Published online Jan 16, 2015. doi: 10.4253/wjge.v7.i1.73
Peer-review started: August 13, 2014
First decision: September 16, 2014
Revised: October 17, 2014
Accepted: October 31, 2014
Article in press: November 3, 2014
Published online: January 16, 2015
Russell bodies are eosinophilic intracytoplasmic globules which are likely the result of disturbed secretion of immunoglobulins that accumulate within the plasma cell. Russell body collections have been identified within the stomach, known as Russell body gastritis. Similar lesions within the duodenum are referred to as Russell body duodenitis, which is rare. Several Russell body gastritis case reports are associated with Helicobacter pylori. However, the etiology of Russell body duodenitis remains unclear. Here we report the first case of Russell body duodenitis with immunoglobulin light chain restriction in a background of peptic duodenitis.
Core tip: Russell body duodenitis is rare and the etiology is unclear. We report a case of Russell body duodenitis with immunoglobulin light chain restriction in a background of peptic duodenitis.
- Citation: Munday WR, Kapur LH, Xu M, Zhang X. Russell body duodenitis with immunoglobulin kappa light chain restriction. World J Gastrointest Endosc 2015; 7(1): 73-76
- URL: https://www.wjgnet.com/1948-5190/full/v7/i1/73.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i1.73
Russell bodies are eosinophilic intracytoplasmic globules which were first described by Russell et al[1] in 1890. These globules are likely the result of disturbed secretion of immunoglobulins that accumulate within the plasma cell. Sixteen case reports have identified abundant collections of Russell bodies in the stomach, known as Russell body gastritis. Similarly, three cases have been reported to occur within the duodenum, with the first in 2011[2]. All three cases presented clinically with upper gastrointestinal symptoms with the subsequent identification of polytypic, Russell body containing plasma cells in the duodenum referred to as Russell body duodenitis[2-4]. Several of the Russell body gastritis case reports are associated with Helicobacter pylori (H. pylori). However, the etiology of Russell body duodenitis remains unclear. Here we report the first case of asymptomatic Russell body duodenitis. Additionally, this is the first reported case showing immunoglobulin light chain restriction.
A 78-year-old female with a past medical history of congestive heart failure, atrial fibrillation, chronic obstructive pulmonary disease, and chronic renal injury, presented to hospital with shortness of breath and lower extremity edema. Past medical history was also significant for diabetes, and hypertension. Past surgical history included sigmoid resection for diverticulitis, rotator cuff repair, and carpal tunnel release. The patient denied alcohol use, and had a history of smoking over sixty pack-years. Upon admission for shortness of breath, the patient was treated with intravenous diuresis and her symptoms subsequently improved.
Further laboratory investigation revealed concomitant iron deficiency anemia and chart review showed progressive decline in hemoglobin over a nine-month period. There was no clinical or laboratory evidence to suggest monoclonal gammopathy.
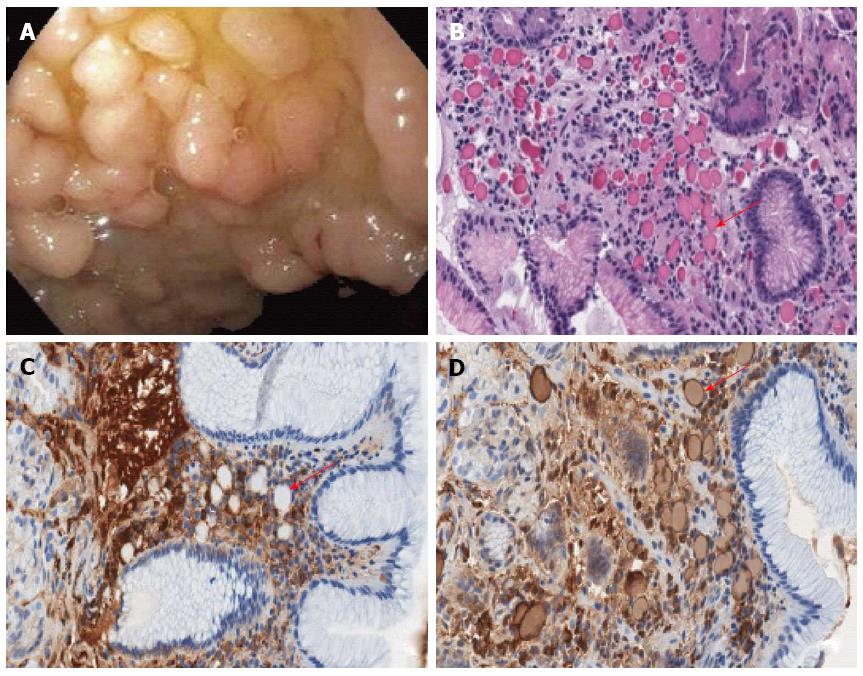
Esophagogastroduodenoscopy and colonoscopy were performed to evaluate the source of anemia. The patient had no prior history of upper or lower gastrointestinal symptoms. Upper endoscopy revealed a few scattered gastric fundic sub-centimeter polyps, and prominent gastric antral folds without evidence of inflammation. In the duodenum, clusters of lobulated polyps (Figure 1A) were located in the duodenal bulb, with a normal appearing second portion of the duodenum. No ulceration was present. Colonoscopy revealed a three centimeter ulcerated, sessile mass at the distal ascending colon, concerning for malignancy.
Random stomach biopsies from the body and antrum showed normal morphology and no evidence of H. pylori. Duodenal biopsies of the lobulated polyps at the duodenal bulb showed numerous eosinophilic globules, or Russell bodies, as well as gastric surface foveolar metaplasia (Figure 1B). CD138 immunostain was positive in plasma cells containing Russell bodies (Figure 1C). Immunoglobulin kappa light chain immunostain showed a dark peripheral rim with light center staining pattern in the Russell bodies (Figure 1D) while lambda immunostain was negative (not shown). The surrounding plasma cells with mature morphology showed polytypic light chain staining pattern. IgH gene rearrangement was negative. Biopsies of the ulcerated, sessile distal colonic mass revealed invasive adenocarcinoma.
Russell bodies are eosinophilic inclusions located in the cytoplasm of plasma cells. While they are typically identified in the setting of several malignancies of hematopoietic origin, they can be seen in some reactive conditions as well. The plasma cells containing Russell bodies, referred to as Mott cells, are often found in the setting of plasma cell myeloma, MALT lymphoma, plasmacytoma, or lymphoplasmacytic lymphoma. Russell body gastritis is a rare reactive condition in which Russell bodies are found within the lamina propria of the gastric mucosa, and so far without a definitive association with H. pylori or malignancy.
Of the sixteen reported cases of Russell body gastritis, several identified monoclonality[5-7]. In these cases, there were no clinical and pathologic features of MALT lymphoma or significant plasma cell neoplasia[5]. One case did show lambda restricted Mott cells, positivity for H. pylori, and concomitant monoclonal gammopathy of undermined significance (MGUS); however, eradication of H. pylori caused the Russell body gastritis to subside while the paraproteinemia remained unaffected[7]. Thus, these cases of monoclonal Mott cell proliferations are either reactive in nature, or possibly, precursor proliferations to more significant conditions, such as MALT lymphoma or plasmacytoma.
Interestingly, the phenomenon of Russell body monoclonality in the presence of mature polytypic plasma cells, as in our case, has been observed before, although outside the gastrointestinal tract. In a biopsy of labial mucosa, Matthews et al[8] identified a patient with monoclonal Russell bodies restricted to IgG and kappa chains in a background of mature plasma cells. Of the twelve patients in their study, this was the only patient diagnosed with a significant medical pathology, namely, Sjogren’s syndrome. B-cell clonality in Sjogren’s syndrome has been hypothesized to alter the salivary or lacrimal gland microenvironment, enabling the progression to lymphoma[9]. Indeed, approximately 5% of patients with Sjogren’s syndrome will develop lymphoma, an incidence 40 times that of the general population[10]. It could be postulated that monotypic Mott cells are similar to monoclonal B-cells in this setting, such that the finding indicates a transient or intermediate step between an inflammatory condition, such as Sjogren’s syndrome, and the progression to malignancy, such as lymphoma.
Further evidence supporting monoclonal Mott cells as an intermediary between inflammatory conditions and malignancy comes from a rare case of gastric Mott cell tumor associated with H. pylori[11]. In this case, abundant monotypic IgG kappa Mott cells were found on gastric biopsy with features suggestive of MALT lymphoma[11]. Furthermore, Mott cells were found in regional lymph nodes[11]. It is possible that H. pylori gastritis, a chronic inflammatory condition, over time stimulated an intermediary monoclonal Mott cell proliferation that subsequently developed malignant transformation and lymph node involvement. Whatever the sequence of events, it may be inferred from this example that monotypic Mott cells harbor malignant potential.
To summarize, the present case shows a unique type of Mott cell monoclonality for several reasons. First, the monoclonal Mott cells were located within the duodenum, of which this is the first reported case at this site. To date, only three cases of Russell body duodenitis have been reported, none of which demonstrate monoclonality[2-4]. Secondly, the monoclonal cells are present in a background of mature, polytypic plasma cells, a finding which is infrequently reported. Lastly, our patient was asymptomatic, the findings of Russell body duodenitis was incidental, and work up for H. pylori was negative. In this case, Russell body duodenitis likely originated from peptic duodenitis, indicated by gastric surface foveolar metaplasia of the overlying duodenal epithelium, and independent of H. pylori. Over time, chronic inflammation at this site may have caused Mott cells to accumulate, which subsequently progressed to monoclonality. It has been suggested that monoclonality of Mott cells may occur secondary to alternations at the immunoglobulin locus, and may be induced by chronic inflammation[7]. Given the low grade nature of MALT lymphomas in the stomach and duodenum, and the likelihood that monotypic Russell body duodenitis is either reactive or pre-malignant, treatment beyond eradication of H. pylori (if present) is likely unnecessary. Further investigation, and the accumulation of additional cases, will be necessary to better understand the clinical significance of monoclonal Russell body duodenitis.
The patient presented with shortness of breath and lower extremity edema. Further laboratory investigation revealed concomitant iron deficiency anemia.
Iron deficiency anemia.
Cause of iron deficiency is unknown. Considering patient’s age, the possibility of gastrointestinal blood loss due to ulcer or malignancy should be ruled out. Esophagogastroduodenoscopy and colonoscopy were performed to evaluate the source of anemia.
Gastric fundic polyps, duodenal polyps and a 3 cm ulcerated, sessile mass at the distal ascending colon.
Russell body duodenitis and colonic invasive adenocarcinoma.
Three cases of polytypic Russell body duodenitis have been reported. Here we report the first case of Russell body duodenitis with immunoglobulin light chain restriction in a background of peptic duodenitis.
Russell body duodenitis is uncommon and the etiology remains unclear. The monotypic Russell body duodenitis is either reactive or pre-malignant, treatment beyond eradication of Helicobacter pylori (if present) is likely unnecessary. Further investigation, and the accumulation of additional cases, will be necessary to better understand the clinical significance of monoclonal Russell body duodenitis.
This is a case report of a rare disease (Russell body duodenitis) described to occur in the duodenum first in 2011.
P- Reviewer: Abu-Zidan F, De Re V S- Editor: Ji FF L- Editor: A E- Editor: Zhang DN
| 1. | Russell W. An Address on a Characteristic Organism of Cancer. Br Med J. 1890;2:1356-1360. [Cited in This Article: ] |
| 2. | Savage NM, Fortson T, Schubert M, Chamberlain S, Lee J, Ramalingam P. Isolated Russell body duodenitis. Dig Dis Sci. 2011;56:2202-2204. [Cited in This Article: ] |
| 3. | Paniz Mondolfi A, Samuel M, Kikhney J, Moter A, Feldman D, Slova D, Filatov A, Theise N. Russell body duodenitis: a histopathological and molecular approach to a rare clinical entity. Pathol Res Pract. 2012;208:415-419. [Cited in This Article: ] |
| 4. | Chen D, Thota P, Liu X. Isolated Russell Body Duodenitis with Concurrent Helicobacter Pylori Gastritis. J Med Cases. 2013;4:166-169. [Cited in This Article: ] |
| 5. | Araki D, Sudo Y, Imamura Y, Tsutsumi Y. Russell body gastritis showing IgM kappa-type monoclonality. Pathol Int. 2013;63:565-567. [Cited in This Article: ] |
| 6. | Coyne JD, Azadeh B. Russell body gastritis: a case report. Int J Surg Pathol. 2012;20:69-70. [Cited in This Article: ] |
| 7. | Wolkersdörfer GW, Haase M, Morgner A, Baretton G, Miehlke S. Monoclonal gammopathy of undetermined significance and Russell body formation in Helicobacter pylori gastritis. Helicobacter. 2006;11:506-510. [Cited in This Article: ] |
| 8. | Matthews JB. The immunoglobulin nature of Russell bodies. Br J Exp Pathol. 1983;64:331-335. [Cited in This Article: ] |
| 9. | Guzmán LM, Castillo D, Aguilera SO. Polymerase chain reaction (PCR) detection of B cell clonality in Sjögren’s syndrome patients: a diagnostic tool of clonal expansion. Clin Exp Immunol. 2010;161:57-64. [Cited in This Article: ] |
| 10. | Quartuccio L, Isola M, Baldini C, Priori R, Bartoloni Bocci E, Carubbi F, Maset M, Gregoraci G, Della Mea V, Salvin S. Biomarkers of lymphoma in Sjögren’s syndrome and evaluation of the lymphoma risk in prelymphomatous conditions: results of a multicenter study. J Autoimmun. 2014;51:75-80. [Cited in This Article: ] |
| 11. | Fujiyoshi Y, lnagaki H, Tateyama H, Murase T, Eimoto T. Mott cell tumor of the stomach with Helicobacter pylori infection. Pathol Int. 2001;51:43-46. [Cited in This Article: ] |