Published online Jan 16, 2015. doi: 10.4253/wjge.v7.i1.53
Peer-review started: September 1, 2014
First decision: November 3, 2014
Revised: December 1, 2014
Accepted: December 16, 2014
Article in press: December 17, 2014
Published online: January 16, 2015
We review the techniques and outcomes of the intragastric resection for gastric submucosal tumors (GSTs) using laparoscope and oral endoscope. In the literature, the mean operation time, intraoperative blood loss, pathological size of the tumor and postoperative hospital stay were 134 min, minimal, 31 mm and 6.4 d, respectively. There were no particular perioperative complications during the follow-up period (mean: 121.3 mo). Intragastric surgery using laparoscopy and oral endoscopy can be considerably beneficial for patients with GSTs locating in the upper third of the stomach between 2-5 cm in diameter and < 8 cm2 in cross-sectional area and located in the upper third of the stomach.
Core tip: The laparoscopic approach for gastric submucosal tumors (GSTs) depends on the characteristics of the submucosal tumors including its location or size. In particular, GSTs located close to the esophagogastric junction or pyloric ring cannot be easily applied the laparoscopic local resection. Therefore, the intragastric approach is adopted for those tumors. This review evaluates the technique and outcomes of the intragastric resection for GSTs using laparoscopy and oral endoscopy. Intragastric surgery using laparoscopy and oral endoscopy can be considerably beneficial for patients with GSTs less than 5 cm in diameter and locating in the upper third of the stomach.
- Citation: Tagaya N, Tatsuoka T, Kubota Y, Takegami M, Sugamata N, Saito K, Okuyama T, Sugamata Y, Oya M. Intragastric surgery using laparoscopy and oral endoscopy for gastric submucosal tumors. World J Gastrointest Endosc 2015; 7(1): 53-58
- URL: https://www.wjgnet.com/1948-5190/full/v7/i1/53.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i1.53
Techniques for the resection of gastric submucosal tumors (GSTs) have seen a shift from an open to an endoscopic approach, and from gastrectomy to local resection[1]. Endoscopic approaches can be divided into oral endoscopic resection and laparoscopic resection. The latter may include the resection from outside, inside or both side, depending on the characteristics of the GST, including its location or size. In particular, GSTs located close to the esophagogastric junction (EGJ) or pyloric ring are not amenable to laparoscopic local resection, and instead an intragastric approach is adopted[2-5]. This review evaluates the techniques and outcomes of intragastric resection for GSTs using laparoscopy and oral endoscopy in a series of patients treated at our institution.
We preoperatively investigated the tumor conditions including the size, location and distance from the EGJ to proximal side of the tumor using an upper gastrointestinal radiological series and endoscopy. Furthermore, endoscopic ultrasound (EUS) was also added to evaluate the location and growing formation of the tumor within the gastric wall[6]. And, EUS-guided fine-needle aspiration biopsy examination was performed when necessary. Computed tomography with contrast medium was added to clarify whether there was any liver metastasis, dissemination, ascites, lymphadenopathy or other comorbidities, as well as the relationship between the tumor and the whole stomach.
The criteria for the use of laparoscopy and oral endoscopy for intragastric resection of GSTs were a tumor between 2-5 cm in diameter and < 8 cm2 in cross-sectional area with the aim of possible removal via the mouth, or an endoscopically evident tendency of the tumor to grow in size during follow-up, and location of the tumor on the posterior wall of the upper third stomach or close to the EGJ[4].
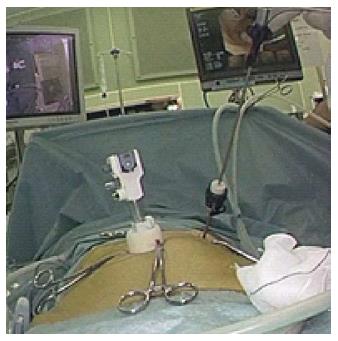
The patient was placed in the supine position under general anesthesia. Initially a 12-mm port was initially introduced into the peritoneal cavity at the umbilicus, using the open laparotomy method. After creating a pneumoperitoneum by Carbon dioxide (CO2) insufflation, and the operative field was kept at 8-10 mmHg of intra-abdominal pressure. The stomach was inflated to confirm the tumor condition using an oral endoscope. When we approached an intragastric technique, the anterior wall of the stomach was lifted up to the abdominal wall using a double-straight needle device (Ideal Lifting: Olympus Medical Systems Co., Tokyo, Japan) to insert the port easily. After this preparation, 5-mm and 12-mm ports were directly inserted into the stomach at the left upper quadrant of the abdominal wall, depending on the tumor location, under the observation of oral endoscope. To obtain the better intragastric operative field, CO2 insufflation was added into the stomach. A linear stapler to minimize the deformity of the stomach and avoid the stenosis of EGJ carried out local resection of the stomach including the lesion with an adequate margin in all directions. The first fire of linear stapler was put on the normal gastric wall near the distal side of the tumor. The direction of the resection line was modified so as not to close the EGJ. The resected specimen with a plastic bag was removed from the mouth by an oral endoscope. If the tumor removal is complicated orally, we made a small gastrostomy enlarging 12-mm port site, and then the specimen extracted from the stomach. We immediately ensured the free margins around the lesion. The entry holes in the stomach were closed using a linear stapler or hand sewing intracorporeally. Finally, the stomach was re-inflated to check the air leakage or bleeding from the closed sites and confirm no stenosis at the EGJ. Abdominal port sites were closed without drainage tube.
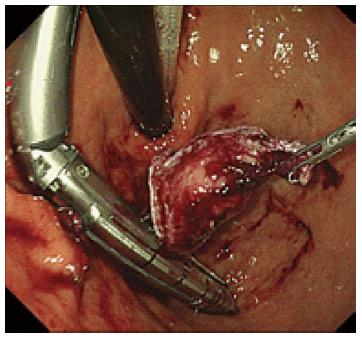
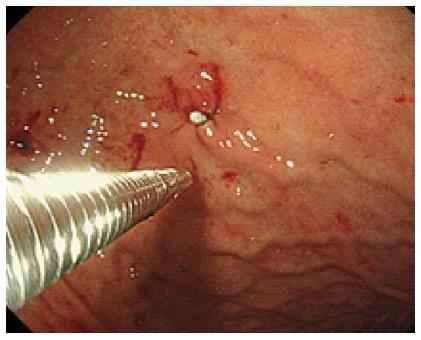
In a modified technique, an initial 12-mm port was introduced at the umbilicus. After checking the intra-abdominal cavity by laparoscope during stomach inflation, the anterior wall of the stomach was pulled out through an umbilical incision, and a 12-mm gastric opening was made. This hole was used for insertion of an Endo-GIA linear stapler or a 10-mm laparoscope. Subsequently a 3-mm port was inserted into the stomach at the left upper quadrant to allow manipulation of the normal gastric mucosa near the tumor (Figure 1). The tumor was resected using a linear stapler under endoscopic guidance (Figure 2). The specimen was retrieved via the mouth. The entry hole in the stomach was directly closed extracorporeally, and the 3-mm hole of the stomach was closed inside the stomach by clipping using an oral endoscope (Figure 3). The skin was only closed at the umbilicus.
Initially a 2.5 cm vertical skin incision was made at the umbilicus, and a X small Alexis Wound Protector (Applied Medical, Rancho Santa Margarita, CA, United States) was attached to the incision. The stomach was pulled out through that incision, and a 2-cm opening was made in the anterior wall of the stomach by laparoscopic coagulating shears. A single port device or surgical glove with 3 or 4 working ports was introduced into the gastric orifice. After the stomach was inflated with CO2 gas, intragastric pressure was maintained between 8 and 10 mmHg. A 10- or 5-mm laparoscope was inserted, and the target tumor was identified. The normal mucosa adjacent to the tumor was initially pulled up with a curved grasper and resected using a 30-mm linear stapler not to expose the tumor itself. Subsequently, the remaining main tumor area was resected continuously using a 45- or 60-mm linear stapler. The specimen was put into the plastic bag and retrieved from the single port site. After the single port device or surgical glove had been removed, the gastric orifice was closed using absorbable sutures. The stomach was re-inflated to confirm no bleeding or air leakage from the repaired site. The umbilical wound was closed without drainage tube.
We postoperatively evaluated the passage condition at the EGJ and the deformity of the residual stomach in all patients on the postoperative day 1 by an upper gastrointestinal radiological series, and followed by gastroscopy every 6 mo thereafter. Further treatment for gastrointestinal stromal tumors (GISTs) was considered according to the results of immunohistochemical tumor staging.
Surgical resections for GSTs are classified into open, endoscopic or laparoscopic procedures. The selection of the procedure depends on the characteristics of the tumor, including its size, location and growth condition. In particular, laparoscopic intragastric resection can be modified for tumors located near the EGJ or pyloric ring, in order to avoid gastrectomy or stomach deformity after resection. From the viewpoint of minimal surgical invasiveness, several laparoscopic intragastric approaches have been reported, but the role of oral endoscopy for intragastric resection of GSTs has been emphasized.
The indications for intragastric resection also depend on the characteristics of the tumor. In general, tumors amenable to this technique are 2-5 cm in size and located on the posterior wall of the upper third of stomach, or close to the EGJ. In our experience, tumors more than 5 cm in size or 8 cm2 in cross-sectional area require an additional gastrostomy for removal of the specimen from the stomach, because those sizes cannot be passed through the EGJ using an oral endoscope. However, when the tumor is less than 2 cm in size, resection depends on the results of FNA. Furthermore, when the tumor is more than 5 cm in size, a transgastric approach is selected for removal[4,5].
The actual resection method involved the use of an endo-linear stapler, coagulating shears or electrocautery. Stapled resection is more beneficial to provide with less operation time and blood loss, and can omit the suture of the resected area. In the resection process using an endo-linear stapler, if the 12-mm port is relatively close to the tumor, or if a tumor is larger than 5 cm, application of an endo-linear stapler is not easy, even if the stomach is inflated, because of the practical movable length of the stapler or the small opening of the stapler jaw[4,5]. Therefore we often use a minimum-length (30-mm) linear stapler, and place the 12-mm port on the greater curvature of the distal stomach under the guidance of oral endoscope. However, for any tumor located on the level of Z-line, submucosal dissection is applied circumferentially using electrocautery to prevent stenosis of the EGJ[9].
For successful intragastric resection of a GST, the use of an oral endoscope is mandatory for defining precisely the location of the tumor, for determining the port placement site in the stomach, for assisting intragastric resection, for confirming hemostasis at the staple line, for retrieval of the specimen via the mouth, and for checking the presence of any air leakage from the resected area after re-inflation of the stomach. Schubert et al[10] have also reported that intraoperative flexible endoscopy has several advantages including facilitation of the trans-illumination of the gastric lesion during laparoscopic observation, elimination of preoperative tattooing of the lesion, and evaluation of the repaired gastric opening for any leakage after resection. Recently, Hiki et al[11] have reported laparoscopic and endoscopic cooperative surgery (LECS) for resection of GISTs. This method makes it possible to obtain an adequate cutting line independently of tumor location, eliminate an unnecessary resection of the gastric wall around the tumor in the setting of exogastric resection, and minimize any deformity of the stomach after resection. However, its indications are limited to intragastric growth-type tumors less than 5 cm in size, those with no direct tumor exposure, and those with no ulceration, in view of the attendant risk of dissemination. It is anticipated that oral endoscopy during laparoscopic procedures will become increasingly important in order to achieve minimal surgical invasiveness.
There are 18 reports covering laparoscopic intragastric resection of GSTs published between 2000 and 2014[5-10,12-23]. Six of them were excluded because their data were mixed with those for exogastric and transgastric procedures, or for single cases. We reviewed previous reports describing laparoscopic intragastric surgery (LIS) for GSTs (Tables 1 and 2)[5,7,9,12-20]. The number of cases ranged from 3 to 13, with a mean of 7 cases. The mean patient age was 62 years (range: 48-77 years). The tumor was located in the upper stomach in almost all cases (96.3%), with the exception of 3 cases. The mean size of the tumor was 31 mm (range: 27-38 mm). The common indications for intragastric resection of GSTs were a tumor location in the upper third of the stomach and posterior wall, intragastric growth, and a tumor diameter of less than 5 cm. The mean operation time was 134 min (range: 75-192 min). There were 4 complications (5.2%), including conversion to open laparotomy in 2 cases, bleeding from the staple line and wound in one case each, respectively. The mean postoperative hospital stay was 6.4 d (range: 4.3-7.7 d). The mean follow-up period was 48.8 mo (range: 8.5-121.7 mo), and only one case of tumor recurrence was recorded. However, the recurrence rate appears to depend on the size of the tumor: Nakamori et al[22] reported that the recurrence rate increased with tumor size, and that the average period until recurrence was 23.6 mo. Evaluation of recurrence required a follow-up period of more than 2 years. This procedure has one limitation of consuming the number of linear staples, approximately 3 being necessary per procedure. When considering the possibility of recurrence, intragastric resection of GSTs using laparoscopy and oral endoscopy is suitable for tumors less than 5 cm in size and located in the upper third of the stomach.
| Ref. | Year | Case | Gender (M/F) | Age (mean) | Location (U/M/L) | Distant from EGJ (mm) | Size (mm) |
| Choi and Oh[12] | 2000 | 9 | NA | NA | 9/0/0 | NA | NA |
| Matthews et al[13] | 2002 | 3 | NA | NA | 3/0/0 | NA | NA |
| Walsh et al[14] | 2003 | 11 | NA | NA | 11/0/0 | NA | 24-85 |
| Pross et al[15] | 2003 | 5 | NA | NA | 5/0/0 | NA | 34 (28-41) |
| Uchikoshi et al[16] | 2004 | 7 | NA | NA | 7/0/0 | NA | 27-75 |
| Li et al[17] | 2008 | 3 | 0/3 | 77 | 2/1/0 | 37 (30-50) | 28 (20-40) |
| Na et al[7] | 2011 | 7 | 3/4 | 65 | 6/1/0 | NA | 27 (23-38) |
| Sahm et al[18] | 2011 | 7 | NA | NA | NA | NA | 38 (28-48) |
| Shim et al[9] | 2011 | 6 | 3/3 | 48 | 7/0/0 | NA | 27 (15-40) |
| Tagaya et al[5] | 2013 | 13 | 5/8 | 61 | 10/3/0 | 40 (10-70) | 27 (10-65) |
| de Vogelaere et al[19] | 2013 | 3 | NA | 68 | 3/0/0 | NA | 38 (27-68) |
| Dong et al[20] | 2014 | 8 | 3/5 | 51 | 6/2/0 | NA | 28 (15-45) |
| Ref. | Year | Operation time (min) | Complication | POHS (d) | Recurrence | Follow up (mo) |
| Choi and Oh[12] | 2000 | 100-140 | Open conversion: 1 | 5.9 | None | Up to 42 |
| Matthews et al[13] | 2002 | NA | NA | NA | NA | NA |
| Walsh et al[14] | 2003 | 186 (120-320) | None | 3.0-8.0 | None | 16.2 (1-32) |
| Pross et al[15] | 2003 | 85-105 | None | 4.0-7.0 | None | NA |
| Uchikoshi et al[16] | 2004 | 141 (95-200) | Open conversion: 1 | 7.6 | 1 in 2 yr | 14-99 |
| Li et al[17] | 2008 | 192 (140-240) | Staple line bleeding: 1 | 7.7 | None | 8-57 |
| Na et al[7] | 2011 | 86 (70-105) | Wound bleeding: 1 | 5.7 | None | 8.5 (1-23.3) |
| Sahm et al[18] | 2011 | NA | None | 6.1 | NA | NA |
| Shim et al[9] | 2011 | 128 (105-145) | None | 4.3 | NA | NA |
| Tagaya et al[5] | 2013 | 176 (132-217) | None | 7.5 | None | 121.7 (1-192) |
| de Vogelaere et al[19] | 2013 | 75 (67-82) | None | 5.0 | None | NA |
| Dong et al[20] | 2014 | 85 (60-130) | None | 7.4 | None | NA |
Transumbilical single-incision laparoscopic abdominal surgery was introduced in 2007 and has since become disseminated worldwide. We have also applied single-incision laparoscopic local resection of the stomach for GSTs showing extragastric growth. There are a few reports[7,8] describing single-port access using a single port devices for tumors showing intragastric growth. Na et al[7] reported that a single-incision intragastric approach did not require the use of intraoperative oral endoscopy or pneumoperitoneum, and that the technique differed in three ways from the conventional approach: the operation time was reduced because of the use of a single gastrostomy and extracorporeal repair, the specimen was easily retrieved from the gastric opening without using an endoscope, and a better cosmetic outcome was achieved at the umbilicus. Morales-Conde et al[8] also reported intragastric endoscopically assisted single-incision surgery for GST at the EGJ. The single-site approach avoids multiple punctures of the stomach, and allows retrieval of larger specimens. However, this approach should be limited to selected cases involving tumors less than 5 cm in diameter without ulceration because of possible tumor rapture due to the complicated procedures employed.
In conclusion, intragastric surgery using laparoscopy and oral endoscopy can be considerably beneficial for patients with GSTs located in the upper third of the stomach. From the viewpoint of minimal surgical invasiveness, the significance of oral endoscopy during laparoscopic procedures is expected to increase for tumors in the stomach.
P- Reviewer: Surlin V S- Editor: Ji FF L- Editor: A E- Editor: Zhang DN
| 1. | Tagaya N, Mikami H, Igarashi A, Ishikawa K, Kogure H, Ohyama O. Laparoscopic local resection for benign nonepithelial gastric tumors. J Laparoendosc Adv Surg Tech A. 1997;7:53-58. [Cited in This Article: ] |
| 2. | Tagaya N, Kita J, Kogure H, Kubota K. Laparoscopic intragastric resection of gastric leiomyoma using needlescopic instruments. Case report. Surg Endosc. 2001;15:414. [Cited in This Article: ] |
| 3. | Tagaya N, Mikami H, Kogure H, Kubota K, Hosoya Y, Nagai H. Laparoscopic intragastric stapled resection of gastric submucosal tumors located near the esophagogastric junction. Surg Endosc. 2002;16:177-179. [Cited in This Article: ] |
| 4. | Tagaya N, Mikami H, Kubota K. Laparoscopic resection of gastrointestinal mesenchymal tumors located in the upper stomach. Surg Endosc. 2004;18:1469-1474. [Cited in This Article: ] |
| 5. | Tagaya N, Kubota Y, Makino N, Takegami M, Saito K, Okuyama T, Yoshiba H, Sugamata Y, Oya M. Gastrointestinal Endoscopy: Laparoscopic intra-gastric resection of gastric sub-mucosal tumors under oral endoscopic guidance. J Gastrointest Dig Syst. 2013;S2:004. [Cited in This Article: ] |
| 6. | Sasaki A, Koeda K, Obuchi T, Nakajima J, Nishizuka S, Terashima M, Wakabayashi G. Tailored laparoscopic resection for suspected gastric gastrointestinal stromal tumors. Surgery. 2010;147:516-520. [Cited in This Article: ] |
| 7. | Na JU, Lee SI, Noh SM. The single incision laparoscopic intragastric wedge resection of gastric submucosal tumor. J Gastric Cancer. 2011;11:225-229. [Cited in This Article: ] |
| 8. | Morales-Conde S, Alarcón I, Ortiz-Moyano C, Barranco A, Padillo FJ, Socas M. Intragastric endoscopic assisted single incision surgery for gastric leiomyoma of the esophagogastric junction. Case Rep Gastrointest Med. 2013;2013:391430. [Cited in This Article: ] |
| 9. | Shim JH, Lee HH, Yoo HM, Jeon HM, Park CH, Kim JG, Song KY. Intragastric approach for submucosal tumors located near the Z-line: a hybrid laparoscopic and endoscopic technique. J Surg Oncol. 2011;104:312-315. [Cited in This Article: ] |
| 10. | Schubert D, Kuhn R, Nestler G, Kahl S, Ebert MP, Malfertheiner P, Lippert H, Pross M. Laparoscopic-endoscopic rendezvous resection of upper gastrointestinal tumors. Dig Dis. 2005;23:106-112. [Cited in This Article: ] |
| 11. | Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729-1735. [Cited in This Article: ] |
| 12. | Choi YB, Oh ST. Laparoscopy in the management of gastric submucosal tumors. Surg Endosc. 2000;14:741-745. [Cited in This Article: ] |
| 13. | Matthews BD, Walsh RM, Kercher KW, Sing RF, Pratt BL, Answini GA, Heniford BT. Laparoscopic vs open resection of gastric stromal tumors. Surg Endosc. 2002;16:803-807. [Cited in This Article: ] |
| 14. | Walsh RM, Ponsky J, Brody F, Matthews BD, Heniford BT. Combined endoscopic/laparoscopic intragastric resection of gastric stromal tumors. J Gastrointest Surg. 2003;7:386-392. [Cited in This Article: ] |
| 15. | Pross M, Wolff S, Nestler G, Schubert D, Kahl S, Lippert H. A technique for endo-organ resection of gastric wall tumors using one intragastric trocar. Endoscopy. 2003;35:613-615. [Cited in This Article: ] |
| 16. | Uchikoshi F, Ito T, Nishida T, Kitagawa T, Endo S, Matsuda H. Laparoscopic intragastric resection of gastric stromal tumor located at the esophago-cardiac junction. Surg Laparosc Endosc Percutan Tech. 2004;14:1-4. [Cited in This Article: ] |
| 17. | Li VK, Hung WK, Chung CK, Ying MW, Lam BY, Kan DM, Chan MC. Laparoscopic intragastric approach for stromal tumours located at the posterior gastric wall. Asian J Surg. 2008;31:6-10. [Cited in This Article: ] |
| 18. | Sahm M, Pross M, Lippert H. Intraluminal resection of gastric tumors using intragastric trocar technique. Surg Laparosc Endosc Percutan Tech. 2011;21:e169-e172. [Cited in This Article: ] |
| 19. | DE Vogelaere K, VAN DE Winkel N, Simoens C, Delvaux G. Intragastric SILS for GIST, a new challenge in oncologic surgery: first experiences. Anticancer Res. 2013;33:3359-3363. [Cited in This Article: ] |
| 20. | Dong HY, Wang YL, Jia XY, Li J, Li GD, Li YQ. Modified laparoscopic intragastric surgery and endoscopic full-thickness resection for gastric stromal tumor originating from the muscularis propria. Surg Endosc. 2014;28:1447-1453. [Cited in This Article: ] |
| 21. | Ludwig K, Weiner R, Bernhardt J. [Minimally invasive resections of gastric tumors]. Chirurg. 2003;74:632-637. [Cited in This Article: ] |