Published online Nov 16, 2012. doi: 10.4253/wjge.v4.i11.479
Revised: October 5, 2012
Accepted: October 26, 2012
Published online: November 16, 2012
Pancreatic fluid collections (PFCs) develop secondary to either fluid leakage or liquefaction of pancreatic necrosis following acute pancreatitis, chronic pancreatitis, surgery or abdominal trauma. Pancreatic fluid collections include acute fluid collections, acute and chronic pancreatic pseudocysts, pancreatic abscesses and pancreatic necrosis. Before the introduction of linear endoscopic ultrasound (EUS) in the 1990s and the subsequent development of endoscopic ultrasound-guided drainage (EUS-GD) procedures, the available options for drainage in symptomatic PFCs included surgical drainage, percutaneous drainage using radiological guidance and conventional endoscopic transmural drainage. In recent years, it has gradually been recognized that, due to its lower morbidity rate compared to the surgical and percutaneous approaches, endoscopic treatment may be the preferred first-line approach for managing symptomatic PFCs. Endoscopic ultrasound-guided drainage has the following advantages, when compared to other alternatives such as surgical, percutaneous and non-EUS-guided endoscopic drainage. EUS-GD is less invasive than surgery and therefore does not require general anesthesia. The morbidity rate is lower, recovery is faster and the costs are lower. EUS-GD can avoid local complications related to percutaneous drainage. Because the endoscope is placed adjacent to the fluid collection, it can have direct access to the fluid cavity, unlike percutaneous drainage which traverses the abdominal wall. Complications such as bleeding, inadvertent puncture of adjacent viscera, secondary infection and prolonged periods of drainage with resultant pancreatico-cutaneous fistulae may be avoided. The only difference between EUS and non-EUS drainage is the initial step, namely, gaining access to the pancreatic fluid collection. All the subsequent steps are similar, i.e., insertion of guide-wires with fluoroscopic guidance, balloon dilatation of the cystogastrostomy and insertion of transmural stents or nasocystic catheters. With the introduction of the EUS-scope equipped with a large operative channel which permits drainage of the PFCs in “one step”, EUS-GD has been increasingly carried out in many tertiary care centers and has expanded the safety and efficacy of this modality, allowing access to and drainage of overly challenging fluid collections. However, the nature of the PFCs determines the outcome of this procedure. The technique and review of current literature regarding EUS-GD of PFCs will be discussed.
- Citation: Fabbri C, Luigiano C, Maimone A, Polifemo AM, Tarantino I, Cennamo V. Endoscopic ultrasound-guided drainage of pancreatic fluid collections. World J Gastrointest Endosc 2012; 4(11): 479-488
- URL: https://www.wjgnet.com/1948-5190/full/v4/i11/479.htm
- DOI: https://dx.doi.org/10.4253/wjge.v4.i11.479
Pancreatic fluid collections (PFCs) develop secondary to either fluid leakage or liquefaction of pancreatic necrosis following acute pancreatitis, chronic pancreatitis, surgery or abdominal trauma[1,2]. PFCs include acute fluid collections, acute and chronic pancreatic pseudocysts, pancreatic abscesses and pancreatic necrosis (Table 1). Up to a few years ago, drainage was recommended if the PFCs were larger than 6 cm, continued to increase in size or did not resolve after 6 wk as well as in asymptomatic patients, in order to avoid subsequent development of complications such as hemorrhage, perforation or secondary infections. Presently, drainage is recommended only for symptomatic collections. Symptomatic PFCs, presenting with pain and mechanical obstruction of the gastric outlet or biliary system, require drainage. Drainage of pancreatic abscesses and infected necrosis is required for the effective control of sepsis[3,4].
| Term | Definition |
| Acute fluid collection | A collection of enzyme-rich pancreatic juice occurring early (within 48 h) in the course of acute pancreatitis, located in or near the pancreas and always lacking a well-defined wall of granulation tissue or fibrous tissue |
| Acute pseudocyst | A collection of pancreatic juice enclosed by a wall of nonepithelialized granulation tissue, arising as a consequence of acute pancreatitis, requiring at least 4 wk to form and devoid of significant solid debris |
| Chronic pseudocyst | A collection of pancreatic juice enclosed by a wall of fibrous or granulation tissue, arising as a consequence of chronic pancreatitis |
| Early pancreatic necrosis | A diffuse or focal area of nonviable pancreatic parenchyma greater than 30% of the gland by CT-scan, typically associated with peripancreatic fat necrosis |
| Late organized pancreatic necrosis | Evolution of acute necrosis to a partially encapsulated, well-defined collection of pancreatic juice and necrotic debris |
| Pancreatic abscess | A circumscribed intra-abdominal collection of pus, usually in proximity to the pancreas, containing little or no pancreatic necrosis, arising as a consequence of acute pancreatitis or pancreatic trauma |
In recent years, it has gradually been recognized that, due to its lower morbidity rate compared to the surgical and percutaneous approaches, endoscopic treatment may be the preferred first-line approach for managing symptomatic PFCs[1,2,4]. Endoscopic ultrasound-guided drainage (EUS-GD) has the following advantages, when compared to other alternatives such as surgical, percutaneous and non-EUS-guided endoscopic drainage[4,5].
EUS-GD is less invasive than surgery and therefore does not require general anesthesia. The morbidity rate is lower, recovery is faster and the costs are lower[4,5]. EUS-GD can avoid local complications related to percutaneous drainage. Because the endoscope is placed adjacent to the fluid collection, it can have direct access to the fluid cavity, unlike percutaneous drainage which traverses the abdominal wall. Complications such as bleeding, inadvertent puncture of adjacent viscera, secondary infection and prolonged periods of drainage with resultant pancreatico-cutaneous fistulae may be avoided. In addition, it is not possible to remove solid necrotic debris through percutaneous drainage, whereas endoscopic necrosectomy may be performed via a transmural approach[4].
The only difference between EUS and non-EUS drainage is the initial step, namely, gaining access to the pancreatic fluid collection. All the subsequent steps are similar, i.e., insertion of guide-wires with fluoroscopic guidance, balloon dilatation of the cystogastrostomy, insertion of transmural stents or nasocystic catheters and endoscopic necrosectomy.
The specific advantages of using EUS-GD include: (1) EUS can distinguish PFCs from masqueraders as cystic tumors, the gallbladder, lymphoceles, true cysts and pseudoaneurysm; (2) EUS can determine the content of the PFC, such as whether it is a simple abscess or if significant necrotic debris is present, which would then require a more aggressive endoscopic approach; (3) EUS can identify interposed blood vessels and potentially reduce the risk of bleeding; (4) EUS can determine the distance between the PFC cavity and gut wall, thus potentially decreasing the risk of perforation; and (5) EUS permits drainage of non-bulging PFCs.
Antibiotic prophylaxis is generally administered in order to reduce the risk of infections. An adequate surgical back-up is mandatory. Drainage can be performed with the patient under moderate sedoanalgesia, although it may be helpful to carry out the procedure under general anaesthesia. Fluoroscopy is necessary, even although it is technically feasible to drain a PFC with a single stent using only EUS guidance.
Drainage should be performed using a linear echoendoscope with a working channel of 3.7 mm or 3.8 mm which allows the insertion of a 10 Fr stent or metallic stent. Echoendoscopes with smaller working channels, such as 2.8 mm or 3.2 mm, permit only the insertion of 7 Fr and 8.5 Fr stents, respectively.
If only echoendoscopes with small working channels are available, the echoendoscope can be exchanged with a therapeutic endoscope over the guide-wire and the drainage can be performed using this endoscope following initial EUS-guided puncture and guide-wire placement into the cavity.
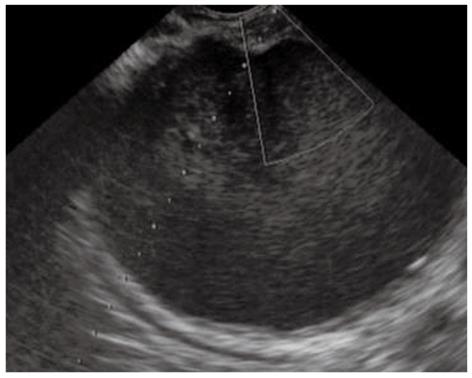
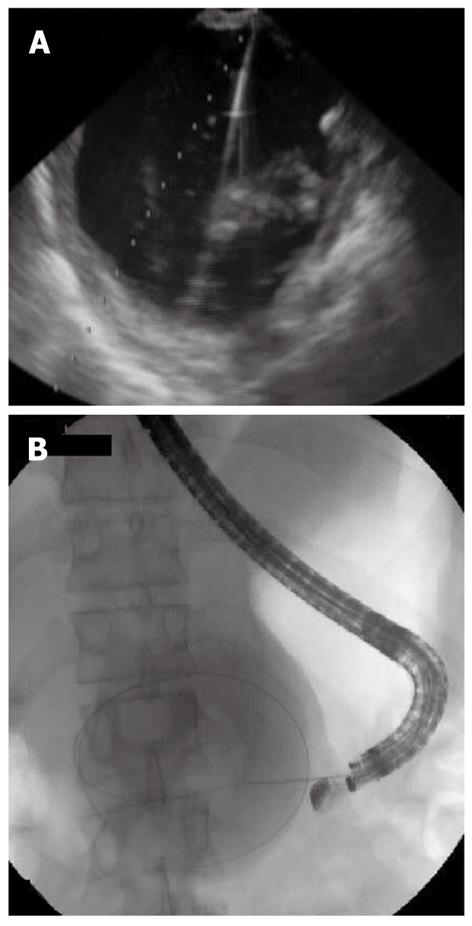
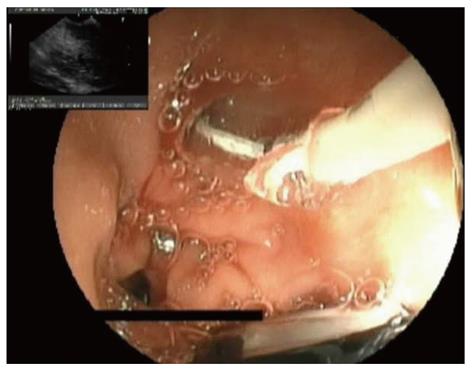
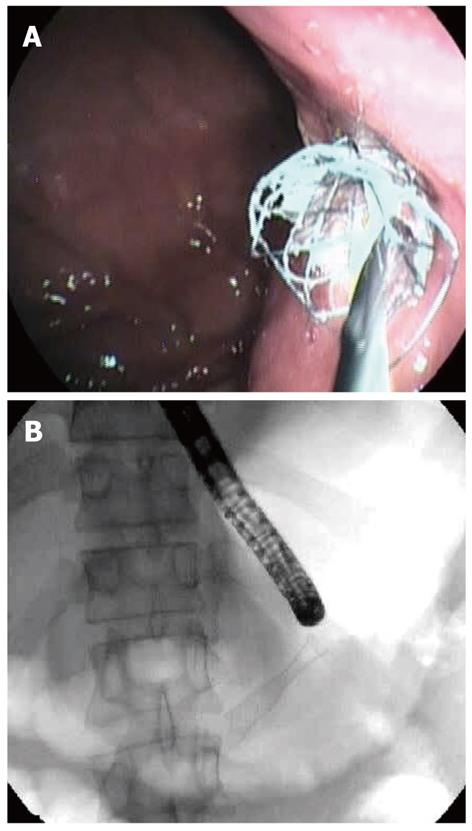
There are several methods for performing EUS-GD of a PFC. The choice of technique is largely based on personal preference and experience, but four general steps are required: (1) Ultrasonographic imagery to identify an appropriate puncture route which has no interposing vessels (Figure 1); (2) Needle puncture of the PFC and insertion of a guide-wire (Figure 2A, B); (3) Dilation of the punctured tract, creating a fistula between gut wall and the PFC (Figure 3); and (4) Insertion of the drainage tubes (Figure 4A, B).
Two different approaches are described, the “single guide-wire approach” and the “double guide-wire approach”[4]. By using the traditional single guide-wire approach, a linear echoendoscope is used to visualize the pancreatic fluid collection; the collection is then punctured with a needle after Doppler US evaluation. Several needles, such as the 19-gauge EUS-fine needle aspiration (FNA) needles, access 19-gauge needle and the cystotome are alternatively employed. A guide-wire is then inserted through the needle into the collection under fluoroscope guidance. Usually, the puncture site is dilated by a balloon catheter to 6 to 8 mm and a double-pigtail transmural stent is then inserted for drainage. When multiple stents or an additional nasocystic catheter is required, the PFC is recannulated by using a catheter and guide-wire, followed by insertion of the second transmural stent or nasocystic catheter.
To circumvent the problem of having to recannulate the PFC after gaining initial transmural access and catheter or transmural stent placement, the concept of a “double-wire” approach, in which 2 guide-wires are inserted through the same catheter before stent placement, has been advocated. Three methods have been described. The initial publication of the procedure used a prototype 3-layer puncture kit which allowed the simultaneous insertion of 2 guide-wires at the initial puncture. This puncture kit consisted of a 6 Fr inner teflon catheter (DuPont, Wilmington, Del) inserted through an outer 8.5 Fr teflon catheter and a 22-gauge FNA needle, which was inserted through the inner catheter. The 6 Fr inner catheter reduces step formation and facilitates the insertion of the assembled kit into the PFC cavity after needle puncture. By using the assembled kit with the needle protruding out at the distal end of the catheter, the PFC is punctured under EUS-guidance, using electrocautery. The assembled inner and outer catheters are then pushed into the cavity. Once entry into the PFC is confirmed by EUS and by aspiration of fluid, the needle and the 6 Fr inner catheter are withdrawn, which leaves behind the 8.5 Fr outer catheter. Two 0.035-inch guide-wires are simultaneously inserted into the PFC cavity. An 8.5 Fr double-pigtail stent and a 7 Fr nasocystic catheter or another stent can be sequentially placed. Other investigators reported inserting either the 10 Fr outer catheter of a cystotome or a 10 Fr Soehendra biliary dilator into the PFC cavity through the single guide-wire inserted at the initial EUS-guided puncture, followed by insertion of a second guide-wire through these catheters. Sequential transmural stent and drainage catheter placement can then be performed without loss of access to the PFC cavity and obviates the need for recannulation, which may be difficult because of a tangential axis of puncture or from poor visibility caused by the fluids flowing from the PFC.
In some cases, EUS-GD fails to reduce the size of the PFC due to the presence of persistent infection with necrotic material. In such cases, endoscopic necrosectomy using a gastroduodenoscope is indicated. A balloon dilator for esophageal strictures is used to dilate the fistula where the stents are inserted. After dilation of the cystogastrostomy or cystoduodenostoma with the balloon dilator, a gastroduodenoscope is directly inserted into the PFC. Mural trabeculation and necrotic substances are then identified in the PFC and necrotic tissue is irrigated by spraying water from a flushing pump and removing the tissue with a basket catheter[4].
One of the most important steps in the development of interventional EUS was taken by Grimm et al[6] in 1992. They created a fistula between the stomach and a cyst with the aid of a linear echoendoscope. Owing to the small working channel of only 2 mm, the EUS-scope had to be exchanged for a regular side-viewing endoscope after a puncture and guide-wire placement in the PFC under EUS guidance. With the introduction of the therapeutic linear EUS-scope with working channels of 3.7 or 3.8 mm, it is now possible to achieve adequate drainage by placing multiple large-bore stents and a nasocystic catheter without changing the endoscope. As a result of these developments, EUS-GD has currently been tested in 1134 published cases and, in experienced hands, is now considered a safe and effective technique for the treatment of PFCs, with a very low complication rate (Table 2)[6-78].
| Ref. | Year | Number of PFCs | Technical success (%) | Clinical success (%) | Complications (%) | Recurrence (%) |
| Grimm et al[6] | 1992 | 1 | 100 | 100 | 0 | 0 |
| Binmoeller et al[7] | 1995 | 27 | 93 | 78 | 52 | 22 |
| Wiersema[8] | 1996 | 1 | 100 | 100 | 0 | 0 |
| Chan et al[9] | 1996 | 1 | 100 | 100 | 0 | 0 |
| Gerolami et al[10] | 1997 | 3 | 100 | 100 | 0 | 0 |
| Ardengh et al[11] | 1998 | 2 | 100 | 100 | 0 | 0 |
| Giovannini et al[12] | 1998 | 6 | 100 | 83 | 0 | 16 |
| Pfaffenbach et al[13] | 1998 | 11 | 91 | 82 | 0 | 18 |
| Vilmann et al[14] | 1998 | 1 | 100 | 100 | 0 | 0 |
| Seifert et al[15] | 2000 | 3 | 100 | 100 | 0 | 0 |
| Seifert et al[16] | 2000 | 6 | 100 | 83 | 0 | 0 |
| Fuchs et al[17] | 2000 | 3 | 100 | 100 | 0 | 0 |
| Baron et al[18] | 2000 | 1 | 100 | 100 | 0 | 0 |
| Wiersema et al[19] | 2001 | 1 | 100 | 100 | 0 | 0 |
| Inui et al[20] | 2001 | 3 | 100 | 100 | 0 | 33 |
| Giovannini et al[21] | 2001 | 35 | 100 | 89 | 3 | 9 |
| Norton et al[22] | 2001 | 14 | 93 | 93 | 14 | 23 |
| Seifert et al[23] | 2001 | 4 | 100 | 75 | 0 | 0 |
| Vosoghi et al[24] | 2002 | 14 | 100 | 93 | 7 | 7 |
| Enya et al[25] | 2003 | 13 | 100 | 85 | 0 | 0 |
| Kakutani et al[26] | 2004 | 1 | 100 | 100 | 0 | 0 |
| Seewald et al[27] | 2005 | 13 | 100 | 85 | 30 | 15 |
| Sriram et al[28] | 2005 | 8 | 100 | 100 | 12 | 0 |
| Benyoumes et al[29] | 2006 | 1 | 100 | 100 | 0 | 0 |
| Raczynski et al[30] | 2006 | 2 | 100 | 100 | 0 | 0 |
| Charnley et al[31] | 2006 | 13 | 100 | 92 | 0 | 0 |
| Hookey et al[32] | 2006 | 32 | 96 | 93 | 11 | 12 |
| Krüger et al[33] | 2006 | 35 | 94 | 88 | 33 | 12 |
| Azar et al[34] | 2006 | 23 | 91 | 82 | 4 | 18 |
| Antillon et al[35] | 2006 | 33 | 94 | 87 | 15 | 4 |
| Kahaleh et al[36] | 2006 | 46 | 100 | 93 | 19 | NA |
| Itoi et al[37] | 2006 | 3 | 100 | 100 | 0 | 0 |
| Rout et al[38] | 2006 | 1 | 100 | 100 | 0 | 0 |
| Seewald et al[39] | 2006 | 8 | 100 | 100 | 0 | 0 |
| Ahlawat et al[40] | 2006 | 11 | 100 | 82 | 18 | 18 |
| Arvanitakis et al[41] | 2007 | 46 | 100 | 94 | 22 | 11 |
| Lopes et al[42] | 2007 | 51 | 94 | 84 | 25 | 17 |
| Jansen et al[43] | 2007 | 8 | 100 | 100 | 0 | 0 |
| Voermans et al[44] | 2007 | 7 | 100 | 100 | 14 | 0 |
| Voermans et al[45] | 2007 | 25 | 100 | 93 | 40 | 7 |
| Kang et al[46] | 2008 | 1 | 100 | 100 | 0 | 0 |
| Escourrou et al[47] | 2008 | 13 | 100 | 100 | 46 | 0 |
| Ardengh et al[48] | 2008 | 77 | 94 | 91 | 6 | 11 |
| Hocke et al[49] | 2008 | 30 | 97 | 83 | 23 | 3 |
| Varadarajulu et al[50] | 2008 | 24 | 100 | 96 | 4 | NA |
| Varadarajulu et al[51] | 2008 | 60 | 95 | 93 | 2 | 4 |
| Barthet et al[52] | 2008 | 28 | 100 | 89 | 25 | NA |
| Jah et al[53] | 2008 | 1 | 100 | 100 | 0 | 0 |
| Reddy et al[54] | 2008 | 6 | 100 | 100 | 16 | 0 |
| Talreja et al[55] | 2008 | 18 | 100 | 95 | 44 | 0 |
| Schrover et al[56] | 2008 | 8 | 100 | 75 | 13 | 0 |
| Mathew et al[57] | 2008 | 6 | 100 | 100 | 16 | 0 |
| Tarantino et al[58] | 2009 | 1 | 100 | 100 | 0 | 0 |
| Park et al[59] | 2009 | 39 | 95 | 100 | 7 | 6 |
| Yasuda et al[60] | 2009 | 26 | 92 | 95 | 0 | 17 |
| Itoi et al[61] | 2009 | 13 | 100 | 100 | 0 | 0 |
| Trevino et al[62] | 2009 | 3 | 100 | 100 | 0 | 0 |
| Varadarajulu et al[63] | 2009 | 10 | 100 | 90 | 0 | 0 |
| Piraka et al[64] | 2009 | 2 | 100 | 100 | 50 | 0 |
| Ang et al[65] | 2009 | 10 | 100 | 100 | 10 | 0 |
| Okabe et al[66] | 2009 | 2 | 100 | 100 | 0 | 0 |
| Antillon et al[67] | 2009 | 1 | 100 | 100 | 0 | 0 |
| Chase et al[68] | 2009 | 1 | 100 | 100 | 0 | 0 |
| Becker et al[69] | 2009 | 7 | 100 | 100 | 57 | 14 |
| Ahn et al[70] | 2010 | 47 | 98 | 100 | 11 | 11 |
| Khashab et al[71] | 2010 | 6 | 100 | NA | 0 | NA |
| Tarantino et al[72] | 2010 | 1 | 100 | 100 | 0 | 0 |
| Koo et al[73] | 2010 | 1 | 100 | 100 | 0 | 0 |
| Pallapothu et al[74] | 2011 | 6 | 100 | 77 | 16 | 16 |
| Jazrawi et al[75] | 2011 | 10 | 100 | 100 | 0 | 10 |
| Larghi et al[76] | 2011 | 1 | 100 | 100 | 0 | 0 |
| Sadik et al[77] | 2011 | 26 | 100 | 88 | 15 | 4 |
| Will et al[78] | 2011 | 132 | 97 | 96 | 29 | 15 |
There are a large number of case reports and case series including PFCs with different characteristics and different methods of drainage and using diverse EUS-scopes and endoscopes.
Recently, a web-based survey was sent to United States and international members of the American Society for Gastrointestinal Endoscopy[79]. Of the 3054 endoscopists to whom the survey was sent, 266 (8.7%) replied: 198 performed pseudocyst drainage [103 (52%)] and the transgastric route was the most commonly used drainage route (65%). The number of stents placed ranged from 1 to 5 and these remained in place for 2 to 30 wk. A CT-scan was used before drainage by 95% of all respondents. EUS imaging was used before drainage by 72 of 103 United States endoscopists (70%) compared with 56 of 95 international endoscopists (59%). EUS-guided drainage was used by 56% of United States endoscopists compared with 43% of international endoscopists. The most common site of transmural entry for drainage of collections appears to be the transgastric route. Although CT-scan is the most commonly used pre-drainage imaging modality, EUS is used before and during transmural drainage of pseudocysts.
However, several nonrandomized case series have suggested that EUS-GD is safer than traditional “blind” techniques[36,80].
Kahaleh et al[36] published the first nonrandomized study which compared endoscopic conventional transmural drainage (CTD) with EUS-GD. In that study, PFCs with bulging and no obvious portal hypertension underwent conventional transmural drainage, while all remaining patients underwent EUS-GD. There were no significant differences between the groups in terms of efficacy or safety. Indirectly, this study supported the concept that EUS-GD is superior, because it can be used to drain PFCs not amenable to CTD, without any increased risks.
Varadarajulu et al[80], in a nonrandomised study, evaluated the CTD with EUS-GD of PFCs in 53 patients. CTD was successful in only 30 patients (57%). To achieve successful drainage, luminal compression was required and at least five puncture attempts were made, potentially increasing the complication risk. On the other hand, EUS allowed a diagnosis of mucinous tumor to be made in two patients and was successful in all cases. Bleeding occurred in one patient who underwent CTD, whereas no complications occurred among those who underwent EUS-GD.
To date only two randomized trials have been published comparing the CTD with EUS-GD of PFCs[50,59]. Varadarajulu et al[50] in 2008 published the first prospective randomized trial to compare the rate of technical success between EUS-GD and CTD of PFCs. Thirty patients were randomised to undergo PFC drainage by EUS (15) or CTD (15) over a 6 mo period. Of the 15 patients randomized to EUS, drainage was not undertaken in one because an alternative diagnosis of biliary cystadenoma was made and this patient was excluded. All 14 patients randomised to an EUS underwent successful drainage (100%), while the procedure was technically successful in only 5 of 15 patients (33%) randomized to CTD. All 10 patients who failed drainage by CTD underwent successful drainage of the PFC on a crossover to EUS. Major procedure-related bleeding was encountered in 2 patients in whom CTD was performed.
Park et al[59] conducted a prospective randomised trial to compare the technical success and clinical outcomes of EUS-GD and CTD for treating pancreatic pseudocysts. A total of 60 consecutive patients with pancreatic pseudocysts were randomly divided into two groups to undergo either EUS-GD (31) or CTD (29). The rate of technical success of the drainage was significantly higher for the EUS group (94 %) than for the CTD group (72 %) (P = 0.039) in intention-to-treat analysis. In cases where CTD failed (8 patients) because the pseudocysts were nonbulging, a crossover was made to EUS-GD, which was successfully performed in all these patients. Complications occurred in 7 % of the EUS group and in 10% of the CTD group (P = 0.67). During follow-up, pseudocyst resolution was achieved in 97% in the EUS group and in 91 % in the CTD group (P = 0.565).
Varadarajulu et al[81] also published the only experience which compares the clinical outcomes of EUS-GD with surgical cyst-gastrostomy for the management of patients with uncomplicated PFCs and a cost analysis of each treatment modality was also performed. Ten patients who underwent surgical cyst-gastrostomy were matched with 20 patients who underwent an EUS-GD. There were no significant differences in rates of treatment success (100% vs 95%), procedural complications (none in either cohort) or reinterventions (10% vs 0%) between surgery and EUS-GD. The mean length of a post-procedure hospital stay for the EUS group was shorter than for the surgical group (2.5 d vs 6.5 d) and the average direct cost per case for EUS-GD was significantly lower when compared with surgical cyst-gastrostomy.
These studies showed that when EUS-GD is performed, the rate of iatrogenic hemorrhage and perforation is lower and the success rate is markedly higher.
Portal hypertension with gastric varices was traditionally a contraindication for endoscopic drainage because of the possibility of iatrogenic hemorrhage. However, in the series by Antillon et al[35], 24% of patients treated had perigastric varices and other groups have also reported a similar ability to perform transenteric drainage in the setting of portal hypertension and intervening perigastric vessels using endoscopic ultrasound-guided drainage[28].
The technical and clinical success mean rates reported for EUS-GD of PFCs in series with more than 10 patients were 97% and 91% respectively and the mean overall recurrence rate was 9%[6-78].
Pancreatic fluid collections that arise in the setting of acute pancreatitis tend to respond better to endoscopic drainage than those arising in patients with chronic pancreatitis; however, in one study, higher success rates were actually seen in those patients with chronic pancreatitis (92% vs 74%), with the difference potentially related to timing of drainage in the acute pancreatitis group[82-84].
For PFCs which contain a clear fluid, such as pseudocysts, the treatment success rates are very high, exceeding 95% and even reaching 100%, while for PFCs in which at EUS the contents can appear to be completely anechoic, with nonfluid/hyperechoic material and a homogenous layer (probably debris) or as focal lesions consistent with necrotic tissue, such as pancreatic abscesses or walled-off pancreatic necrosis, the results for clinical resolution are generally poorer than pseudocyst.
Hookey et al[32] compared etiologies, drainage techniques and outcomes in 116 patients (32 EUS-GD) who underwent endoscopic drainage of PFCs. Of the 116 patients, 8 patients had pancreatic necrosis and 9 had pancreatic abscesses. In this study, drainage of organized necrosis was associated with a significantly higher failure rate than other collections. Drainage of necrosis resulted in clinical success in only 25% of cases and technical success in 50%. Six of eight patients had a nasocystic catheter placed and one patient experienced recurrence. There were two procedure-related complications in this subgroup. Nine patients underwent endoscopic drainage for pancreatic abscesses. Seven of nine patients had a nasocystic catheter placed. All procedures were technically successful and eight of nine (88.9%) patients had clinical success. One abscess recurred and there were no procedure related complications.
Seifert et al[15] were the first group to describe the combination of EUS-GD transmural puncture in necrotizing pancreatitis or abscess followed by tract dilation and repeated, direct endoscopic debridements of the lesser sac. In this series, fenestration of the gastric wall and debridement of infected necrosis by direct retroperitoneal endoscopy was performed on three patients. This strategy led to rapid clinical improvement and no serious complications.
In 2001, Giovannini et al[21] reported their experience with EUS-guided drainage of pancreatic pseudocysts and pancreatic abscesses in 35 patients. Twenty of these patients had pancreatic abscesses, located either in the tail of the pancreas (17 patients) or adjacent to the gastric wall (3 patients). Placement of a 7 Fr nasocystic drain was successful in 18 of 20 patients. The remaining two patients required surgery. Over a mean follow-up period of 27 mo, two relapses occurred.
In 2005, Seewald et al[27] performed a retrospective study of the outcome of patients with pancreatic necrosis and abscesses, all unfit to undergo surgery. The treatment included synchronous EUS-GD procedures followed by balloon dilation of the cystogastrostomy or cystoduodenostoma, daily endoscopic necrosectomy and saline solution washing, and sealing of pancreatic fistulae by N-butyl-2-cyanoacrylate. This study was performed over a 7 year period with 13 consecutive patients, 5 with infected pancreatic necrosis and 8 with pancreatic abscesses. Endoscopic therapy was successful in resolving the infected necrosis or abscess in 12 of 13 patients over a median follow-up period of 9.5 mo. One patient required additional surgery to evacuate necrosis that extended into the paracolic gutter. Two patients with a disconnected duct gland syndrome developed recurrent fluid collections after 2 and 4 mo. These patients ultimately required pancreatic head resections. Two patients had their persistent ductal leaks glued. Complications included three episodes of locally controlled bleeding. The median number of daily necrosectomy and lavage was 7 (range 2-23) and 12 (range 2-41), respectively.
In 2007, Lopes et al[42] performed a retrospective analysis of 51 patients who underwent EUS-GD of PFCs. Twenty-six of these patients had pancreatic abscesses. What is notable in this study regarding pancreatic abscesses is that the endoscopic approach was not more hazardous for abscesses in regard to the complications rate when compared to other pancreatic fluid collections. Placement of a nasocystic drain did not reduce the complication rate but the placement of two stents narrowed the rate of complications.
Recently, Sadik et al[77] compared the outcome for EUS-GD of clear fluid pancreatic pseudocysts (15 patients) with the outcome for abscess drainage (10 patients). The EUS-GD drainage was successful in 94% of the pseudocysts and in 80% of the abscesses (P = 0.04). The complication rate in pseudocysts was 6% and in abscesses was 30% (P = 0.02).
Will et al[78] have published the largest series reported in the literature with 81 abscess and 34 infected necrosis drained transluminally with EUS, with an overall clinical success rate of 97% and 94% and a recurrence rate of 16% and 18%, respectively.
However, EUS-GD for PFCs presents some challenges and disadvantages. One of the challenges encountered during EUS-GD, especially in infected PFCs, is the process of sequential transgastric stenting and nasocystic catheter placement, which may be difficult because of the collapse of the cystic cavity, the presence of a notable quantity of fluid or pus being emitted by the cavity which obscures the endoscopic view and the tangential axis of the punctured tract[39].
Other challenges encountered, especially when the content of the collection is non-fluid, are: (1) the small diameter of the 10 Fr plastic stents used which limits the efficacy in draining; (2) the need, in some cases, to place more stents for drainage, which has been associated with the need for multiple revisions in 17.7% to 27% of cases due to obstruction; and (3) when the placement of a naso-cystic catheter is required, patient discomfort and dislodgement of the catheter are often reported[3,4].
For the above reasons, the type of stent used for endoscopic drainage is currently a major area of interest. In a small number of cases, covered self-expandable metal stents (CSEMSs), with different diameters and different endoscopic techniques of placement, have recently been adopted for drainage[55,58,67,72].
Talreja et al[55] evaluated the efficacy and safety of transenteric drainage of PFCs by using CSEMSs. In that study, 18 patients underwent drainage of PFCs and a median of 1 session was required to achieve drainage. The technical and clinical success rates were 100% and 95%, respectively; with 14 patients (78%) achieving complete resolution of their PFC. The mean follow-up period until final resolution was 77 d and complications included superinfection (5), bleeding (2) and inner migration (1).
Antillon et al[67] report a case in which they used a large diameter removable metallic esophageal stent to facilitate drainage of infected pancreatic necrosis after multiple failed conventional necrosectomies.
A CSEMS can be an alternative to conventional drainage with plastic stents because it offers the option of a larger diameter access fistula for drainage and may increase the final success rate while it reduces the time to PFC resolution. A larger prospective randomised study should be carried out to compare this technique with conventional drainage with plastic stents in order to validate these findings.
The main potential complications of concern are superinfection, bleeding and perforation.
The complication rate reported ranges between 0% and 52% (Table 2), with a mean overall complication rate of 15% in series with more than 10 patients[6-78].
To minimise risk, only collections with a mature wall and within 1 cm of the gastrointestinal lumen should undergo endoscopic drainage. Any coagulopathy, if present, should be corrected. Patients with pseudocysts undergoing drainage should also receive prophylactic antibiotics in order to prevent secondary infection of a sterile collection.
The risk of complication is low thus far in the context of EUS-GD of PFCs without endoscopic necrosectomy and debridement. Perforation rates ranging between 3%-5% were reported in the context of endoscopic necrosectomy[45,49,78]. The risk can be reduced by adhering to key principles, such as draining only a collection with a mature wall, performing stepwise balloon dilatation of the cystogastrostomy, avoiding over-insufflations of the cavity with air and performing gentle debridement using saline lavage and aspiration, baskets, soft snares and retrieval nets when required.
In conclusion, the availability of curved linear array echoendoscopes has resulted in EUS-GD of PFCs as a credible alternative to drainage via the surgical or percutaneous route. The development of new instruments and devices is the basis for alternative less invasive approaches to various pathologies. Further progress in instrumentation is required to make this technique safer and more effective. In the meantime, the endoscopic approach should be dictated by local expertise and individual patient presentation.
In addition, it must be recognized that not all endosonographers have the technical expertise to perform such complex procedures. Apart from the ability to perform linear EUS, a background in endoscopic retrograde cholangiopancreatography is important and additional exposure and specific training are required. EUS-GD is mandatory for non-bulging PFCs and in high-risk patients, such as those with portal hypertension.
Peer reviewers: Viktor Ernst Eysselein, MD, Professor of Medicine, Division of Gastroenterology, Harbor-UCLA Medical Center, 1000 W. Carson Street, Box 483, Torrance, CA 90509, United States; Fauze Maluf-Filho, MD, Hospital das Clínicas, São Paulo University School of Medicine, 488 Olegario Mariano, São Paulo 05508-010, Brazil
S- Editor Song XX L- Editor Roemmele A E- Editor Zhang DN
| 1. | Jacobson BC, Baron TH, Adler DG, Davila RE, Egan J, Hirota WK, Leighton JA, Qureshi W, Rajan E, Zuckerman MJ. ASGE guideline: The role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas. Gastrointest Endosc. 2005;61:363-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Habashi S, Draganov PV. Pancreatic pseudocyst. World J Gastroenterol. 2009;15:38-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 174] [Cited by in F6Publishing: 154] [Article Influence: 10.3] [Reference Citation Analysis (4)] |
| 3. | Chak A. Endosonographic-guided therapy of pancreatic pseudocysts. Gastrointest Endosc. 2000;52:S23-S27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Seewald S, Ang TL, Kida M, Teng KY, Soehendra N. EUS 2008 Working Group document: evaluation of EUS-guided drainage of pancreatic-fluid collections (with video). Gastrointest Endosc. 2009;69:S13-S21. [PubMed] [Cited in This Article: ] |
| 5. | Seewald S, Ang TL, Teng KY, Groth S, Zhong Y, Richter H, Imazu H, Omar S, Polese L, Seitz U. Endoscopic ultrasound-guided drainage of abdominal abscesses and infected necrosis. Endoscopy. 2009;41:166-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Grimm H, Binmoeller KF, Soehendra N. Endosonography-guided drainage of a pancreatic pseudocyst. Gastrointest Endosc. 1992;38:170-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 155] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Binmoeller KF, Soehendra N. Endoscopic ultrasonography in the diagnosis and treatment of pancreatic pseudocysts. Gastrointest Endosc Clin N Am. 1995;5:805-816. [PubMed] [Cited in This Article: ] |
| 8. | Wiersema MJ. Endosonography-guided cystoduodenostomy with a therapeutic ultrasound endoscope. Gastrointest Endosc. 1996;44:614-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 89] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Chan AT, Heller SJ, Van Dam J, Carr-Locke DL, Banks PA. Endoscopic cystgastrostomy: role of endoscopic ultrasonography. Am J Gastroenterol. 1996;91:1622-1625. [PubMed] [Cited in This Article: ] |
| 10. | Gerolami R, Giovannini M, Laugier R. Endoscopic drainage of pancreatic pseudocysts guided by endosonography. Endoscopy. 1997;29:106-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Ardengh JC, Della Libera E, Ferrari AP. Endosonography-guided drainage of pancreatic pseudocyst without gastric or duodenal compression. Endoscopy. 1998;30:S71-S72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Giovannini M, Bernardini D, Seitz JF. Cystogastrotomy entirely performed under endosonography guidance for pancreatic pseudocyst: results in six patients. Gastrointest Endosc. 1998;48:200-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 142] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Pfaffenbach B, Langer M, Stabenow-Lohbauer U, Lux G. [Endosonography controlled transgastric drainage of pancreatic pseudocysts]. Dtsch Med Wochenschr. 1998;123:1439-1442. [PubMed] [Cited in This Article: ] |
| 14. | Vilmann P, Hancke S, Pless T, Schell-Hincke JD, Henriksen FW. One-step endosonography-guided drainage of a pancreatic pseudocyst: a new technique of stent delivery through the echo endoscope. Endoscopy. 1998;30:730-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Seifert H, Wehrmann T, Schmitt T, Zeuzem S, Caspary WF. Retroperitoneal endoscopic debridement for infected peripancreatic necrosis. Lancet. 2000;356:653-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 259] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Seifert H, Dietrich C, Schmitt T, Caspary W, Wehrmann T. Endoscopic ultrasound-guided one-step transmural drainage of cystic abdominal lesions with a large-channel echo endoscope. Endoscopy. 2000;32:255-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Fuchs M, Reimann FM, Gaebel C, Ludwig D, Stange EF. Treatment of infected pancreatic pseudocysts by endoscopic ultrasonography-guided cystogastrostomy. Endoscopy. 2000;32:654-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Baron TH, Wiersema MJ. EUS-guided transesophageal pancreatic pseudocyst drainage. Gastrointest Endosc. 2000;52:545-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Wiersema MJ, Baron TH, Chari ST. Endosonography-guided pseudocyst drainage with a new large-channel linear scanning echoendoscope. Gastrointest Endosc. 2001;53:811-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Inui K, Yoshino J, Okushima K, Miyoshi H, Nakamura Y, Watanabe S, Takashima T, Nakazawa S, Hattori T. EUS-guided one-step drainage of pancreatic pseudocysts: experience in 3 patients. Gastrointest Endosc. 2001;54:87-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Giovannini M, Pesenti C, Rolland AL, Moutardier V, Delpero JR. Endoscopic ultrasound-guided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echo endoscope. Endoscopy. 2001;33:473-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 220] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Norton ID, Clain JE, Wiersema MJ, DiMagno EP, Petersen BT, Gostout CJ. Utility of endoscopic ultrasonography in endoscopic drainage of pancreatic pseudocysts in selected patients. Mayo Clin Proc. 2001;76:794-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Seifert H, Faust D, Schmitt T, Dietrich C, Caspary W, Wehrmann T. Transmural drainage of cystic peripancreatic lesions with a new large-channel echo endoscope. Endoscopy. 2001;33:1022-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Vosoghi M, Sial S, Garrett B, Feng J, Lee T, Stabile BE, Eysselein VE. EUS-guided pancreatic pseudocyst drainage: review and experience at Harbor-UCLA Medical Center. MedGenMed. 2002;4:2. [PubMed] [Cited in This Article: ] |
| 25. | Enya M, Yasuda I, Tomita E, Shirakami Y, Otsuji K, Shinoda T, Moriwaki H. Endoscopic ultrasound-guided drainage of pancreatic pseudocysts using a large-channel echoendoscope and a conventional polypectomy snare. Dig Endosc. 2003;15:323-328. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Kakutani H, Hino S, Ikeda K, Uchiyama Y, Sumiyama K, Kuramochi A, Kawamura M, Tajiri H. A case of pancreatic pseudocyst successfully treated with endoscopic cystgastrostomy using a new therapeutic echoendoscope. Dig Endosc. 2004;16:337–339. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Seewald S, Groth S, Omar S, Imazu H, Seitz U, de Weerth A, Soetikno R, Zhong Y, Sriram PV, Ponnudurai R. Aggressive endoscopic therapy for pancreatic necrosis and pancreatic abscess: a new safe and effective treatment algorithm (videos). Gastrointest Endosc. 2005;62:92-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 249] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 28. | Sriram PV, Kaffes AJ, Rao GV, Reddy DN. Endoscopic ultrasound-guided drainage of pancreatic pseudocysts complicated by portal hypertension or by intervening vessels. Endoscopy. 2005;37:231-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Benyoumes M, Deprez PH. A single therapeutic echo endoscope used for combined transgastric and transpapillary drainage of a pancreatic abscess. Endoscopy. 2006;38:98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Raczynski S, Teich N, Borte G, Wittenburg H, Mössner J, Caca K. Percutaneous transgastric irrigation drainage in combination with endoscopic necrosectomy in necrotizing pancreatitis (with videos). Gastrointest Endosc. 2006;64:420-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Charnley RM, Lochan R, Gray H, O'Sullivan CB, Scott J, Oppong KE. Endoscopic necrosectomy as primary therapy in the management of infected pancreatic necrosis. Endoscopy. 2006;38:925-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 32. | Hookey LC, Debroux S, Delhaye M, Arvanitakis M, Le Moine O, Devière J. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63:635-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 253] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 33. | Krüger M, Schneider AS, Manns MP, Meier PN. Endoscopic management of pancreatic pseudocysts or abscesses after an EUS-guided 1-step procedure for initial access. Gastrointest Endosc. 2006;63:409-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Azar RR, Oh YS, Janec EM, Early DS, Jonnalagadda SS, Edmundowicz SA. Wire-guided pancreatic pseudocyst drainage by using a modified needle knife and therapeutic echoendoscope. Gastrointest Endosc. 2006;63:688-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Antillon MR, Shah RJ, Stiegmann G, Chen YK. Single-step EUS-guided transmural drainage of simple and complicated pancreatic pseudocysts. Gastrointest Endosc. 2006;63:797-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Kahaleh M, Shami VM, Conaway MR, Tokar J, Rockoff T, De La Rue SA, de Lange E, Bassignani M, Gay S, Adams RB. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 250] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 37. | Itoi T, Tsuchiya T, Sofuni A, Itokawa F, Kurihara T, Moriyasu F, Kawai T. Endoscopic ultrasonography-guided simultaneous internal and external drainage of infected pancreatic pseudocysts. Dig Endosc. 2006;18:71-74. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Rout S, Rahman SH, Sheridan MB, Guillou PJ, Menon KV. Endoscopic ultrasound guided transgastric stenting of traumatic pancreatic pseudocyst. JOP. 2006;7:423-426. [PubMed] [Cited in This Article: ] |
| 39. | Seewald S, Thonke F, Ang TL, Omar S, Seitz U, Groth S, Zhong Y, Yekebas E, Izbicki J, Soehendra N. One-step, simultaneous double-wire technique facilitates pancreatic pseudocyst and abscess drainage (with videos). Gastrointest Endosc. 2006;64:805-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Ahlawat SK, Charabaty-Pishvaian A, Jackson PG, Haddad NG. Single-step EUS-guided pancreatic pseudocyst drainage using a large channel linear array echoendoscope and cystotome: results in 11 patients. JOP. 2006;7:616-624. [PubMed] [Cited in This Article: ] |
| 41. | Arvanitakis M, Delhaye M, Bali MA, Matos C, De Maertelaer V, Le Moine O, Devière J. Pancreatic-fluid collections: a randomized controlled trial regarding stent removal after endoscopic transmural drainage. Gastrointest Endosc. 2007;65:609-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 42. | Lopes CV, Pesenti C, Bories E, Caillol F, Giovannini M. Endoscopic-ultrasound-guided endoscopic transmural drainage of pancreatic pseudocysts and abscesses. Scand J Gastroenterol. 2007;42:524-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 43. | Jansen JM, Hanrath A, Rauws EA, Bruno MJ, Fockens P. Intracystic wire exchange facilitating insertion of multiple stents during endoscopic drainage of pancreatic pseudocysts. Gastrointest Endosc. 2007;66:157-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Voermans RP, Eisendrath P, Bruno MJ, Le Moine O, Devière J, Fockens P. Initial evaluation of a novel prototype forward-viewing US endoscope in transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2007;66:1013-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Voermans RP, Veldkamp MC, Rauws EA, Bruno MJ, Fockens P. Endoscopic transmural debridement of symptomatic organized pancreatic necrosis (with videos). Gastrointest Endosc. 2007;66:909-916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 46. | Kang SG, Park do H, Kwon TH, Park JY, Park SH, Park JH, Lee SH, Chung IK, Kim HS, Kim SJ. Transduodenal endoscopic necrosectomy via pancreaticoduodenal fistula for infected peripancreatic necrosis with left pararenal space extension (with videos). Gastrointest Endosc. 2008;67:380-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Escourrou J, Shehab H, Buscail L, Bournet B, Andrau P, Moreau J, Fourtanier G. Peroral transgastric/transduodenal necrosectomy: success in the treatment of infected pancreatic necrosis. Ann Surg. 2008;248:1074-1080. [PubMed] [Cited in This Article: ] |
| 48. | Ardengh JC, Coelho DE, Coelho JF, de Lima LF, dos Santos JS, Módena JL. Single-step EUS-guided endoscopic treatment for sterile pancreatic collections: a single-center experience. Dig Dis. 2008;26:370-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Hocke M, Will U, Gottschalk P, Settmacher U, Stallmach A. Transgastral retroperitoneal endoscopy in septic patients with pancreatic necrosis or infected pancreatic pseudocysts. Z Gastroenterol. 2008;46:1363-1368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2008;68:1102-1111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 51. | Varadarajulu S, Tamhane A, Blakely J. Graded dilation technique for EUS-guided drainage of peripancreatic fluid collections: an assessment of outcomes and complications and technical proficiency (with video). Gastrointest Endosc. 2008;68:656-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 52. | Barthet M, Lamblin G, Gasmi M, Vitton V, Desjeux A, Grimaud JC. Clinical usefulness of a treatment algorithm for pancreatic pseudocysts. Gastrointest Endosc. 2008;67:245-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 53. | Jah A, Jamieson N, Huguet E, Griffiths W, Carroll N, Praseedom R. Endoscopic ultrasound-guided drainage of an abdominal fluid collection following Whipple's resection. World J Gastroenterol. 2008;14:6867-6868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Reddy DN, Gupta R, Lakhtakia S, Jalal PK, Rao GV. Use of a novel transluminal balloon accessotome in transmural drainage of pancreatic pseudocyst (with video). Gastrointest Endosc. 2008;68:362-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Talreja JP, Shami VM, Ku J, Morris TD, Ellen K, Kahaleh M. Transenteric drainage of pancreatic-fluid collections with fully covered self-expanding metallic stents (with video). Gastrointest Endosc. 2008;68:1199-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 56. | Schrover IM, Weusten BL, Besselink MG, Bollen TL, van Ramshorst B, Timmer R. EUS-guided endoscopic transgastric necrosectomy in patients with infected necrosis in acute pancreatitis. Pancreatology. 2008;8:271-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | Mathew A, Biswas A, Meitz KP. Endoscopic necrosectomy as primary treatment for infected peripancreatic fluid collections (with video). Gastrointest Endosc. 2008;68:776-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Tarantino I, Barresi L, Fazio V, Di Pisa M, Traina M. EUS-guided self-expandable stent placement in 1 step: a new method to treat pancreatic abscess. Gastrointest Endosc. 2009;69:1401-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Park DH, Lee SS, Moon SH, Choi SY, Jung SW, Seo DW, Lee SK, Kim MH. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy. 2009;41:842-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 60. | Yasuda I, Iwata K, Mukai T, Iwashita T, Moriwaki H. EUS-guided pancreatic pseudocyst drainage. Dig Endosc. 2009;21 Suppl 1:S82-S86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Itoi T, Itokawa F, Tsuchiya T, Kawai T, Moriyasu F. EUS-guided pancreatic pseudocyst drainage: simultaneous placement of stents and nasocystic catheter using double-guidewire technique. Dig Endosc. 2009;21 Suppl 1:S53-S56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Trevino JM, Christein JD, Varadarajulu S. EUS-guided transesophageal drainage of peripancreatic fluid collections. Gastrointest Endosc. 2009;70:793-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Varadarajulu S, Trevino JM, Christein JD. EUS for the management of peripancreatic fluid collections after distal pancreatectomy. Gastrointest Endosc. 2009;70:1260-1265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Piraka C, Shah RJ, Fukami N, Chathadi KV, Chen YK. EUS-guided transesophageal, transgastric, and transcolonic drainage of intra-abdominal fluid collections and abscesses. Gastrointest Endosc. 2009;70:786-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Ang TL, Teo EK, Fock KM. Endoscopic drainage and endoscopic necrosectomy in the management of symptomatic pancreatic collections. J Dig Dis. 2009;10:213-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Okabe Y, Kaji R, Ishida Y, Sakamoto T, Maeda A, Kikuma K, Tsuruta O, Sata M. Trans-gastric endoscopic drainage using a large balloon for pancreatic necrosis and abscess - two case reports. Dig Endosc. 2009;21 Suppl 1:S71-S74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 67. | Antillon MR, Bechtold ML, Bartalos CR, Marshall JB. Transgastric endoscopic necrosectomy with temporary metallic esophageal stent placement for the treatment of infected pancreatic necrosis (with video). Gastrointest Endosc. 2009;69:178-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | Chase MP, Yarze JC, Gumustop B, Leach RP. Endoscopic ultrasound-guided aspiration and oral antibiotic therapy as definitive treatment of an asymptomatic pancreatic abscess. Pancreas. 2009;38:475-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 69. | Becker V, Huber W, Meining A, Prinz C, Umgelter A, Ludwig L, Bajbouj M, Gaa J, Schmid RM. Infected necrosis in severe pancreatitis--combined nonsurgical multi-drainage with directed transabdominal high-volume lavage in critically ill patients. Pancreatology. 2009;9:280-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | Ahn JY, Seo DW, Eum J, Song TJ, Moon SH, Park do H, Lee SS, Lee SK, Kim MH. Single-Step EUS-Guided Transmural Drainage of Pancreatic Pseudocysts: Analysis of Technical Feasibility, Efficacy, and Safety. Gut Liver. 2010;4:524-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 71. | Khashab MA, Lennon AM, Singh VK, Shin EJ, Canto MI, Kalloo AN, Okolo PI, Giday SA. EUS-guided pseudocyst drainage as a one-step procedure using a novel multiple wire insertion technique. Endoscopy. 2010;42 Suppl 2:E292-E293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 72. | Tarantino I, Traina M, Barresi L, Volpes R, Gridelli B. Transgastric plus transduodenal necrosectomy with temporary metal stents placement for treatment of large pancreatic necrosis. Pancreas. 2010;39:269-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Koo JE, Park do H, Oh J, Lee YH, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-Guided Multitransgastric Endoscopic Necrosectomy for Infected Pancreatic Necrosis with Noncontagious Retroperitoneal and Peritoneal Extension. Gut Liver. 2010;4:140-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Pallapothu R, Earle DB, Desilets DJ, Romanelli JR. NOTES(®) stapled cystgastrostomy: a novel approach for surgical management of pancreatic pseudocysts. Surg Endosc. 2011;25:883-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Jazrawi SF, Barth BA, Sreenarasimhaiah J. Efficacy of endoscopic ultrasound-guided drainage of pancreatic pseudocysts in a pediatric population. Dig Dis Sci. 2011;56:902-908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 76. | Larghi A, Seerden TC, Galasso D, Perri V, Uchida N, Carnuccio A, Costamagna G. EUS-guided cystojejunostomy for drainage of a pseudocyst in a patient with Billroth II gastrectomy. Gastrointest Endosc. 2011;73:169-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 77. | Sadik R, Kalaitzakis E, Thune A, Hansen J, Jönson C. EUS-guided drainage is more successful in pancreatic pseudocysts compared with abscesses. World J Gastroenterol. 2011;17:499-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 42] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (2)] |
| 78. | Will U, Wanzar C, Gerlach R, Meyer F. Interventional ultrasound-guided procedures in pancreatic pseudocysts, abscesses and infected necroses - treatment algorithm in a large single-center study. Ultraschall Med. 2011;32:176-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Yusuf TE, Baron TH. Endoscopic transmural drainage of pancreatic pseudocysts: results of a national and an international survey of ASGE members. Gastrointest Endosc. 2006;63:223-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 80. | Varadarajulu S, Wilcox CM, Tamhane A, Eloubeidi MA, Blakely J, Canon CL. Role of EUS in drainage of peripancreatic fluid collections not amenable for endoscopic transmural drainage. Gastrointest Endosc. 2007;66:1107-1119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 81. | Varadarajulu S, Lopes TL, Wilcox CM, Drelichman ER, Kilgore ML, Christein JD. EUS versus surgical cyst-gastrostomy for management of pancreatic pseudocysts. Gastrointest Endosc. 2008;68:649-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 82. | Baron TH, Harewood GC, Morgan DE, Yates MR. Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc. 2002;56:7-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 383] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 83. | Johnson MD, Walsh RM, Henderson JM, Brown N, Ponsky J, Dumot J, Zuccaro G, Vargo J. Surgical versus nonsurgical management of pancreatic pseudocysts. J Clin Gastroenterol. 2009;43:586-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 84. | Morton JM, Brown A, Galanko JA, Norton JA, Grimm IS, Behrns KE. A national comparison of surgical versus percutaneous drainage of pancreatic pseudocysts: 1997-2001. J Gastrointest Surg. 2005;9:15-20; discussion 20-1. [PubMed] [Cited in This Article: ] |