Published online Jan 16, 2013. doi: 10.4253/wjge.v5.i1.6
Revised: September 26, 2012
Accepted: December 1, 2012
Published online: January 16, 2013
Gastric antral vascular ectasia (GAVE) is an uncommon but often severe cause of upper gastrointestinal (GI) bleeding, responsible of about 4% of non-variceal upper GI haemorrhage. The diagnosis is mainly based on endoscopic pattern and, for uncertain cases, on histology. GAVE is characterized by a pathognomonic endoscopic pattern, mainly represented by red spots either organized in stripes radially departing from pylorus, defined as watermelon stomach, or arranged in a diffused-way, the so called honeycomb stomach. The histological pattern, although not pathognomonic, is characterized by four alterations: vascular ectasia of mucosal capillaries, focal thrombosis, spindle cell proliferation and fibrohyalinosis, which consist of homogeneous substance around the ectatic capillaries of the lamina propria. The main differential diagnosis is with Portal Hypertensive Gastropathy, that can frequently co-exists, since about 30% of patients with GAVE co-present a liver cirrhosis. Autoimmune disorders, mainly represented by Reynaud’s phenomenon and sclerodactyly, are co-present in about 60% of patients with GAVE; other autoimmune and connective tissue disorders are occasionally reported such as Sjogren’s syndrome, systemic lupus erythematosus, primary biliary cirrhosis and systemic sclerosis. In the remaining cases, GAVE syndrome has been described in patients with chronic renal failure, bone marrow transplantation and cardiac diseases. The pathogenesis of GAVE is still obscure and many hypotheses have been proposed such as mechanical stress, humoural and autoimmune factors and hemodynamic alterations. In the last two decades, many therapeutic options have been proposed including surgical, endoscopic and medical choices. Medical therapy has not clearly shown satisfactory results and surgery should only be considered for refractory severe cases, since this approach has significant mortality and morbidity risks, especially in the setting of portal hypertension and liver cirrhosis. Endoscopic therapy, particularly treatment with Argon Plasma Coagulation, has shown to be as effective and also safer than surgery, and should be considered the first-line treatment for patients with GAVE-related bleeding.
- Citation: Fuccio L, Mussetto A, Laterza L, Eusebi LH, Bazzoli F. Diagnosis and management of gastric antral vascular ectasia. World J Gastrointest Endosc 2013; 5(1): 6-13
- URL: https://www.wjgnet.com/1948-5190/full/v5/i1/6.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i1.6
Gastric antral vascular ectasia (GAVE) is an uncommon but often severe cause of upper gastrointestinal (GI) bleeding, responsible of about 4% of non-variceal upper GI hemorrhage[1]. This disease was first described in 1953 by Ryder et al[2], but deeply investigated only 25 years later, in 1978, by Van Vliet et al[3]. Since then, a better but still incomplete knowledge of this condition has been reach; however, the exact prevalence is not known, the pathogenesis remains unclear and the best therapeutic approach has not yet been defined. The aim of this paper is to review the current findings about GAVE and to contribute to a better understanding of this often mis-diagnosed disease and critically review the current therapeutics options.
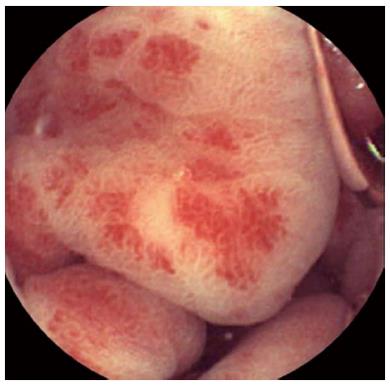
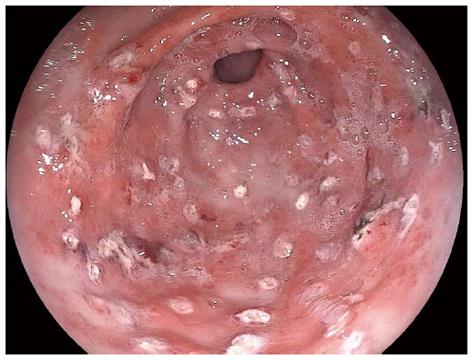
GAVE is characterized by a pathognomonic endoscopic pattern, mainly represented by red spots either organized in stripes radially departing from pylorus, defined as watermelon stomach, or arranged in a diffused-way, the so called honeycomb stomach[4] (Figures 1 and 2).
GAVE is typically located in the gastric antrum, however it may be rarely found also in other areas of the GI tract, including cardia[5,6], duodenum, jejunum[7] and rectum[8,9]. The involvement of the proximal part of the stomach is almost rare and generally located within a diaphragmatic hernia[10]. At the endoscopic ultrasound (EUS), the gastric antrum appears hyperthrophic with a spongy appearance of the mucosa and submucosa and a well-preserved muscularis propria[11,12].
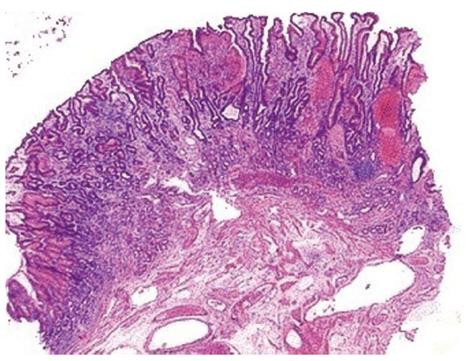
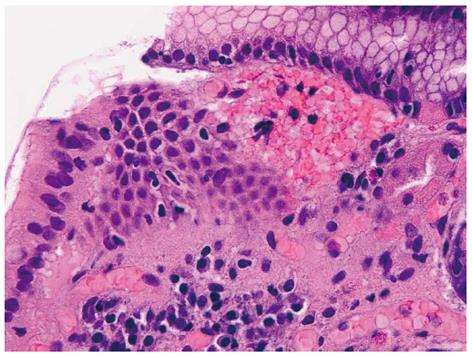
The histological pattern, although not pathognomonic, is characterized by four alterations: vascular ectasia of mucosal capillaries, focal thrombosis, spindle cell proliferation (= smooth muscle cell and myofibroblast hyperplasia) and fibrohyalinosis, which consist of homogeneous substance around the ectatic capillaries of the lamina propria[13-15] (Figures 3 and 4). In 1989, Gilliam et al[14] proposed a score system to diagnose GAVE, which considered only two histological criteria: the co-presence of ectasia and/or fibrin thrombi and spindle cell proliferation (Gilliam’s score). Subsequently, a third parameter, fibrohyalinosis, was added to improve both sensibility and specificity[15]. This latter score, called “GAVE score”, showed a higher diagnostic accuracy (80%) to differentiate GAVE from Portal Hypertensive Gastropathy, which may be present in patients with co-existing portal hypertension. Table 1 summarized both the histological scores, the Gilliam’s score and the GAVE score.
| Gastric antral vascular ectasia score(range 0-5) | Gilliam’s score(range 0-4) | ||
| Score | Fibrin thrombi and/or vascular ectasia | Spindle cell proliferation | Fibrohyalinosis |
| 0 | Both absent | Absent | Absent |
| 1 | One present | Increased | Present |
| 2 | Both present | Marked increased | - |
Patients with portal hypertension often present an endoscopic pattern called portal hypertensive gastropathy (PHG), which needs to be distinct from the GAVE pattern, since they represent two separate entities in the setting of liver cirrhosis. The differential diagnosis is mainly based on the endoscopic appearance and, in the doubtful cases, by the histological pattern.
PHG occurs only in patients with portal hypertension and typically involves the fundus and the corpus of the stomach; the endoscopic pattern is characterized by a combination of four main characteristics: a mosaic-like pattern, presence of red point lesions, cherry red spots and black-brown spots[16]. The histological findings may clarify the uncertain cases by the assessment of the “GAVE score”, indeed, a GAVE score > 3 is considered highly diagnostic for the presence of GAVE (Table 1)[15]. The main aspects to consider in the differential diagnosis between GAVE and PHG are summarised in Table 2. The importance to distinguish these two clinical entities is mainly related to the different therapeutic approach; the reduction of portal pressure by using drugs (beta-blockers, somatostatin, octreotide), trans-jugular intra-hepatic porto-systemic shunt (TIPS) or surgery (portocaval shunts) are not effective for the treatment of GAVE[17,18].
| Features | Portal hypertensive gastropathy | Gastric antral vascular ectasia |
| Site | Fundus-corpus | Antrum |
| Endoscopic pattern | Combination of: Mosaic-like pattern Red point lesions Cherry red spots Black-brown spots | Red spots organised: Striped-pattern (watermelon-stomach) Diffused-pattern (honeycomb-stomach) |
| Histological pattern | Not specific | Highly specific |
| Response to | Present | Absent |
| β-Blockers/transjugular intrahepatic portosystemic shunt/portocaval shunts | ||
GAVE syndrome can complicate the course of many diseases (Table 3). Autoimmune disorders, mainly represented by Reynaud’s phenomenon and sclerodactyly, are co-present in about 60% of patients with GAVE[10]; other autoimmune and connective tissue disorders are occasionally reported such as Sjogren’s syndrome[19], systemic lupus erythematosus[20], primary biliary cirrhosis and systemic sclerosis[21]. In this latter case, it has been reported that GAVE can even represent the presenting syndrome, preceding the development of the autoimmune disorders by several months[21].
| Associated disease | Prevalence (%) | Ref. |
| Autoimmune diseases | 60 | |
| Raynaud's phenomenon | [10] | |
| Sclerodactyly | [10] | |
| Sjogren's syndrome | [19] | |
| Systemic sclerosis | [21,32] | |
| Primary biliary cirrhosis | [10,32] | |
| Systemic lupus erythematosus | [20] | |
| Liver cirrhosis and/or portal hypertension | 30 | [22-24] |
| Others | 10 | |
| Chronic renal failure | [10] | |
| Bone marrow transplantation | [25] | |
| Cardiac diseases | [10,26] |
About 30% of patients with GAVE co-present a liver cirrhosis[22-24], whatever etiology (viral, autoimmune, toxic-metabolic). In the remaining cases, GAVE syndrome has been described in patients with chronic renal failure[10], bone marrow transplantation[25] and cardiac diseases[10,26].
Non-cirrhotic patients more frequently present the typical endoscopic watermelon-, striped-pattern and are mainly represented by middle-aged women whereas the honeycomb-, diffuse-pattern prevails in patients with liver failure[1,4,27]. However, the endoscopic appearance is not related to the patient’s outcome[4] but could reflect a different pathogenesis.
GAVE syndrome is an acquired disease rather than a congenital alteration. The pathogenesis of GAVE is still obscure and many hypotheses have been proposed such as mechanical stress, humoural and autoimmune factors and hemodynamic alterations.
Mechanical stress represented by strong gastric peristalsis has been supposed to induce prolapse and trauma of antral mucosa and intermittent obstruction of blood vessels, which can lead to fibro-muscular hyperplasia and vascular ectasia[28]. These latter are typical findings of GAVE and other gastrointestinal lesions due to repeated traumas and mucosal prolapse (i.e., stomas and prolapsed haemorrhoids)[13]. Furthermore, a subset of patients with liver cirrhosis and GAVE has been showen to have antro-pyloric dysfunction with abnormal antral motor response to meals[29].
Many authors have assumed a pivotal role of humoral factors as gastrin, vasoactive inhibitory peptide (VIP), 5-hydroxytryptamine, glucagon, catecholamines, prostanoid and other undefined vasoactive substances. GAVE syndrome has been associated with both increased[28] and decreased levels of gastrinemia[15] and these conflicting data reduced the importance initially ascribed to this hormone, which was hypothesised to induce spindle cell proliferation, hyperplasia, prolonged sphincter relaxation and also capillary and venous dilatation. A possible role of both VIP and 5-hydroxytryptamine has been proposed after the evidence of the presence of actively proliferating neuroendocrine cells surrounding the ectasic vessels in the lamina propria of patients with GAVE[30]. The release of these substances seems to be responsible for the local vasodilatation and the tendency to bleed. On the other hand, glucagon and catecholamines do not seem to play any role in the pathogenesis of GAVE, since concentrations of these metabolites have shown to be similar in cirrhotics with or without GAVE. However, prostaglandin E2, a prostanoid with vaso-dilatator and acid-inhibitory effect, showed significantly higher levels in patients with GAVE[31].
Up to 60% of patients with GAVE have also an autoimmune associated disease and show the presence of autoantibodies[10], therefore an autoimmune pathogenesis has been suggested. Indeed, several autoantibodies have been detected in patients with GAVE; Watson et al[32] found that all patients with systemic sclerosis and GAVE were positive for antinuclear antibodies and, in some cases, were also positive for anti-centromere antibodys. This antibody was subsequently demonstrated to recognize a specific and formerly unknown centromeric protein, involved in the cell growth process[33]. Garcia et al[34] and Valdez et al[35] found in the sera of a patients with GAVE an antinucleolar antibody that specifically recognized a RNA helicase II (RH-II). It has been speculated that these autoantibodies could cross-react with specific proteins present in the vessels of the gastric mucosa and sub-mucosa inducing the typical alterations. However, the exact role played by these autoantibodies is still unknown and only the development of an animal model will probably provide further information.
It is now evident that portal hypertension does not play a role in the GAVE development, since it is not present in up to 70% of patients, and the reduction of portal hypertension does not affect the course of the disease[17]. Moreover, it has been shown that liver transplantation despite persistent portal hypertension induces complete disappearance of the antral vascular lesions[36]. It could be speculated that liver failure, at least in a subset of patients, and not portal hypertension, could have a role in the pathogenesis of GAVE altering the metabolism of not yet identified substances.
Finally, GAVE syndrome could have a multifactorial pathogenesis, with the driven process strictly related to the different clinical settings (i.e., autoimmune or liver failure setting), thus explaining the dissimilar endoscopic appearance (watermelon- or honeycomb-pattern).
In the last two decades, many therapeutic options have been proposed including surgical, endoscopic and medical choices and the best approach is still to be defined.
The surgical approach, most commonly represented by antrectomy, has a clear clinical efficacy in the management of GAVE-related bleeding, since none of the patients surgically treated has recurrence of bleeding in the post-operative period[37]. However, this approach has significant mortality and morbidity risks, especially in the setting of portal hypertension and liver cirrhosis. Novitsky et al[37] reviewed 45 reported surgical cases and found that antrectomy was the most frequently performed surgical approach (89% of cases) with a 30-d mortality rate of 6.6% and the principal cause of death was multiorgan failure. As previously mentioned, porto-caval shunts, including TIPS, have no role in the treatment of GAVE syndrome[17].
A wide variety of drugs have been used to try to control GAVE-related bleeding, however no one has clearly shown satisfactory results in order to consider medical therapy as a valid alternative to an invasive approach.
Hormonal therapy - estrogen-progesterone - has been shown to control bleeding related to GI vascular malformations, including GAVE, by undefined mechanisms[38,39]. However, since the vascular lesions persist despite cessation of bleeding, a dose-reduction is usually related to bleeding relapse[40-42]. Moreover, the long-term treatment with hormonal-therapy can induce severe side effects, such as menorrhagia and gynaecomastia, and increase the risk of endometrial and breast cancer[43].
Octreotide, a long-acting somatostatin analogue, has been shown to effectively control chronic bleeding related to vascular abnormalities. Nardone and co-workers treated 3 patients with GAVE-related bleeding with octreotide (0.1 mg subcutaneous three times a day) for 6 mo, obtaining a transient reduction of bleeding in one case and cessation in the others two patients, with partial and total regression of the lesions[44]. This result can be partly explained by several effects exerted by this hormone such as the inhibitory effect on both neuroendocrine cells surrounding the ectatic vessels and on smooth muscle cells, and the anti-angiogenic effect. However, other authors have not confirmed these results[45] and the role played by octreotide needs to be further investigated in larger sample size studies.
Few case-reports have suggested a potential benefit from the use of tranexamic acid but reported severe side effects (central venous stasis retinopathy; deep venous thrombosis and pulmonary embolism) limit its use[46-48].
A case-report showed complete resolution of GAVE with intravenous infusion of methylprednisolone and cyclophosfamide in a patient with associated systemic sclerosis and pernicious anaemia[49]; but, such result has not been yet confirmed in larger series.
In conclusion, drug therapies have no definite role in the cure of GAVE-related bleeding and should be considered an experimental therapeutic approach in the setting of controlled clinical trials.
The endoscopic treatment principally represented by laser photoablation and, more recently, by Argon Plasma Coagulation (APC) has shown a similar and safer effect as surgery.
Neodymium-yttrium-aluminum garnet (Nd: YAG) laser coagulation has been successfully used to control GAVE-related bleeding. All series have confirmed the efficacy of this endoscopic thermal therapy by reducing or abolishing the need of blood transfusions in about 50% to 80% of cases, with a mean of 3 treatment sessions (range 1-10)[50-53].
The most serious complication described after laser therapy, even if rare, is represented by gastric perforation. Two weeks after almost all laser therapy sessions, a gastric ulceration is frequently observed, even when the laser treatment session has been performed with an energy power sufficient to induce only superficial scarring without deep tissue necrosis[54]. Another complication observed after repeated treatment sessions, is pyloric stenosis, that can induce either delayed gastric emptying or true obstruction[54,55]. Up to 8% of patients developed this complication, that can be resolved by balloon dilation[55]. Moreover, after multiple, high energy, laser therapy sessions, patients may develop hyperplastic polyps, even after 20 mo of follow-up[56]. These polyps can reach large dimensions and induce recurrent anaemia without evidence of recurrence of vascular abnormalities[56]. Their development is thought to be secondary to reactive foveolar hyperplasia and no focal malignancy has been detected. However, Bernstein and co-workers presented a case-report of a multifocal gastric cancer developed after repeated sessions of laser therapy over a five-year period[57].
Other important disadvantages of laser endoscopic therapy are the high cost and the need of a long training period, since most severe complications, such as perforation and death, happen more frequently when the endoscopist is not sufficiently skilled with the procedure[51,54].
Argon plasma coagulation (APC) is a noncontact technique with a controllable depth of coagulation (0.5-3 mm). High-frequency current is applied to the tissue through ionized and electrically conductive gas, called argon plasma; the diverging gas flow allows an axial, radial and retrograde application (Figure 5). In comparison to Nd: YAG laser therapy, APC is easier to use, more manageable, cheaper and, most importantly, safer; nevertheless, randomized trials comparing the two endoscopic procedures are lacking.
The complications are rare and mostly mild. The most frequently reported complication is represented by intestinal gas distension related to argon flow, which can leave the patient with a feeling of discomfort after the endoscopic session. Wall emphysema and intestinal pneumatosis have been described, but these conditions are usually reversible[58]. More serious adverse events described after APC treatment are asymptomatic antral stenosis[59] and upper GI hemorrhage. One severe case of sepsis, which conduced to death due to infectious peritonitis, has also been described[60]. The risk of intestinal perforation is very low and limited to very thin-walled structures (i.e., caecum)[58,61]; notably, no case of perforation during APC treatment of GAVE has been described.
The largest case series of APC treatment reported an efficacy ranging from 90%[60] to 100%[62], with no further need for blood transfusions and an increase of hemoglobin level from 2.3 g/dL[62] to 5.5 g/dL[58] in almost all patients. The setting of argon gas flow usually ranges between 0.8 L/min and 2.5 L/min, the electrical power from 40 W to 100 W and, generally, a mean of 2.5 sessions are needed to achieve complete eradication[58,62,63].
Several other endoscopic therapies have been proposed in the last years, such as cryotherapy, band ligation and radiofrequency ablation.
A small, prospective pilot study, based on 12 patients, investigated the efficacy of cryotherapy for the treatment of GAVE-related bleeding achieving a complete response to treatment (i.e., no need for blood transfusion) in 50% of cases[64]. Cryotherapy is based on the rapid decrease of temperature due to the rapid expansion of carbon dioxide (CO2) released by the spray catheter; such sudden decrease of temperature causes superficial necrosis of the mucosa and of the superficial submucosal, with eradication of antral teleangiectasias, and subsequent re-epithelialization. The need for specialized equipment and for specific training, represents Cryotherapy’s main limitations; furthermore, the need of an overtube placed to enable passive venting of CO2, might add technical difficulty and risk to the procedure.
Several case-reports and one observational comparative study have reported the use of band ligation for patients with GAVE related bleeding[65-67]. Based on the small, retrospective study that compared endoscopic band ligation with endoscopic thermal therapy, band ligation showed a significant higher rate of bleeding cessation, fewer treatment sessions required to achieve cessation of bleeding, a greater increase in hemoglobin values and reduction of the need for transfusions[67]. The higher efficacy compared to standard thermal therapy is probably due to a more reliable eradication of the abnormal vasculature in the mucosa and submucosal.
Finally, a pilot study has investigated the role of radiofrequency ablation for the treatment of GAVE[68]; 6 patients with transfusion-dependent GAVE-related bleeding were enrolled and after 1 to 3 treatments, all but one no longer needed transfusions during the 6 mo follow up, without reporting adverse events.
Although cryotherapy, endoscopic band ligation and radiofrequency ablation have provided encouraging results, well-performed, larger, prospective studies are needed before providing any definitive conclusion.
GAVE is an infrequent but severe cause of upper gastrointestinal bleeding, characterized by a pathognomonic endoscopic pattern of red spots organized either in stripes or randomly distributed in the gastric antrum. GAVE can develop in the setting of many diseases, mainly represented by autoimmune diseases and liver cirrhosis. Although many hypotheses, such as mechanical stress, humoral/immunological factors and haemodynamics alterations, have been proposed, the pathogenesis of GAVE remains still obscure and probably different pathways occur in different clinical settings. The therapy is limited to surgical or endoscopic approach, since most drug therapies have shown conflicting results. Surgery has the advantage to be a definitive therapy but with high morbidity and mortality risks, especially in patients with severe co-morbidities, such as liver cirrhosis. Endoscopic therapy, particularly treatment with APC, has shown to be as effective and also a safer than surgery, and should be considered the first-line treatment for patients with GAVE-related bleeding.
P- Reviewers Rodrigo L, Eisenbach C S- Editor Song XX L- Editor A E- Editor Zhang DN
| 1. | Dulai GS, Jensen DM, Kovacs TO, Gralnek IM, Jutabha R. Endoscopic treatment outcomes in watermelon stomach patients with and without portal hypertension. Endoscopy. 2004;36:68-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Rider JA, Klotz AP, Kirsner JB. Gastritis with veno-capillary ectasia as a source of massive gastric hemorrhage. Gastroenterology. 1953;24:118-123. [PubMed] [Cited in This Article: ] |
| 3. | van Vliet AC, ten Kate FJ, Dees J, van Blankenstein M. Abnormal blood vessels of the prepyloric antrum in cirrhosis of the liver as a cause of chronic gastrointestinal bleeding. Endoscopy. 1978;10:89-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Ito M, Uchida Y, Kamano S, Kawabata H, Nishioka M. Clinical comparisons between two subsets of gastric antral vascular ectasia. Gastrointest Endosc. 2001;53:764-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Stotzer PO, Willén R, Kilander AF. Watermelon stomach: not only an antral disease. Gastrointest Endosc. 2002;55:897-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Shaffer RA, Scobey MW. Ring around the cardia: a watermelon stomach variant. Gastrointest Endosc. 2003;57:280-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Calès P, Voigt JJ, Payen JL, Bloom E, Berg P, Vinel JP, Pradère B, Broussy P, Pascal JP. Diffuse vascular ectasia of the antrum, duodenum, and jejunum in a patient with nodular regenerative hyperplasia. Lack of response to portosystemic shunt or gastrectomy. Gut. 1993;34:558-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Gürsoy M, Baysal C, Demirhan B, Moray G, Boyacioğlu S. Rectal vascular ectasia associated with watermelon stomach. Gastrointest Endosc. 1999;50:854-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Singh D, Shill M, Kaur H. The watermelon rectum. J Clin Gastroenterol. 2001;33:164-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Gostout CJ, Viggiano TR, Ahlquist DA, Wang KK, Larson MV, Balm R. The clinical and endoscopic spectrum of the watermelon stomach. J Clin Gastroenterol. 1992;15:256-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 180] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Parente F, Petrillo M, Vago L, Bianchi Porro G. The watermelon stomach: clinical, endoscopic, endosonographic, and therapeutic aspects in three cases. Endoscopy. 1995;27:203-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 12. | Shudo R, Yazaki Y, Sakurai S, Uenishi H, Yamada H, Sugawara K. Diffuse antral vascular ectasia: EUS after argon plasma coagulation. Gastrointest Endosc. 2001;54:623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Suit PF, Petras RE, Bauer TW, Petrini JL. Gastric antral vascular ectasia. A histologic and morphometric study of “the watermelon stomach”. Am J Surg Pathol. 1987;11:750-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 86] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Gilliam JH, Geisinger KR, Wu WC, Weidner N, Richter JE. Endoscopic biopsy is diagnostic in gastric antral vascular ectasia. The “watermelon stomach”. Dig Dis Sci. 1989;34:885-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 74] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Payen JL, Calès P, Voigt JJ, Barbe S, Pilette C, Dubuisson L, Desmorat H, Vinel JP, Kervran A, Chayvialle JA. Severe portal hypertensive gastropathy and antral vascular ectasia are distinct entities in patients with cirrhosis. Gastroenterology. 1995;108:138-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 121] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Carpinelli L, Primignani M, Preatoni P, Angeli P, Battaglia G, Beretta L, Bortoli A, Capria A, Cestari R, Cosentino F. Portal hypertensive gastropathy: reproducibility of a classification, prevalence of elementary lesions, sensitivity and specificity in the diagnosis of cirrhosis of the liver. A NIEC multicentre study. New Italian Endoscopic Club. Ital J Gastroenterol Hepatol. 1997;29:533-540. [PubMed] [Cited in This Article: ] |
| 17. | Spahr L, Villeneuve JP, Dufresne MP, Tassé D, Bui B, Willems B, Fenyves D, Pomier-Layrargues G. Gastric antral vascular ectasia in cirrhotic patients: absence of relation with portal hypertension. Gut. 1999;44:739-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 138] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Kamath PS, Lacerda M, Ahlquist DA, McKusick MA, Andrews JC, Nagorney DA. Gastric mucosal responses to intrahepatic portosystemic shunting in patients with cirrhosis. Gastroenterology. 2000;118:905-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Goel A, Christian CL. Gastric antral vascular ectasia (watermelon stomach) in a patient with Sjögren’s syndrome. J Rheumatol. 2003;30:1090-1092. [PubMed] [Cited in This Article: ] |
| 20. | Archimandritis A, Tsirantonaki M, Tzivras M, Hatzis G, Davaris P. Watermelon stomach in a patient with vitiligo and systemic lupus erythematosus. Clin Exp Rheumatol. 1996;14:227-228. [PubMed] [Cited in This Article: ] |
| 21. | Elkayam O, Oumanski M, Yaron M, Caspi D. Watermelon stomach following and preceding systemic sclerosis. Semin Arthritis Rheum. 2000;30:127-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Payen JL, Calès P. [Gastric modifications in cirrhosis]. Gastroenterol Clin Biol. 1991;15:285-295. [PubMed] [Cited in This Article: ] |
| 23. | Jabbari M, Cherry R, Lough JO, Daly DS, Kinnear DG, Goresky CA. Gastric antral vascular ectasia: the watermelon stomach. Gastroenterology. 1984;87:1165-1170. [PubMed] [Cited in This Article: ] |
| 24. | Lee FI, Costello F, Flanagan N, Vasudev KS. Diffuse antral vascular ectasia. Gastrointest Endosc. 1984;30:87-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 49] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Tobin RW, Hackman RC, Kimmey MB, Durtschi MB, Hayashi A, Malik R, McDonald MF, McDonald GB. Bleeding from gastric antral vascular ectasia in marrow transplant patients. Gastrointest Endosc. 1996;44:223-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Arendt T, Barten M, Lakner V, Arendt R. Diffuse gastric antral vascular ectasia: cause of chronic gastrointestinal blood loss. Endoscopy. 1987;19:218-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Fuccio L, Zagari RM, Serrani M, Eusebi LH, Grilli D, Cennamo V, Laterza L, Asioli S, Ceroni L, Bazzoli F. Endoscopic argon plasma coagulation for the treatment of gastric antral vascular ectasia-related bleeding in patients with liver cirrhosis. Digestion. 2009;79:143-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Quintero E, Pique JM, Bombi JA, Bordas JM, Sentis J, Elena M, Bosch J, Rodes J. Gastric mucosal vascular ectasias causing bleeding in cirrhosis. A distinct entity associated with hypergastrinemia and low serum levels of pepsinogen I. Gastroenterology. 1987;93:1054-1061. [PubMed] [Cited in This Article: ] |
| 29. | Charneau J, Petit R, Calès P, Dauver A, Boyer J. Antral motility in patients with cirrhosis with or without gastric antral vascular ectasia. Gut. 1995;37:488-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 63] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Lowes JR, Rode J. Neuroendocrine cell proliferations in gastric antral vascular ectasia. Gastroenterology. 1989;97:207-212. [PubMed] [Cited in This Article: ] |
| 31. | Saperas E, Perez Ayuso RM, Poca E, Bordas JM, Gaya J, Pique JM. Increased gastric PGE2 biosynthesis in cirrhotic patients with gastric vascular ectasia. Am J Gastroenterol. 1990;85:138-144. [PubMed] [Cited in This Article: ] |
| 32. | Watson M, Hally RJ, McCue PA, Varga J, Jiménez SA. Gastric antral vascular ectasia (watermelon stomach) in patients with systemic sclerosis. Arthritis Rheum. 1996;39:341-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | He D, Zeng C, Woods K, Zhong L, Turner D, Busch RK, Brinkley BR, Busch H. CENP-G: a new centromeric protein that is associated with the alpha-1 satellite DNA subfamily. Chromosoma. 1998;107:189-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Garcia MC, Zhou J, Henning D, Arnett FC, Valdez BC. Unique epitopes in RNA helicase II/Gu protein recognized by serum from a watermelon stomach patient. Mol Immunol. 2000;37:351-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Valdez BC, Henning D, Busch RK, Woods K, Flores-Rozas H, Hurwitz J, Perlaky L, Busch H. A nucleolar RNA helicase recognized by autoimmune antibodies from a patient with watermelon stomach disease. Nucleic Acids Res. 1996;24:1220-1224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Vincent C, Pomier-Layrargues G, Dagenais M, Lapointe R, Létourneau R, Roy A, Paré P, Huet PM. Cure of gastric antral vascular ectasia by liver transplantation despite persistent portal hypertension: a clue for pathogenesis. Liver Transpl. 2002;8:717-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Novitsky YW, Kercher KW, Czerniach DR, Litwin DE. Watermelon stomach: pathophysiology, diagnosis, and management. J Gastrointest Surg. 2003;7:652-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | van Cutsem E, Rutgeerts P, Vantrappen G. Treatment of bleeding gastrointestinal vascular malformations with oestrogen-progesterone. Lancet. 1990;335:953-955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 209] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 39. | Manning RJ. Estrogen/progesterone treatment of diffuse antral vascular ectasia. Am J Gastroenterol. 1995;90:154-156. [PubMed] [Cited in This Article: ] |
| 40. | Schoonbroodt D, Horsmans Y, Hoang P, Poncelet-Maton E, Laka A, Geubel A. [Vascular gastric anomalies, CREST syndrome and primary biliary cirrhosis: efficacy of ethinyl estradiol-norethisterone combination]. Gastroenterol Clin Biol. 1994;18:649-651. [PubMed] [Cited in This Article: ] |
| 41. | Moss SF, Ghosh P, Thomas DM, Jackson JE, Calam J. Gastric antral vascular ectasia: maintenance treatment with oestrogen-progesterone. Gut. 1992;33:715-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Tran A, Villeneuve JP, Bilodeau M, Willems B, Marleau D, Fenyves D, Parent R, Pomier-Layrargues G. Treatment of chronic bleeding from gastric antral vascular ectasia (GAVE) with estrogen-progesterone in cirrhotic patients: an open pilot study. Am J Gastroenterol. 1999;94:2909-2911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, Ernster VL, Cummings SR. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016-1037. [PubMed] [Cited in This Article: ] |
| 44. | Nardone G, Rocco A, Balzano T, Budillon G. The efficacy of octreotide therapy in chronic bleeding due to vascular abnormalities of the gastrointestinal tract. Aliment Pharmacol Ther. 1999;13:1429-1436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Barbara G, De Giorgio R, Salvioli B, Stanghellini V, Corinaldesi R. Unsuccessful octreotide treatment of the watermelon stomach. J Clin Gastroenterol. 1998;26:345-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Park RH, Danesh BJ, Upadhyay R, Howatson AG, Lee FD, Russell RI. Gastric antral vascular ectasia (watermelon stomach)--therapeutic options. Postgrad Med J. 1990;66:720-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | McCormick PA, Ooi H, Crosbie O. Tranexamic acid for severe bleeding gastric antral vascular ectasia in cirrhosis. Gut. 1998;42:750-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Woo KS, Tse LK, Woo JL, Vallance-Owen J. Massive pulmonary thromboembolism after tranexamic acid antifibrinolytic therapy. Br J Clin Pract. 1989;43:465-466. [PubMed] [Cited in This Article: ] |
| 49. | Lorenzi AR, Johnson AH, Davies G, Gough A. Gastric antral vascular ectasia in systemic sclerosis: complete resolution with methylprednisolone and cyclophosphamide. Ann Rheum Dis. 2001;60:796-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Gostout CJ, Ahlquist DA, Radford CM, Viggiano TR, Bowyer BA, Balm RK. Endoscopic laser therapy for watermelon stomach. Gastroenterology. 1989;96:1462-1465. [PubMed] [Cited in This Article: ] |
| 51. | Sargeant IR, Loizou LA, Rampton D, Tulloch M, Bown SG. Laser ablation of upper gastrointestinal vascular ectasias: long term results. Gut. 1993;34:470-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Potamiano S, Carter CR, Anderson JR. Endoscopic laser treatment of diffuse gastric antral vascular ectasia. Gut. 1994;35:461-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Bourke MJ, Hope RL, Boyd P, Gillespie PE, Ward M, Cowen AE, Williams SJ. Endoscopic laser therapy for watermelon stomach. J Gastroenterol Hepatol. 1996;11:832-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Mathou NG, Lovat LB, Thorpe SM, Bown SG. Nd: YAG laser induces long-term remission in transfusion-dependent patients with watermelon stomach. Lasers Med Sci. 2004;18:213-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Liberski SM, McGarrity TJ, Hartle RJ, Varano V, Reynolds D. The watermelon stomach: long-term outcome in patients treated with Nd: YAG laser therapy. Gastrointest Endosc. 1994;40:584-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Geller A, Gostout CJ, Balm RK. Development of hyperplastic polyps following laser therapy for watermelon stomach. Gastrointest Endosc. 1996;43:54-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Bernstein CN, Pettigrew N, Wang KK, Greenberg H, Lipschitz J. Multifocal gastric neoplasia after recurrent laser therapy for the watermelon stomach. Can J Gastroenterol. 1997;11:403-406. [PubMed] [Cited in This Article: ] |
| 58. | Wahab PJ, Mulder CJ, den Hartog G, Thies JE. Argon plasma coagulation in flexible gastrointestinal endoscopy: pilot experiences. Endoscopy. 1997;29:176-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 130] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 59. | Probst A, Scheubel R, Wienbeck M. Treatment of watermelon stomach (GAVE syndrome) by means of endoscopic argon plasma coagulation (APC): long-term outcome. Z Gastroenterol. 2001;39:447-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Roman S, Saurin JC, Dumortier J, Perreira A, Bernard G, Ponchon T. Tolerance and efficacy of argon plasma coagulation for controlling bleeding in patients with typical and atypical manifestations of watermelon stomach. Endoscopy. 2003;35:1024-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Schmeck-Lindenau HJ, Kurtz W, Heine M. Inflammatory polyps: an unreported side effect of argon plasma coagulation. Endoscopy. 1998;30:S93-S94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Yusoff I, Brennan F, Ormonde D, Laurence B. Argon plasma coagulation for treatment of watermelon stomach. Endoscopy. 2002;34:407-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | Sebastian S, McLoughlin R, Qasim A, O’Morain CA, Buckley MJ. Endoscopic argon plasma coagulation for the treatment of gastric antral vascular ectasia (watermelon stomach): long-term results. Dig Liver Dis. 2004;36:212-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Cho S, Zanati S, Yong E, Cirocco M, Kandel G, Kortan P, May G, Marcon N. Endoscopic cryotherapy for the management of gastric antral vascular ectasia. Gastrointest Endosc. 2008;68:895-902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Sinha SK, Udawat HP, Varma S, Lal A, Rana SS, Bhasin DK. Watermelon stomach treated with endoscopic band ligation. Gastrointest Endosc. 2006;64:1028-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Kumar R, Mohindra S, Pruthi HS. Endoscopic band ligation: a novel therapy for bleeding gastric antral vascular ectasia. Endoscopy. 2007;39 Suppl 1:E56-E57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Wells CD, Harrison ME, Gurudu SR, Crowell MD, Byrne TJ, Depetris G, Sharma VK. Treatment of gastric antral vascular ectasia (watermelon stomach) with endoscopic band ligation. Gastrointest Endosc. 2008;68:231-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 68. | Gross SA, Al-Haddad M, Gill KR, Schore AN, Wallace MB. Endoscopic mucosal ablation for the treatment of gastric antral vascular ectasia with the HALO90 system: a pilot study. Gastrointest Endosc. 2008;67:324-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |