Published online Jun 16, 2021. doi: 10.4253/wjge.v13.i6.161
Peer-review started: January 27, 2021
First decision: April 6, 2021
Revised: April 14, 2021
Accepted: May 21, 2021
Article in press: May 21, 2021
Published online: June 16, 2021
Acute pancreatitis is of one the most common gastroenterology-related indications for hospital admissions worldwide. With the widespread reliance on endoscopic retrograde cholangiopancreatography (ERCP) for the management of pancreaticobiliary conditions, post-ERCP pancreatitis (PEP) has come to represent an important etiology of acute pancreatitis. Despite many studies aiming to better understand the pathogenesis and prevention of this iatrogenic disorder, findings have been heterogeneous, and considerable variation in clinical practice exists. Herein, we review the literature regarding PEP with the goal to raise awareness of this entity, discuss recent data, and present evidence-based best practices. We believe this manuscript will be useful for gastrointestinal endoscopists as well as other specialists involved in the management of patients with PEP.
Core Tip: Post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP) represents an important etiology of acute pancreatitis and is the most common major adverse event post-ERCP. Nevertheless, gaps in knowledge remain, as do large variations in clinical practice. Best practices with respect to the prevention of PEP continue to evolve as new evidence becomes available. Herein, we review the literature regarding PEP to increase awareness of this entity, facilitate best practices in PEP prevention and subsequent management, and ultimately improve clinical outcomes.
- Citation: Weissman S, Ahmed M, Baniqued MR, Ehrlich D, Tabibian JH. Best practices for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. World J Gastrointest Endosc 2021; 13(6): 161-169
- URL: https://www.wjgnet.com/1948-5190/full/v13/i6/161.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i6.161
Acute pancreatitis is an acute, inflammatory disease of the pancreas, responsible for over 100000 hospital admissions annually in the United States[1,2]. It represents a major cause of morbidity and healthcare consumption in the United States and indeed worldwide[1-3]. There are numerous established etiologies of acute pancreatitis, among which gallstones and alcohol are generally the most common[4]. A number of other etiologies have been elucidated and better appreciated over the last several decades, including acute pancreatitis which arises as an adverse event (AE) following endoscopic retrograde cholangiopancreatography (ERCP), i.e. post-ERCP pancreatitis (PEP)[5]. PEP is the most common major AE of ERCP and has garnered significant interest from the biomedical community. However, its pathogenesis has yet to be fully understood, and its clinical management remains heterogeneous[1,6]. Identifying those at high-risk for PEP is critical to formulating an individualized prophylactic and therapeutic approach[6,7]. A multitude of pharmacological and endoscopic measures have been studied to mitigate the risk of PEP[7], include the use of rectal non-steroidal anti-inflammatory drugs (NSAIDs), aggressive intravenous (IV) hydration, and pancreatic duct stenting[8]; which of these is most effective or appropriate, however, remains a subject of ongoing study and debate. Herein, we review the current prophylactic and therapeutic measures for the prevention and management of PEP in attempt to provide evidence-based clinical guidance for best practices.
The pathogenesis of acute pancreatitis is centered around direct acinar cell injury with subsequent activation of proteolytic pancreatic enzymes. Inciting injuries include obstruction (e.g., from stone or tumor), alcohol and other toxins, and trauma, among others[9]. In PEP, activation of inflammatory pathways can occur for multiple reasons, which similarly include mechanical obstruction, direct trauma, or toxic injury[9,10]. When bile duct cannulation is difficult, prolonged papillary manipulation and repeat instrumentation can lead to mechanical injury and edema, impairing flow of pancreatic enzymes from the exocrine pancreas into the small intestine[8]. Electrocautery can also cause edema and similarly impair flow of pancreatic enzymes. Hydrostatic injury can occur secondary to intraductal water or contrast injection[8]. Contrast agents themselves can potentially cause chemical injury (even without significant changes in hydrostatic pressure); however, their role in this regard in the pathogenesis of PEP remains controversial and may depend on the chemical properties of the specific contrast agent[11]. The ensuing sequence of inflammation and recruitment of cytokines can manifest locally or go on to activate a systemic inflammatory response syndrome, resulting in higher severity of acute pancreatitis.
The diagnosis of acute pancreatitis (of any etiology) can be made with at least two of the following three criteria: (1) Typical epigastric abdominal pain (often radiating to the back); (2) Serum pancreatic enzyme levels > 3 × the upper limit of normal; and (3) Imaging findings consistent with acute pancreatitis (Table 1), as indicated by the revised Atlanta classification[8]. Although this criteria will accurately lead to the diagnosis of acute pancreatitis from other etiologies, these criteria are not always accurate in patients following ERCP. As a result of the biliary trauma caused by ERCP, many times these patients will meet two of these criteria but in reality lack acute pancreatitis. Nevertheless, the revised Atlanta criteria has been shown to more accurately predict PEP severity as compared to the consensus criteria[9]. The Cotton criteria used to diagnose PEP was developed in 1991 and has since been modified to specify whether the post-procedural abdominal pain is “new or worsened” (Table 1)[8]. Additional criteria to be classified as mild PEP includes an amylase level > 3 × the upper limit of normal within 24 h post-procedure and any hospitalization of at least 2 d, while moderate disease requires 4-10 d. Severe PEP is characterized by: (1) Hospitalization for > 10 d; (2) The development of a complication (e.g., necrosis/abscess); or (3) The need for intervention (surgery)[8].
| Revised Atlanta classification | Cotton criteria | |
| Mild | Requires 2 out of 3: Epigastric abdominal pain; amylase/lipase > 3 × normal limit; abdominal image findings; no organ failure; no local or systemic complications | New or worsened abdominal pain and amylase > 3 × upper limit of normal within 24 h after the procedure and requiring hospital stay/extension by 2-3 d |
| Moderate | Transient organ failure (resolves within 48 h). Local or systemic complications without persistent organ failure | All the above with requiring 4-10 d hospitalization |
| Severe | Persistent organ failure (> 48 h). Single/multiple organ failure | > 10 d hospitalization or requiring intervention. Development of a complication (pseudocyst, necrosis) or Need for surgical intervention |
Of note, the diagnosis of PEP in the post-ERCP patient can sometimes be challenging, potentially leading to over- or under-diagnosis. In acute pancreatitis, epigastric pain is typically constant and radiates to the back; conversely, bowel distention and painful spasms occurring after ERCP are episodic and fleeting in nature, though the two may be difficult to distinguish. Elevations in serum pancreatic enzyme levels can occur post-ERCP in the absence of abdominal pain or imaging features of acute pancreatitis, rendering routine post-ERCP ordering of these tests of unclear (or no) clinical significance; however, marked elevations of serum amylase and/or lipase > 1000 units/L at two hours after ERCP are highly predictive of PEP[8,10-12]. The adoption of a uniform definition for the diagnosis of PEP will not only aid in its early diagnosis but also impact its subsequent treatment, though an individualized management approach would likely still be needed given the potential nuances of such procedures.
Predicting which patients are at high risk for PEP is crucial. Several factors have been regarded as important predictors of a patient’s risk of developing PEP. These risk factors are additive and can be categorized as: (1) Patient-; (2) Procedure-; or (3) Operator-related[8]. Patient-related risk factors include age (younger and older), female sex, normal serum bilirubin, recurrent pancreatitis, prior PEP, or those with sphincter of Oddi dysfunction[13]. While controversy surrounds age as risk factor for PEP, data have illustrated that pancreatitis in the elderly population could present differently and even be asociated with different outcomes[14,15]. Of note, patients with pancreas divisum may be at higher risk of acute pancreatitis which might influence clinical decision making with regard to the prophylactic measures taken to prevent PEP in this population[16]. Procedure-rated factors include difficult cannulations, pancreatic duct injection, sphincter of Oddi manometry, or precut sphincterotomy. Hospital and endoscopist procedure volume also seems to correlate with outcomes[17]. In fact, a database study involving nearly 200000 ERCPs performed in the inpatient setting found a significantly lower procedural failure rate and shorter length of stay in hospitals performing ≥ 200 ERCPs per year[4]. Additional factors such as pancreatotoxic drugs, biliary stents, or bile duct stones may influence the risk of PEP but their roles are not yet fully established (Table 2)[13].
| Risk factors for post-ERCP pancreatitis by category | ||
| Patient-related | Procedure-related | Operator-related |
| Sphincter of Oddi dysfunction | Pancreatic sphincterotomy | Endoscopist inexperience |
| Age (young or old) | Recent sphincter of Oddi manometry | Lower ERCP case volume |
| Normal bilirubin | Difficult biliary cannulation | Poor fluoroscopic imaging |
| Female sex | Papillary balloon dilation | Aggressive attempts at cannulation |
| History of PEP | Numerous pancreatic duct cannulations | Poor ancillary services |
| History of pancreatitis | Inadvertent/high-pressure pancreatography | Unfamilarity with preventative methods |
Prophylactic measures that may help curtail PEP[18]. Several well-designed meta-analyses have found an association between early needle-knife precutting and lower rates of PEP, as compared to persistent attempts at cannulation[19,20]. A recent study showed that prophylactic pancreatic stenting following a double-guide wire technique reduces the rate of PEP, as double-guidewire technique alone was associated with higher PEP[21]. As such, international endoscopic societies recommend early needle-knife precut sphincterotomy (or papillotomy) and double-guide wire technique with prophylactic pancreatic duct stenting, especially in difficult biliary cannulation, to prevent ERCP-related AEs[2,18,22-29].
The use of IV fluids, in particular aggressive periprocedural IV hydration, has been recommended for the prevention of PEP[18,22]. Two meta-analyses found that the use of aggressive hydration with lactated Ringer’s Solution, 35-45 mL/kg administered over 8-10 h, decreased the incidence of PEP[30,31]. Another more recent study found similar results when comparing aggressive to standard IV hydration[32]. There is evidence that suggests lactated Ringer’s solution may be preferable as compared to normal saline[33,34]. Of note, aggressive hydration should be tempered in patients that are at risk of fluid overload (those with heart failure, anisarca, poor renal function, ascites etc.) and may be less impactful in those that have a prophylactic pancreatic duct stent placed[18].
Numerous pharmacological approaches have been studied as a means to preventing (or decreasing the severity of) PEP. These include: NSAIDs, somatostatin, protease inhibitors, antibiotics, nitrates, heparin, and others. Prophylactic NSAIDs are perhaps the most studied pharmacological tool found to help prevent PEP[35-42]. Indeed, numerous meta-analyses have examined the effect of NSAIDs, and while the overwhelming majority found a significantly lower incidence of PEP — a few found a nonsignificant difference[35-42]. As such, it has been recommended to use 100 mg of diclofenac or indomethacin (per rectum) before ERCP in all patients who do not have a contraindication[18]. Of note, the use of NSAIDs in combination with other pharmacologic measures to prevent PEP is not recommended by the European of society of gastrointestinal endoscopy[18]. However, recommendations from other societies do not support or deny the use of NSAIDs with other pharmacological measures[2,43]. Studies to better understand the role and optimal timing, route, and dose of NSAIDs in this regard are ongoing[44].
Somatostatin is a cyclic peptide that has an inhibitory effect on multiple systems of the body[45]. There are a few studies that have shown that its use is associated with an overall reduction in the incidence of PEP; however, these studies may be biased by a small sample size and have had conflicting results with other studies[18]. Additionally, octreotide, a somatostatin analogue, was shown to have no significant difference in PEP incidence when compared to a placebo, unless used at a dose higher that 0.5 mg[46]. Thus, this somatostatin is not recommended for PEP prophylaxis.
Protease inhibitors can be used to inhibit the activation of proteolytic enzymes that are released from the pancreas and play a role on the pathogenesis of PEP[47]. However, at this time the results of its usefulness in PEP prevention are inconclusive[18]. Notably, a study from 2010 found that the main protease inhibitors, gabexate mesylate and ulinastatin, had no effect on PEP[48]. As such, it is not recommended to administer protease inhibitors for PEP prophylaxis[2,18,43].
Nitrates can also be used as a form of prophylaxis, with sublingual administration being the best studied route[49]. This most recent meta-analysis showed that the use of glyceryl trinitrate reduces the overall incidence of PEP, which was consistent with four previously published meta-analyses[49-53]. It is currently recommended that sublingual glyceryl trinitrate be considered in patients with a contraindication to NSAIDs or to aggressive hydration for prevention of PEP[18].
Epinephrine has also been proposed as a method for PEP prevention. It is administered by spraying the papilla to reduce the edema and prevent PEP. However, there are conflicting results in two randomised controlled trials which compared epinephrine and saline[54,55]. Topical administration of epinephrine onto the papilla for PEP prophylaxis is not recommended[18].
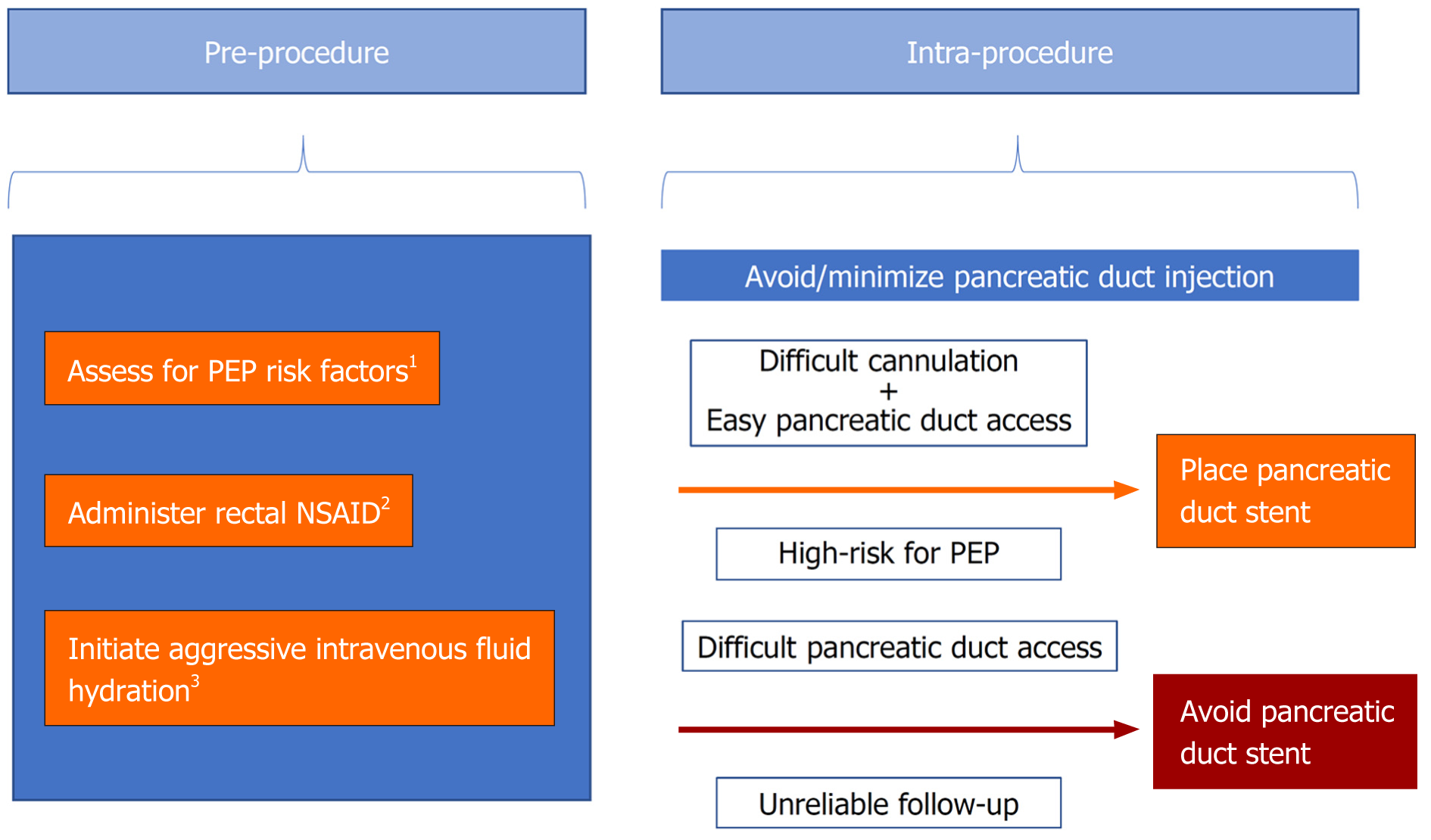
Best practice with respect to the prevention of PEP continues to progress as the literature evolves and new evidence becomes available. First, we suggest that prior to ERCP, clinicians should conduct a thorough assessment for possible risk factors for PEP. Second, rectal indomethacin (or diclofenac) should be considered for all patients undergoing ERCP. Third, IV fluids (lactated Ringer's solution or alternatively normal saline) should be given pre-, intra-, and post-procedure to those who do not have a contraindication to high-volume hydration, particularly in those with a contraindication to NSAIDs. Fourth, pancreatic duct stenting should be performed prophylactically in cases of difficult cannulation and when pancreatic duct access is readily achieved. Fifth, in patients without a prior sphincterotomy who are at high-risk for PEP, cannulation with needle-knife precut techniques (e.g., suprapapillary fistulotomy) should be progressed to early or considered as a primary approach so as to avoid trauma to the pancreatic duct orifice. Finally, pancreatic duct injections should be minimized (Figure 1).
Despite advances in collective knowledge of the mechanisms of and risk factors for PEP, it remains the most common major AE of ERCP and incompletely understood. Best practice with regards to prevention is through careful patient selection, sound endoscopic technique, and evidence-based prophylactic measures. Thoughtful attention to risk factors for PEP is vital in order to guide specific procedural and other preventative techniques and to optimize outcomes. Preventive measures include administration of (rectal) NSAIDs, aggressive IV hydration, various procedural techniques aimed at avoiding trauma to the papillary region, pancreatic duct stenting, and avoiding contrast injection into the pancreatic duct. The optimal choice and/or combination of these measures often requires individualized decision-making. Future high-quality studies are needed to better evaluate these and other approaches and thereby decrease the incidence and severity of PEP.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Covino M, Nakamura K S-Editor: Zhang H L-Editor: A P-Editor: Zhang YL
| 1. | Fagenholz PJ, Castillo CF, Harris NS, Pelletier AJ, Camargo CA Jr. Increasing United States hospital admissions for acute pancreatitis, 1988-2003. Ann Epidemiol. 2007;17:491-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 2. | Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019; 156: 254-272. e11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 776] [Cited by in F6Publishing: 891] [Article Influence: 178.2] [Reference Citation Analysis (0)] |
| 3. | Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-15; 1416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1232] [Cited by in F6Publishing: 1254] [Article Influence: 114.0] [Reference Citation Analysis (3)] |
| 4. | Yang AL, Vadhavkar S, Singh G, Omary MB. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168:649-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 5. | Kwon CI, Song SH, Hahm KB, Ko KH. Unusual complications related to endoscopic retrograde cholangiopancreatography and its endoscopic treatment. Clin Endosc. 2013;46:251-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Morales SJ, Sampath K, Gardner TB. A Review of Prevention of Post-ERCP Pancreatitis. Gastroenterol Hepatol (N Y). 2018;14:286-292. [PubMed] [Cited in This Article: ] |
| 7. | Maranki J, Yeaton P. Prevention of post-ERCP pancreatitis. Curr Gastroenterol Rep. 2013;15:352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1890] [Cited by in F6Publishing: 1934] [Article Influence: 58.6] [Reference Citation Analysis (1)] |
| 9. | Smeets X, Bouhouch N, Buxbaum J, Zhang H, Cho J, Verdonk RC, Römkens T, Venneman NG, Kats I, Vrolijk JM, Hemmink G, Otten A, Tan A, Elmunzer BJ, Cotton PB, Drenth J, van Geenen E. The revised Atlanta criteria more accurately reflect severity of post-ERCP pancreatitis compared to the consensus criteria. United European Gastroenterol J. 2019;7:557-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4134] [Cited by in F6Publishing: 3692] [Article Influence: 335.6] [Reference Citation Analysis (38)] |
| 11. | Sasahira N, Kawakami H, Isayama H, Uchino R, Nakai Y, Ito Y, Matsubara S, Ishiwatari H, Uebayashi M, Yagioka H, Togawa O, Toda N, Sakamoto N, Kato J, Koike K. Early use of double-guidewire technique to facilitate selective bile duct cannulation: the multicenter randomized controlled EDUCATION trial. Endoscopy. 2015;47:421-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Freeman ML, Guda NM. Prevention of post-ERCP pancreatitis: a comprehensive review. Gastrointest Endosc. 2004;59:845-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 305] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 13. | Cotton PB, Garrow DA, Gallagher J, Romagnuolo J. Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years. Gastrointest Endosc. 2009;70:80-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 426] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 14. | Quero G, Covino M, Fiorillo C, Rosa F, Menghi R, Simeoni B, Potenza A, Ojetti V, Alfieri S, Franceschi F. Acute pancreatitis in elderly patients: a single-center retrospective evaluation of clinical outcomes. Scand J Gastroenterol. 2019;54:492-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Deutsch L, Matalon S, Phillips A, Leshno M, Shibolet O, Santo E. Older age, longer procedures and tandem endoscopic-ultrasound as risk factors for post-endoscopic retrograde cholangiopancreatography bacteremia. World J Gastroenterol. 2020;26:6402-6413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Kuzel AR, Lodhi MU, Rahim M. Pancreatic Divisum: An Unusual Cause of Chronic Pancreatitis in a Young Patient. Cureus. 2017;9:e1856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Tse F, Yuan Y, Moayyedi P, Leontiadis GI. Guide wire-assisted cannulation for the prevention of post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy. 2013;45:605-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Dumonceau JM, Kapral C, Aabakken L, Papanikolaou IS, Tringali A, Vanbiervliet G, Beyna T, Dinis-Ribeiro M, Hritz I, Mariani A, Paspatis G, Radaelli F, Lakhtakia S, Veitch AM, van Hooft JE. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2020;52:127-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 328] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 19. | Tang Z, Yang Y, Yang Z, Meng W, Li X. Early precut sphincterotomy does not increase the risk of adverse events for patients with difficult biliary access: A systematic review of randomized clinical trials with meta-analysis and trial sequential analysis. Medicine (Baltimore). 2018;97:e12213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Chen J, Wan JH, Wu DY, Shu WQ, Xia L, Lu NH. Assessing Quality of Precut Sphincterotomy in Patients With Difficult Biliary Access: An Updated Meta-analysis of Randomized Controlled Trials. J Clin Gastroenterol. 2018;52:573-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Tse F, Yuan Y, Moayyedi P, Leontiadis GI, Barkun AN. Double-guidewire technique in difficult biliary cannulation for the prevention of post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy. 2017;49:15-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Mine T, Morizane T, Kawaguchi Y, Akashi R, Hanada K, Ito T, Kanno A, Kida M, Miyagawa H, Yamaguchi T, Mayumi T, Takeyama Y, Shimosegawa T. Clinical practice guideline for post-ERCP pancreatitis. J Gastroenterol. 2017;52:1013-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Mangiavillano B, Montale A, Frazzoni L, Bianchetti M, Sethi A, Repici A, Fuccio L. Endoscopic biliary self-expandable metallic stent in malignant biliary obstruction with or without sphincterotomy: systematic review and meta-analysis. Endosc Int Open. 2019;7:E26-E35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Sawas T, Arwani N, Al Halabi S, Vargo J. Sphincterotomy with endoscopic biliary drainage for severe acute cholangitis: a meta-analysis. Endosc Int Open. 2017;5:E103-E109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Manes G, Paspatis G, Aabakken L, Anderloni A, Arvanitakis M, Ah-Soune P, Barthet M, Domagk D, Dumonceau JM, Gigot JF, Hritz I, Karamanolis G, Laghi A, Mariani A, Paraskeva K, Pohl J, Ponchon T, Swahn F, Ter Steege RWF, Tringali A, Vezakis A, Williams EJ, van Hooft JE. Endoscopic management of common bile duct stones: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2019;51:472-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 286] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 26. | Yang XM, Hu B. Endoscopic sphincterotomy plus large-balloon dilation vs endoscopic sphincterotomy for choledocholithiasis: a meta-analysis. World J Gastroenterol. 2013;19:9453-9460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 36] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | de Clemente Junior CC, Bernardo WM, Franzini TP, Luz GO, Dos Santos MEL, Cohen JM, de Moura DTH, Marinho FRT, Coronel M, Sakai P, de Moura EGH. Comparison between endoscopic sphincterotomy vs endoscopic sphincterotomy associated with balloon dilation for removal of bile duct stones: A systematic review and meta-analysis based on randomized controlled trials. World J Gastrointest Endosc. 2018;10:130-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 13] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Xu L, Kyaw MH, Tse YK, Lau JY. Endoscopic sphincterotomy with large balloon dilation versus endoscopic sphincterotomy for bile duct stones: a systematic review and meta-analysis. Biomed Res Int. 2015;2015:673103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Berry R, Han JY, Tabibian JH. Difficult biliary cannulation: Historical perspective, practical updates, and guide for the endoscopist. World J Gastrointest Endosc. 2019;11:5-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 28] [Cited by in F6Publishing: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Wu D, Wan J, Xia L, Chen J, Zhu Y, Lu N. The Efficiency of Aggressive Hydration With Lactated Ringer Solution for the Prevention of Post-ERCP Pancreatitis: A Systematic Review and Meta-analysis. J Clin Gastroenterol. 2017;51:e68-e76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Zhang ZF, Duan ZJ, Wang LX, Zhao G, Deng WG. Aggressive Hydration With Lactated Ringer Solution in Prevention of Postendoscopic Retrograde Cholangiopancreatography Pancreatitis: A Meta-analysis of Randomized Controlled Trials. J Clin Gastroenterol. 2017;51:e17-e26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Park CH, Paik WH, Park ET, Shim CS, Lee TY, Kang C, Noh MH, Yi SY, Lee JK, Hyun JJ. Aggressive intravenous hydration with lactated Ringer's solution for prevention of post-ERCP pancreatitis: a prospective randomized multicenter clinical trial. Endoscopy. 2018;50:378-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1080] [Cited by in F6Publishing: 921] [Article Influence: 83.7] [Reference Citation Analysis (3)] |
| 34. | Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, Smith B, Banks PA, Conwell DL. Lactated Ringer's solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9: 710-717. e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 297] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 35. | Feng Y, Navaneethan U, Zhu X, Varadarajulu S, Schwartz I, Hawes R, Hasan M, Yang A. Prophylactic rectal indomethacin may be ineffective for preventing post-endoscopic retrograde cholangiopancreatography pancreatitis in general patients: A meta-analysis. Dig Endosc. 2017;29:272-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | He X, Zheng W, Ding Y, Tang X, Si J, Sun LM. Rectal Indomethacin Is Protective against Pancreatitis after Endoscopic Retrograde Cholangiopancreatography: Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2018;2018:9784841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Yu LM, Zhao KJ, Lu B. Use of NSAIDs via the Rectal Route for the Prevention of Pancreatitis after ERCP in All-Risk Patients: An Updated Meta-Analysis. Gastroenterol Res Pract. 2018;2018:1027530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Yang C, Zhao Y, Li W, Zhu S, Yang H, Zhang Y, Liu X, Peng N, Fan P, Jin X. Rectal nonsteroidal anti-inflammatory drugs administration is effective for the prevention of post-ERCP pancreatitis: An updated meta-analysis of randomized controlled trials. Pancreatology. 2017;17:681-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Inamdar S, Han D, Passi M, Sejpal DV, Trindade AJ. Rectal indomethacin is protective against post-ERCP pancreatitis in high-risk patients but not average-risk patients: a systematic review and meta-analysis. Gastrointest Endosc. 2017;85:67-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 40. | Hou YC, Hu Q, Huang J, Fang JY, Xiong H. Efficacy and safety of rectal nonsteroidal anti-inflammatory drugs for prophylaxis against post-ERCP pancreatitis: a systematic review and meta-analysis. Sci Rep. 2017;7:46650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Yaghoobi M, Pauls Q, Durkalski V, Romagnuolo J, Fogel EL, Tarnasky PR, Aliperti G, Freeman ML, Kozarek RA, Jamidar PA, Wilcox CM, Elta GH, Hawes RH, Wood-Williams A, Cotton PB. Incidence and predictors of post-ERCP pancreatitis in patients with suspected sphincter of Oddi dysfunction undergoing biliary or dual sphincterotomy: results from the EPISOD prospective multicenter randomized sham-controlled study. Endoscopy. 2015;47:884-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Akbar A, Abu Dayyeh BK, Baron TH, Wang Z, Altayar O, Murad MH. Rectal nonsteroidal anti-inflammatory drugs are superior to pancreatic duct stents in preventing pancreatitis after endoscopic retrograde cholangiopancreatography: a network meta-analysis. Clin Gastroenterol Hepatol. 2013;11:778-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 43. | Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018;154:1096-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 437] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 44. | A Randomized Controlled Trial of IV Ketorolac to Prevent Post-ERCP Pancreatitis. [accessed 2021 Jan 26] In: ClinicalTrials.gov [Internet]. NIH: U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02465138 ClinicalTrials.gov Identifier: NCT02465138. [Cited in This Article: ] |
| 45. | O'Toole TJ, Sharma S. Physiology, Somatostatin. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2020. [Cited in This Article: ] |
| 46. | Zhang Y, Chen QB, Gao ZY, Xie WF. Meta-analysis: octreotide prevents post-ERCP pancreatitis, but only at sufficient doses. Aliment Pharmacol Ther. 2009;29:1155-1164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Seta T, Noguchi Y, Shikata S, Nakayama T. Treatment of acute pancreatitis with protease inhibitors administered through intravenous infusion: an updated systematic review and meta-analysis. BMC Gastroenterol. 2014;14:102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Zhang ZF, Yang N, Zhao G, Zhu L, Zhu Y, Wang LX. Preventive effect of ulinastatin and gabexate mesylate on post-endoscopic retrograde cholangiopancreatography pancreatitis. Chin Med J (Engl). 2010;123:2600-2606. [PubMed] [Cited in This Article: ] |
| 49. | Ding J, Jin X, Pan Y, Liu S, Li Y. Glyceryl trinitrate for prevention of post-ERCP pancreatitis and improve the rate of cannulation: a meta-analysis of prospective, randomized, controlled trials. PLoS One. 2013;8:e75645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Shao LM, Chen QY, Chen MY, Cai JT. Nitroglycerin in the prevention of post-ERCP pancreatitis: a meta-analysis. Dig Dis Sci. 2010;55:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Chen B, Fan T, Wang CH. A meta-analysis for the effect of prophylactic GTN on the incidence of post-ERCP pancreatitis and on the successful rate of cannulation of bile ducts. BMC Gastroenterol. 2010;10:85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Bai Y, Xu C, Yang X, Gao J, Zou DW, Li ZS. Glyceryl trinitrate for prevention of pancreatitis after endoscopic retrograde cholangiopancreatography: a meta-analysis of randomized, double-blind, placebo-controlled trials. Endoscopy. 2009;41:690-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Bang UC, Nøjgaard C, Andersen PK, Matzen P. Meta-analysis: Nitroglycerin for prevention of post-ERCP pancreatitis. Aliment Pharmacol Ther. 2009;29:1078-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Xu LH, Qian JB, Gu LG, Qiu JW, Ge ZM, Lu F, Wang YM, Li YM, Lu HS. Prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis by epinephrine sprayed on the papilla. J Gastroenterol Hepatol. 2011;26:1139-1144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Matsushita M, Takakuwa H, Shimeno N, Uchida K, Nishio A, Okazaki K. Epinephrine sprayed on the papilla for prevention of post-ERCP pancreatitis. J Gastroenterol. 2009;44:71-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |