Published online Sep 28, 2017. doi: 10.4254/wjh.v9.i27.1115
Peer-review started: February 8, 2017
First decision: March 27, 2017
Revised: April 6, 2017
Accepted: June 12, 2017
Article in press: June 13, 2017
Published online: September 28, 2017
To investigate the role of glutathione S-transferase T1 donor-specific T lymphocytes in plasma cell-rich rejection of liver allografts.
The study group included 22 liver transplant patients. Among them, 18 patients were mismatched for the glutathione S-transferase T1 (GSTT1) alleles (don+/rec-), and 4 were matched (don+/rec+). Seven of the mismatched patients produced anti-GSTT1 antibodies and developed plasma cell-rich rejection (former de novo immune hepatitis). For the detection of specific T lymphocytes, peripheral blood mononuclear cells were collected and stored in liquid nitrogen. The memory T cell response was studied by adding to the cell cultures to a mix of 39 custom-made, 15-mer overlapping peptides, which covered the entire GSTT1 amino acid sequence. The specific cellular response to peptides was analyzed by flow cytometry using the markers CD8, CD4, IL-4 and IFNγ.
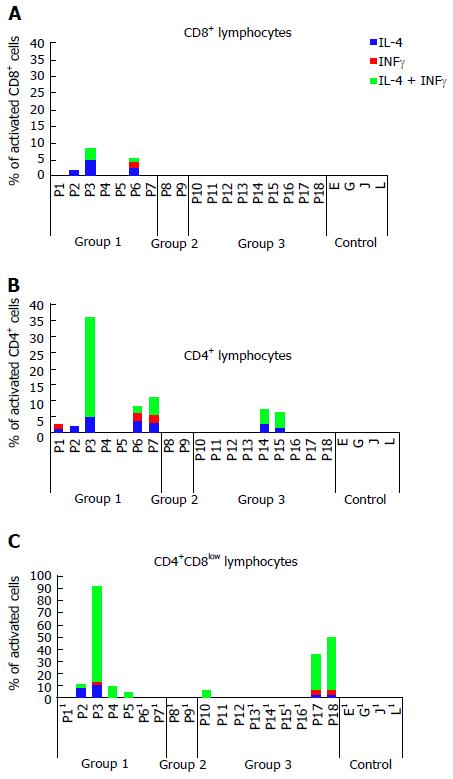
Activation of CD8+ T cells with different peptides was observed exclusively in the group of patients with plasma-cell rich rejection (3 out of 7), with production of IL-4 and/or IFNγ at a rate of 1%-4.92% depending on the peptides. The CD4+ response was most common and not exclusive for patients with the disease, where 5 out of 7 showed percentages of activated cells from 1.24% to 31.34%. Additionally, two patients without the disease but with the mismatch had cells that became stimulated with some peptides (1.45%-5.18%). Highly unexpected was the finding of a double positive CD4+CD8low T cell population that showed the highest degree of activation with some of the peptides in 7 patients with the mismatch, in 4 patients with plasma cell-rich rejection and in 3 patients without the disease. Unfortunately, CD4+CD8low cells represent 1% of the total number of lymphocytes, and stimulation could not be analyzed in 9 patients due to the low number of gated cells. Cells from the 4 patients included as controls did not show activation with any of the peptides.
Patients with GSTT1 mismatch can develop a specific T-cell response, but the potential role of this response in the pathogenesis of plasma cell-rich rejection is unknown.
Core tip: In solid organ transplants, donor recipient mismatch of glutathione S-transferase T1 (GSTT1) alleles triggers a specific immune response with the production of IgG antibodies. In a proportion of mismatched liver and kidney transplants, the clinical outcome is rejection. However, detection of GSTT1-specific T lymphocytes has not been documented. We provide the first evidence of T cells able to become activated by GSTT1 peptides in patients who develop plasma cell-rich (PC-rich) rejection after GSTT1-mismatch liver transplantation. Interestingly, not only CD8+ or CD4+ cells but also double positive CD4+CD8low cells reacted to the antigenic stimulation in vitro.
- Citation: Martínez-Bravo MJ, Sánchez B, Sousa JM, Acevedo MJ, Gómez-Bravo MA, Núñez-Roldán A, Aguilera I. T-cell allorecognition of donor glutathione S-transferase T1 in plasma cell-rich rejection. World J Hepatol 2017; 9(27): 1115-1124
- URL: https://www.wjgnet.com/1948-5182/full/v9/i27/1115.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i27.1115
In the context of liver transplantation, both glutathione S-transferase T1 (GSTT1) mismatch and the presence of GSTT1 antibodies have been associated with the development of de novo immune hepatitis[1-3], recently accepted as a rejection of the liver allograft in which allogeneic hepatocytes that express GSTT1 constitutively in their cytoplasm are the main targets of the immune response. The Banff Working Group on Liver Allograft Pathology has recently updated the terminologies of post-transplant complications and encourages the use of “plasma cell-rich rejection” instead of the former “de novo autoimmune hepatitis”[4]. Therefore, in this manuscript, we will use the new terminology.
Plasma cell-rich (PC-rich) rejection is a liver disorder of unclear pathogenesis that is usually diagnosed within the first two years after liver transplantation. A common feature of all the patients diagnosed in our hospital is the presence of GSTT1 antibodies due to the recognition of GSTT1 as a foreign antigen expressed in the graft when the recipient lacks this gene. Although it is a very specific anti-donor response, it is unclear whether these antibodies have a pathogenic effect since some patients with sustained antibody-titers will never develop PC-rich rejection.
Pregnancy, transfusion and transplantation are circumstances where the host immune system is able to recognize foreign major and minor histocompatibility antigens. This is the case for GSTT1, a drug metabolizing enzyme that is abundantly expressed in the liver and kidney. Recipients who lack this gene (GSTT1*0/0) might generate antibodies against GSTT1 after blood transfusion and/or organ transplantation from GSTT1-positive donors (GSTT1*A/0 or *A/A)[5,6]. It has been reported that the GSTT1 protein is able to induce a memory B cell response in GSTT1*0/0 women after pregnancy with GSTT1-positive offspring[6]. Moreover, it has been demonstrated that GSTT1-specific plasma cells are quickly activated when a GSTT1-positive patient receives an infusion of hematopoietic cells from an HLA-identical sensitized donor[7].
The liver is a very special organ with a variety of important cell types able to function as APCs. Hepatocytes, which represent 60% of the liver cells, express MHC class I at low levels and have the ability to serve as antigen presenting cells (APCs). Furthermore, under some pathological circumstances in a pro-inflammatory environment, parenchymal cells and biliary epithelial cells can express MHC class II antigens[8]. Some studies in mouse models have indicated that both CD4+ and CD8+ T cells can independently initiate hepatocyte rejection, more rapidly in the case of CD8+ cells, somehow preceding the CD4+ mediated response[9]. In humans, patients with chronic allograft failure of kidney grafts have significantly higher frequencies of CD4+ T cells indirectly activated by allogeneic peptides when compared with controls, whereas CD4+ T cells activated in a direct manner reduced the cytotoxic T cell response[10]. However, there are variables such as immunosuppression therapy that can alter the immunological response in different ways.
In this study, we aim to explore the role of T cells in the context of PC-rich rejection. We have compared the T cell response in PBMCs collected from 18 GSTT1-mismatched liver transplant patients, 7 of which had a diagnosis of PC-rich rejection, with 4 GSTT1-matched transplanted patients after re-stimulation in vitro with the whole set of GSTT1 peptides. In summary, we have the first evidence of GSTT1-specific memory T cells ready to become activated after recall with the antigen, but further studies will be needed to test the potential role of these cells in the pathogenesis of PC-rich rejection.
The study group included 22 liver transplant patients, 10 females and 12 males, who had transplants between June 1996 and April 2011. Eighteen of the patients lacked the GSTT1 gene and received a liver from a GSTT1 positive donor (rec-/don+). Consequently, all of them were candidates to develop a specific immune response against this foreign antigen. Four additional patients without the GSTT1 mismatch (rec+/don+) were included as a control group. Within the mismatched patients, we observed three different types of immune and clinical responses regarding the GSTT1 antigen. Group 1 consisted of 7 patients who produced anti-GSTT1 antibodies and developed PC-rich rejection. Group 2 included 2 patients who produced anti-GSTT1 antibodies but did not develop PC-rich rejection. Group 3 included 9 patients who did not produce anti-GSTT1 antibodies (which always precede clinical manifestations) and consequently did not develop the disease. Written informed consent was obtained from all of the participants, and the procedures were in accordance with the Helsinki Declaration. The study protocol was approved by the Ethics Committee of the University Hospital Virgen del Rocío, Seville, Spain. Patient characteristics are described in Table 1. Baseline immunosuppression was cyclosporine in 13 cases and tacrolimus in 9 cases, either alone or combined with mycophenolate mofetil and steroids during the first months. Cells were obtained at a mean time of 6.68 years after the transplant (1-16). Changes in the immunosuppression therapy at the time of cell extraction are described in Table 1. Six of the patients with PC-rich rejection were also receiving prednisone as a specific treatment, and one patient was not adequately diagnosed and died in 2014.
| Group | Patient | Gender | LT date | Original disease | Baseline IS | PBMC extraction date | Years after Tx | Treatment at PBMC extraction |
| 1 | 1 | M | 06-05-99 | Alcoholic cirrhosis | CYA (N), MMF, ST | 12-04-12 | 13 | CYA (N), MMF, ST |
| 2 | F | 07-05-07 | Cirrhosis probably autoimmune | CYA, MMF, ST | 16-04-12 | 5 | CYA (N), MMF, ST | |
| 3 | F | 02-07-00 | HCV cirrhosis | CYA (N), ST, BASILISIMAB | 13-03-12 | 12 | TAC, AZA, ST | |
| 4 | F | 18-09-03 | Alcoholic cirrhosis | CYA, MMF, ST | 09-05-12 | 9 | TAC, MMF, ST | |
| 5 | M | 02-11-01 | HCV + alcoholic cirrhosis | CYA (N), ST, BASILISIMAB | 14-06-12 | 11 | MMF, ST | |
| 6 | F | 27-03-09 | Primary biliary cirrhosis | CYA, MMF | 12-04-12 | 3 | MMF, ST | |
| 7 | F | 18-11-06 | Secondary biliary cirrhosis | CYA (N), MMF, ST | 08-05-12 | 6 | CYA, MMF | |
| 2 | 8 | M | 23-11-96 | HBV cirrhosis | CYA (N), MMF, ST | 19-06-12 | 16 | CYA (N), MMF |
| 9 | F | 03-06-96 | Agenesis of the bile ducts | CYA, ST | 21-05-12 | 16 | TAC | |
| 3 | 10 | M | 12-7-06 | Alcoholic cirrhosis + hepatocarcinoma | TAC, MMF, ST | 16-04-12 | 6 | MMF, SIR |
| 11 | M | 12-02-09 | HBV cirrhosis | TAC, MMF, ST | 16-04-12 | 3 | MMF, SIR | |
| 12 | M | 06-07-10 | Non-alcoholic steatohepatitis | TAC (10 d) CYA, RAPA | 17-04-12 | 2 | MMF, SIR | |
| 13 | M | 19-04-11 | HCV cirrhosis+ hepatocarcinoma | CYA, ST | 18-04-12 | 1 | CYA, ST | |
| 14 | M | 18-01-09 | HCV cirrhosis | TAC, MMF, ST | 02-05-12 | 3 | TAC | |
| 15 | F | 18-06-04 | Alcoholic cirrhosis | TAC | 07-05-12 | 8 | MMF, EVE | |
| 16 | M | 30-07-08 | HCV cirrhosis | TAC, MMF, ST | 08-05-12 | 4 | MMF, EVE | |
| 17 | M | 20-12-04 | HCV cirrhosis | TAC, MMF, ST | 22-05-12 | 7 | TAC, MMF | |
| 18 | F | 20-09-99 | HCV cirrhosis | CYA, ST | 18-06-12 | 13 | CYA | |
| 4 | E | F | 09-03-09 | Alcoholic cirrhosis | CYA, ST | 21-03-12 | 3 | CYA, MMF |
| G | M | 16-11-08 | HCV cirrhosis | TAC, DACLIZUMAB | 30-04-12 | 3 | CYA | |
| J | F | 22-05-10 | Hepatocarcinoma | CYA, ST | 02-05-12 | 2 | CYA | |
| L | M | 28-07-11 | Primary biliary cirrhosis | TAC, MMF, ST | 19-06-12 | 1 | TAC |
Peripheral blood samples from the patients and their donors were collected, and genomic DNA was purified using the QIAmp DNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Primers and conditions for the GSTT1 PCR reaction have been described in detail elsewhere[11].
Following the manufacturer’s protocol, total IgG antibodies in sera were analyzed using a commercially available ELISA, which contains the human GSTT1 recombinant protein (Biomedal, Seville, Spain).
We selected 15-mer peptides that overlapped by 9 amino acids and spanned the GSTT1 protein. In total, there were 39 peptides (Table 2). Peptides were synthesized by Innovative Peptide Solutions, JPT (Berlin, Germany). Peptide purity was higher than 80%, as assayed by HPLC, and the peptide composition was verified by mass spectrometry. Lyophilized peptides were dissolved at 10 mg/mL in DMSO, aliquoted, and stored at -20 °C.
| Pool | Amino acid # | Amino acid sequence |
| 1 | 1-15 | MGLELYLDLLSQPCR |
| 7-21 | LDLLSQPCRAVYIFA | |
| 13-27 | PCRAVYIFAKKNDIP | |
| 19-33 | IFAKKNDIPFELRIV | |
| 25-39 | DIPFELRIVDLIKGQ | |
| 2 | 31-45 | RIVDLIKGQHLSDAF |
| 37-51 | KGQHLSDAFAQVNPL | |
| 43-57 | DAFAQVNPLKKVPAL | |
| 49-63 | NPLKKVPALKDGDFT | |
| 55-69 | PALKDGDFTLTESVA | |
| 3 | 61-75 | DFTLTESVAILLYLT |
| 67-81 | SVAILLYLTRKYKVP | |
| 73-87 | YLTRKYKVPDYWYPQ | |
| 79-93 | KVPDYWYPQDLQARA | |
| 85-99 | YPQDLQARARVDEYL | |
| 4 | 91-105 | ARARVDEYLAWQHTT |
| 97-111 | EYLAWQHTTLRRSCL | |
| 103-117 | HTTLRRSCLRALWHK | |
| 109-123 | SCLRALWHKVMFPVF | |
| 115-129 | WHKVMFPVFLGEPVS | |
| 5 | 119-133 | MFPVFLGEPVSPQTL |
| 125-139 | GEPVSPQTLAATLAE | |
| 131-145 | QTLAATLAELDVTLQ | |
| 137-151 | LAELDVTLQLLEDKF | |
| 143-157 | TLQLLEDKFLQNKAF | |
| 6 | 149-163 | DKFLQNKAFLTGPHI |
| 155-169 | KAFLTGPHISLADLV | |
| 161-175 | PHISLADLVAITELM | |
| 167-181 | DLVAITELMHPVGAG | |
| 173-187 | ELMHPVGAGCQVFEG | |
| 7 | 179-193 | GAGCQVFEGRPKLAT |
| 185-199 | FEGRPKLATWRQRVE | |
| 191-205 | LATWRQRVEAAVGED | |
| 197-211 | RVEAAVGEDLFQEAH | |
| 203-217 | GEDLFQEAHEVILKA | |
| 8 | 209-223 | EAHEVILKAKDFPPA |
| 215-229 | LKAKDFPPADPTIKQ | |
| 221-235 | PPADPTIKQKLMPWV | |
| 226-240 | TIKQKLMPWVLAMIR |
Post-transplant PBMCs were isolated using BD Vacutainer CPT ficoll tubes (BD Biosciences, CA, United States), frozen in FCS containing 10% DMSO, and stored in liquid nitrogen. For stimulation experiments, 3-4 × 105 cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Biochrom AG, Berlin, Germany), Penicillin/Streptomicin (100 U penicillin/mL, 100 μg streptomycin/mL), 1 mmol/L Na-Piruvate (Sigma Aldrich, MI, EEUU) and L-Glutamine (2 mmol/L, Irvine Scientific, Wicklow, Ireland) in the presence of 8 pools, each one containing 5 peptides and the last one containing only 4 (10 μg/mL each peptide). Next, 10 μg/mL anti-CD28/CD49d (BD Biosciences, CA, United States) was added for 48 h at 37 °C 5% CO2, and 10 μg/mL Brefeldin A was added to the samples during the last four hours (Golgi Plug: BD Biosciences). A negative control (without peptide but with the proportional amount of DMSO) and a positive activation control with 10 ng/mL PMA + 1 μg/mL ionomycin (Sigma Aldrich) were included in each assay. Pre-transplant samples were not available.
HLA class I and II binding affinity to GSTT1 peptides was analyzed by the Immune Epitope Database (IEDB) and Analysis Resources NetMHCI/IIpan.
Immunofluorescence staining was performed after fixation and permeabilization using lysing solution (BD Biosciences, CA, United States) with the following surface and intracellular markers: Anti-human CD4-PerCP/CD8-APC/IFNγ-FITC/IL-4-PE (Becton Dickinson BD Biosciences, CA, United States). Lymphocyte cytokine release patterns were analyzed by flow cytometry (FACSort; BD Biosciences) using CELLQuest software. The specific cellular response to the different pools was calculated by subtracting the percentage of activation of T cells cultured without GSTT1 peptides (negative control). Typically, 50000 events were collected using FL3 (CD4PerCP-Cy5) or FL4 (CD8-APC) as a fluorescent trigger. A second set of gating was drawn to include CD8- or CD8low and IFNγ and IL-4 expression.
We have categorized as positive the populations with more than 1% of activated cells. All of the patients who showed stimulation revealed a polyclonal T cell response since we observed stimulation with more than one peptide. For simplicity reasons, we have represented the highest percentage of cell activation among the positive values obtained with each peptide (Figure 1 and Table 3). The group of patients with PC-rich rejection (group 1) was the only group in which activation of CD8+ T cells was detected in 3 patients, expressing IL-4, IFNγ or both cytokines simultaneously. This group also shows the most abundant and diverse patterns of CD4+ T cell activation exhibiting Th0 (IL-4/ IFNγ), Th1 (IFNγ) and Th2 (IL-4) pathways, although cellular activation is not exclusive of group 1 and was also observed in two patients included in group 3 (Table 3). The most striking result was the presence of CD4+CD8low double positive (DP) cells that seem to be enriched in GSTT1-specific cells, especially cells with a secretion profile of both cytokines tested (3.44% patient 2, 78.95% patient 3, 9.54% patient 4, 4.56% patient 5). Unfortunately, DP cells are not abundant, and only 4 of the 7 patients with PC-rich rejection could be analyzed due to the low number of double positive cells gated in the remaining 3 cases.
| Group | Pat # | CD8+ | CD4+ | CD4+CD8low | ||||||
| IL-4 | IFNγ | IL-4/IFNγ | IL-4 | IFNγ | IL-4/IFNγ | IL-4 | IFNγ | IL-4/IFNγ | ||
| 1 | 1 | - | - | - | 1.24% | 1.41% | - | Δ | Δ | Δ |
| 2 | 1.7% | - | - | 2.04% | - | - | 8.23% | - | 3.44% | |
| 3 | 4.92% | - | 3.54% | 4.93% | - | 31.34% | 10.77% | 2.25% | 78.95% | |
| 4 | - | - | - | - | - | - | - | - | 9.54% | |
| 5 | - | - | - | - | - | - | - | - | 4.56% | |
| 6 | 2.36% | 2.03% | 1.15% | 3.71% | 2.45% | 2.14% | Δ | Δ | Δ | |
| 7 | - | - | - | 3.19% | 2.35% | 5.63% | Δ | Δ | Δ | |
| 2 | 8 | - | - | - | - | - | - | Δ | Δ | Δ |
| 9 | - | - | - | - | - | - | Δ | Δ | Δ | |
| 3 | 10 | - | - | - | - | - | - | - | - | 6.63% |
| 11 | - | - | - | - | - | - | - | - | - | |
| 12 | - | - | - | - | - | - | - | - | - | |
| 13 | - | - | - | - | - | - | Δ | Δ | Δ | |
| 14 | - | - | - | 2.65% | - | 4.71% | Δ | Δ | Δ | |
| 15 | - | - | - | 1.45% | - | 5.18% | Δ | Δ | Δ | |
| 16 | - | - | - | - | - | - | Δ | Δ | Δ | |
| 17 | - | - | - | - | - | - | 1.68% | 4.6% | 29.58% | |
| 18 | - | - | - | - | - | - | 2.51% | 4.13% | 43.05% | |
| Control | E | - | - | - | - | - | - | Δ | Δ | Δ |
| G | - | - | - | - | - | - | Δ | Δ | Δ | |
| J | - | - | - | - | - | - | Δ | Δ | Δ | |
| L | - | - | - | - | - | - | Δ | Δ | Δ | |
The patients with antibodies but without PC-rich rejection (group 2) did not show CD4+ or CD8+ T cell activation, whereas five of the 9 patients included in group 3, without antibodies and therefore without disease, exhibited stimulation with some peptides. Again, the higher percentages of activation occurred in the double positive CD4+CD8low cells (6.63% patient 10, 29.58% patient 17 and 43.05% patient 18), although in some cases the number of double positive CD4+CD8low cells was too low to perform further analysis (Table 3). The four patients included as the control group with recipients and donors that were matched for the GSTT1 positive allele did not become activated with any of the peptides assayed.
In summary, 12 out of 18 liver transplant recipients with the GSTT1 mismatch showed different degrees of T lymphocyte activation upon exposure to the GSTT1 peptides. Although we could not test for memory markers, the short time of stimulation (48 h) indicates that this is not a primary response but a reactivation of pre-existing GSTT1-specific lymphocytes. There are 3 cell types involved, including CD4+, CD8+ and CD4+CD8low cells, all of them with diverse cytokine expression patterns whose role is not easy to interpret, although DP cells are known to appear in situations of long-term exposure to antigens.
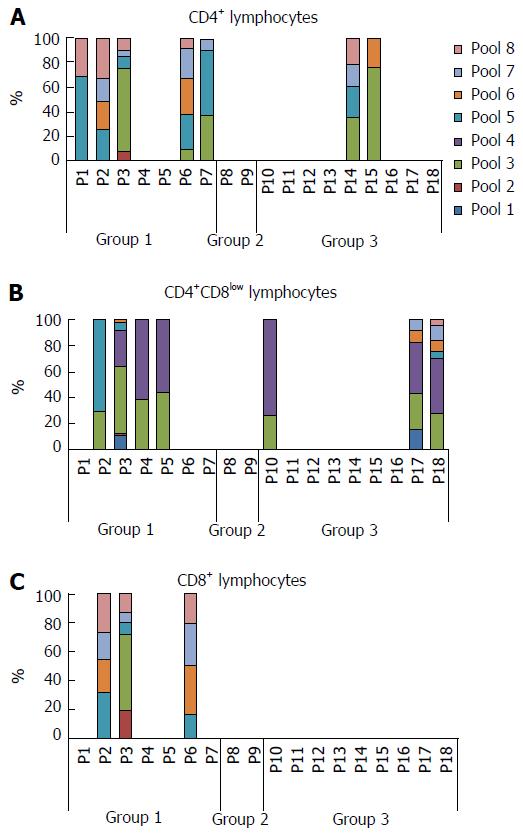
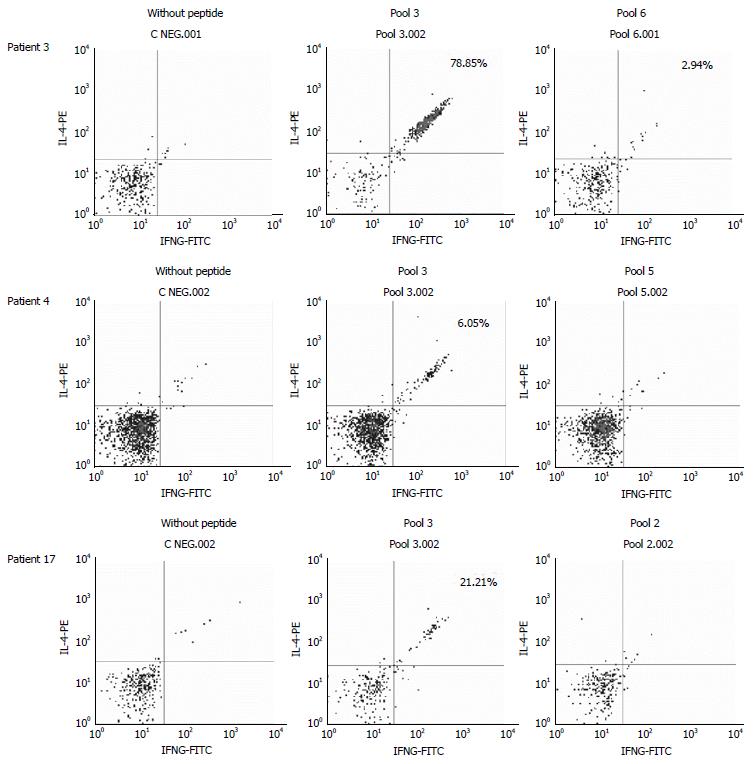
When we analyzed the relative contribution of each pool to the activation of T lymphocytes in each patient, we found that pools 3 and 4 seemed especially antigenic for the DP cells, whereas pool 4 did not stimulate any of the single CD4+ or CD8+ T cells of any patient in which other pools seem to have a more relevant role (Figure 2). A representative plot of flow cytometry data with cells gated on CD4+ first and then CD8, selecting those cells with a low expression of CD8, is shown in Figure 3. We have selected 3 patients with different degrees of activation after stimulation with the pools of peptides; the negative control (without peptide) is also shown and was subtracted to obtain the final values (Figure 3).
These results have to be interpreted in the context of an indirect allo-recognition pathway since the experiments were performed only in the presence of recipient cells. The recipients’ HLA genotypes are described in Table 4, highlighting in bold HLA class I and II alleles with the best percentile ranks for presentation of GSTT1 peptides, as concluded from the in silico analysis. We found a good correlation with part of the experimental results of T cell activation measured in terms of IL-4 and/or IFNγ production by CD8+, CD4+ and CD4+CD8low T cells upon exposure to GSTT1 peptides. However, the fact that HLA genotyping was performed by low resolution methods constitutes a limitation of the analysis. When we placed the in silico-proposed peptides along the GSTT1 amino acid sequence, we were able to define very clearly a highly antigenic zone of the protein that basically shared amino acids from positions 60 to 80 (Table 5). Interestingly, the selected peptides are long, not only for HLA class II, as expected, but also for HLA class I alleles.
| Pat # | HLA-A* | HLA-B* | DRB1* | |||
| 1 | 01 | 03 | 07 | 57 | 11 | 15 |
| 2 | 01 | 66 | 08 | 41 | 03 | 13 |
| 3 | 11 | 29 | 07 | 35 | 07 | 13 |
| 4 | 02 | 11 | 51 | 60 | 04 | 13 |
| 5 | 30 | - | 13 | 18 | 03 | 07 |
| 6 | 02 | 11 | 35 | 44 | 07 | 08 |
| 7 | 26 | 29 | 38 | 44 | 01 | 03 |
| 8 | 23 | 24 | 14 | 52 | 01 | 11 |
| 9 | 11 | 68 | 35 | 44 | 01 | 14 |
| 10 | 02 | 33 | 14 | 35 | 01 | 07 |
| 11 | 01 | 29 | 57 | 61 | 01 | 04 |
| 12 | 01 | 33 | 44 | 64 | 01 | 07 |
| 13 | 01 | - | 08 | 18 | 04 | 07 |
| 14 | 29 | - | 44 | - | 07 | - |
| 15 | 01 | 30 | 08 | 51 | 03 | 07 |
| 16 | 02 | 29 | 39 | 44 | 07 | 11 |
| 17 | 03 | 32 | 37 | 44 | 03 | 12 |
| 18 | 03 | 11 | 14 | 49 | 07 | - |
| Recipient’s HLA | Peptide sequence | Aa position | Length | Percentile rank |
| Class I | ||||
| A*01:01 | FTLTESVAILLY1 | 62-73 | 12 | 0.1 |
| A*02:01 | YIFAKKNDIPFEL | 18-30 | 13 | 0.1 |
| CLRALWHKVMFPV | 110-122 | 13 | 0.1 | |
| IKQKLMPWVLAMI | 227-239 | 13 | 0.1 | |
| A*03:01 | SVAILLYLTRKYK1 | 67-79 | 13 | 0.2 |
| A*11:01 | ESVAILLYLTRK1 | 66-77 | 12 | 0.1 |
| A*29:02 | FLTESVAILLY1 | 62-73 | 12 | 0.2 |
| B*07:02 | SPQTLAATLAEL | 129-140 | 12 | 0.1 |
| RPKLATWRQRVEAA | 188-201 | 14 | 0.2 | |
| B*08:01 | FAQVNPLKKVPAL | 45-57 | 13 | 0.2 |
| LAWQHTTLRRSCL | 99-111 | 13 | 0.2 | |
| DPTIKQKLMPWVL | 224-236 | 13 | 0.2 | |
| B*35:01 | YPQDLQARARVDEY | 85-98 | 14 | 0.1 |
| B*44:02 | TESVAILLY1 | 65-73 | 9 | 0.15 |
| AELDVTLQL | 138-146 | 9 | 0.15 | |
| Class II | ||||
| DRB1*01:03 | GDFTLTESVAILLYL1 | 60-74 | 15 | 0.6 |
| DRB1*07:01 | ALKDGDFTLTESVAI1 | 56-70 | 15 | 0.4 |
| DRB1*11:01 | AILLYLTRKYKVPDY1 | 69-83 | 15 | 0.5 |
| DRB1*12:01 | SVAILLYLTRKYKVP1 | 67-81 | 15 | 0.72 |
| DRB1*13:01 | LTESVAILLYLTRKY1 | 64-78 | 15 | 0.17 |
| DRB1*14:01 | SVAILLYLTRKYKVP1 | 67-81 | 15 | 0.48 |
| DRB1*15:01 | ESVAILLYLTRKYKV1 | 66-80 | 15 | 0.41 |
In this study, we have demonstrated the existence of memory T cells specific for the GSTT1 antigen in patients with PC-rich rejection after GSTT1-mismatched liver transplants. The results support our initial hypothesis in which both specific B and T cells are required to function simultaneously in the development of the immune response leading to PC-rich rejection. In fact, only patients diagnosed with the disease showed a combined T and B cell response, whereas those patients with specific T cells but lacking the humoral response never experienced this type of rejection.
The fact that GSTT1 is a drug metabolizing enzyme found in the cytoplasm of hepatocytes and cholangiocytes makes it difficult to explain a pathogenic role of anti-GSTT1 antibodies. Although it cannot be assumed that cytosolic antigens are never expressed on the cell surface[12], the presence of antibodies in all of the patients with a diagnosis of PC-rich rejection is evidence of specific B cells capable of presentation of GSTT1 to specific T cells. B cells are known to be critical for alloreactive T cells to differentiate into memory T cells[13,14]. In fact, a very interesting report by Zeng et al[15] demonstrated in an animal model of chronic allograft vasculopathy (CAV) that mice deficient in both B cells and antibodies were protected from CAV, while mice that were deficient for antibodies but not for B cells developed CAV. The conclusion was that B cells contributed to CAV by enhancing T-cell responses[15]. Very recently, Shiu et al[16] demonstrated that B cells are involved in supporting T-cell responses in patients with antibody-mediated rejection in a B-cell-dependent indirect T-cell alloreactivity.
CD4+ cells seem to have a predominant role in the context of GSTT1 mismatch in the patients described in this study. Mouse models have provided evidence of the role of CD4+ T cells acting as effectors that directly mediate injury in renal allografts, while CD8+ T cells had very little influence in promoting graft dysfunction[17]. Similarly, CD4+ cells were sufficient to mediate rapid rejection of a cardiac allograft through the indirect pathway of alloantigen recognition[18]. Hence, CD4+ specific T cells are key elements for the progression of allograft immunity, especially within the CD4+ T cell indirect response. In the liver of mice with clinical manifestations of hepatitis, MHC class II-expressing hepatocytes are able to act as APCs and activate specific CD4+ T lymphocytes[19].
The pathogenic role of GSTT1-specific CD8+ T cells in PC-rich rejection has not been explored. The results obtained in this study reveal the existence of reactive CD8+ cells in the group of patients with PC-rich rejection, with percentages of activation that range from 1.1% to 8.46%, which is not as low as expected in immunosuppressed patients. A substantial difference between the percentages of IFNγ-producing CD8+ T cells at diagnosis and during treatment with prednisolone has been demonstrated in patients with type 2 autoimmune hepatitis[20]. We should keep in mind that the patients with PC-rich rejection described in this study are under successful treatment with prednisone that has to be maintained throughout life. It would be very interesting to know the level of stimulation of cells obtained at diagnosis, before initiation of the treatment, since cells from immunosuppressed patients exhibit much lower levels of activation than immunocompetent cells. For that reason, it is even more remarkable that certain types of T lymphocytes from the patients with PC-rich rejection showed high percentages of activation.
The results of this study leave many questions about the function of GSTT1-specific CD4+CD8low T cells in the context of transplantation. Subgroups of CD4hiCD8low T cells have been described in chronic viral infections, with antigen specificity and memory phenotype[21,22], or in parasitic infections where the frequency of CD4+CD8low T cells was higher in Chagasic patients than in healthy donors[23]. In a study performed with human cells from CMV-seropositive patients, the CD4+CD8low population contained a two- to eight-fold higher frequency of antigen-specific IFNγ+ cells than the CD4+CD8- population[24]. It seems that this type of cell appears in chronic processes, mainly in viral infections, but this scenario could also be extended to the transplant setting where sustained expression of a foreign antigen, such as GSTT1, might lead to chronic rejection.
The terminologies used to describe post-transplant clinical situations with overlapping manifestations might be confusing. Late rejection, de novo autoimmune/alloimmune hepatitis or idiopathic post-transplant hepatitis may all be part of immune-mediated injury[25]. The underlying pathology of the formerly called de novo autoimmune hepatitis was poorly understood, and diagnoses were based mainly on histological findings such as the presence of plasma cell rich infiltrates or hepatocyte rosette formation; however, because rosettes are poorly reproducible, some groups do not consider them a diagnostic feature[26].
Although we did not have enough samples to check for memory markers, based on the short time of stimulation in vitro (48 h), we can say that GSTT1-specific lymphocytes are memory cells. It is still too soon to propose a model, as we have not yet tested what would be the response when recipients’ cells are confronted with GSTT1 peptides presented via the direct pathway. Apparently, there is not a predominant HLA class I or II allele among the donors of the patients with PC-rich rejection that could explain why some patients develop rejection and others do not. Given that donor cells are not available, in future studies we will have to design strategies to demonstrate the existence of donor HLA-restricted GSTT1-specific T lymphocytes through the use of artificial molecules such as pentamers, as well as cytotoxicity assays on “donor-like” target cells.
Antibody-mediated rejection of the liver allografts has never been considered a main problem after liver transplantation until now. The Banff Working Group on Liver Allograft pathologies published last year a new report in which the role of HLA as well as glutathione S-transferase T1 (GSTT1) donor specific antibodies is discussed. In this report, they have included new criteria and have suggested changes in the terminology of post-transplant complications. The process termed de novo autoimmune hepatitis is now defined as plasma cell-rich rejection.
The authors’ group has studied de novo immune hepatitis for years. The authors identified the target antigen as a donor protein expressed in the graft but absent from the donor. A genetic mismatch between a GSTT1+ donor and a GSTT1- recipient constitutes a risk factor to produce GSTT1 antibodies and to develop PC-rich rejection (former de novo immune hepatitis) but the pathogenic mechanisms leading to this type of rejection are still unknown.
The existence of T lymphocytes specific for GSTT1 in patients with PC-rich rejection has never been explored. The immune response requires collaboration between GSTT1-specific B and T lymphocytes. The hypothesis contemplated that the patients might have memory T cells able to become activated after recall with the antigenic stimulus. This is the first study in which GSTT1-specific T cells have been found in patients with PC-rich rejection in conjunction with anti-GSTT1 antibodies.
Although ultimate diagnosis of PC-rich rejection relies on histological examination, the fact that some histological features are common to different post-transplant outcomes makes a reliable diagnosis a complicated task. Understanding the mechanisms leading to PC-rich rejection would contribute to a correct diagnosis and appropriate therapy.
Glutathione S-transferase T1 is a drug metabolizing enzyme highly expressed in liver and kidney.
This manuscript investigated the role of GSTT1 donor-specific T lymphocytes in plasma cell-rich rejection of liver allografts in patients, and found that T cells were able to become activated by GSTT1 peptides in patients who develop plasma cell-rich rejection after GSTT1-mismatch liver transplantation. The research design and detecting methods are reasonable, data analysis is correct, writing is fluent, written informed consent was obtained from all of the participants, and the study protocol was approved.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: He ST S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Aguilera I, Wichmann I, Sousa JM, Bernardos A, Franco E, García-Lozano JR, Núñez-Roldán A. Antibodies against glutathione S-transferase T1 (GSTT1) in patients with de novo immune hepatitis following liver transplantation. Clin Exp Immunol. 2001;126:535-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Aguilera I, Sousa JM, Gavilán F, Bernardos A, Wichmann I, Nuñez-Roldán A. Glutathione S-transferase T1 mismatch constitutes a risk factor for de novo immune hepatitis after liver transplantation. Liver Transpl. 2004;10:1166-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Rodriguez-Mahou M, Salcedo M, Fernandez-Cruz E, Tiscar JL, Bañares R, Clemente G, Vicario JL, Alvarez E, Rodriguez-Sainz C. Antibodies against glutathione S-transferase T1 (GSTT1) in patients with GSTT1 null genotype as prognostic marker: long-term follow-up after liver transplantation. Transplantation. 2007;83:1126-1129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Demetris AJ, Bellamy C, Hübscher SG, O'Leary J, Randhawa PS, Feng S, Neil D, Colvin RB, McCaughan G, Fung JJ. 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am J Transplant. 2016;16:2816-2835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 359] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 5. | Aguilera I, Wichmann I, Gentil MA, Gonzalez-Escribano F, Nuñez-Roldan A. Alloimmune response against donor glutathione S-transferase T1 antigen in renal transplant recipients. Am J Kidney Dis. 2005;46:345-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Wichmann I, Aguilera I, Sousa JM, Bernardos A, García Núñez EJ, Vigil E, Magariño R, Magariño I, Torres A, Núñez-Roldán A. Antibodies against glutathione S-transferase T1 in non-solid organ transplanted patients. Transfusion. 2006;46:1505-1509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Aguilera I, Espigado I, Martinez-Bravo MJ, Tallon I, Urbano-Ispizua A, Nuñez-Roldan A. Glutathione S-transferase T1 is a potential new target for the hepatic component of graft vs host disease after HSCT. Bone Marrow Transplant. 2010;45:774-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Bogdanos DP, Gao B, Gershwin ME. Liver immunology. Compr Physiol. 2013;3:567-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | Bumgardner GL, Orosz CG. Unusual patterns of alloimmunity evoked by allogeneic liver parenchymal cells. Immunol Rev. 2000;174:260-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Cook HT, Lechler RI. Loss of direct and maintenance of indirect alloresponses in renal allograft recipients: implications for the pathogenesis of chronic allograft nephropathy. J Immunol. 2001;167:7199-7206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Martínez-Bravo MJ, Calderón-Cabrera C, Márquez-Malaver FJ, Rodríguez N, Guijarro M, Espigado I, Núñez-Roldán A, Pérez-Simón JA, Aguilera I. Mismatch on glutathione S-transferase T1 increases the risk of graft-versus-host disease and mortality after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:1356-1362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Rose ML. Activation of autoimmune B cells and chronic rejection. Transplantation. 2005;79:S22-S24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165:5558-5565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 179] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Ng YH, Oberbarnscheidt MH, Chandramoorthy HC, Hoffman R, Chalasani G. B cells help alloreactive T cells differentiate into memory T cells. Am J Transplant. 2010;10:1970-1980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Zeng Q, Ng YH, Singh T, Jiang K, Sheriff KA, Ippolito R, Zahalka S, Li Q, Randhawa P, Hoffman RA. B cells mediate chronic allograft rejection independently of antibody production. J Clin Invest. 2014;124:1052-1056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Shiu KY, McLaughlin L, Rebollo-Mesa I, Zhao J, Semik V, Cook HT, Roufosse C, Brookes P, Bowers RW, Galliford J. B-lymphocytes support and regulate indirect T-cell alloreactivity in individual patients with chronic antibody-mediated rejection. Kidney Int. 2015;88:560-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Gaughan A, Wang J, Pelletier RP, Nadasdy T, Brodsky S, Roy S, Lodder M, Bobek D, Mofatt-Bruce S, Fairchild RL. Key role for CD4 T cells during mixed antibody-mediated rejection of renal allografts. Am J Transplant. 2014;14:284-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Honjo K, Xu Xy, Bucy RP. CD4+ T-cell receptor transgenic T cells alone can reject vascularized heart transplants through the indirect pathway of alloantigen recognition. Transplantation. 2004;77:452-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Herkel J, Jagemann B, Wiegard C, Lazaro JF, Lueth S, Kanzler S, Blessing M, Schmitt E, Lohse AW. MHC class II-expressing hepatocytes function as antigen-presenting cells and activate specific CD4 T lymphocyutes. Hepatology. 2003;37:1079-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Longhi MS, Hussain MJ, Bogdanos DP, Quaglia A, Mieli-Vergani G, Ma Y, Vergani D. Cytochrome P450IID6-specific CD8 T cell immune responses mirror disease activity in autoimmune hepatitis type 2. Hepatology. 2007;46:472-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Nascimbeni M, Shin EC, Chiriboga L, Kleiner DE, Rehermann B. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood. 2004;104:478-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Nascimbeni M, Pol S, Saunier B. Distinct CD4+ CD8+ double-positive T cells in the blood and liver of patients during chronic hepatitis B and C. PLoS One. 2011;6:e20145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Giraldo NA, Bolaños NI, Cuellar A, Guzman F, Uribe AM, Bedoya A, Olaya N, Cucunubá ZM, Roa N, Rosas F. Increased CD4+/CD8+ double-positive T cells in chronic Chagasic patients. PLoS Negl Trop Dis. 2011;5:e1294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Suni MA, Ghanekar SA, Houck DW, Maecker HT, Wormsley SB, Picker LJ, Moss RB, Maino VC. CD4(+)CD8(dim) T lymphocytes exhibit enhanced cytokine expression, proliferation and cytotoxic activity in response to HCMV and HIV-1 antigens. Eur J Immunol. 2001;31:2512-2520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 25. | Hübscher SG. What is the long-term outcome of the liver allograft? J Hepatol. 2011;55:702-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Sebagh M, Castillo-Rama M, Azoulay D, Coilly A, Delvart V, Allard MA, Dos Santos A, Johanet C, Roque-Afonso AM, Saliba F. Histologic findings predictive of a diagnosis of de novo autoimmune hepatitis after liver transplantation in adults. Transplantation. 2013;96:670-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |