Published online Jun 10, 2015. doi: 10.4253/wjge.v7.i6.652
Peer-review started: November 26, 2014
First decision: December 12, 2014
Revised: January 3, 2015
Accepted: March 16, 2015
Article in press: March 18, 2015
Published online: June 10, 2015
AIM: To assess the double-balloon enteroscopy (DBE) role in malignant small bowel tumors (MSBT).
METHODS: This is a retrospective descriptive study performed in a single center. All consecutive patients who underwent a DBE with final diagnosis of a malignant neoplasm from 2004 to 2014 in our referral center were included. Patient demographic and clinical pathological characteristics were recorded and reviewed. MSBT diagnosis was achieved either by DBE directed biopsy with multiple tissue sampling, endoscopic findings or histological analysis of surgical specimen. We have analyzed double-balloon enteroscopy impact in outcome and clinical course of these patients.
RESULTS: Of 627 patients, 28 (4.5%) (mean age = 60 ± 17.3 years) underwent 30 procedures (25 anterograde, 5 retrograde) and were diagnosed of a malignant tumor. Patients presented with obscure gastrointestinal bleeding (n = 19, 67.9%), occlusion syndrome (n = 7, 25%) and diarrhea (n = 1, 3.6%). They were diagnosed by DBE biopsy (n = 18, 64.3%), histological analysis of surgical specimen (n = 7, 25%) and unequivocal endoscopic findings (n = 2, 7.1%). Gastrointestinal stromal tumor (n = 8, 28.6%), adenocarcinoma (n = 7, 25%), lymphoma (n = 4, 14.3%), neuroendocrine tumor (n = 4, 14.3%), metastatic (n = 3, 10.7%) and Kaposi sarcoma (n = 1, 3.6%) were identified. DBE modified outcome in 7 cases (25%), delaying or avoiding emergency surgery (n = 3), modifying surgery approach (n = 2) and indicating emergency SB partial resection instead of elective approach (n = 2).
CONCLUSION: DBE may be critical in the management of MSBT providing additional information that may be decisive in the clinical course of these patients.
Core tip: Malignant small bowel tumors (MSBT) are a heterogeneous and relatively rare group of neoplasms. Double balloon enteroscopy (DBE) may have a critical role in the management of MSBT because of its diagnosis and therapeutic capabilities. DBE procedure may delay or avoid emergency surgery, clarifying the tumor location and characteristics. We have assessed DBE impact in these lesions in a large series of patients of a single referral center.
- Citation: Robles EPC, Delgado PE, Conesa PB, Andrés BM, Guggiana MF, Mateos EA, Caballero MF, Agudo JLR, Martínez SC, Latorre R, Soria F, Gutiérrez JMH, Martínez EPC. Role of double-balloon enteroscopy in malignant small bowel tumors. World J Gastrointest Endosc 2015; 7(6): 652-658
- URL: https://www.wjgnet.com/1948-5190/full/v7/i6/652.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i6.652
Small bowel tumors (SBT) are relatively rare, accounting for 3% to 6% of all gastrointestinal neoplasms[1]. Malignant SBTs (MSBT) are described in the 3.6%-14.5% of patients of double balloon enteroscopy (DBE) series[2-4]. The difference in incidence in these studies is because many authors considered benign and malignant tumors and included duodenal neoplasms. MSBT are a heterogeneous group with different predominant histological types within different studies[5-10]. We will focus on malignant primitive SB tumors such as adenocarcinoma, stromal, neuroendocrine, lymphoproliferative and metastatic tumors. Moreover, these lesions may have a poor prognosis in its natural course so that early diagnosis and treatment may be critical[11,12]. These tumors are often diagnosed late because of their nonspecific clinical presentation[13-16], when they have few therapeutic possibilities[17,18].
Obscure gastrointestinal bleeding (OGIB) is the most common clinical presentation in some studies[4,19,20], while a suspected mass is reported to be the first DBE indication by other authors[2,21]. Therefore, MSBT represent a real diagnostic challenge for the physician. DBE is a well-established procedure in diagnosis and treatment of SB disorders. However, there are few data to date reporting DBE role in MSBT[20,22-24]. Our study was conducted to assess the impact of DBE in these neoplasms.
This is a retrospective descriptive study. All consecutive patients with MSBT who underwent a DBE procedure in our institution were investigated. Patient demographic and clinical pathological characteristics were recorded. MSBT diagnosis was achieved either by DBE directed biopsy with multiple tissue sampling, endoscopic findings or histological analysis of surgical specimen.
DBE procedure (Fuji Film, Saitama, Japan) was performed by expert endoscopists as described by Yamamoto et al[25]. Fujinon EN-450 P5, EN-450 T5 and EN-580T enteroscopes were used. There was no special preparation for the anterograde approach besides an 8-12 h fast. For the retrograde approach, bowel preparation was performed as in colonoscopy. All patients provided written consent to undergo DBE under general anesthesia or deep sedation. Capsule endoscopy (CE) and radiological studies such as CT scan were also considered, when available.
Endoscopists were aware of prior findings reported by CE or other techniques. DBE approach was selected based on the information prior to DBE procedure including previous CE, clinical and/or radiological findings. When the location was uncertain, the oral approach was preferred.
DBE data including indication, approach, endoscopic findings, tumor location, time of the procedure, biopsy histological diagnosis, therapeutics and complications were collected. Tattoo injection was performed to mark the maximum length of bowel inspected or the location of the lesion and to guide the elective or emergency surgery.
Finally, we analyzed how DBE procedure influenced MSBT management and outcome. In this sense, avoiding or modifying the elective or emergency surgery approach was considered as the main evaluation criterion.
Descriptive statistics were used to describe clinical pathological features, endoscopic and radiological findings. Categorical variables were calculated as percentages and continuous variables were expressed as mean values (SD).
Of 627 consecutive patients who underwent 880 DBEs from January 2004 to September 2014 at our referral center, 89 (14.2%) were confirmed to have a SBT. Twenty-eight patients (4.5%) (mean age ± SD: 60 ± 17.3 years) underwent 30 DBEs (25 anterograde, 5 retrograde) (median time: 65 min, range 20-160) and were diagnosed of a MSBT. We only include the malignant tumors localized distal to Treitz. There was a male preponderance in gender (n = 20, 71.4%).
Patient’s characteristics are shown in Table 1. The most common clinical indication was OGIB (67.9%). Patients presented with overt-OGIB (n = 9, 32.1%), occult-OGIB (n = 10, 35.7%), occlusion syndrome (n = 8, 28.6%) and diarrhea (n = 1, 3.6%). In addition, 10 patients (35.7%) had weight loss and 6 patients (21.4%) transfusion requirements.
| GIST | Adenocarcinoma | Lymphoma | Neuroendocrine tumor | |
| No. patients (% of MSBT) | 8 (28.6%) | 7 (25%) | 4 (14.3%) | 4 (14.3%) |
| Sex (M/F) | 7/1 | 3/4 | 2/2 | 4/0 |
| Mean age (SD) (yr) | 64 ± 15 | 59 ± 16 | 48 ± 22 | 55 ± 24 |
| Clinical presentation | ||||
| Overt- obscure OGIB | 4 | 1 | 1 | 1 |
| Occult- obscure OGIB | 3 | 3 | 2 | 2 |
| Diarrhea | 0 | 0 | 0 | 1 |
| Occlusion syndrome | 1 | 3 | 1 | 0 |
| Duodenum/ jejunum/ ileum | 0/7/1 | 0/6/1 | 0/3/1 | 0/0/4 |
DBE was indicated following CE in 17 cases (60.7%) and this procedure confirmed the MSBT in 14 cases (82.4%). The capsule was retained in 4 cases due to SB stenosis identifying the tumor in two of them and retrieved by DBE in all patients. CT scan (n = 8, 28.6%) and other radiological studies (n = 2, 7.1%) were previously performed and a suspected mass was identified in 6 cases (21.4%). CT scan also detected a SB complete stenosis in four cases and DBE clarified that only in three of them there was a complete stenosis without overpassing it with the endoscope. Among patients with obstructive symptoms, radiological imaging was the first SB study in 6 (75%) cases and direct DBE was performed in 2 (25%) patients.
DBE directed-biopsy was attempted in 25 patients (89.3%) and benign/reactive mucosa was found in 5 of them (1 midgut neuroendocrine tumor, 1 adenocarcinoma and 3 GIST) so that 20 patients (71.4%) were finally confirmed to have a MSBT by DBE biopsy (Table 2). Two patients (7.1%) had moderate bleeding after DBE biopsy that stopped after endoscopic treatment. Directed-biopsy by DBE was not attempted in 3 patients (10.7%) with GIST (n = 1), neuroendocrine tumor (n = 1) and metastatic disease (n = 1) because of active bleeding that required emergency surgery within GIST and neuroendocrine tumors and because it was considered unnecessary for diagnosis in the other case. In addition, histological analysis of surgical specimen and endoscopic findings lead to diagnosis in 7 (25%) and 1 (3.6%) patients, respectively.
| DBE biopsy | Final diagnosis (% of MSBTs) | |
| MSBT | 20/25 (80%) | 28 |
| GIST | 4/7 (57.1%) | 8 (28.6%) |
| Adenocarcinoma | 6/7 (85.7%) | 7 (25%) |
| Lymphoma | 4/4 (100%) | 4 (14.3%) |
| Neuroendocrine tumor | 2/3 (66.7%) | 4 (14.3%) |
| Metastatic | 3/3 (100%) | 4 (14.3%) |
| Kaposi Sarcoma | 1/1 (100%) | 1 (3.6%) |
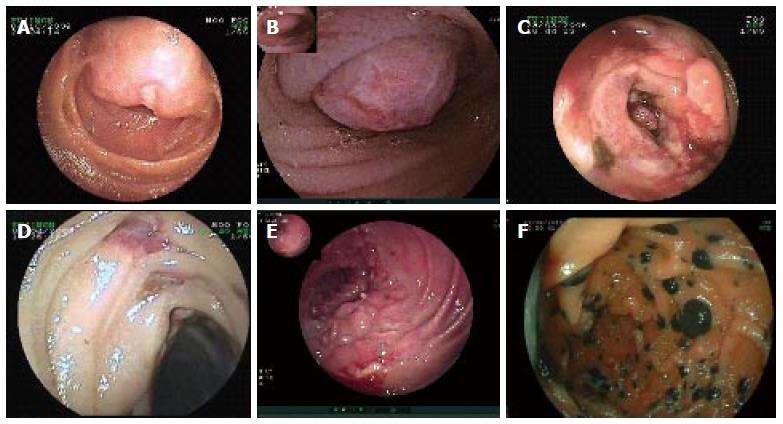
Seven different histological types of MSBT were found. Most of them were located in jejunum (n = 20, 71.4%) followed by ileum (n = 8, 28.6%). We have only included malignant tumors located between distal to Treitz and terminal ileum. Endoscopic findings of different MSBT are shown in Figure 1.
The most common malignant tumor was GIST (n = 8, 28.6%) followed by adenocarcinoma (n = 7, 25%). GIST was also the most common MSBT within OGIB patients (36.8%). In two GIST an enteric fistula was identified by DBE with passage of contrast into the peritoneum so that emergency surgery was indicated after tattoo injection. One of them deceased in the intensive care unit. Another patient with severe anemia and transfusion requirements underwent a DBE that confirmed an ulcerated jejunal GIST with active bleeding. Argon plasma coagulation was successfully performed so that emergency surgery was delayed. The positive detection rate by directed-biopsy within GIST was 57.1%.
Adenocarcinoma was mainly located in jejunum (n = 5, 71.4%). One patient underwent DBE because of one CT suspected jejunal mass and chronic anemia. Finally two synchronic jejunal adenocarcinomas were found modifying the surgery approach. Three patients with adenocarcinoma (42.9%) had impassable SB stenosis despite multiples endoscopic maneuvers and an enteral stent was successfully placed in one case[26]. There were 2 patients with liver metastasis at diagnosis and 3 patients did not underwent surgery because of comorbidities. Finally, 4 patients (57.1%) with adenocarcinoma underwent elective surgery.
Among lymphoma tumors (n = 4, 14.3%), there were 2 MALT, 1 non-Hodking diffuse large B lymphoma and 1 Burkitt lymphoma. One patient with a jejunal MALT lymphoma had refractory celiac disease. Three patients were treated by chemotherapy and the remaining patient refused treatment.Half of patients (n = 2) with neuroendocrine tumors had multiple small tumors besides the main ileal lesion undiagnosed by previous enhanced CT scan.
A jejunal Kaposi sarcoma actively bleeding was identified in a patient with acute overt-OGIB and endoscopic hemostasis was successfully performed. All patients with SB metastasis had the primary lesion already diagnosed (colonic adenocarcinoma, choriocarcinoma, lung adenocarcinoma and melanoma). DBE modified surgical approach in one patient with clinical occlusion syndrome suspected because of metastasis in whom a total DBE confirmed that the obstruction was due to adherences.
Tattoo injection was performed in 21 cases (75%) and guided elective (n = 8, 28.6%) or emergency surgery (n = 5, 17.9%). There was no complication related to therapeutics.
In summary, DBE modified the clinical course and outcome in 7 patients (25%), delaying or avoiding emergency surgery (n = 3), modifying surgery approach (n = 2) and indicating emergency SB partial resection instead of elective approach (n = 2). It’s interesting to note that within these 7 patients, in only 3 cases (42.9%) surgery was delayed or avoided due to endoscopic therapy. Two patients with actively bleeding GIST and Kaposi sarcoma in whom argon plasma coagulation was successfully performed and one patient with a stenosing adenocarcinoma who underwent a DBE with an enteral prosthesis placed.
Regarding the diagnostic performance of deep enteroscopy in SB tumors, Chen et al[3] in an Asiatic retrospective study reported 440 DBEs in 400 patients, diagnosing 67 SB tumors by DBE, with 16.8% overall diagnostic yield. Eleven patients with negative DBE were diagnosed of a SBT by CE or surgery. The positive detection rate among the 78 patients with SBT was higher with DBE than with CT scan (85.9% vs 72.9%, respectively). Adenocarcinoma (29.5%), GIST (24.4%) and lymphoma (15.4%) were the most common tumors reported by this author. They were mostly located at the jejunum (60.3%), and the MSBT detection rate was 14.5%. Cangemi et al[2], in an American research study, with 1652 DBE performed in 1106 patients reported a SBT detection rate of 12.1%. However, the MSBT rate was about 5%. The most common lesions were neuroendocrine tumor (19.4%), GIST (7.5%) and lymphoma (7.5%).
A study from United States[27] analyzes the impact on incidence and survival rates for SBT after the emergence of CE and deep enteroscopy. In order to assess the potential impact of this technology, they compared the incidence rates from 1992-2000 and 2001-2009 to determine if there were different diagnostic yields between both periods. SBT remain uncommon in United States, and its incidence significantly increased from 2.5 during the 1992-2000 time frame to 3.1 per 100000/year in the later period of time (P < 0.004). The survival was significantly better in the 2001-2009 cohort (52.6% vs 63.1% 5-year survival, P < 0.001). Stage-specific analysis showed a significant rise in more distant disease only in African-Americans after 2000, which may reflect factors in tumor biology, treatment, and/or access to care of these patients.
In the present study, we reported on 28 patients (4.5%) with MSBTs, all distal to Treitz. When DBE was carried out, there was a suspicion of SBT in all cases. The histological type distribution is quite different between different countries. Adenocarcinoma[3,20,28], neuroendocrine SBT[1,4] and lymphoma[21] have been reported to be the most frequent histological type by different authors. These differences are probably due to the different geographical distributions and clinical presentations of different studies of patient’s populations. In our study, GIST was the most common MSBT followed by adenocarcinoma.
DBE allowed histopathological diagnosis in most patients (71.4%), except in GI stromal tumors. The histological detection rate in GIST was low (57.4%) but higher than reported by other authors[22,29]. In addition, there were some extremely rare tumors detected, such as jejunal Kaposi sarcoma.
MSBTs were more common among men (71.4%). These tumors may be presented with complete SB stenosis and/or acute overt OGIB, requiring early management by emergency DBE[30-32] or surgery[33]. This procedure may define the characteristics of SB stenosis or bleeding in order to make a surgery decision and/or perform endoscopic treatment[34,35].
There has been recently reported[5] that in patients presented with OGIB, DBE following a positive CE may be the first option, but direct surgery may also be indicated. Interventional digital subtraction angiography has also been reported to be effective in GIST with bleeding[36].
Among patients with a high clinical suspicion of a SBT in the setting of a negative CE result, radiological imaging or deep enteroscopy are equally indicated. CT scan or MR is the preferred initial test in patients with obstructive symptoms. We have performed a DBE following a positive CE in all cases to have a histological and endoscopic diagnosis.
We are also convinced that the entire exploration of the SB in selected cases such as patients with neuroendocrine tumors may be crucial, because this may impact further management. In our series, we have reported multiple adenocarcinomas or neuroendocrine tumors in the same patient. In addition, the histological analysis may have different diagnostic yields within different lesions of the same MSBT. In other cases, to achieve the primary MSBT location for histological and endoscopic diagnosis may be enough.
Thus, DBE has proven to be accurate in management of MSBT. In our study, DBE modified the outcome of 7 patients (25%), not only because of diagnosis capabilities but also of therapeutics interventions.
However, there were some limitations of our study as the retrospective design and potential referral bias.
In conclusion, DBE is critical in the management of MSBT and may have an impact delaying or avoiding emergency surgery. This procedure clarifies the tumor location and characteristics allowing tattoo injection to guide a possible surgery and provides additional information to other procedures that may be decisive in the clinical course of these patients.
Malignant small bowel tumors (MSBT) are a heterogeneous group of rare tumors. However, the incidence of these neoplasms is increasing correlated to the expansion of deep enteroscopy and video capsule endoscopy.
There’re different histological subtypes of MSBT with different prognosis and management. The real incidence of each histological type and clinical characteristics are not well-established. Studies to date have reported different distributions of these neoplasms depending on the geographical area. Recently, double-balloon enteroscopy (DBE) following capsule endoscopy was confirmed as a valid strategy in patients with a suspected MSBT presenting with obscure gastrointestinal bleeding (OGIB).
Most of studies to date report series from Asia or United States. There’re few large European reports of MSBT. In addition, there’s no consensus regarding the most common histological type or clinical presentation by different authors. The present study represents a large series of a referral center in DBE. The authors have considered only patients with jejunal of ileal tumors in order to clarify the DBE role in these cases. OGIB was the most common clinical presentation and gastrointestinal stromal tumors the most common type.
This study clarifies the DBE role in MSBT. The present data might suggest that DBE might impact in about 25% of patients with MSBT by modifying surgery approach.
DBE is an endoscopic technique originally described by Yamamoto that allows the entire examination of the small bowel, with two balloons fitted onto the tips of the scope and over tube.
This study clarifies the role of DBE in the management of MSBT on proper scientific level.
P- Reviewer: Hoffman A, Kashida H, Luo HS, Tepes B S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Gay G, Delvaux M. Small-bowel endoscopy. Endoscopy. 2008;40:140-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Cangemi DJ, Patel MK, Gomez V, Cangemi JR, Stark ME, Lukens FJ. Small bowel tumors discovered during double-balloon enteroscopy: analysis of a large prospectively collected single-center database. J Clin Gastroenterol. 2013;47:769-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Chen WG, Shan GD, Zhang H, Li L, Yue M, Xiang Z, Cheng Y, Wu CJ, Fang Y, Chen LH. Double-balloon enteroscopy in small bowel tumors: a Chinese single-center study. World J Gastroenterol. 2013;19:3665-3671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 26] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Partridge BJ, Tokar JL, Haluszka O, Heller SJ. Small bowel cancers diagnosed by device-assisted enteroscopy at a U.S. referral center: a five-year experience. Dig Dis Sci. 2011;56:2701-2705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Islam RS, Leighton JA, Pasha SF. Evaluation and management of small-bowel tumors in the era of deep enteroscopy. Gastrointest Endosc. 2014;79:732-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 418] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 7. | Haselkorn T, Whittemore AS, Lilienfeld DE. Incidence of small bowel cancer in the United States and worldwide: geographic, temporal, and racial differences. Cancer Causes Control. 2005;16:781-787. [PubMed] [Cited in This Article: ] |
| 8. | Goodman MT, Matsuno RK, Shvetsov YB. Racial and ethnic variation in the incidence of small-bowel cancer subtypes in the United States, 1995-2008. Dis Colon Rectum. 2013;56:441-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Aparicio T, Zaanan A, Svrcek M, Laurent-Puig P, Carrere N, Manfredi S, Locher C, Afchain P. Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis. 2014;46:97-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 10. | Reynolds I, Healy P, Mcnamara DA. Malignant tumours of the small intestine. Surgeon. 2014;12:263-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Gill SS, Heuman DM, Mihas AA. Small intestinal neoplasms. J Clin Gastroenterol. 2001;33:267-282. [PubMed] [Cited in This Article: ] |
| 12. | Rondonotti E, Pennazio M, Toth E, Menchen P, Riccioni ME, De Palma GD, Scotto F, De Looze D, Pachofsky T, Tacheci I. Small-bowel neoplasms in patients undergoing video capsule endoscopy: a multicenter European study. Endoscopy. 2008;40:488-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Yang YS, Huang QY, Wang WF, Sun G, Peng LH. Primary jejunoileal neoplasmas: a review of 60 cases. World J Gastroenterol. 2003;9:862-864. [PubMed] [Cited in This Article: ] |
| 14. | Kim CH, Kye BH, Lee JI, Kim SH, Kim HJ, Kang WK, Oh ST. Clinicopathological features of primary jejunoileal tumors. J Korean Soc Coloproctol. 2010;26:334-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Yang WL, Zhang XC, Yan ZQ, Zhang HM, Zhao Z, Zhang JG, Wang YJ. Clinical analysis of primary small intestinal neoplasms in 305 cases. Zhonghua Zhongliu Zazhi. 2007;29:781-783. [PubMed] [Cited in This Article: ] |
| 16. | Guo X, Mao Z, Su D, Jiang Z, Bai L. The clinical pathological features, diagnosis, treatment and prognosis of small intestine primary malignant tumors. Med Oncol. 2014;31:913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Williamson JM, Williamson RC. Small bowel tumors: pathology and management. J Med Assoc Thai. 2014;97:126-137. [PubMed] [Cited in This Article: ] |
| 18. | Sánchez-Ramón A, Cerino-Palomino V, Medina-Franco H. [Small bowel tumors: experience at the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”]. Rev Gastroenterol Mex. 2012;77:181-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Lee BI, Choi H, Choi KY, Byeon JS, Jang HJ, Eun CS, Cheon JH, Shin SJ, Kim JO, Lee MS. Clinical characteristics of small bowel tumors diagnosed by double-balloon endoscopy: KASID multi-center study. Dig Dis Sci. 2011;56:2920-2927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Yamagami H, Oshitani N, Hosomi S, Suekane T, Kamata N, Sogawa M, Okazaki H, Watanabe K, Tominaga K, Watanabe T. Usefulness of double-balloon endoscopy in the diagnosis of malignant small-bowel tumors. Clin Gastroenterol Hepatol. 2008;6:1202-1205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Mitsui K, Tanaka S, Yamamoto H, Kobayashi T, Ehara A, Yano T, Goto H, Nakase H, Tanaka S, Matsui T. Role of double-balloon endoscopy in the diagnosis of small-bowel tumors: the first Japanese multicenter study. Gastrointest Endosc. 2009;70:498-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Nakatani M, Fujiwara Y, Nagami Y, Sugimori S, Kameda N, Machida H, Okazaki H, Yamagami H, Tanigawa T, Watanabe K. The usefulness of double-balloon enteroscopy in gastrointestinal stromal tumors of the small bowel with obscure gastrointestinal bleeding. Intern Med. 2012;51:2675-2682. [PubMed] [Cited in This Article: ] |
| 23. | Almeida N, Figueiredo P, Lopes S, Gouveia H, Leitão MC. Double-balloon enteroscopy and small bowel tumors: a South-European single-center experience. Dig Dis Sci. 2009;54:1520-1524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Takeshita N, Otsuka Y, Nara S, Noie T, Ito K, Harihara Y, Furushima K, Konishi T. Utility of preoperative small-bowel endoscopy for hemorrhagic lesions in the small intestine. Surg Today. 2012;42:536-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Yamamoto H, Kita H, Sunada K, Hayashi Y, Sato H, Yano T, Iwamoto M, Sekine Y, Miyata T, Kuno A. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol. 2004;2:1010-1016. [PubMed] [Cited in This Article: ] |
| 26. | Pérez-Cuadrado E, Carballo F, Latorre R, Soria F, López-Albors O. An endoscopic technique for treating symptomatic distal jejunum obstruction by leaving the overtube in place. Rev Esp Enferm Dig. 2013;105:107-109. [PubMed] [Cited in This Article: ] |
| 27. | Lui FH, Gerson LB. Impact of Small Bowel Imaging on Incidence and Survival Rates of Small Bowel Cancer: Analysis With the SEER Database. Gastrointest Endosc. 2014;40:AB14. [Cited in This Article: ] |
| 28. | Imaoka H, Higaki N, Kumagi T, Miyaike J, Ohmoto M, Yamauchi K, Murakami T, Murakami H, Ikeda Y, Yokota T. Characteristics of small bowel tumors detected by double balloon endoscopy. Dig Dis Sci. 2011;56:2366-2371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | He Q, Bai Y, Zhi FC, Gong W, Gu HX, Xu ZM, Cai JQ, Pan DS, Jiang B. Double-balloon enteroscopy for mesenchymal tumors of small bowel: nine years’ experience. World J Gastroenterol. 2013;19:1820-1826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Mönkemüller K, Neumann H, Meyer F, Kuhn R, Malfertheiner P, Fry LC. A retrospective analysis of emergency double-balloon enteroscopy for small-bowel bleeding. Endoscopy. 2009;41:715-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Büschel P, Mönkemüller K, von Falkenhausen U, Fry LC, Malfertheiner P, Lippert H, Meyer F. Emergency double balloon enteroscopy: a feasible and promising diagnostic as well as possible therapeutic option in recurrent midgut bleeding. BMJ Case Rep. 2011;2011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Pérez-Cuadrado Robles E, Bebia Conesa P, Esteban Delgado P, Zamora Nava LE, Martínez Andrés B, Rodrigo Agudo JL, López Higueras A, López Martin A, Latorre R, Soria F. Emergency double-balloon enteroscopy combined with real-time viewing of capsule endoscopy: a feasible combined approach in acute overt-obscure gastrointestinal bleeding? Dig Endosc. 2015;27:338-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Tan KK, Bang SL, Ho CK. Surgery for perforated small bowel malignancy: a single institution’s experience over 4 years. Surgeon. 2012;10:6-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Lee H, Park JC, Shin SK, Lee SK, Lee YC. Preliminary study of enteroscopy-guided, self-expandable metal stent placement for malignant small bowel obstruction. J Gastroenterol Hepatol. 2012;27:1181-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Honda W, Ohmiya N, Hirooka Y, Nakamura M, Miyahara R, Ohno E, Kawashima H, Itoh A, Watanabe O, Ando T. Enteroscopic and radiologic diagnoses, treatment, and prognoses of small-bowel tumors. Gastrointest Endosc. 2012;76:344-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Chen YT, Sun HL, Luo JH, Ni JY, Chen D, Jiang XY, Zhou JX, Xu LF. Interventional digital subtraction angiography for small bowel gastrointestinal stromal tumors with bleeding. World J Gastroenterol. 2014;20:17955-17961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |