Published online Dec 16, 2021. doi: 10.4253/wjge.v13.i12.638
Peer-review started: August 19, 2021
First decision: September 29, 2021
Revised: October 4, 2021
Accepted: November 25, 2021
Article in press: November 25, 2021
Published online: December 16, 2021
Polyps are precursors to colorectal cancer, the third most common cancer in the United States. Large polyps, i.e.,, those with a size ≥ 20 mm, are more likely to harbor cancer. Colonic polyps can be removed through various techniques, with the goal to completely resect and prevent colorectal cancer; however, the management of large polyps can be relatively complex and challenging. Such polyps are generally more difficult to remove en bloc with conventional methods, and depending on level of expertise, may consequently be resected piecemeal, leading to an increased rate of incomplete removal and thus polyp recurrence. To effectively manage large polyps, endoscopists should be able to: (1) Evaluate the polyp for characteristics which predict high difficulty of resection or incomplete removal; (2) Determine the optimal resection technique (e.g., snare polypectomy, endoscopic mucosal resection, endoscopic submucosal dissection, etc.); and (3) Recognize when to refer to colleagues with greater expertise. This review covers important considerations in this regard for referring and receiving endoscopists and methods to best manage large colonic polyps.
Core Tip: Large polyps, often defined as ≥ 20 mm in size, are generally more challenging to resect than smaller polyps with regard to both difficulty of complete removal and risk of adverse events. To effectively manage large polyps, endoscopists should be able to evaluate them for characteristics which may increase the difficulty of endoscopic resection, determine the optimal resection technique, and recognize when to refer to colleagues for more advanced approaches. Herein, we review important considerations and methods to best manage large colonic polyps.
- Citation: Markarian E, Fung BM, Girotra M, Tabibian JH. Large polyps: Pearls for the referring and receiving endoscopist. World J Gastrointest Endosc 2021; 13(12): 638-648
- URL: https://www.wjgnet.com/1948-5190/full/v13/i12/638.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i12.638
Colonic polyps have a risk of developing into colorectal cancer (CRC), the third most common cause of cancer-related deaths in the United States[1]. Prior studies have demonstrated that the removal of adenomatous polyps during a colonoscopy is associated with a significant reduction in CRC-related death[2,3]. However, achieving complete resection of a polyp can be challenging, especially with larger polyps. Previous studies have reported that 70%-90% of CRCs are preventable with routine screening colonoscopy and polypectomy[3]; however, 7%-9% are reported to occur despite being up-to-date with colonoscopy[4]. This subset of CRCs is thought to be likely due to either missed polyps or incompletely removed polyps.
The risk of incomplete polyp removal has been reported to increase with increasing polyp size[5]. “Large polyps” are generally defined as being ≥ 20 mm in size (though other cut offs may also be used) and carry a greater likelihood of underlying advanced dysplasia and carcinoma[6]. Indeed, the term “advanced adenoma”[7] has been introduced to stress the clinical and histopathological significance of polyps ≥ 10 mm in size. With advances in polyp removal techniques, management of large polyps has shifted away from surgery and towards endoscopic resection, using novel methods like endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR). In this review, we expound key considerations and techniques to best manage large colonic polyps from the perspective of both the referring and the receiving endoscopist.
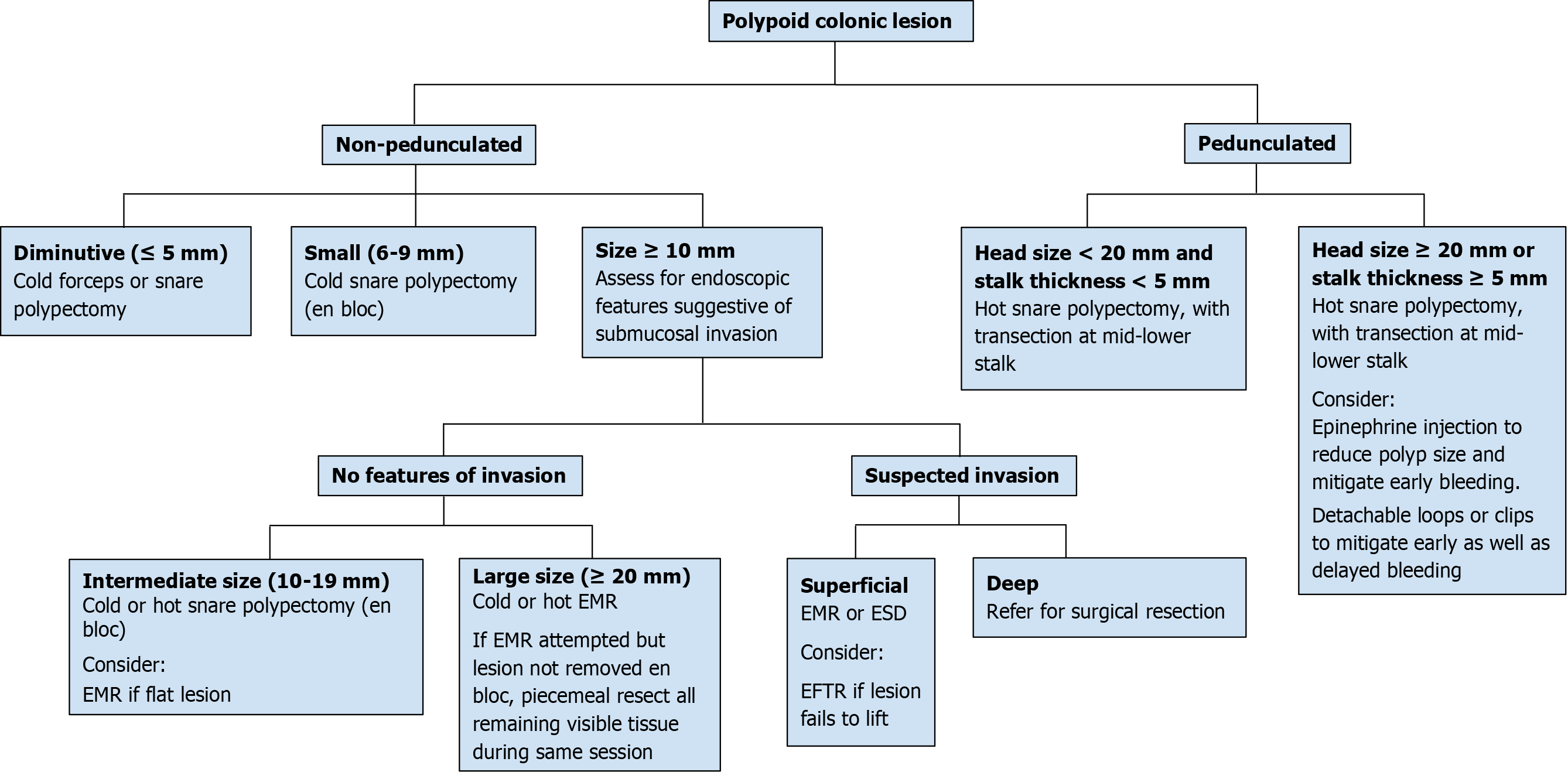
When a polyp is detected, a decision must be made whether endoscopic resection is possible[8,9], and if so, what the best method of resection may be (Figure 1). Certain features, including large size, can pose a technical challenge for complete resection and may indicate a need for advanced endoscopic techniques, as discussed in forthcoming sections, or surgical resection[10]. In addition to polyp size, features including morphology, location, and associated local features are all important determinants in gauging endoscopic resectability[10]. For instance, pedunculated polyps tend to be, on average, easier to grasp (along the peduncle or “stalk”) and resect as opposed to sessile polyps[11,12]. Polyp location also influences resectability, as right-sided lesions tend to be more difficult to resect due to the presence of colonic folds which can impede visualization and maneuverability, increasing the risk of incomplete removal, among other factors[13]. Surface characteristics, discussed in the next section, can also predict submucosal invasion, which may prevent safe resection. Invasive cancers are associated with polyps that fail to lift with submucosal injection, a non-granular surface, depressed subtype, firmness, and redness[14-16]. However, non-lifting does not always predict invasion, as a failure to lift can also be seen in previously biopsied or partially resected polyps with associated tissue fibrosis. Finally, associated local features can impact endoscopic resection; for instance, severe refractory colitis can impede large polyp resection and potentially result in the need for a colectomy[17]. Endoscopic ultrasound (EUS) can be used to evaluate rectal polyps (in particular T stage) and determine feasibility of endoscopic resection when the endoscopic appearance is concerning for possible deep invasion[18,19]. When EUS is not available or feasible (e.g., polyps proximal to the rectosigmoid), cross-sectional imaging such as magnetic resonance or computed tomography can be considered.
Size, morphology, site, access (SMSA) is a scoring system used to predict the difficulty encountered during polyp resection[20]. The scoring is as follows: size (1-9 points), morphology (1-3 points), site (1-2 points), and access (1-3 points). Based on the total score, polyps are classified as Level 1 (4-5), Level 2 (6-9), Level 3 (10-12), or Level 4 (> 12). This system provides an objective assessment of the complexity of a polyp with higher scores suggesting increased complexity. Endoscopists should be aware of complex (and usually large) polyps scored under this system and consider the level of expertise needed to deal with these difficult polyps, referring the patient in necessary cases. Endoscopically unresectable polyps are generally referred to surgery, and are often managed with segmental colectomy, though studies have reported success using hybrid laparoendoscopic approaches i.e.,, combined endoscopic laparoscopic surgery (CELS), to avoid colon resection[21,22].
In addition to the features mentioned thus far, critically important here is determining whether a polyp is benign or premalignant, and within the latter, the degree of dysplasia that may be harbored within. There are several validated systems that can help to characterize and classify polyps in this regard, including the Paris classification[23], the narrow-band imaging international colorectal endoscopic (NICE) classification[24], and the Kudo pit pattern classification[25]. The Paris classification classifies polyps as pedunculated (1p), sessile (1s), flat (IIa, IIb, IIc), or ulcerated (III)[24]. It also classifies surface morphology as granular or non-granular for non-pedunculated polyps (1s and II). However, recent studies have questioned the validity of the Paris classification because of interobserver variability, recommending the system not be used for routine practice[26,27]. The NICE classification classifies polyps as hyperplastic or sessile serrated polyps (SSP) (type 1), conventional adenomas (type 2), or deep submucosal invasive cancer (type 3) based on color, associated vessels, and surface patterns[24]. The Kudo classification classifies polyps based on mucosal surface analysis. Also called the pit-pattern system, it requires magnification during colonoscopy to evaluate the pit pattern of polyps. This classification system classifies pit patterns as round (Type I), papillary/stellar (Type II), tubular or small round (Type III-S), large tubular or round (Type III-L), gyrus/branch-like (Type IV), non-structured/amorphous (Type V-I), and decrease of amorphous pits (Type V-N). Type I and II polyps are considered benign while types III-V are considered to show neoplastic and malignant changes[28]. Despite the existence of the above classification systems, it is important to note that there is significant variability and agreement as to what the optimal method of classifying polyps should be.
The emergence of artificial intelligence (AI) applications has direct implications in colonoscopy practices. The use of computer-aided detection (CADe) software has been demonstrated to decrease the polyp miss rate[29], especially for non-polypoid lesions in the right colon. AI has also been used to characterize polyps, also known as colonoscopy practice-polyp characterization (CADx). This can improve the accuracy of polyp diagnosis and reduce unnecessary resection of non-dysplastic polyps[29]. Although data on the outcomes of AI for polyp detection are evolving rapidly, the few completed studies have demonstrated a significant increase in the detection of adenomas and polyps[30,31]. However, the detection of more polyps does not necessarily improve outcomes; one study found that non-advanced adenomas were detected to a greater extent using AI-colonoscopies while identification of advanced adenomas was not substantially improved[32]. More research is needed to determine the value of AI systems in polyp detection and characterization.
Studies have shown that incomplete polyp removal in daily clinical practice, especially in the case of large polyps, can contribute to future interval cancers[33]. Consequently, appropriate technique and complete resection of large colonic polyps is essential in preventing CRC (Figure 1). Incomplete removal renders future endoscopic resection more challenging; therefore, an endoscopist should aim for complete resection on the first attempt. For polyps ≥ 20 mm in size, the United States Multi-Society Task Force (USMSTF) recommends that an endoscopist be experienced in advanced polyp resection techniques to ensure complete resection[9]. Although polyps that are endoscopically resectable are occasionally sent for surgery, studies show that only about 5-10% of patients subsequently require surgery if they undergo endoscopic resection first[34]. Knowing your expertise and comfort level is particularly important on a variety of levels in the case of polyps that may be challenging to resect; for instance, it is relevant to ensuring the best outcome for the patient, peace of mind for the performing provider, and to avoid potential medical professional liability. Referring to a more experienced provider for a complete resection is thus generally recommended over attempting to complete a polypectomy but failing to achieve complete resection, especially if thermal energy is applied in the process and/or when the a priori probability of incomplete removal seems high. In addition, biopsies of the polyp should be performed with caution so as to avoid scarring and complicating future endoscopic resection. If a biopsy is needed, the biopsy should be performed cold and avoid flat areas of the lesion[35].
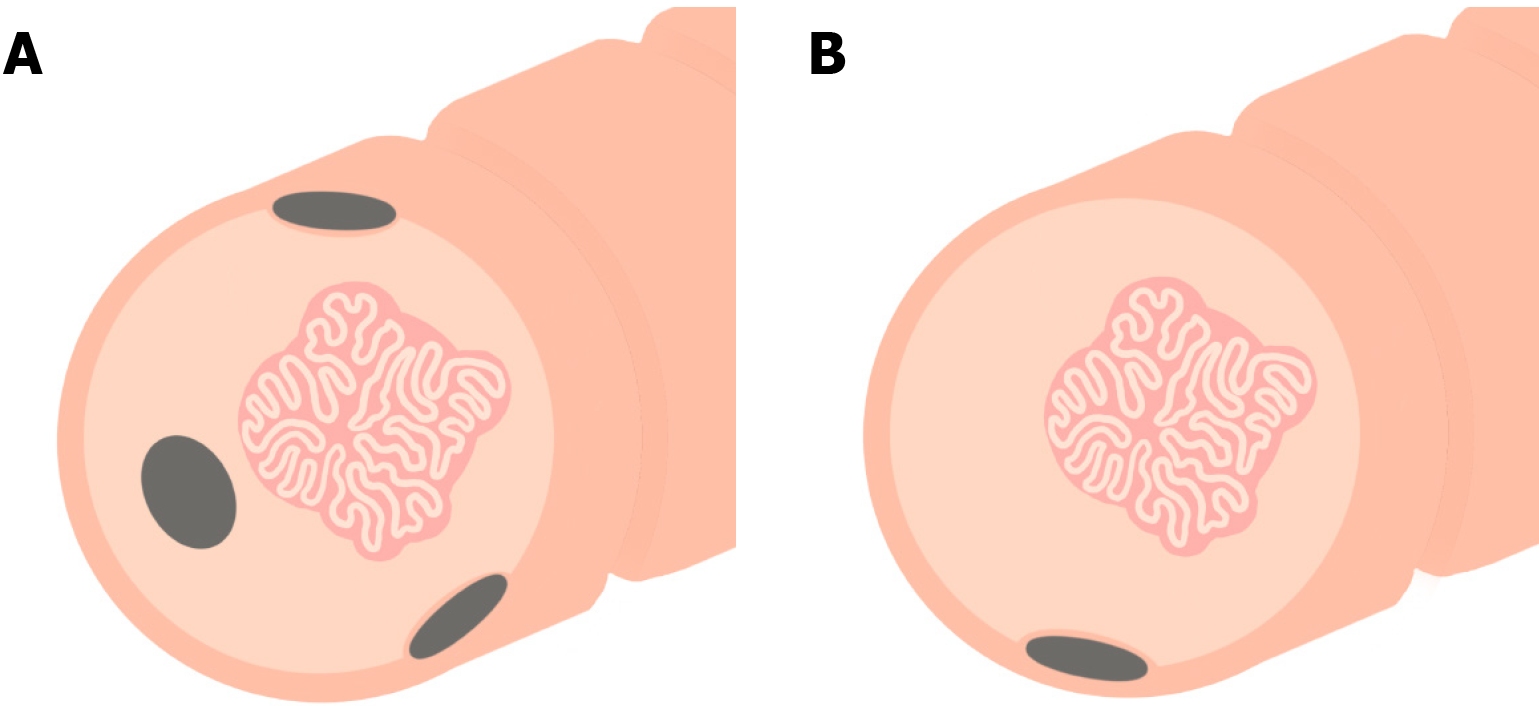
If a polyp is deemed unresectable by a provider, it is often advised to tattoo so it can be easily recognized by the receiving provider. Currently, India Ink, a compound known commercially as “Spot Ex,” is most commonly used for endoscopic tattooing[36]. With respect to tattoo location and number of tattoos, best practice depends in large part on whether the polyp is planned for referral to a surgeon or to an advanced endoscopist, as shown in Figure 2[37,38]. Generally speaking, a tattoo should be placed a) immediately distal to the polyp and circumferentially in multiple quadrants to facilitate intraoperative visualization when planning to refer for surgical resection or b) in one quadrant 3-5 cm distal to the polyp, with care to not inject into or under the polyp, when planning to refer for advanced endoscopic resection. Tattoo placement may not be necessary if the polyp is in the cecum or distal rectum, as these locations are typically easily identifiable on future examinations, but this may vary based on individual (e.g., anatomical) and institutional (e.g., surgeon or advanced endoscopist preference) factors[9]. Irrespective of such factors, photodocumentation and clear description regarding tattoo placement are critical[39,40].
With respect to tattoo injection technique, a few options exist. The “bleb” method is one which is considered reliable for the placement of tattoos[41], wherein, 0.5 to 1.0 mL of saline is placed into the submucosa, followed by a needle inserted into the saline bleb to inject the tattoo agent. The bleb method ensures that the tattoo only enters the submucosal space and not into extracolonic tissue. A second method involves directly injecting the tattoo into the submucosa and lifting the needle toward the center of the lumen, although this technique requires greater expertise[36]. Of note, analogous to polypectomy snares, different length and caliber injection needles are available, the appropriate choice of which may, depending on polyp location and other considerations, best facilitate tattoo placement[42-44]; for instance, a shorter, smaller caliber needle may be opted for when tattooing a right colonic polyp in a coagulopathic patient (as opposed to a standard/larger length and caliber needle for a rectal polyp).
Adverse events (AEs) associated with endoscopic tattooing, albeit rare, have been reported. For example, tattooing can cause submucosal fibrosis (Figure 3) and consequent muscle injury during future endoscopic resection if the tattoo ink spreads underneath the polyp, e.g., if injection is performed too close to or into the polyp or if an excess volume of ink is injected (which can later dissipate laterally to involve the submucosa below the polyp)[40]. Thus, when a polyp is planned for referral for endoscopic resection, the closer the tattoo is to the polyp, the less tattoo volume should be used. Reports of inflammatory responses, localized necrosis from an inflammatory pseudotumor, and rectus muscle abscess have also been described[45-47]. These potential AEs should be taken into account when placing an endoscopic tattoo and accordingly established techniques should be followed.
The endoscopic resection technique that is used largely depends on the morphology of the polyp, in particular its size and whether it is pedunculated or not, as discussed below[9].
Large polyps can be pedunculated or non-pedunculated. For pedunculated polyps ≥ 10 mm in size, hot snare polypectomy (HSP), in which electrocoagulation is used for resection, is suggested[9]. For larger pedunculated polyps, epinephrine injection into the head or stalk can also be considered to reduce the polyp size and make resection easier[48]. Other strategies include using a detachable loop or placing clips at the polyp stalk before resection. Cold snare polypectomy (CSP) may also be used for resection and has been reported to have a lower rate of post-polypectomy bleeding[49]; however, the rate of complete resection may be higher with HSP compared with CSP when resecting large pedunculated polyps[50].
Endoscopic mucosal resection: The majority of non-pedunculated (i.e., sessile) polyps can be removed by endoscopic mucosal resection (EMR). In this technique, fluid is injected submucosally to lift the polyp and facilitate resection. Many variations of this technique have been developed, such as hot snare EMR, cold snare EMR, and underwater EMR.
In the hot snare EMR (HS-EMR) technique, the underlying submucosa is first injected with a contrast dye, such as methylene blue, to achieve lifting of the polyp, which allows optimal placement of a snare to grab the polyp away from the mucosa, followed by resection with application of electrocautery. Polyps < 20 mm in size can be removed entirely (en bloc resection), while larger polyps can be removed in segments (piecemeal resection). Because HS-EMR utilizes electrocautery, it can minimize intraprocedural bleeding of cut tissue due to its coagulation effect and also destroy the polyp margins, thus leading to a lower recurrence rate[9]. However, the use of electrocautery is also associated with a higher risk of post-procedural bleeding and perforation, compared to the cold snare technique[51].
Cold snare EMR (CS-EMR) allows for large polyp resection without use of electrocautery. In this variation of EMR, the submucosa may be injected to raise the polyp, similar to HS-EMR, after which the snare is then opened slightly larger than the area of the polyp (resecting some normal tissue margin) to remove it en bloc or piecemeal. As previously mentioned, this technique is associated with lower rates of post-procedural bleeding and perforation compared to HS-EMR. Studies of CS-EMR have shown low rates of polyp recurrence and AEs with excellent resection rates[52-54]. Although HS-EMR is currently the standard of care in endoscopic resections, CS-EMR represents an equally effective and safe resection method for large polyps.
Given that complete en bloc resection rates decrease in polyps ≥ 10 mm using traditional EMR techniques (which in turn increases the rate of recurrence), underwater EMR (UEMR) has been proposed as an alternative effective strategy to resect large polyps[18,19]. This method avoids the use of submucosal injection by aspirating gas and instilling water into the colonic lumen, which raises the mucosal pathology (polyp) away from the underlying submucosa, allowing safer and complete resection of the polyp. Especially useful in the case of large polyps, UEMR has shown significantly increased rates of R0 resections for polyps 10-20 mm in size without increasing the rate of AEs[55]. This variant of EMR represents a viable alternative to traditional resection techniques for large polyps that are difficult to remove completely.
Endoscopic submucosal dissection: Endoscopic submucosal dissection (ESD) allows for the complete removal of polyps too large for EMR (≥ 20 mm in size) and/or that are strongly suspicious for cancer. ESD is also utilized in cases with suspected submucosal invasion, local early carcinoma, or laterally spreading polyps/tumors[56]. Studies have demonstrated that ESD may have better outcomes for larger polyps, as EMR often requires piecemeal removal which has an increased rate of recurrence (about 20%)[57].
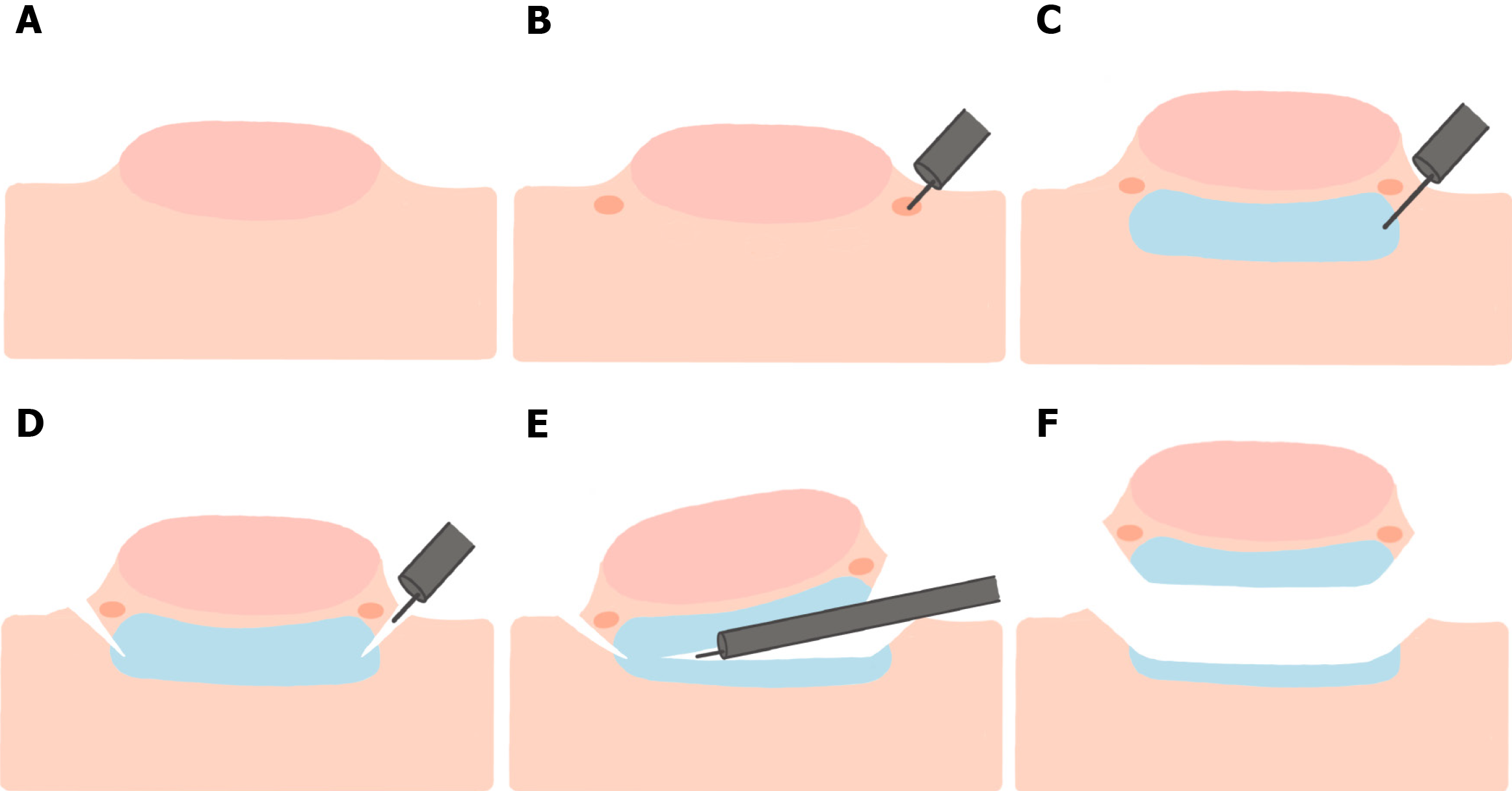
In the ESD technique, the area underneath the polyp is first injected to lift the polyp, followed by creation of an incision into the mucosa using an ESD knife. The submucosal edges are trimmed to allow access to the submucosal plane where the dissection is performed (Figure 4), resulting in an en bloc resection of large polyps/tumors. While ESD has excellent rates of en bloc resection, it has higher rates of AEs compared to EMR, including perforation, bleeding, and hospitalization related to the procedure[58]. Low-voltage coagulation (“soft” ESD) can be performed after resecting the polyp to reduce the risk of post-resection bleeding[59].
Endoscopic full-thickness resection: Endoscopic full-thickness resection (EFTR) is a novel approach which enables all layers of the colon wall to be removed[60,61]. This technique is often used for polyps < 30 mm in size which either fail to lift after submucosal injection or that are difficult to resect with conventional EMR techniques. Multiple studies have shown the efficacy and safety of EFTR[59], in both animal models and human patients, with excellent resection rates for non-lifting adenomas and low rates of AEs (about 14%)[62]. The technique uses a full-thickness resection device (FTRD®), which has been shown to enable complete resection of polyps beneath the mucosa[63]. At this time, EFTR is not widely practiced as few endoscopists are trained in this technique.
Endoscopic clipping: Bleeding, the most common AE after EMR, is more likely to occur in patients undergoing resection of large polyps, polyps ≥ 10 mm with a thick stalk, right-sided polyps, and in patients on anticoagulation/antiplatelet agents or with comorbid conditions that increase the risk of bleeding[64,65]. Clipping can be used to effectively stop or prevent bleeding through mechanical pressure. In one study, endoscopic clipping significantly reduced the risk of bleeding after resection of large polyps (≥ 20 mm), with 7.6% of subjects without clipping having bleeding compared to 4.3% with clipping[66]. In addition, clip placement is often utilized to close post-polypectomy mucosal defects[67].
Surveillance: After complete resection of large polyps, close surveillance is recommended to detect disease recurrence and/or metachronous colorectal polyps. Surveillance is important for early detection of asymptomatic and resectable recurrences, which increases patients’ chances for curative therapy[68]. The USMSTF recommends that colonoscopy should be performed within 1 year after resection to look for metachronous polyps. If this examination is normal, a subsequent examination should be performed after 3 years, and then 5 years (if the second examination is also normal). However, shorter examination intervals may also be used if additional polyps are found[68]. Shorter examinations are also favored in the case of piecemeal resection of a large polyp because of the significantly increased risk of residual polyp tissue and recurrence. Thus, a period of 2-6 mo is typically the recommended interval for surveillance colonoscopy in such cases[69].
As endoscopic resection techniques have evolved, there has been a shift in the management of large colonic polyps from being referred for colon surgery to endoscopic resection. Effective resection of these large polyps can be complex, but success has been documented using methods like EMR and ESD. Endoscopists should be comfortable at recognizing large colonic polyps through classification systems such as the NICE or Paris classification, and these polyps should be resected by endoscopists experienced with advanced resection techniques. Standardized practices coupled with clear communication can help ensure optimal outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao W S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2268] [Cited by in F6Publishing: 2753] [Article Influence: 688.3] [Reference Citation Analysis (2)] |
| 2. | Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3107] [Cited by in F6Publishing: 3009] [Article Influence: 97.1] [Reference Citation Analysis (1)] |
| 3. | Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1952] [Cited by in F6Publishing: 2085] [Article Influence: 173.8] [Reference Citation Analysis (1)] |
| 4. | Pohl H, Robertson DJ. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol. 2010;8:858-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, Levy LC, Toor A, Mackenzie TA, Rosch T, Robertson DJ. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74-80.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 504] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 6. | De Ceglie A, Hassan C, Mangiavillano B, Matsuda T, Saito Y, Ridola L, Bhandari P, Boeri F, Conio M. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: A systematic review. Crit Rev Oncol Hematol. 2016;104:138-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson DJ. Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153:307-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 426] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 8. | Kaltenbach T, Anderson JC, Burke CA, Dominitz JA, Gupta S, Lieberman D, Robertson DJ, Shaukat A, Syngal S, Rex DK. Endoscopic Removal of Colorectal Lesions: Recommendations by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2020;115:435-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 9. | Shaukat A, Kaltenbach T, Dominitz JA, Robertson DJ, Anderson JC, Cruise M, Burke CA, Gupta S, Lieberman D, Syngal S, Rex DK. Endoscopic Recognition and Management Strategies for Malignant Colorectal Polyps: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020;92:997-1015.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Aarons CB, Shanmugan S, Bleier JI. Management of malignant colon polyps: current status and controversies. World J Gastroenterol. 2014;20:16178-16183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 70] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (2)] |
| 11. | Pickhardt PJ, Pooler BD, Kim DH, Hassan C, Matkowskyj KA, Halberg RB. The Natural History of Colorectal Polyps: Overview of Predictive Static and Dynamic Features. Gastroenterol Clin North Am. 2018;47:515-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Tholoor S, Tsagkournis O, Basford P, Bhandari P. Managing difficult polyps: techniques and pitfalls. Ann Gastroenterol. 2013;26:114-121. [PubMed] [Cited in This Article: ] |
| 13. | Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol. 2006;101:2866-2877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Matsuda T, Parra-Blanco A, Saito Y, Sakamoto T, Nakajima T. Assessment of likelihood of submucosal invasion in non-polypoid colorectal neoplasms. Gastrointest Endosc Clin N Am. 2010;20:487-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Uno Y, Munakata A. The non-lifting sign of invasive colon cancer. Gastrointest Endosc. 1994;40:485-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 170] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Ishiguro A, Uno Y, Ishiguro Y, Munakata A, Morita T. Correlation of lifting vs non-lifting and microscopic depth of invasion in early colorectal cancer. Gastrointest Endosc. 1999;50:329-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Rubin PH, Friedman S, Harpaz N, Goldstein E, Weiser J, Schiller J, Waye JD, Present DH. Colonoscopic polypectomy in chronic colitis: conservative management after endoscopic resection of dysplastic polyps. Gastroenterology. 1999;117:1295-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 260] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Binmoeller KF, Weilert F, Shah J, Bhat Y, Kane S. "Underwater" EMR without submucosal injection for large sessile colorectal polyps (with video). Gastrointest Endosc. 2012;75:1086-1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 19. | Binmoeller KF, Hamerski CM, Shah JN, Bhat YM, Kane SD, Garcia-Kennedy R. Attempted underwater en bloc resection for large (2-4 cm) colorectal laterally spreading tumors (with video). Gastrointest Endosc. 2015;81:713-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Gupta S, Miskovic D, Bhandari P, Dolwani S, McKaig B, Pullan R, Rembacken B, Rutter MD, Riley S, Valori R, Vance ME, Faiz OD, Saunders BP, Thomas-Gibson S. The ‘SMSA’ Scoring System for Determining the Complexity of a Polyp. Gut. 2011;60:A129-A129. [DOI] [Cited in This Article: ] |
| 21. | Mal F, Perniceni T, Levard H, Boudet MJ, Levy P, Gayet B. [Colonic polyps considered unresectable by endoscopy. Removal by combinations of laparoscopy and endoscopy in 65 patients]. Endoscopy. 2006;38:A1433. [DOI] [Cited in This Article: ] |
| 22. | Aslani N, Alkhamesi NA, Schlachta CM. Hybrid Laparoendoscopic Approaches to Endoscopically Unresectable Colon Polyps. J Laparoendosc Adv Surg Tech A. 2016;26:581-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1117] [Cited by in F6Publishing: 1198] [Article Influence: 57.0] [Reference Citation Analysis (3)] |
| 24. | Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, Soetikno R, Rex DK. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599-607.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 376] [Cited by in F6Publishing: 384] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 25. | Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 721] [Cited by in F6Publishing: 655] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 26. | van Doorn SC, Hazewinkel Y, East JE, van Leerdam ME, Rastogi A, Pellisé M, Sanduleanu-Dascalescu S, Bastiaansen BA, Fockens P, Dekker E. Polyp morphology: an interobserver evaluation for the Paris classification among international experts. Am J Gastroenterol. 2015;110:180-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Gupta S. Trouble in Paris (classification): polyp morphology is in the eye of the beholder. Am J Gastroenterol. 2015;110:188-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Patino-Barrientos S, Sierra-Sosa D, Garcia-Zapirain B, Castillo-Olea C, Elmaghraby A. Kudo’s Classification for Colon Polyps Assessment Using a Deep Learning Approach. Appl Sci. 2020;10:501. [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Vinsard DG, Mori Y, Misawa M, Kudo SE, Rastogi A, Bagci U, Rex DK, Wallace MB. Quality assurance of computer-aided detection and diagnosis in colonoscopy. Gastrointest Endosc. 2019;90:55-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 30. | Wang P, Berzin TM, Glissen Brown JR, Bharadwaj S, Becq A, Xiao X, Liu P, Li L, Song Y, Zhang D, Li Y, Xu G, Tu M, Liu X. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut. 2019;68:1813-1819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 450] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 31. | Aziz M, Fatima R, Dong C, Lee-Smith W, Nawras A. The impact of deep convolutional neural network-based artificial intelligence on colonoscopy outcomes: A systematic review with meta-analysis. J Gastroenterol Hepatol. 2020;35:1676-1683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Barua I, Vinsard DG, Jodal HC, Løberg M, Kalager M, Holme Ø, Misawa M, Bretthauer M, Mori Y. Artificial intelligence for polyp detection during colonoscopy: a systematic review and meta-analysis. Endoscopy. 2021;53:277-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 115] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 33. | Bronzwaer MES, Koens L, Bemelman WA, Dekker E, Fockens P; COPOS study group. Volume of surgery for benign colorectal polyps in the last 11 years. Gastrointest Endosc. 2018;87:552-561.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Moss A, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Burgess NG, Sonson R, Byth K, Bourke MJ. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015;64:57-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 330] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 35. | Rutter MD, Chattree A, Barbour JA, Thomas-Gibson S, Bhandari P, Saunders BP, Veitch AM, Anderson J, Rembacken BJ, Loughrey MB, Pullan R, Garrett WV, Lewis G, Dolwani S. British Society of Gastroenterology/Association of Coloproctologists of Great Britain and Ireland guidelines for the management of large non-pedunculated colorectal polyps. Gut. 2015;64:1847-1873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 36. | Rex DK. The Appropriate Use and Techniques of Tattooing in the Colon. Gastroenterol Hepatol (N Y). 2018;14:314-317. [PubMed] [Cited in This Article: ] |
| 37. | Grimm IS, McGill SK. Look, but don't touch: what not to do in managing large colorectal polyps. Gastrointest Endosc. 2019;89:479-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Kim HG, Thosani N, Banerjee S, Chen A, Friedland S. Effect of prior biopsy sampling, tattoo placement, and snare sampling on endoscopic resection of large nonpedunculated colorectal lesions. Gastrointest Endosc. 2015;81:204-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Nahid M, Shrestha AK, Imtiaz MR, Basnyat PS. Endoscopic tattooing for colorectal lesions: impact on quality of care and patient outcomes. Ann R Coll Surg Engl. 2020;102:594-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Yang M, Pepe D, Schlachta CM, Alkhamesi NA. Endoscopic tattoo: the importance and need for standardised guidelines and protocol. J R Soc Med. 2017;110:287-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Barquero D, González V, García O, Fernández A, Blasco A, Navarro M, Bargalló García A, Martín M, Erice E, Ariza X, Hernández C, Vascónez C, Castellví J, Mata A. Ways to perform an endoscopic tattoo. Prospective and randomized study in patients with colorectal neoplasm. Rev Esp Enferm Dig. 2021;113:519-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | NeedleMaster Injection Needles. Olympus America. [cited 20 February 2021]. Available from: https://medical.olympusamerica.com/products/needlemaster-injection-needles. [Cited in This Article: ] |
| 43. | AcuJect® Variable Injection Needle. Cook Medical. [cited 20 February 2021]. Available from: https://www.cookmedical.com/products/esc_vin_webds/. [Cited in This Article: ] |
| 44. | InterjectTM Sclerotherapy Needles. Boston Scientific. [cited 20 February 2021]. Available from: https://www.bostonscientific.com/en-US/products/needles/interject-sclerotherapy-needles.html. [Cited in This Article: ] |
| 45. | Coman E, Brandt LJ, Brenner S, Frank M, Sablay B, Bennett B. Fat necrosis and inflammatory pseudotumor due to endoscopic tattooing of the colon with india ink. Gastrointest Endosc. 1991;37:65-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 87] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Gianom D, Hollinger A, Wirth HP. [Intestinal perforation after preoperative colonic tattooing with India ink]. Swiss Surg. 2003;9:307-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Alba LM, Pandya PK, Clarkston WK. Rectus muscle abscess associated with endoscopic tattooing of the colon with India ink. Gastrointest Endosc. 2000;52:557-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Tanaka S, Oka S, Kaneko I, Hirata M, Mouri R, Kanao H, Yoshida S, Chayama K. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 356] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 49. | Kawamura T, Takeuchi Y, Asai S, Yokota I, Akamine E, Kato M, Akamatsu T, Tada K, Komeda Y, Iwatate M, Kawakami K, Nishikawa M, Watanabe D, Yamauchi A, Fukata N, Shimatani M, Ooi M, Fujita K, Sano Y, Kashida H, Hirose S, Iwagami H, Uedo N, Teramukai S, Tanaka K. A comparison of the resection rate for cold and hot snare polypectomy for 4-9 mm colorectal polyps: a multicentre randomised controlled trial (CRESCENT study). Gut. 2018;67:1950-1957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 50. | Yamamoto T, Suzuki S, Kusano C, Yakabe K, Iwamoto M, Ikehara H, Gotoda T, Moriyama M. Histological outcomes between hot and cold snare polypectomy for small colorectal polyps. Saudi J Gastroenterol. 2017;23:246-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 51. | Lorenzo-Zúñiga V, Boix J, Moreno-de-Vega V, de-la-Ossa ND, Òdena G, Bartolí R. Microperforation of the colon: animal model in rats to reproduce mucosal thermal damage. J Surg Res. 2014;188:415-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Choksi N, Elmunzer BJ, Stidham RW, Shuster D, Piraka C. Cold snare piecemeal resection of colonic and duodenal polyps ≥1 cm. Endosc Int Open. 2015;3:E508-E513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Piraka C, Saeed A, Waljee AK, Pillai A, Stidham R, Elmunzer BJ. Cold snare polypectomy for non-pedunculated colon polyps greater than 1 cm. Endosc Int Open. 2017;5:E184-E189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 54. | Tutticci NJ, Hewett DG. Cold EMR of large sessile serrated polyps at colonoscopy (with video). Gastrointest Endosc. 2018;87:837-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 55. | Yamashina T, Uedo N, Akasaka T, Iwatsubo T, Nakatani Y, Akamatsu T, Kawamura T, Takeuchi Y, Fujii S, Kusaka T, Shimokawa T. Comparison of Underwater vs Conventional Endoscopic Mucosal Resection of Intermediate-Size Colorectal Polyps. Gastroenterology. 2019;157:451-461.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 56. | Tanaka S, Saitoh Y, Matsuda T, Igarashi M, Matsumoto T, Iwao Y, Suzuki Y, Nishida H, Watanabe T, Sugai T, Sugihara K, Tsuruta O, Hirata I, Hiwatashi N, Saito H, Watanabe M, Sugano K, Shimosegawa T; Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol. 2015;50:252-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 57. | Draganov PV, Wang AY, Othman MO, Fukami N. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol. 2019;17:16-25.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 244] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 58. | Fujiya M, Tanaka K, Dokoshi T, Tominaga M, Ueno N, Inaba Y, Ito T, Moriichi K, Kohgo Y. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:583-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 236] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 59. | Yoshida N, Yagi N, Naito Y, Yoshikawa T. Safe procedure in endoscopic submucosal dissection for colorectal tumors focused on preventing complications. World J Gastroenterol. 2010;16:1688-1695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 70] [Cited by in F6Publishing: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Schmidt A, Bauerfeind P, Gubler C, Damm M, Bauder M, Caca K. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy. 2015;47:719-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 61. | Vitali F, Naegel A, Siebler J, Neurath MF, Rath T. Endoscopic full-thickness resection with an over-the-scope clip device (FTRD) in the colorectum: results from a university tertiary referral center. Endosc Int Open. 2018;6:E98-E103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 62. | Kuellmer A, Mueller J, Caca K, Aepli P, Albers D, Schumacher B, Glitsch A, Schäfer C, Wallstabe I, Hofmann C, Erhardt A, Meier B, Bettinger D, Thimme R, Schmidt A; FTRD study group. Endoscopic full-thickness resection for early colorectal cancer. Gastrointest Endosc. 2019;89:1180-1189.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 63. | Albrecht H, Raithel M, Braun A, Nagel A, Stegmaier A, Utpatel K, Schäfer C. Endoscopic full-thickness resection (EFTR) in the lower gastrointestinal tract. Tech Coloproctol. 2019;23:957-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Buddingh KT, Herngreen T, Haringsma J, van der Zwet WC, Vleggaar FP, Breumelhof R, Ter Borg F. Location in the right hemi-colon is an independent risk factor for delayed post-polypectomy hemorrhage: a multi-center case-control study. Am J Gastroenterol. 2011;106:1119-1124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 65. | Kim HS, Kim TI, Kim WH, Kim YH, Kim HJ, Yang SK, Myung SJ, Byeon JS, Lee MS, Chung IK, Jung SA, Jeen YT, Choi JH, Choi KY, Choi H, Han DS, Song JS. Risk factors for immediate postpolypectomy bleeding of the colon: a multicenter study. Am J Gastroenterol. 2006;101:1333-1341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 66. | Luba D, Raphael M, Zimmerman D, Luba J, Detka J, DiSario J. Clipping prevents perforation in large, flat polyps. World J Gastrointest Endosc. 2017;9:133-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 67. | Cereatti F, Grassia R, Drago A, Conti CB, Donatelli G. Endoscopic management of gastrointestinal leaks and fistulae: What option do we have? World J Gastroenterol. 2020;26:4198-4217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 31] [Cited by in F6Publishing: 30] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 68. | Kahi CJ, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Lieberman D, Levin TR, Robertson DJ, Rex DK. Colonoscopy Surveillance after Colorectal Cancer Resection: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2016;111:337-46; quiz 347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 69. | Rex DK, Kahi CJ, Levin B, Smith RA, Bond JH, Brooks D, Burt RW, Byers T, Fletcher RH, Hyman N, Johnson D, Kirk L, Lieberman DA, Levin TR, O'Brien MJ, Simmang C, Thorson AG, Winawer SJ; American Cancer Society; US Multi-Society Task Force on Colorectal Cancer. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2006;130:1865-1871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 239] [Article Influence: 13.3] [Reference Citation Analysis (0)] |