 | 0.0% | 0.661 |
| Regimen | Doublet | 134 | 12.0 | 5.3% | 26.3 | 17.4% | ||
| Triple | 20 | 13.0 | 5.3% | 29.7 | 18.5% | |||
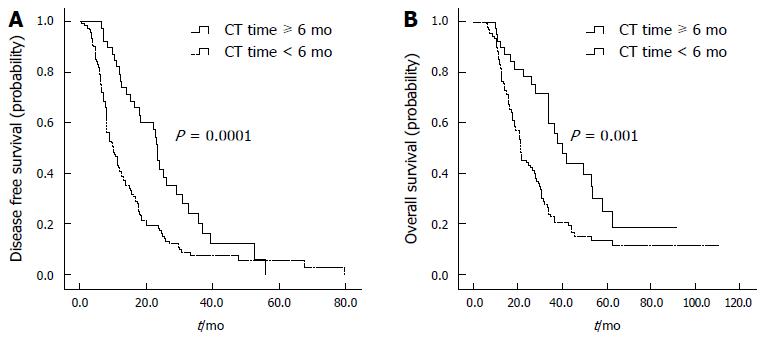
| Adjuvant chemotherapy time | ≥ 6 mo | 39 | 23.2 | 20.2% | 0.000 | 40.2 | 25.0% | 0.001 |
| < 6 mo | 125 | 9.9 | 7.3% | 21.6 | 13.4% | |||
In our study, various chemotherapeutic agents, including platinum-, taxane-, epirubicin-based regimens, did not show any significant differences in survival benefit (data not shown).
The median number of cycles of adjuvant chemotherapy was six, and the median time of adjuvant chemotherapy was 4.2 mo. Thirty-nine (22.1%) of the 176 patients received adjuvant chemotherapy for longer than six mo, as shown in Table 5. A longer duration of adjuvant chemotherapy was significantly associated with a better prognosis; the median OS was prolonged to 40.2 mo (95%CI: 30.6-48.2) in patients given adjuvant chemotherapy for longer than six months, compared with 21.6 mo (95%CI: 19.1-24.0) in patients given adjuvant chemotherapy for less than six months (P = 0.001). The median DFS was 23.2 mo (95%CI: 21.5-24.9) in patients given adjuvant chemotherapy for longer than six months, compared with 9.9 mo (95%CI: 7.6-12.3) in patients receiving adjuvant chemotherapy for less than six months (P = 0.0001) (Table 5, Figure 3). The patient characteristics were similar between the two groups.
Of the 164 patients who received adjuvant chemotherapy, only 39 patients continued the treatment for over six months. The most common reasons for withdrawal of treatment included the refusal of the patients to continue treatment due to inadequate social support (32%), adverse events (28%), the detection of relapse or metastasis (14.6%), or other factors (25.4%). A total of 114 patients (69.5%) required dose modifications or chemotherapy delays, including 24/39 (61.5%) in the chemotherapy ≥ 6 mo group and 90/125 (72.0%) in the chemotherapy < 6 mo group. Of the 154 patients who received doublet or triplet regimens, 20 patients (13.0%) switched to monotherapy because of adverse events or upon their request.
Adverse events, including hematologic and non-hematologic toxic effects, were analyzed. The most frequent grade 3 or 4 adverse events were neutropenia (20.3%), nausea and vomiting (7.3%), anorexia (6.7%), and diarrhea (3.7%). Overall, 44 patients (26.8%) developed grade 3 or 4 toxicities (data not shown).
The aim of this retrospective study was to provide evidence for clinical treatment of T4bN1-3M0/TxN3bM0 GC patients after curative resection. This population is at the most advanced stage of GC at which resection is possible; therefore, R0 resection is difficult, and the risk of recurrence is high. Currently, controversy exists regarding whether prolonging the duration of adjuvant chemotherapy, intensifying adjuvant chemotherapy, or undergoing preoperative chemotherapy will improve the prognosis for these patients. More efforts to explore appropriate adjuvant therapy modalities are necessary for clinical practice.
Despite undergoing standardized adjuvant chemotherapy followed by curative resection performed by experienced surgeons in our high-volume GC centers, patients with T4bN1-3M0/TxN3bM0 GC had a high risk of recurrence and a poor prognosis. The 5-year OS of the entire group was 16.8%, which is significantly lower than that of patients with stage III disease, ranging between 40%-70% in most phase 3 trials[3,9]. Patients at stage IIIC accounted for 88.6% of our study population; the 5-year OS for these patients was far lower than that of patients with stage IIIC GC reported in another study (14.0% vs 30.2%)[10]. Moreover, a Korean study[6] showed that the 5-year OS rate of the patients who received adjuvant chemotherapy with T4bN1-3M0/TxN3bM0 GC was 39.6%; only 61.7% of these patients experienced recurrence[11]. However, the 5-year OS of patients in our study who received adjuvant chemotherapy for longer than 6 mo was only 25%, and 147 (83.5%) of the 176 patients experienced recurrence.
Several factors may be responsible for the poor prognosis of patients in our study. First, new diagnostic modalities such as endoscopic ultrasound (EUS), positron emission tomography/computed tomography (PET/CT), magnetic resonance imaging (MRI), and laparoscopic staging, were not used for preoperative staging of patients treated during the early part of the study, which may have reduced the accuracy of staging and led to the advanced gastric cancer be treated as resectable gastric cancer improperly[12-14]. Therefore, patients included in this study may be mixed with advanced patients actually, and these errors can be avoided using new staging approach. Second, the risk of non-regional lymph node metastases is increased in patients with N3b, although all tumors with T4bN1-3M0/TxN3bM0 are staged regardless of the M1 category; additionally, without appropriate clinical information, surgical pathologists may be unaware that particular lymph node metastases are already distant metastases and they may be classified as N3b instead of M1. Third, Korean and Japanese surgeons have performed more D2+ lymphadenectomies, total gastrectomies, multivisceral resections, and Billroth II digestive tract reconstructions than their Chinese counterparts; indeed, the OS of Korean patients was longer than that of Chinese patients, especially for those with stage III disease[15]. Fourth, 39 patients in our study underwent limited lymph node dissections, whereas only 4 patients received postoperative chemoradiotherapy, as the INT 0116 study established postoperative chemoradiotherapy as a standard of care for patients who undergo < D2 dissections[16]. These facts reflect the medical status in China and contribute to a new understanding of T4bN1-3M0/TxN3bM0 patients, who mostly belong to stage IIIC, while they are distinct from conventional stage IIIC GC patients with regard to the biological behavior and prognosis of the disease.
In our study, the most common pattern of first recurrence was distant metastasis; sites of distant metastasis and locoregional recurrence accounted for 45.6% and 22.5%, respectively, of patients with T4bN1-3M0/TxN3bM0 recurrent GC. Patients with locoregional recurrence showed a better prognosis than patients with distant metastasis, suggesting that systemic therapy, rather than local therapy, was more likely to benefit patients with T4bN1-3M0/TxN3bM0 GC. According to the results of the ACTS-GC and CLASSIC trials[3,9], adjuvant chemotherapy with one year of S1 or 6 mo of the XELOX regimen after a D2 gastrectomy was confirmed to be the standard adjuvant treatment for locally advanced gastric cancer. Without definitive data favoring combined therapy over monotherapy, especially in GC patients with the most advanced stage of T4bN1-3M0/TxN3bM0, it remains unclear whether an intensified or longer duration of adjuvant chemotherapy provides an additional benefit.
In our study, triple adjuvant chemotherapy showed no significant survival benefit compared with a doublet regimen. Recently, the SAMIT study and the ITACA-S study, both of which compared poly-chemotherapy vs monotherapy, failed to show any benefit for patients in an adjuvant setting[5,17]. Intensifying adjuvant chemotherapy is almost considered too difficult to provide additional benefit. It is of note that patients who received adjuvant chemotherapy for longer than six months in our study benefited significantly from the treatment, with the median OS prolonged to 40.2 mo. In contrast, the median OS was 21.6 mo for patients who received chemotherapy for less than six months. It is therefore suggested that prolonged adjuvant chemotherapy may improve the outcomes for patients at a high risk of distant recurrence. However, only 22.1% of the patients completed all six months of chemotherapy, which may be explained by the frailty of GC patients after surgery, along with the toxicity of adjuvant poly-chemotherapy. In this case, active dose modification based on the adverse events of chemotherapy should to be performed to ensure adequate chemotherapy time and additional benefit from the treatment.
While preoperative chemotherapy may theoretically be superior to postoperative chemotherapy for several reasons[18-20], preoperative chemotherapy has been widely used for patients with T4bN1-3M0/TxN3bM0 GC in clinical practice. However, whether perioperative or postoperative chemotherapy is more beneficial for T4bN1-3M0/TxN3bM0 patients lacks data supported by prospective studies; the ongoing RESOLVE study (NCT01534546) to compare perioperative chemotherapy of SOX vs SOX/XELOX as postoperative chemotherapy in locally advanced gastric cancer with D2 dissection may provide additional evidence. Moreover, patients in arm C of the RESOLVE study will receive 8 cycles of perioperative SOX followed by 3 cycles of S-1 monotherapy, which may provide evidence for prolonged adjuvant chemotherapy.
Based on the classification and statistical analysis, 26 patients with T4b disease were excluded from our study because they had a positive resection margin, which indicates that at least one-third of T4b patients according to preoperative staging failed to eventually undergo R0 resection. Preoperative chemoradiotherapy (CRT) may increase resectability and improve the outcomes of T4b patients. The role of CRT continues to be evaluated in many ongoing clinical trials worldwide, such as the Trial of Preoperative Therapy for Gastric and Esophagogastric Junction Adenocarcinoma (TOPGEAR, NCT01924819) and the ARTIST-II trial in patients with lymph node-positive GC after D2 gastrectomy.
Due to the small sample sizes and the heterogeneity of therapy administered over a long period, the results in this study have been mixed and biased. Although this study was conducted based on retrospective data, we think that the bias may be reduced by the fact that the surgeries were performed in our high-volume GC centers and patients had access to good medical care. Indeed, this study is the largest retrospective analysis of the effect of adjuvant therapy on patients with T4bN1-3M0/TxN3bM0 GC; the results reflect the current medical situation for the treatment of gastric cancer in China and are complementary to those of large-scale phase III prospective trials.
Undoubtedly, along with an in-depth understanding of molecular and gene profiling, personalized precision medicine as well as adjuvant and perioperative multimodal therapies[21] will be crucial for improving the outcomes of conventional adjuvant chemotherapeutic treatments in the future.
In conclusion, patients with T4bN1-3M0/TxN3bM0 gastric cancer showed a poor prognosis, with the most common pattern of first recurrence being distant metastasis rather than locoregional recurrence. Adjuvant chemotherapy for longer than six months may improve the outcomes of this patient group. However, a prospective randomized controlled study will be required to confirm these findings and to improve the outcomes for patients with T4bN1-3M0/TxN3bM0 gastric cancer.
In view of the limited evidence regarding T4bN1-3M0/TxN3bM0 GC, as well as the difficulty of achieving R0 resection and the high risk of disease recurrence, this retrospective study is complementary to large-scale phase III prospective trials and may provide implications for clinical practice.
The population targeted in our study is difficult to treat with no accepted standard of care. This study is the largest retrospective analysis of the effect of adjuvant therapy on patients with T4bN1-3M0/TxN3bM0 GC. Furthermore, our study explored the patterns of recurrence and their relationships to the prognosis of these patients.
To provide evidence regarding the postoperative treatment of patients with T4bN1-3M0/TxN3bM0 gastric cancer, for which guidelines have not been established.
Patients who had undergone curative resection between 1996 and 2014 with a pathological stage of T4bN1-3M0/TxN3bM0 for gastric cancer were retrospectively analyzed; staging was based on the 7th edition of the American Joint Committee on Cancer staging system. The clinicopathological characteristics, administration of adjuvant chemotherapy, and patterns of recurrence were studied. Univariate and multivariate analyses of prognostic factors were conducted. The chemotherapeutic agents mainly included fluorouropyrimidine, platinum and taxanes, used as monotherapy, doublet, or triplet regimens. Patterns of first recurrence were categorized as locoregional recurrence, peritoneal dissemination, or distant metastasis.
The 5-year overall survival (OS) of the whole group (n = 176) was 16.8%, and the median OS was 25.7 mo (95%CI: 20.9-30.5). Lymphovascular invasion and a node positive rate (NPR) ≥ 0.8 were associated with a poor prognosis (P = 0.01 and P = 0.048, respectively). One hundred forty-seven (83.5%) of the 176 patients eventually experienced recurrence; the most common pattern of the first recurrence was distant metastasis. The prognosis was best for patients with locoregional recurrence and worst for those with peritoneal dissemination. Twelve (6.8%) of the 176 patients did not receive adjuvant chemotherapy, while 164 (93.2%) patients received adjuvant chemotherapy. Combined chemotherapy, including doublet and triplet regimens, was associated with a better prognosis than monotherapy, with no significant difference in 5-year OS (17.5% vs 0%, P = 0.613). The triplet regimen showed no significant survival benefit compared with the doublet regimen for 5-year OS (18.5% vs 17.4%, P = 0.661). Thirty-nine (22.1%) patients received adjuvant chemotherapy for longer than six months; the median OS in patients who received adjuvant chemotherapy for longer than six months was 40.2 mo (95%CI: 30.6-48.2), significantly longer than the 21.6 mo (95%CI: 19.1-24.0) in patients who received adjuvant chemotherapy for less than six months (P = 0.001).
Patients with T4bN1-3M0/TxN3bM0 gastric cancer showed a poor prognosis, with the most common pattern of first recurrence being distant metastasis rather than locoregional recurrence. Adjuvant chemotherapy for longer than six months may improve the outcomes of this patient group.
To date, few retrospective studies have analyzed the survival and prognosis factors for T4bN1-3M0/TxN3bM0 GC patients; however, due to the small sample sizes and different treatment regimens, the results have been mixed. No meta-analyses have been conducted on this topic. However, a prospective randomized controlled study will be required to confirm these findings and to improve the outcomes for patients with T4bN1-3M0/TxN3bM0 gastric cancer.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kopljar M, Merrett ND S- Editor: Kong JX L- Editor: A E- Editor: Wang CH
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20108] [Cited by in F6Publishing: 19774] [Article Influence: 2197.1] [Reference Citation Analysis (17)] |
| 2. | Gee DW, Rattner DW. Management of gastroesophageal tumors. Oncologist. 2007;12:175-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 869] [Cited by in F6Publishing: 1002] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 4. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1055] [Cited by in F6Publishing: 1176] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 5. | Tsuburaya A, Yoshida K, Kobayashi M, Yoshino S, Takahashi M, Takiguchi N, Tanabe K, Takahashi N, Imamura H, Tatsumoto N. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): a phase 3 factorial randomised controlled trial. Lancet Oncol. 2014;15:886-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Ha TK, Jung MS, Lee KH, Lee KG, Kwon SJ. The effect of adjuvant chemotherapy on stage IV (T4N1-3M0 and T1-3N3M0) gastric cancer. Cancer Res Treat. 2009;41:19-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Greene FL PD, Fleming ID. AJCC Cancer Staging Manual: TNM Classification of Malignant Tumors, 6th ed. New York: Springer-Verlag; 2002; . [Cited in This Article: ] |
| 8. | Edge SB BD, Compton CC. AJCC Cancer Staging Handbook. New York: Springer-Verlag; 2010; . [DOI] [Cited in This Article: ] |
| 9. | Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 690] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 10. | Jung H, Lee HH, Song KY, Jeon HM, Park CH. Validation of the seventh edition of the American Joint Committee on Cancer TNM staging system for gastric cancer. Cancer. 2011;117:2371-2378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Xue YW, Wei YZ. The relationship of prognosis to surgery and pathologic characteristics of stage IV (M0) gastric cancer patients. Chin J Cancer. 2010;29:355-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Bentrem D, Wilton A, Mazumdar M, Brennan M, Coit D. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol. 2005;12:347-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol. 2007;25:2107-2116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 14. | Weber WA, Ott K. Imaging of esophageal and gastric cancer. Semin Oncol. 2004;31:530-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Shen ZL, Song KY, Ye YJ, Xie QW, Liang B, Jiang K, Park CH, Wang S. Significant differences in the clinicopathological characteristics and survival of gastric cancer patients from two cancer centers in china and Korea. J Gastric Cancer. 2015;15:19-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, Gunderson LL, Goldman B, Martenson JA, Jessup JM. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327-2333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 521] [Cited by in F6Publishing: 588] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 17. | Bajetta E, Floriani I, Di Bartolomeo M, Labianca R, Falcone A, Di Costanzo F, Comella G, Amadori D, Pinto C, Carlomagno C, Nitti D, Daniele B, Mini E, Poli D, Santoro A, Mosconi S, Casaretti R, Boni C, Pinotti G, Bidoli P, Landi L, Rosati G, Ravaioli A, Cantore M, Di Fabio F, Aitini E, Marchet A; ITACA-S (Intergroup Trial of Adjuvant Chemotherapy in Adenocarcinoma of the Stomach Trial) Study Group. Randomized trial on adjuvant treatment with FOLFIRI followed by docetaxel and cisplatin versus 5-fluorouracil and folinic acid for radically resected gastric cancer. Ann Oncol. 2014;25:1373-1378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210-5218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 511] [Cited by in F6Publishing: 496] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 19. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1216] [Cited by in F6Publishing: 1361] [Article Influence: 104.7] [Reference Citation Analysis (0)] |
| 20. | Yoshikawa T, Rino Y, Yukawa N, Oshima T, Tsuburaya A, Masuda M. Neoadjuvant chemotherapy for gastric cancer in Japan: a standing position by comparing with adjuvant chemotherapy. Surg Today. 2014;44:11-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Cohen DJ, Leichman L. Controversies in the treatment of local and locally advanced gastric and esophageal cancers. J Clin Oncol. 2015;33:1754-1759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |