Published online Aug 25, 2015. doi: 10.4253/wjge.v7.i11.1023
Peer-review started: May 16, 2015
First decision: June 18, 2015
Revised: June 24, 2015
Accepted: August 13, 2015
Article in press: August 14, 2015
Published online: August 25, 2015
Endoscopic retrograde pancreatography (ERP) is an accurate imaging modality in the diagnosis of pancreatobiliary diseases. However, its use has been substantially reduced due to the invasiveness of procedure, the risk of complications and the widespread availability of non-invasive cross-section imaging techniques (computed tomography, magnetic resonance imaging, and endoscopic ultrasound). Since the introduction of endoscopic sphincterotomy, ERP has transformed from diagnostic method to an almost exclusively therapeutic procedure. Pancreatic duct injection substantially increased the risk of post-ERP pancreatitis (1.6%-15.7%); therefore, according to international guidelines ERP is recommended only in cases where biliary intervention is required. However, the role of ERP in the management of pancreatic diseases is currently not clearly defined, but in some cases the filling of pancreatic duct may provide essential information complementing the results of non-invasive imaging techniques. The aim of this publication is to systematically summarize the literature dealing with the diagnostic yield of ERP. We would like to define the precise indications of ERP and overview a diagnostic protocol of pancreatic diseases depending on international guidelines and the opinion of Hungarian experts, because it may improve the diagnostic accuracy, minimize of burden of patients and reduce the risk of procedure related complications.
Core tip: Since the development and widespread availability of non-invasive imaging techniques the importance of diagnostic endoscopic pancreatography (ERP) has substantially reduced. However, in some complicated cases or during pancreatic interventional endoscopic procedures such as minor papilla sphincterotomy, pancreatic sphincterotomy, pancreatic stent implantation, ERP may provide essential information. This article seeks to summarize the results of previous studies and recommendations of international guidelines to define the diagnostic yield and correct indications of ERP.
- Citation: Bor R, Madácsy L, Fábián A, Szepes A, Szepes Z. Endoscopic retrograde pancreatography: When should we do it? World J Gastrointest Endosc 2015; 7(11): 1023-1031
- URL: https://www.wjgnet.com/1948-5190/full/v7/i11/1023.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i11.1023
Endoscopic retrograde cholangiopancreatography (ERCP) is an invasive procedure that provides radiological visualization of the detailed structure and the pathological changes of the biliary tree and pancreatic ducts by injection of contrast agent into the common bile duct (CBD) and the main pancreatic duct (MPD). Since its development in 1968, it has become a widely used and accurate imaging modality in the diagnosis of pancreatobiliary diseases[1]. Since the introduction of endoscopic sphincterotomy in 1974[2], ERCP has become the most important minimal invasive treatment method for various biliary and pancreatic diseases including bile duct or pancreatic duct stones (choledocholithiasis or wirsungolithiasis), benign and malignant biliary and pancreatic duct obstructions. Recently ERCP has transformed from a diagnostic method to an almost exclusively therapeutic procedure due to the widespread availability of noninvasive cross-section imaging techniques such as abdominal ultrasound (AUS), computed tomography (CT), magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound (EUS)[3]. Numerous studies emphasize the disadvantages of ERCP such as post-ERCP complications and the burden to patients. In a meta-analysis of 21 prospective trials the incidence of mild-to-moderate complications reached 5.17%, and that of severe events up to 1.67%[4] (Table 1). Post-ERCP pancreatitis (PEP) is the most frequent complication with approximately 3.5% but its incidence ranges widely (1.6%-15.7%) depending on the patient selection and the definition of pancreatitis[5-7]. Pancreatic duct injection substantially increased the risk of PEP, therefore the role of diagnostic endoscopic pancreatography (ERP) gradually decreased. International guidelines recommend ERCP only in cases where biliary intervention is required[3-8], but the indication of ERP is not clearly defined. According to the current guidelines routine rectal administration of 100 mg diclofenac or indomethacin immediately before or after ERCP is strongly recommended to prevent PEP. In patients with MPD filling and increased patient or procedure related risk factors for PEP temporary application of prophylactic small caliber pancreatic stents is also recommended to reduce the risk of severe PEP[9].
| Mild to moderate | Severe | Death | |
| Pancreatitis | 3.07% | 0.40% | 0.11% |
| Bleeding | 0.95% | 0.39% | 0.05% |
| Perforation | 0.60% | 0.06% | |
| Infection | 1.15% | 0.28% | 0.11% |
| Total | 5.17% | 1.67% | 0.33% |
The aim of this article is to systematically review the literature dealing with the diagnostic yield of ERP in various pancreatic diseases, and to define the principles and indications of ERP depending on the recommendations of international guidelines and the opinion of Hungarian experts (Tables 2 and 3) .
| Indicated | Slightly indicated | Not indicated | Description | |
| Pancreas divisum | 83.6% | 16.7% | 0% | During therapeutic intervention |
| Acute pancreatitis | 16.7% | 50% | 33.3% | Recurrent "idiopathic" acute pancreatitis |
| Chronic pancreatitis | 83.3% | 16.7% | 0% | Complicated chronic pancreatitis (MPD stricture, pancreatic duct stones, chronic abdominal pain, obstructive jaundice) |
| Autoimmune pancreatitis | 66.7% | 33.3% | 0% | Suspicion of autoimmune pancreatitis which has not identified by noninvasive imaging techniques |
| Pancreatic neoplasia | 0% | 50% | 50% | Suspicion of pancreatic neoplasia with obstructive jaundice |
| Pancreatic cystic neoplasia | 0% | 16.7% | 83.3% | In case of IPMN ERP associated with high risk of complications Pancreatic cysts and pseudocysts generally do not communicate with the pancreatic duct therefore the ERP cannot identify them |
| Pancreatic injury | 100% | 0% | 0% | Suspicion of pancreatic ductal injury in stable patients Suspicion of pancreatic fistula Suspicion of fistula formation |
| Postoperative pancreatic fistula | 100% | 0% | 0% |
| Indicated | Not indicated | Description |
| 50% | 50% | ERP may help differentiate between cholangiocarcinoma and pancreatic illnesses |
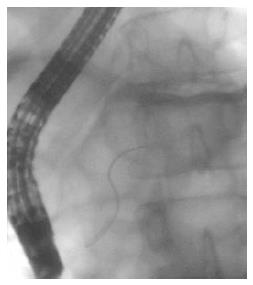
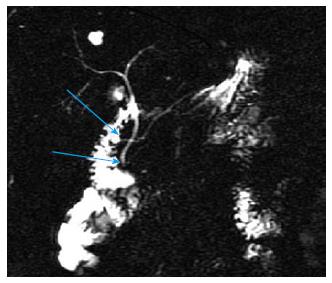
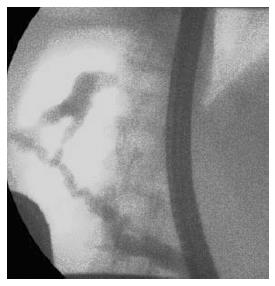
Pancreas divisum (PD) is the most common congenital anomaly of the pancreas in which the dorsal and ventral pancreatic duct drain separately into the duodenum. Recently ERP has been the gold standard imaging modality for the diagnosis of PD due to its high diagnostic accuracy[10,11], but the rate of complete pancreatography and the success of minor papilla cannulation significantly influence the sensitivity of ERP[12] (Figure 1). The high rate of complications is the greatest disadvantage of ERP, therefore noninvasive procedures, such as MRCP and EUS are increasingly spreading worldwide in this indication as well. Sensitivity and specificity of MRCP in the detection of PD is 52%-73.3% and 96.8%-97%, and the diagnostic accuracy can further be improved with the use of secretin stimulation (73.3%-86% and 97%)[13,14] (Figure 2). A comparison study carried out by Lai et al[15]. has shown that adequate evaluation of the pancreatic duct by EUS is possible in 78% of cases, and the sensitivity, specificity, and positive and negative predictive values for EUS are 95%, 97%, 86%, and 99%.
ERP has an important therapeutic role in the endoscopic treatment (including minor papillotomy with or without pancreatic duct stenting) of patients with symptomatic PD. There is no prospective randomized controlled trial comparing endoscopic and surgical therapy, but previous retrospective studies could not detect any differences between the pooled overall response rates of the two treatment groups (endoscopic vs surgical treatment 54.3-79.2 vs 51.4-83.3 depending on the indication)[16].
The importance of ERCP in the identification of the etiology of acute pancreatitis (AP) has rapidly decreased in the recent decades due to the widespread availability of noninvasive imaging modalities[17]. The diagnosis of uncomplicated AP is mainly based on the clinical symptoms, elevated serum levels of pancreatic enzymes (amylase, lipase) and the morphological changes in the pancreas on the AUS, CT or MRI images[18]. Therapeutic ERCP with biliary sphincterotomy and removal of CBD stones can effectively improve the outcome, and according to the recent international guidelines it is indicated in acute biliary pancreatitis within 72 h, if noninvasive examinations prove the presence of acute cholangitis or raise the suspicion of CBD obstruction in association with acute pancreatitis[19,20]. On the contrary, failed biliary cannulation and repeated MPD filling in patients with acute biliary pancreatitis may worsen the overall outcome and therefore some data suggest that small caliber prophylactic pancreatic stents may be applied as a bridging procedure to prevent complications in this group of patients[21].
In 10%-15% of patients with recurrent acute pancreatitis if the complete noninvasive diagnostic evaluation could not reveal the exact cause and etiology, and as a consequence the diagnosis of “idiopathic” acute pancreatitis may arise. Therefore in patients with idiopathic acute pancreatitis, after the cessation of an acute inflammatory attack an ERCP with biliary and/or pancreatic sphincter of Oddi manometry, an endoscopic ultrasound, and secretin enhanced MRCP may leads to a diagnosis of biliary microlithiasis, sphincter of Oddi dysfunction, PD, cystic fibrosis, a choledochocele, annular pancreas, an anomalous pancreatobiliary junction, small pancreatobiliary tumors, or early stage of chronic pancreatitis[22,23].
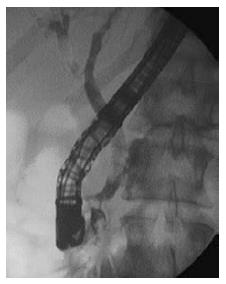
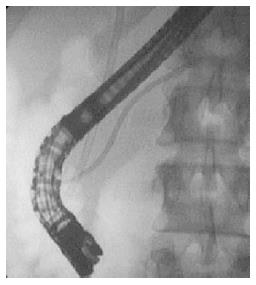
Chronic pancreatitis (CP) is a progressive fibroinflammatory disorder with irreversible destruction of the pancreatic parenchyma and ducts. Frequently the complications, such as bile duct stenosis, obstructive jaundice, diabetes mellitus or malabsorption call the attention for the presence of the disease[24]. In advanced stages the recognition of parenchymal fibrosis and moreover calcification is relatively easy with AUS, CT, MRI and EUS, and typical ductal alterations with ERCP or MRCP[25]. The early recognition of CP and its differentiation from pancreatic cancer (PC) sometimes represents a real diagnostic challenge[26]. Currently ERCP has been replaced by EUS (especially with elastography), MRI, CT, and MRCP in the early diagnosis of CP. However, ERCP plays an essential role in the more precise identification of complications such as obstructive jaundice, pancreatic stones, MPD strictures, chronic abdominal pain, and also gives the opportunity for the minimally invasive treatment (pancreatic sphincterotomy or balloon dilatation, pancreatic duct stenting, etc.)[27] (Figure 3). The European Society of Gastroenterology recommends the endoscopic treatment as the first-line therapy for painful uncomplicated CP, and highlights its effectivity in the management of obstructive jaundice and pancreatic stones associated with CP[3] (Figures 4 and 5). In cases of complicated CP the long-term efficacy of surgical intervention is superior to endoscopy in most patients[28,29]. Despite the fact, that repeated pancreatography is usually necessary during the endoscopic intervention of the pancreatic duct, the risk of PEP is significantly reduced in CP as compared to the general population. However, the role of ERP as first examination in the diagnosis of suspected complicated CP is questionable[6]. Therefore, in our clinical practice, we perform ERCP in CP patients only in case of chronic pancreatic pain and suspected MPD obstruction (stricture with prestenotic dilatation) based on MRCP or EUS. In these patients, pancreatic sphincterotomy, pancreatic stricture dilatation and multiple plastic or self-expanding metal stenting during ERP proved to be useful to achieve long term symptomatic improvement.
Autoimmune pancreatitis (AIP) is an uncommon inflammatory disorder of the pancreas with a presumed autoimmune etiology[30]. It may present with a wide variety of clinical and morphological features including painless obstructive jaundice, asymptomatic focal mass or diffuse enlargement of the pancreas which mimic PC[31]. The diagnosis of AIP requires a multidisciplinary approach including imaging studies, histology, serology, assessment of other organ involvement and the therapeutic response to steroid treatment[32,33]. There were differences in the diagnostic approach and the techniques used between different countries. For instance, ERP is usually ignored in Western counties to avoid PEP in contrast to Japan where this examination is usually performed[34]. The correct diagnosis requires detailed information equally about the pancreatic parenchyma and ducts. In typical cases of AIP a diffusely enlarged or “sausage shaped” pancreas with featureless borders and/or loss of lobular architecture can be detected with AUS, CT and MRI[35]. In 30%-40% of the cases focal mass is found, which can lead to false diagnosis of pancreatic malignancy[36,37]. Ductal imaging, ERP and MRCP may show a long, narrow ductal stricture (greater than one-third the length of the MPD) or multiple, non-continuous strictures without marked upstream dilation, and side branches arising from the stricture[38-40]. However, given that ERCP is an invasive method which debit the patient and can cause adverse effects (pancreatitis, bleeding), the noninvasive MRCP is becoming the first choice examination for pancreatobiliary diseases. Previous comparison studies have shown that MRCP is less sensitive in the differentiation of focal form of AIP and PC, therefore cannot completely replace ERCP for the diagnostic evaluation of AIP[41,42]. The multicenter study carried out by Suguma et al[43]. has highlighted the ability of ERP to diagnose AIP based on ERP feature alone is limited, but taken together with clinical symptoms, serology and/or histology it can be useful.
Previously ERCP was the gold standard in the diagnosis of PC. Localized MPD stenosis with focal ductal branch dilation and with distal dilation of MPD (“double duct” sign) were the most frequently detectable morphological changes[44,45]. The current role of ERCP is therapeutic rather than diagnostic. In cases of inoperable locally advanced and metastatic pancreatic malignancy the development of obstructive jaundice constitutes an absolute indication of ERCP[46]. Malignant biliary stenosis may be treated with plastic, but preferably with self-expandable metallic stent implantation[3]. Pancreatography, ERCP-guided brush cytological sampling and/or biopsy of the pancreatic duct may be useful to prove malignancy, but EUS-guided fine needle aspiration (EUS-FNA) is the first-choice sampling procedure in suspected unresectable pancreatic solid and cystic lesions due to minimal invasiveness, lower complication rate and higher sensitivity compared to ERCP sampling[47]. A meta-analysis performed by Li et al[48]. showed that ERCP combined with EUS was associated with a high diagnostic yield compared to ERCP or EUS alone, but the complete length of procedures substantially increased, however, it can be reduced if the two examination are performed under the same sedation, but the rate of complication not changed[49].
Cystic pancreatic lesions represent a great diagnostic problem because of the morphological similarities between benign and malignant cysts and because of the possibility of malignant transformation[50] and the increasing number of the detected lesions due to the improvement of the abdominal imaging modalities and their availabilities. The differentiation between the four types of pancreatic cystic neoplasms (PCN) substantially may influence the therapeutic approach. Serous cystadenomas (SCA) and solid pseudopapillary neoplasms (SPN) are associated with lower malignant potential compared to intraductal papillary mucinous neoplasms (IPMN) and mucinous cystic neoplasms (MCN). Previously ERP was the gold standard diagnostic procedure in the identification and classification of IPMN. Diffuse or segmental dilation of the MPD or its side branches connected to the cyst can be recognized on the ERP images, with no other cause of the dilatation. The pathognomonic characteristic of IPMN is the gaping orifice of Vater papilla with thick mucus oozing (fish mouth papilla)[51]. The international consensus guidelines do not recommend the routine ERP for the morphological and cytological diagnosis of IPMN (fluid sampling or brushing of MPD) due to the invasiveness of the procedure and the high risk of complications. Currently MRCP, EUS and EUS-guided sampling are most preferred[52,53]. The other malignant cyst type and the pancreatic pseudocysts generally do not communicate with the pancreatic duct, therefore the ERP cannot identify them.
Blunt pancreatic trauma can frequently lead to acute pancreatitis with or without MPD disruption. Pancreatic injuries caused by blunt abdominal trauma are relatively rare with an overall incidence of 0.2%-12%[54]. Pancreatic injury occurs as a result of the traumatic compression of the pancreas between the vertebral column and the anterior abdominal wall. Pancreatic injury is more common in children and young adults because of decreased protective intra-abdominal fat. CT is the primary imaging modality of choice in patients with blunt abdominal trauma, with the sensitivity for pancreatic parenchymal injury between 67%-85%[55]. Although pancreatic ductal injury can frequently be detected with non-invasive MRCP, ERCP is the most accurate diagnostic tool for the assessment of ductal injury[56]. Besides, it can also provide endoscopic treatment. Delays in ERCP have led to significantly higher complication rates[57]. Although ERCP is the most useful procedure for the diagnosis of pancreatic ductal injury in stable patients, surgery should be considered without hesitation if the patient’s condition is unstable. Recently, some case series proved that pancreatic duct plastic stent placement with and without pancreatic sphincterotomy can be an effective endoscopic therapy in resolving pancreatic duct disruption and preventing chronic fistula formation[58]. Although stent implantation can improve the clinical condition and resolve fistula and pseudocyst, stent induced ductal stricture is a major long-term complication.
Postoperative pancreatic fistula (POPF) formation is a frequent and severe complication of pancreatic surgery[59,60]. Its incidence ranges from 2% to 51% depending on the definition used. POPF was defined by International Study Group on Pancreatic Fistula as a measurable drain output on or after postoperative day 3 with an amylase content greater than 3 times the serum amylase activity[61]. In the early postoperative phase the upper abdominal discomfort associated with fever, tachycardia, slower recovery and persistently high drain output raises the suspicion of postoperative complication, such as pancreatic fistula (Figure 6). The amylase level of drain fluid is extremely elevated in a typical case[62]. ERCP and MRCP are the two most widely used imaging modality in the confirmation of POPF with high diagnostic accuracy. In case of pancreaticopleural fistula their sensitivity may reach to 78% and 80%[63]. Recently ERCP was the most preferred investigation for confirming the diagnosis of POPF, but its use is reduced due to invasive nature and elevated risk of infective complications arising from fistula filling. However, it has the advantage of direct visualization of MPD and precise location of fistula, and the ability to simultaneously perform endoscopic therapeutic maneuvers[64].
Pancreatobiliary maljunction (PBM) is a rare congenital malformation in which the CBD and the pancreatic duct are united outside the duodenal wall with or without dilation of CBD[65]. The sphincter of oddi is located in the distal part of the common channel, therefore it cannot properly regulate the outflow of biliopancreatic juice, resulting regurgitation of bile into the MPD and pancreatic juice into the CBD. The elevated intraductal pressure often causes dilatation of CBD, and the chronic biliopancreatic reflux increases the risk of development of malignancy. The diagnosis of PBM is based on the identification of the anomalous union between the pancreatic and bile ducts by ERCP, MRCP, EUS or intraductal ultrasound. ERCP is the most accurate imaging method, and it provides an opportunity for the biliary intervention (biliary stone extraction, stent implantation) and bile sampling as well. High biliary levels of pancreatic enzymes are suggestive of regurgitation of pancreatic juice into the common bile duct[66]. In atypical PBM cases with relatively short common channel, the diagnostic accuracy of MRCP and EUS is lower, but they are very effective in the detection of PBM associated pancreatobiliary cancers at an early stage[67].
ERP is still one of the most accurate diagnostic procedures in patients with suspected pancreatic ductal disorders, including idiopathic acute recurrent pancreatitis, chronic pancreatitis, pancreatic ductal injuries and fistula formation, pancreatic cystic neoplasms and early pancreatic cancer. However, before performing ERP, endoscopists should carefully evaluate the extent of the clinically necessary pancreatogram, if there any, to establish the diagnosis. Increasingly widespread application of noninvasive methods for the diagnosis of pancreatobiliary diseases (such as MRCP and EUS), and less frequent use of diagnostic ERP could dramatically decrease post-ERCP complications. In contrast, pancreatic interventional endoscopic procedures, such as pancreatic sphincterotomy, dilatations and pancreatic stent implantation are necessitates for complete pancreatic ductal contrast filling and analysis of digitally enhanced pancreatogram with fluoroscopy to completely understand the anatomy and intraductal pathology before the initiation of endoscopic therapy.
In case of distal biliary obstruction, when the non-invasive imaging modalities are available we do not recommend the filling of pancreatic duct, selective biliary drainage is proposed. ERP should be considered in case of suspected pancreatic ductal abnormalities, such as pancreatic injury, fistula or congenital malformation, and when pancreatic ductal intervention is necessary.
P- Reviewer: Kubota K, Zhu YL S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | McCune WS, Shorb PE, Moscovitz H. Endoscopic cannulation of the ampulla of vater: a preliminary report. Ann Surg. 1968;167:752-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 348] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Kawai K, Akasaka Y, Murakami K, Tada M, Koli Y. Endoscopic sphincterotomy of the ampulla of Vater. Gastrointest Endosc. 1974;20:148-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 438] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Adler DG, Baron TH, Davila RE, Egan J, Hirota WK, Leighton JA, Qureshi W, Rajan E, Zuckerman MJ, Fanelli R. ASGE guideline: the role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc. 2005;62:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 4. | Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, Pilotto A, Forlano R. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781-1788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 669] [Cited by in F6Publishing: 695] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 5. | Chen JJ, Wang XM, Liu XQ, Li W, Dong M, Suo ZW, Ding P, Li Y. Risk factors for post-ERCP pancreatitis: a systematic review of clinical trials with a large sample size in the past 10 years. Eur J Med Res. 2014;19:26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Hauser G, Milosevic M, Stimac D, Zerem E, Jovanović P, Blazevic I. Preventing post-endoscopic retrograde cholangiopancreatography pancreatitis: what can be done. World J Gastroenterol. 2015;21:1069-1080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Dumonceau JM, Andriulli A, Elmunzer BJ, Mariani A, Meister T, Deviere J, Marek T, Baron TH, Hassan C, Testoni PA. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - updated June 2014. Endoscopy. 2014;46:799-815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 362] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 8. | American Gastroenterological Association (AGA) Institute on “Management of Acute Pancreatits” Clinical Practice and Economics Committee; AGA Institute Governing Board. AGA Institute medical position statement on acute pancreatitis. Gastroenterology. 2007;132:2019-2021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Dumonceau JM, Delhaye M, Tringali A, Dominguez-Munoz JE, Poley JW, Arvanitaki M, Costamagna G, Costea F, Devière J, Eisendrath P. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2012;44:784-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 225] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 10. | Morgan DE, Logan K, Baron TH, Koehler RE, Smith JK. Pancreas divisum: implications for diagnostic and therapeutic pancreatography. AJR Am J Roentgenol. 1999;173:193-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Takács T, Czakó L, Madácsy L, Nagy F, Lonovics J. [Endoscopic therapy of pancreas divisum]. Orv Hetil. 1998;139:2761-2764. [PubMed] [Cited in This Article: ] |
| 12. | Rustagi T, Golioto M. Diagnosis and therapy of pancreas divisum by ERCP: a single center experience. J Dig Dis. 2013;14:93-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Rustagi T, Njei B. Magnetic resonance cholangiopancreatography in the diagnosis of pancreas divisum: a systematic review and meta-analysis. Pancreas. 2014;43:823-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Mosler P, Akisik F, Sandrasegaran K, Fogel E, Watkins J, Alazmi W, Sherman S, Lehman G, Imperiale T, McHenry L. Accuracy of magnetic resonance cholangiopancreatography in the diagnosis of pancreas divisum. Dig Dis Sci. 2012;57:170-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Lai R, Freeman ML, Cass OW, Mallery S. Accurate diagnosis of pancreas divisum by linear-array endoscopic ultrasonography. Endoscopy. 2004;36:705-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Liao Z, Gao R, Wang W, Ye Z, Lai XW, Wang XT, Hu LH, Li ZS. A systematic review on endoscopic detection rate, endotherapy, and surgery for pancreas divisum. Endoscopy. 2009;41:439-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Cherian JV, Selvaraj JV, Natrayan R, Venkataraman J. ERCP in acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2007;6:233-240. [PubMed] [Cited in This Article: ] |
| 18. | Hritz I, Czakó L, Dubravcsik Z, Farkas G, Kelemen D, Lásztity N, Morvay Z, Oláh A, Pap Á, Párniczky A. [Acute pancreatitis. Evidence-based practice guidelines, prepared by the Hungarian Pancreatic Study Group]. Orv Hetil. 2015;156:244-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Besselink M, van Santvoort H, Freeman M, Gardner T, Mayerle J, Vege SS, Werner J, Banks P, McKay C, Fernandez-del Castillo C. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1080] [Cited by in F6Publishing: 920] [Article Influence: 83.6] [Reference Citation Analysis (3)] |
| 20. | Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-1415; 1416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1232] [Cited by in F6Publishing: 1245] [Article Influence: 113.2] [Reference Citation Analysis (3)] |
| 21. | Fejes R, Kurucsai G, Székely A, Székely I, Altorjay A, Madácsy L. Feasibility and safety of emergency ERCP and small-caliber pancreatic stenting as a bridging procedure in patients with acute biliary pancreatitis but difficult sphincterotomy. Surg Endosc. 2010;24:1878-1885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Fischer M, Hassan A, Sipe BW, Fogel EL, McHenry L, Sherman S, Watkins JL, Schmidt S, Lazzell-Pannell L, Lehman GA. Endoscopic retrograde cholangiopancreatography and manometry findings in 1,241 idiopathic pancreatitis patients. Pancreatology. 2010;10:444-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Sajith KG, Chacko A, Dutta AK. Recurrent acute pancreatitis: clinical profile and an approach to diagnosis. Dig Dis Sci. 2010;55:3610-3616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Ammann RW. Diagnosis and management of chronic pancreatitis: current knowledge. Swiss Med Wkly. 2006;136:166-174. [PubMed] [Cited in This Article: ] |
| 25. | Choueiri NE, Balci NC, Alkaade S, Burton FR. Advanced imaging of chronic pancreatitis. Curr Gastroenterol Rep. 2010;12:114-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | He YX, Xu HW, Sun XT, Ye Z, Wang W, Lai XW, Wang XT, Hu LH, Sun C, Liao Z. Endoscopic management of early-stage chronic pancreatitis based on M-ANNHEIM classification system: a prospective study. Pancreas. 2014;43:829-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Seicean A, Vultur S. Endoscopic therapy in chronic pancreatitis: current perspectives. Clin Exp Gastroenterol. 2015;8:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Ahmed Ali U, Pahlplatz JM, Nealon WH, van Goor H, Gooszen HG, Boermeester MA. Endoscopic or surgical intervention for painful obstructive chronic pancreatitis. Cochrane Database Syst Rev. 2015;3:CD007884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Cahen DL, Gouma DJ, Nio Y, Rauws EA, Boermeester MA, Busch OR, Stoker J, Laméris JS, Dijkgraaf MG, Huibregtse K. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med. 2007;356:676-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 579] [Cited by in F6Publishing: 444] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 30. | Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561-1568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1044] [Cited by in F6Publishing: 891] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 31. | Kamisawa T, Chari ST, Giday SA, Kim MH, Chung JB, Lee KT, Werner J, Bergmann F, Lerch MM, Mayerle J. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas. 2011;40:809-814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 32. | Okazaki K, Kawa S, Kamisawa T, Shimosegawa T, Tanaka M. Japanese consensus guidelines for management of autoimmune pancreatitis: I. Concept and diagnosis of autoimmune pancreatitis. J Gastroenterol. 2010;45:249-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010-1016; quiz 934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 722] [Cited by in F6Publishing: 756] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 34. | Naitoh I, Nakazawa T, Hayashi K, Miyabe K, Shimizu S, Kondo H, Yoshida M, Yamashita H, Umemura S, Hori Y. Clinical evaluation of international consensus diagnostic criteria for type 1 autoimmune pancreatitis in comparison with Japanese diagnostic criteria 2011. Pancreas. 2013;42:1238-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Wakabayashi T, Kawaura Y, Satomura Y, Watanabe H, Motoo Y, Okai T, Sawabu N. Clinical and imaging features of autoimmune pancreatitis with focal pancreatic swelling or mass formation: comparison with so-called tumor-forming pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2679-2687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 36. | Kanno A, Masamune A, Shimosegawa T. Endoscopic approaches for the diagnosis of autoimmune pancreatitis. Dig Endosc. 2015;27:250-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Szepes Z, Dobra M, Góg C, Zábrák E, Makula É, Tiszlavicz L, Kiss T, Molnár T, Nagy F, Czakó L. Pancreatic cancer or autoimmune pancreatitis: endosonography as a diagnostic reviser. Orv Hetil. 2013;154:62-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Nakazawa T, Ohara H, Sano H, Aoki S, Kobayashi S, Okamoto T, Imai H, Nomura T, Joh T, Itoh M. Cholangiography can discriminate sclerosing cholangitis with autoimmune pancreatitis from primary sclerosing cholangitis. Gastrointest Endosc. 2004;60:937-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 39. | Moon SH, Kim MH. The role of endoscopy in the diagnosis of autoimmune pancreatitis. Gastrointest Endosc. 2012;76:645-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Horiuchi A, Kawa S, Hamano H, Hayama M, Ota H, Kiyosawa K. ERCP features in 27 patients with autoimmune pancreatitis. Gastrointest Endosc. 2002;55:494-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A, Kodama M, Kamata N. Can MRCP replace ERCP for the diagnosis of autoimmune pancreatitis. Abdom Imaging. 2009;34:381-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Takuma K, Kamisawa T, Tabata T, Inaba Y, Egawa N, Igarashi Y. Utility of pancreatography for diagnosing autoimmune pancreatitis. World J Gastroenterol. 2011;17:2332-2337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 24] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Sugumar A, Levy MJ, Kamisawa T, Webster GJ, Kim MH, Enders F, Amin Z, Baron TH, Chapman MH, Church NI. Endoscopic retrograde pancreatography criteria to diagnose autoimmune pancreatitis: an international multicentre study. Gut. 2011;60:666-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 44. | Iwata T, Kitamura K, Yamamiya A, Ishii Y, Sato Y, Nomoto T, Ikegami A, Yoshida H. Evaluation of diagnostic cytology via endoscopic naso-pancreatic drainage for pancreatic tumor. World J Gastrointest Endosc. 2014;6:366-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Hanada K, Okazaki A, Hirano N, Izumi Y, Teraoka Y, Ikemoto J, Kanemitsu K, Hino F, Fukuda T, Yonehara S. Diagnostic strategies for early pancreatic cancer. J Gastroenterol. 2015;50:147-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 46. | Baron TH, Mallery JS, Hirota WK, Goldstein JL, Jacobson BC, Leighton JA, Waring JP, Faigel DO. The role of endoscopy in the evaluation and treatment of patients with pancreaticobiliary malignancy. Gastrointest Endosc. 2003;58:643-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Weilert F, Bhat YM, Binmoeller KF, Kane S, Jaffee IM, Shaw RE, Cameron R, Hashimoto Y, Shah JN. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc. 2014;80:97-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 48. | Li H, Hu Z, Chen J, Guo X. Comparison of ERCP, EUS, and ERCP combined with EUS in diagnosing pancreatic neoplasms: a systematic review and meta-analysis. Tumour Biol. 2014;35:8867-8874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Aslanian HR, Estrada JD, Rossi F, Dziura J, Jamidar PA, Siddiqui UD. Endoscopic ultrasound and endoscopic retrograde cholangiopancreatography for obstructing pancreas head masses: combined or separate procedures. J Clin Gastroenterol. 2011;45:711-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Mimura T, Masuda A, Matsumoto I, Shiomi H, Yoshida S, Sugimoto M, Sanuki T, Yoshida M, Fujita T, Kutsumi H. Predictors of malignant intraductal papillary mucinous neoplasm of the pancreas. J Clin Gastroenterol. 2010;44:e224-e229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 51. | Kang MJ, Jang JY, Kim SJ, Lee KB, Ryu JK, Kim YT, Yoon YB, Kim SW. Cyst growth rate predicts malignancy in patients with branch duct intraductal papillary mucinous neoplasms. Clin Gastroenterol Hepatol. 2011;9:87-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 52. |
Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM; International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. |
| 53. | Clores MJ, Thosani A, Buscaglia JM. Multidisciplinary diagnostic and therapeutic approaches to pancreatic cystic lesions. J Multidiscip Healthc. 2014;7:81-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Mayer JM, Tomczak R, Rau B, Gebhard F, Beger HG. Pancreatic injury in severe trauma: early diagnosis and therapy improve the outcome. Dig Surg. 2002;19:291-297; discussion 297-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Bigattini D, Boverie JH, Dondelinger RF. CT of blunt trauma of the pancreas in adults. Eur Radiol. 1999;9:244-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Fulcher AS, Turner MA, Yelon JA, McClain LC, Broderick T, Ivatury RR, Sugerman HJ. Magnetic resonance cholangiopancreatography (MRCP) in the assessment of pancreatic duct trauma and its sequelae: preliminary findings. J Trauma. 2000;48:1001-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 57. | Kim HS, Lee DK, Kim IW, Baik SK, Kwon SO, Park JW, Cho NC, Rhoe BS. The role of endoscopic retrograde pancreatography in the treatment of traumatic pancreatic duct injury. Gastrointest Endosc. 2001;54:49-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 58. | Huckfeldt R, Agee C, Nichols WK, Barthel J. Nonoperative treatment of traumatic pancreatic duct disruption using an endoscopically placed stent. J Trauma. 1996;41:143-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | El Nakeeb A, Salah T, Sultan A, El Hemaly M, Askr W, Ezzat H, Hamdy E, Atef E, El Hanafy E, El-Geidie A. Pancreatic anastomotic leakage after pancreaticoduodenectomy. Risk factors, clinical predictors, and management (single center experience). World J Surg. 2013;37:1405-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 60. | Dugalic VD, Knezevic DM, Obradovic VN, Gojnic-Dugalic MG, Matic SV, Pavlovic-Markovic AR, Dugalic PD, Knezevic SM. Drain amylase value as an early predictor of pancreatic fistula after cephalic duodenopancreatectomy. World J Gastroenterol. 2014;20:8691-8699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3282] [Cited by in F6Publishing: 3393] [Article Influence: 178.6] [Reference Citation Analysis (0)] |
| 62. | Butturini G, Daskalaki D, Molinari E, Scopelliti F, Casarotto A, Bassi C. Pancreatic fistula: definition and current problems. J Hepatobiliary Pancreat Surg. 2008;15:247-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 63. | Malleo G, Pulvirenti A, Marchegiani G, Butturini G, Salvia R, Bassi C. Diagnosis and management of postoperative pancreatic fistula. Langenbecks Arch Surg. 2014;399:801-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 64. | Facy O, Chalumeau C, Poussier M, Binquet C, Rat P, Ortega-Deballon P. Diagnosis of postoperative pancreatic fistula. Br J Surg. 2012;99:1072-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Kamisawa T, Ando H, Suyama M, Shimada M, Morine Y, Shimada H. Japanese clinical practice guidelines for pancreaticobiliary maljunction. J Gastroenterol. 2012;47:731-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 66. | Kamisawa T, Takuma K, Itokawa F, Itoi T. Endoscopic diagnosis of pancreaticobiliary maljunction. World J Gastrointest Endosc. 2011;3:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 18] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Takuma K, Kamisawa T, Tabata T, Hara S, Kuruma S, Inaba Y, Kurata M, Honda G, Tsuruta K, Horiguchi S. Importance of early diagnosis of pancreaticobiliary maljunction without biliary dilatation. World J Gastroenterol. 2012;18:3409-3414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 27] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |