Published online Nov 28, 2016. doi: 10.4254/wjh.v8.i33.1442
Peer-review started: June 1, 2016
First decision: July 20, 2016
Revised: July 28, 2016
Accepted: September 13, 2016
Article in press: September 18, 2016
Published online: November 28, 2016
To develop a simplified bioartificial liver (BAL) device prototype, suitable to use freshly and preserved liver Microorgans (LMOs) as biological component.
The system consists of 140 capillary fibers through which goat blood is pumped. The evolution of hematocrit, plasma and extra-fiber fluid osmolality was evaluated without any biological component, to characterize the prototype. LMOs were cut and cold stored 48 h in BG35 and ViaSpan® solutions. Fresh LMOs were used as controls. After preservation, LMOs were loaded into the BAL and an ammonia overload was added. To assess LMOs viability and functionality, samples were taken to determine lactate dehydrogenase (LDH) release and ammonia detoxification capacity.
The concentrations of ammonia and glucose, and the fluids osmolalities were matched after the first hour of perfusion, showing a proper exchange between blood and the biological compartment in the minibioreactor. After 120 min of perfusion, LMOs cold preserved in BG35 and ViaSpan® were able to detoxify 52.9% ± 6.5% and 53.6% ± 6.0%, respectively, of the initial ammonia overload. No significant differences were found with Controls (49.3% ± 8.8%, P < 0.05). LDH release was 6.0% ± 2.3% for control LMOs, and 6.2% ± 1.7% and 14.3% ± 1.1% for BG35 and ViaSpan® cold preserved LMOs, respectively (n = 6, P < 0.05).
This prototype relied on a simple design and excellent performance. It’s a practical tool to evaluate the detoxification ability of LMOs subjected to different preservation protocols.
Core tip: This work describes the development of a simplified bioartiticial liver prototype (BAL, suitable to use rat liver Microorgans (LMOs) as biological component, and the evaluation of these tissue slices performance in this new model. We demonstrate that the minibioreactor constructed allows a good performance of fresh and cold preserved LMOs, showing the importance of architecture and model configuration on these devices design. Besides its application as BAL, this minibioreactor could serve as a suitable laboratory tool to evaluate the behavior and functionality of LMOs subjected to different preservation protocols due to its simple design and the utilization of standard materials.
- Citation: Pizarro MD, Mediavilla MG, Quintana AB, Scandizzi ÁL, Rodriguez JV, Mamprin ME. Performance of cold-preserved rat liver Microorgans as the biological component of a simplified prototype model of bioartificial liver. World J Hepatol 2016; 8(33): 1442-1451
- URL: https://www.wjgnet.com/1948-5182/full/v8/i33/1442.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i33.1442
To date, acute liver failure continues to be a defeating syndrome in the clinical practice due to its rapid development and its high risk of mortality. Patients always require a multidisciplinary approach for adequate management and subsequent organ transplantation. Unfortunately, the scarcity of donor organs often limits liver transplantation in time. Among the different approaches that have been tested to maintain the patients until transplantation and/or to facilitate self-regeneration of the damaged liver is the bioartificial liver (BAL)[1]. In BAL devices, the plasma of the patient is treated by its circulation through a bioreactor that accommodates a biologically active component which performs the diminished or lacking hepatic metabolic functions. Ammonia detoxification is one key task this biological component must carry out because increased blood levels of this metabolite are toxic to the central nervous system[2].
Investigations concerning the development of BAL devices containing normal hepatocytes are still being conducted[3,4]. Some researchers have chosen to employ immortalized hepatocytes[5] while others have focused their efforts in preparing bioreactors housing isolated hepatocyte with or without extra-cellular matrix and structural components[6,7].
Our group has already reported the construction of a minibioreactor (MBR) consisting in a hollow fiber based cartridge with blood flowing through the fiber lumens. Rat isolated hepatocytes were used as the biological component, showing an effective ammonia depuration rate[8]. Since it is thought that the “ideal” biological component for a BAL should contain all the constituents present in a liver lobule in order to obtain maximal function, we became interested in evaluating the performance of rat liver Microorgans (LMOs). These are thin fragments of tissue that retain the basic micro-architecture of the liver lobe, including cell to cell contact and cell to cell communication[9,10].
On the other hand, in order to become a useful clinical tool, any BAL device must be ready to use when a patient needs it. This means the biological component should be not only available but viable and functional. In a previous work we have presented BG35 [Bes-Gluconate-Polyetyleneglycol (PEG) 35 kDa], a novel preservation solution, that exhibited an efficacy similar to that of the ViaSpan® to give protection to LMOs against injury produced by the ischemia followed by reoxygenation suffered as a consequence of cold preservation[11].
The objectives of this work were to develop a simplified prototype BAL suitable to use LMOs as biological component, and to evaluate the performance of fresh and cold preserved rat LMOs in this model.
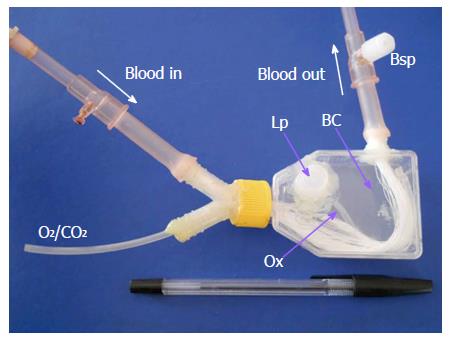
The MBR (Figure 1) was constructed using a 25 cm2 culture flask, adding a loading port (Lp) on top, a Y-polypropylene connector (Nalgene cat. 6152-0375) onto its lid and a simple connector at one side. One hundred and forty PolyamixTM hollow fibers (Gambro, Hechingen, Germany) are assembled to these two connectors and sealed with epoxy glue. The diverse parts that made up the MBR can be appreciated in Figure 1.
In the MBR, two main compartments can be distinguished: The hollow fibers internal lumen constitutes the blood compartment, while the biological compartment (BC) comprises the space outside the hollow fibers (total volume of 50 cm3). A silicone tube (Ox, oxygenator) enters to the BC through the Y connector and allows the oxygenation of the BC fluid. The LMOs were placed in the biological compartment through the Lp port and released on the flat surface of the device. This allows a homogeneous distribution of LMOs and a better oxygenation and exchange of solutes.
The components of the perfusion system used are detailed in Figure 2. The blood reservoir, that contains a clot filter, and the MBR are immersed in a water bath at 37 °C. The peristaltic pump (model 7554-60, Cole Parmer, United States) allows the recirculation of heparinized goat blood (total volume: 35 mL) through all the system at a constant flow of 9 mL/min.
In all the experiments performed, we first filled the system with goat blood via the inlet tube and then inoculated 1 g of LMOs into the BC (or Krebs-Henseleit Reoxygenation media (KHR) alone, in the experiments done to characterize system operation, which composition is shown in page 11). The silicone tube was used to oxygenate the BC compartment with carbogen gas (95% O2/5% CO2) at a stable pressure of 85 mmHg. The blood pH was kept at 7.40 ± 0.50 adding 8.4% sodium bicarbonate if necessary.
To test ammonia detoxification capability of the rat LMOs, we added an aliquot of an ammonium chloride solution (approximate concentration: 350 mmol/L) to the blood in order to achieve an initial ammonia plasma concentration of 1.06 ± 0.12 mmol/L, n = 6 (blood sample t = 0). Then, we initiated blood perfusion and took blood and BC fluid samples after 60 and 120 min of operation to perform the different assays detailed below.
In order to characterize the operation of the system “in vitro”, i.e., without LMOs, different MBR were perfused for 120 min with only KHR solution inside the BC compartment and the following parameters were evaluated:
Hematocrit, to determine the probable rupture of some fibers with the concomitant passage of blood to the BC, and to study the possible hemolytic action of the peristaltic pump. Blood samples were taken from blood sample port at different perfusion times and were centrifuged (1000 x g - 3 min, Rolco CH24 centrifuge). The hematocrit was calculated using the next equation:
Hematocrit (%) = red blood cells volume/blood total volume.
Plasma and extra-fiber fluid osmolality, were measured in order to monitor the correct transfer of fluids between blood and the BC, using a freezing point osmometer (Osmomat 030, Gonotec, GmbH, Berlin, Germany).
Protein analysis using fast protein liquid chromatography (FPLC), in order to study the diffusive properties of the hollow fibers used in the construction of the MBR and to determine the possible passage of plasmatic proteins towards the BC, especially those belonging to the immune system that could damage the biological component (described below).
Metabolite concentration in both compartments, such as glucose and ammonia, to determine their correct distribution in the MBR (described below).
Samples of plasma were taken after 0, 60 and 120 min of perfusion and hemoglobin concentration was determined using the oxyhemoglobin method[12].
To calculate the percentage of hemolysis, we used the following equation, described by Arnaud[13]:
Hemolysis (%) = 100 × {[HbS× (1 - Ht)] ÷ HbT}
where HbS is the hemoglobin content, expressed in g/100 mL, of the different samples; HbT is the total hemoglobin content (in whole blood), and Ht is the hematocrit value measured after 0, 60 or 120 min of perfusion.
Samples of basal plasma and BC fluid were taken after 60 and 120 min of perfusion and analyzed by Gel Filtration Chromatography. They were centrifuged (12100 ×g - 5 min), filtered and 100 μL were seeded in a Tricorn Superdex-200 column (30 × 1 cm, GE Healthcare, Sweden), equilibrated with 50 mmol/L Tris, 150 mmol/L NaCl buffer, pH 7.00, previously degassed by vacuum filtration. The column was manipulated using an ÄKTA-Prime equipment (GE Healthcare, Sweden), at a constant flow of 0.5 mL/min. Each sample was analyzed in duplicate. Chromatograms were registered measuring absorbance at 280 nm and, to determine the protein molecular weight, a standard calibration curve was made using a “Molecular Weights 29000-700000” kit, following the supplier’s instructions (Sigma-Aldrich, St Louis, Missouri, United States).
The livers were obtained from male Wistar rats weighing 250-300 g. Animals had access to regular laboratory food for rodents and water ad libitum. Animals were cared in conformity with the principles and recommendations for the care and utilization of laboratory animals, suggested by the National Academy of Sciences. The rats were adapted to experimental laboratory environment for fourteen days before to experimentation. All experimental procedures were authorized by the School of Biochemical and Pharmaceutical Sciences Institutional Animal Care and Use Committee (Res No. 139/2011).
LMOs were manually cut from rat livers into slices of 338 ± 27 μm thickness, n = 25. They were cut using a microtome blade attached to a plastic handle. We performed all the manipulations on ice (at 0 °C) to decrease tissue injury, and on top of a paper filter to avoid the pieces of livers from sliding what could impede the correct cutting of the tissue[14].
Subsequently, LMOs were allocated in various solutions. Control group (non-preserved or fresh) LMOs were suspended in KHR and directly put in the MBR perfusion. KHR buffer was composed as follows: 114 mmol/L NaCl, 25 mmol/L NaHCO3, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 4.8 mmol/L KCl, 1.5 mmol/L CaCl2, 10 mmol/L HEPES, 25 mmol/L glucose, 5 mmol/L fructose, 1 mmol/L allopurinol, 3 mmol/L glycine, 10 μmol/L adenosine, 6 mmol/L ornithine, 10 mmol/L sodium lactate; pH 7.40, 328 ± 7 mOsm/kg water (n = 6)[15]. Preserved LMOs were stored 48 h in BG35 and ViaSpan® solutions (Table 1) before MBR perfusion as explained in the next section.
| ViaSpan® | BG35 | |
| Impermeants (mmol/L) | ||
| Lactobionate | 100 | |
| Gluconate | 100 | |
| Raffinose | 30 | |
| Buffers (mmol/L) | ||
| KH2PO4 | 25 | 2.5 |
| BES | 50 | |
| Substrates (mmol/L) | ||
| Allopurinol | 1 | 1 |
| Glutathione | 3 | 3 |
| Adenosine | 5 | 5 |
| Glycine | 15 | |
| MgSO4 | 5 | 5 |
| Colloids (g/L) | ||
| HES | 50 | |
| PEG 35000 | 40 | |
| pH | 7.40 | 7.40 |
| Osm (mOsm/kg water) | 320 ± 4 | 339 ± 4 |
As it was stated, in the case of the preserved groups, LMOs were stored in two different preservation solutions. Fifty LMOs were preserved during 48 h at 0 °C[11] in a crystal flask immerse in 50 mL of one of these preservation solutions: (1) ViaSpan® (Bristol-Myers Squibb Pharmaceutical Limited; ViaSpan® group); and (2) BG35 (Bes-Gluconate plus 4% PEG 35 kDa; BG35 group).
The composition of the preservation solutions used are shown in Table 1. A period of 48 h of preservation was selected since in initial investigations (data not exposed) the viability evaluated by lactate dehydrogenase (LDH) leakage was modestly changed by 1 d of cold ischemia, but a pronounced increase was observed after 2 d.
After 48 h of cold preservation, LMOs were completely rinsed with a flush solution earlier reported by our group[16] to fully eliminate residual cold preservation solution. After that, LMOs were placed into the MBR.
Viability of LMOs was tested by LDH release. LDH activity was determined in the BC fluid and the slices as earlier explained[17]. Data are shown as the percentage of the total enzyme activity released into the incubation medium.
Samples of blood and BC fluid were taken at different periods of time (0, 60 and 120 min), blood samples were centrifuged (12000 ×g, 3 min) and all samples were conserved in liquid nitrogen until the determinations were performed. Ammonia was measured using the van Anken enzymatic determination in a volume of 0.8 mL consisting of 66.7 mmol/L phosphate buffer, pH 8.30, 0.14 mmol/L, NADPH, 6.5 mmol/L sodium-ketoglutarate, 2.5 mmol/L ADP, 120 UI/mL glutamate dehydrogenase (cat. #G2626, Sigma Aldrich St. Louis, MO, United States)[18].
The following equations were then used to calculate ammonia mass balance:
QB,t = [(A)B,t× VB,t] - [(A)B,Bas× VB,t]
QBC,t = [(A)BC,t× VBC,t] - [(A)BC,Bas× VBC,t]
QT,t = QB,t + QBC,t
Where: QB,t and QBC,t represent the ammonia mass at time t in blood and the BC fluid respectively; (A)B,t and (A)BC,t are the ammonia concentrations in blood and BC fluid at different times; (A)B,Bas is basal blood ammonia concentration; VB,t and VBC,t are the blood and BC fluid volumes, and QT,t is the total ammonia mass at different times.
The ammonia detoxification capacity is expressed as the % of the initial dose detoxified at different times and was calculated using the following equation.
% Dose = 100 - [(QT,t x 100)/QT,0]
Where QT,0 is total ammonia mass at time 0.
Glucose was determined using a commercial kit (“Glicemia Enzimática AA”, Wiener Laboratories, Rosario, Argentina) and following the manufacturer’s instructions.
Samples of livers from all experimental groups were fixed in 10% formaldehyde, dehydrated, embedded in paraffin, sectioned with a micrometer, stained with hematoxylin-eosin and mounted. Sections were microscopically analyzed and some aspects of the hepatic parenchyma were taken into consideration: Hepatic cell plate organization, the form of endothelial cells and hepatocytes, presence of necrotic areas and blebs in the plasmatic membrane of the hepatocytes. To perform the analyses, we used a light field microscope (Olympus Co, LTD. Model U-MDOB), equipped with a digital camera (Olympus model D-360 Zoom-3.2 megapixels of resolution).
Chemicals were purchased from Sigma (St. Louis, Missouri, United States) and were analytical grade pure.
Results are presented as mean ± SD. We performed a one-way or multifactor analysis of variance with Scheffe’s multiple range test as post-test to establish the statistical significance of the differences between means. P values smaller than 0.05 were taken as statistically significant. The statistical review of the study was performed by a biomedical statistician.
In order to characterize the “in vitro system” operation, different MBR were perfused for 120 min, without any biological component. The mean data of six individual runs are shown in Table 2. The plasma/BC relationship did not change during the experiments. Plasma and KHR solution osmolalities were arrived to equilibrium after the first hour of perfusion, demonstrating a proper exchange of solutes between the two compartments. No significant variation of the hematocrits was observed during the function of the system, but a minimum breakup of the erythrocytes was generated after 120 min of perfusion by the activity of the peristaltic pump. Ammonia concentration became equal in both compartments after the first hour of perfusion and the total mass (Q) of this metabolite remained constant during the whole experiment, indicating that no loss or interactions with any system component occurred. Similar behavior was observed for glucose distribution.
| Perfusion time | (Osm)B/(Osm)BC | Hto (%) | Hemolysis (%) | (Glucose)B/(Glucose)BC | (NH4+)B/(NH4+)BC | QNH4+ (μmol) |
| 0 min | 0.94 ± 0.02 | 47 ± 3 | 0.27 ± 0.09 | 0.09 ± 0.04 | 52.8 ± 4.0 | 36.3 ± 1.6 |
| 60 min | 1.00 ± 0.02 | 44 ± 5 | 0.59 ± 0.10 | 0.77 ± 0.07 | 1.1 ± 0.1 | 36.1 ± 1.6 |
| 120 min | 1.00 ± 0.01 | 45 ± 3 | 0.79 ± 0.12 | 0.90 ± 0.06 | 1.2 ± 0.3 | 36.1 ± 1.5 |
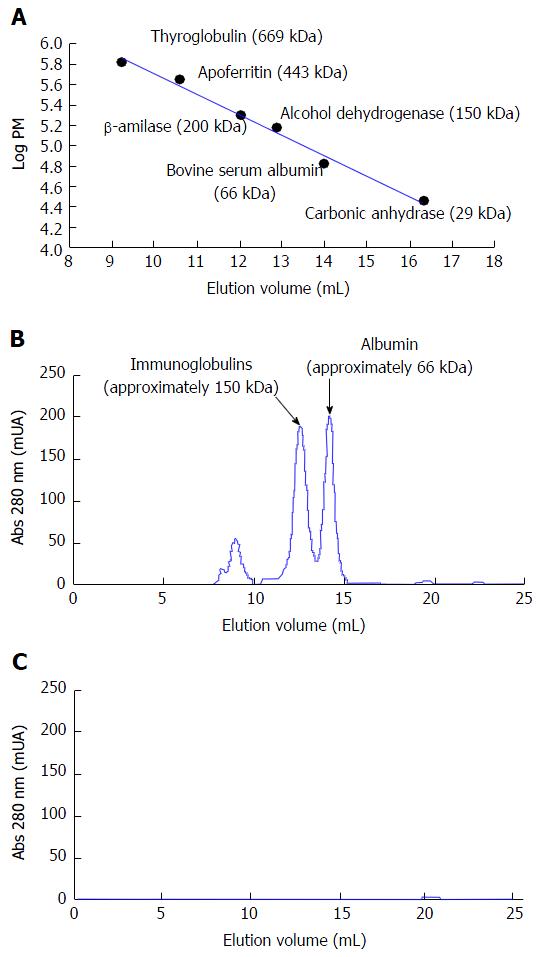
The protein analysis by gel filtration chromatography is shown in Figure 3. In the chromatogram obtained for a sample of basal plasma (Figure 3B) we can observe the presence of two main peaks. Based on the calibration curve obtained (Figure 3A), they can be assigned to the major plasma proteins: Albumin [elution volume (Ve) = 14.15 mL] and immunoglobulins (mainly IgG, Ve = 12.52 mL). Two minor peaks are also appreciated (Ve < 9 mL) that correspond to proteins of high molecular weight (MW > 700 kDa). These could be α2-macroglobulin (MW = 725 kDa, Ve = 8.92 mL) and the pentameric form of IgM (MW = 950 kDa, Ve = 8.22 mL). Figure 3C shows the chromatogram obtained for a sample of the BC fluid after 120 min of blood perfusion (the same result was obtained after 60 min). It can be noticed that none of the plasma proteins was capable of crossing the membrane of the hollow fibers used in the construction of the MBR.
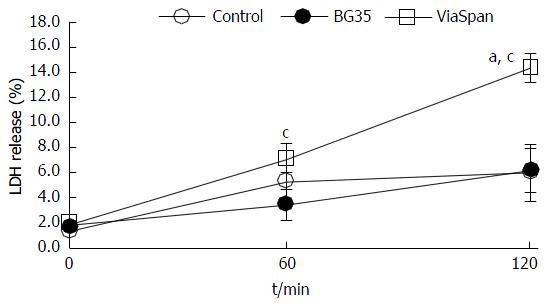
Figure 4 exposes the time changes in LMOs viability (determined by LDH release) throughout the two hours of the experiments performed. One gram of fresh LMOs (controls) or LMOs cold preserved in BG35 and ViaSpan® solutions was loaded into the BC and the MBR was then perfused during 120 min. The amount of enzyme released by fresh LMOs and LMOs cold preserved in BG35 showed a minor increase after two hours of perfusion. However, LMOs preserved in ViaSpan® solution showed a statistically significant raise in this parameter as perfusion time increased. The values of LDH release reached after 120 min in the MBR were: 6.0% ± 2.3% for controls; 6.2% ± 1.7% for LMOs cold preserved in BG35 and 14.3% ± 1.1% for the group cold preserved in ViaSpan®, (P < 0.05, n = 6).
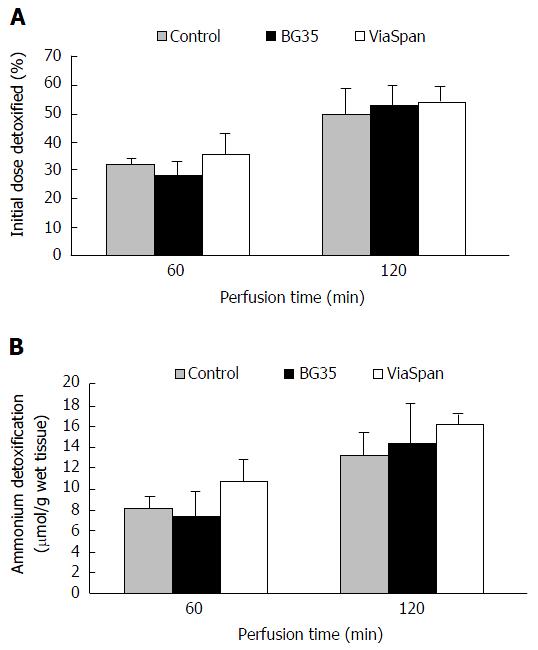
The ammonia detoxification capability of the device was evaluated by measuring the time course evolution of plasma ammonia concentration: An ammonia overload was added to the blood to obtain an ammonia plasma concentration of 1.06 ± 0.12 mmol/L, n = 6. We determined the ammonia content in blood and BC fluid samples obtained before initiating the perfusion (time 0) and after the first and second hour of operation (time 60 and 120 min, respectively). In Figure 5A it can be appreciated the LMOs ammonia detoxification capacity during two hours of MBR functioning. It can be observed that both preserved groups were able to detoxify a percentage of ammonium initial doses similar to control group, during the whole experiment. After two hours, the percentage of the initial dose detoxified (Figure 5A) was 49.3% ± 8.8% for controls LMOs; 52.9 ± 6.5 for BG35 and 53.6 ± 6.0 for ViaSpan® preserved LMOs (n = 6). To get a better knowledge about the amount of ammonia that LMOs were able to metabolize in the MBR, Figure 5B shows the µmols of this compound detoxified per gram of wet tissue. The values reached at the end of the perfusion period were: Control: 13.2 ± 2.2; BG35: 14.2 ± 3.8, and ViaSpan® 16.0 ± 1.1 μmol of NH4+ detoxified/g wet tissue (n = 6).
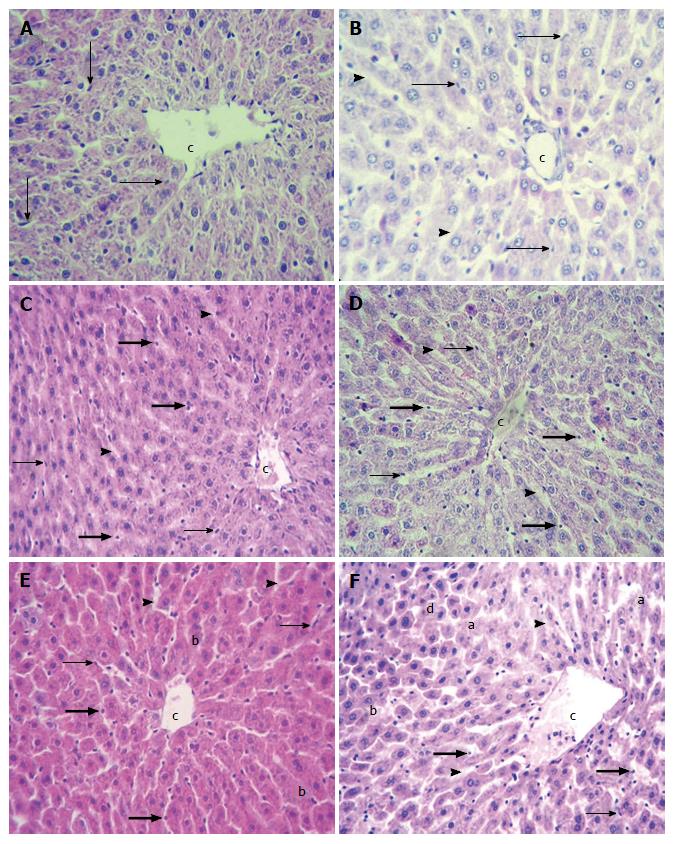
Control and cold preserved LMOs (48 h in BG35 and ViaSpan® solutions) were morphologically analyzed to assess hepatic tissue integrity, at the beginning and after 2 h of perfusion in the MBR.
Control LMOs showed normal hepatocyte cords with fusiform endothelial cells attached to the extracellular matrix of perisinusioidal space (EMPS), both at 0 min and at 120 min of perfusion period in the MBR (Figure 6A and B).
LMOs preserved in BG35 had organized hepatocyte cords with sinusoids slightly dilated and endothelial cells with two different morphology patterns: Fusiform or rounded, both attached to EMPS (Figure 6D) at 0 min. After 120 min, morphological features changed. Heptocyte cords continued to be organized but sinusoids were dilated with abundant rounded endothelial cells either attached to EMPS or seen inside sinusoidal lumen (Figure 6E).
At 0 min, LMOs preserved in ViaSpan® solution showed balonized hepatocytes and abundant rounded endothelial cells. Endothelial cells were attached to EMPS and sinusoidal lumen was dilated (Figure 6D). At 120 min LMOs had abundant blebs and areas of disrupted hepatocyte cords (Figure 6E).
The goal of this study was the development of a simplified BAL prototype suitable to use LMOs as biological component, and the evaluation of fresh and cold preserved rat LMOs performance in this model.
Our simple hollow fiber MBR was constructed to enable the control of LMOs performance (i.e., viability and detoxification, but also suitable for the measurement of other parameters such as synthesis functions specific of liver) and sampling of blood and BC fluid during operation. In a first stage, we characterized this simplified prototype by setting different functional parameters without the biological component. We observed an optimum exchange of fluids and metabolites. The PolyamixTM hollow fibers used allow adequate diffusive and convective mass transport. In order to evaluate the performance of these fibers against large size molecules we determined their permeability to plasma proteins. The experiments using FPLC showed that the pore size of the membranes used, with a cutoff value of 50 kDa, blocks the transfer of plasma proteins into the BC thus preventing damage of LMOs by the hypothetical patient’s immune system proteins (antibodies, complement system).
After checking the system operation without any biological component, as a final step of the “in vitro” characterization of our BAL model, a validation step was performed, evaluating the performance of control and preserved LMOs in the MBR designed. The architecture chosen for the BAL we present here was not trivial; the BAL system in use in our laboratory, with isolated hepatocytes as biological component, was not suitable for LMOs which almost did not detoxify ammonia when applied to it (data not shown). As LMOs detoxification of ammonia on flat plates[11] was satisfactory, we decided to construct a “flat bottom” BAL to allow accommodating the tissue slices in a less crowded manner. In BAL devices designed to use LMOs, it is essential that the bioreactor architecture ensures a good viability of this biological component during the blood detoxification performance.
We observed that LDH releasing from LMOs cold preserved in Viaspan® was increasing with the perfusion time and this phenomenon was not observed for LMOs preserved in BG35 solution or controls. This fact can be attributed to a protective effect exerted by PEG 35000 kDa (key component of BG35 solution) on cell membranes[14,19,20].
Observation of ammonia depuration is an evidence of hepatic synthetic function and is an important feature to propose the device we present here for clinical application[21,22]. When this MBR was challenged with an ammonia overload it showed an effective detoxification of this detrimental metabolite, either when cold preserved or fresh LMOs were examined. LMOs cold preserved in both preservation solutions were able to detoxify a similar percentage of the initial dose as compared to the control group. Although LMOs cold preserved in ViaSpan® showed higher levels of LDH release after 120 min of reperfusion they were able to detoxify an ammonium overload as well as control and cold preserved in BG35 solution LMOs did. Our group had already shown that, immediately after 48 h of cold preservation, ATP levels were severely decreased but they were actively replenished during reperfusion[23,24]. This fact can explain the good ammonium detoxification performance observed and constitute an indication of LMOs conserved mitochondrial function after cold preservation. Histological evaluation of LMOs showed that although BG35 protect hepatic morphology better than ViaSpan® solution, both cold preservation solutions proved to be useful to preserve the biological component integrity in our flat-plate model of MBR.
To provide a clear idea of the amount of ammonia that LMOs were able to metabolize, we also determined the amount (μmol) of this compound detoxified per gram of wet tissue during reperfusion. Once again we found similar levels of ammonium detoxification between control LMOs and LMOs cold preserved in Viaspan® or BG35 solution. The ammonium concentration in blood of patients with acute liver failure (ALF) could be greater than 0.2 mmol/L and it should be considered that also there is a continuous infusion of this metabolite to blood flow. In our in vitro experiments we used a higher concentration (1 mmol/L) since we worked with a single initial dose of ammonium. In addition, Calligaris et al[25] showed that neither cell viability nor ammonium detoxification capacity of freshly isolated hepatocyte suspensions were affected by the concentration of the initial ammonium overload.
It is important to consider that in this work we tested two preservation solutions: ViaSpan® which is the gold standard in liver preservation[26,27] and BG35 that was design by our group specifically to suit cold preservation of LMOs, and the entire liver in the future. The use of BG35 solution for the cold storage of LMOs may facilitate liver research since one litter of ViaSpan® is about 3 time more expensive than the same volume of BG35[11,14].
The experimental MBR presented in this study relied on a simple design and was constructed using standard materials available in most laboratories. Due to these facts we foresee its employment as a useful tool to study the performance of LMOs submitted either to preservation protocols or any other treatment or condition. Taking into account all the results previously shown, we have demonstrated that LMOs could be used as the biological component of the MBR designed, showing an adequate capacity to detoxify ammonia. We have also optimized the techniques to cold preserve this biocomponent to ensure its continuous availability, which is essential for any BAL to become a useful therapeutic tool for patients with ALF. As future prospects, these results encourage us to study other important liver functions, as transcription of albumin and clotting factors during reperfusion and to challenge it to treat acute liver failure of small animal models which will allow the measurement of bilirubin conjugation, blood clotting functions or intracranial pressure all important clinical prognostic predictors for ALF patients[28-30]. Also, to scale this MBR up and evaluate it in big animal models of ALF such as pigs.
The authors would like to thanks Dr. German Rosano for his technical support in FPLC analysis.
Acute liver failure is a condition that sometimes is resolved spontaneously but in most cases requires liver transplantation. The regeneration capacity of the liver seems to be behind the cases were, after the first insult has disappeared, the organ recovers by itself. This has been shown to be a consequence of the amount of viable mass remaining in terms of tissue capacity to cope with the detoxification of harmful metabolites produced by the damage and to provide the needed quantities of essential liver produced molecules and factors. This is why many attempts have been pursued to help the patient´s liver to transit this acute failure and either recover or extend the time frame for a liver transplantation to be practical. In this sense, bioartificial liver (BAL) is thought as the better choice to accomplish this job but till now it is only performed by medical care teams that are able to obtain the biological component in the same unit making the practice limited to very few centers in the world.
A choice for the optimal biological component for BAL devices, as looking forward to develop a tool ready to use worldwide, is not straightforward. Hepatic derived cell lines, whole animal livers (even “humanized” organs) and primary human or animal hepatocytes have been proposed and tested but none have proven to be easily translatable to health centers reality. The work presented here proposes the use of tissue slices [liver Microorgans (LMOs)] and their preservation for at least 48 h in a preservation solution designed by their group. The obtainment of this biological component presents much less technical difficulty than isolation of viable hepatocyes and it bares all the cellular types and a conserved micro-architecture compared to the liver itself. The authors also show the extension of the period of use of these LMOs from few hours to 2 d and they are certain that it could be increased more by tuning the composition of their BG35 solution further.
To date the reports found in the literature inform the use of isolated cells, either primary hepatocytes or continuous cell lines, or even whole pig livers and attempts have been made to cultivate the cellular component on artificial scaffolds mimicking extracellular matrices and micro-architecture. This biological components are used either fresh isolated or obtained directly by in vitro culturing. To the best of our knowledge, the authors are the only group using and combining tissue slices and cold preservation techniques to successfully apply these LMOs onto BAL devices. The authors are still working with the dimensions of a mini-prototype that should be scaled up to be used for human patients and this is the future challenge the authors have to undertake.
It follows that the application of their results would be the design of a BAL accessible on demand at low cost in health care centers for the treatment of patients, with either acute or chronic liver failure, for their recovery, or as a support until organ transplantation, and to ameliorate their quality of life in the process.
This is an in vitro study for demonstration of the bio-artificial liver with detoxification. An interesting study for research design and innovation of the device.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chiu KW S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Wang Y, Susando T, Lei X, Anene-Nzelu C, Zhou H, Liang LH, Yu H. Current development of bioreactors for extracorporeal bioartificial liver (Review). Biointerphases. 2010;5:FA116-FA131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Strain AJ, Neuberger JM. A bioartificial liver--state of the art. Science. 2002;295:1005-1009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 206] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Han B, Shi XL, Zhang Y, Chu XH, Gu JY, Xiao JQ, Ren HZ, Tan JJ, Gu ZZ, Ding YT. Microbiological safety of a novel bio-artificial liver support system based on porcine hepatocytes: a experimental study. Eur J Med Res. 2012;17:13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Giri S, Acikgöz A, Bader A. Isolation and Expansion of Hepatic Stem-like Cells from a Healthy Rat Liver and their Efficient Hepatic Differentiation of under Well-defined Vivo Hepatic like Microenvironment in a Multiwell Bioreactor. J Clin Exp Hepatol. 2015;5:107-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Pan X, Wang Y, Yu X, Li J, Zhou N, Du W, Zhang Y, Cao H, Zhu D, Chen Y. Establishment and characterization of an immortalized human hepatic stellate cell line for applications in co-culturing with immortalized human hepatocytes. Int J Med Sci. 2015;12:248-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Kostadinova R, Boess F, Applegate D, Suter L, Weiser T, Singer T, Naughton B, Roth A. A long-term three dimensional liver co-culture system for improved prediction of clinically relevant drug-induced hepatotoxicity. Toxicol Appl Pharmacol. 2013;268:1-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 206] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 7. | Ebrahimkhani MR, Neiman JA, Raredon MS, Hughes DJ, Griffith LG. Bioreactor technologies to support liver function in vitro. Adv Drug Deliv Rev. 2014;69-70:132-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Rodriguez JV, Pizarro MD, Scandizzi AL, Guibert EE, Almada LL, Mamprin ME. Construction and performance of a minibioreactor suitable as experimental bioartificial liver. Artif Organs. 2008;32:323-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Gershonowitz A, Itach EG, Shouval D, Mitrani D, Ilan Y, Mitrani E. Development of a scaled up liver device incorporating cryo-preserved pig liver micro-organs. J Hepatol. 2004;41:950-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Guan N, Blomsma SA, van Midwoud PM, Fahy GM, Groothuis GM, de Graaf IA. Effects of cryoprotectant addition and washout methods on the viability of precision-cut liver slices. Cryobiology. 2012;65:179-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Pizarro MD, Mediavilla MG, Berardi F, Tiribelli C, Rodríguez JV, Mamprin ME. Cold storage of liver microorgans in ViaSpan and BG35 solutions: study of ammonia metabolism during normothermic reoxygenation. Ann Hepatol. 1979;13:256-264. [PubMed] [Cited in This Article: ] |
| 12. | Rodkey FL, Hill TA, Pitts LL, Robertson RF. Spectrophotometric measurement of carboxyhemoglobin and methemoglobin in blood. Clin Chem. 1979;25:1388-1393. [PubMed] [Cited in This Article: ] |
| 13. | Arnaud FG, Khirabadi BS, Fahy GM. Normothermic blood perfusion of isolated rabbit kidneys. III. In vitro physiology of kidneys after perfusion with Euro-Collins solution or 7.5 M cryoprotectant (VS4). Transpl Int. 2002;15:278-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Mandolino C, Pizarro MD, Quintana AB, Rodríguez JV, Mamprin ME. Hypothermic preservation of rat liver microorgans (LMOs) in bes-gluconate solution. Protective effects of polyethyleneglycol (PEG) on total water content and functional viability. Ann Hepatol. 2011;10:196-206. [PubMed] [Cited in This Article: ] |
| 15. | Mamprin ME, Vega F, Rodriguez JV. Adenosine 5’triphosphate transport and accumulation during the cold preservation of rat hepatocytes in University of Wisconsin solution. World J Gastroenterol. 2005;11:1957-1964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Mamprin ME, Guibert EE, Rodriguez JV. Glutathione content during the rinsing and rewarming process of rat hepatocytes preserved in University of Wisconsin solution. Cryobiology. 2000;40:270-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Olinga P, Merema MT, Hof IH, De Jager MH, De Jong KP, Slooff MJ, Meijer DK, Groothuis GM. Effect of cold and warm ischaemia on drug metabolism in isolated hepatocytes and slices from human and monkey liver. Xenobiotica. 1998;28:349-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | van Anken HC, Schiphorst ME. A kinetic determination of ammonia in plasma. Clin Chim Acta. 1974;56:151-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 98] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Faure JP, Hauet T, Han Z, Goujon JM, Petit I, Mauco G, Eugene M, Carretier M, Papadopoulos V. Polyethylene glycol reduces early and long-term cold ischemia-reperfusion and renal medulla injury. J Pharmacol Exp Ther. 2002;302:861-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Giraud S, Bon D, Neuzillet Y, Thuillier R, Eugene M, Hauet T, Barrou B. Concentration and chain length of polyethylene glycol in islet isolation solution: evaluation in a pancreatic islet transplantation model. Cell Transplant. 2012;21:2079-2088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Shi XL, Gao Y, Yan Y, Ma H, Sun L, Huang P, Ni X, Zhang L, Zhao X, Ren H. Improved survival of porcine acute liver failure by a bioartificial liver device implanted with induced human functional hepatocytes. Cell Res. 2016;26:206-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Gerlach JC. Development of a hybrid liver support system: a review. Int J Artif Organs. 1996;19:645-654. [PubMed] [Cited in This Article: ] |
| 23. | Mamprin ME, Petrocelli S, Guibert E, Rodriguez J. A novel BES-gluconate-sucrose (BGS) solution for cold storage of isolated hepatocytes. Cryo Letters. 2008;29:121-33. [PubMed] [Cited in This Article: ] |
| 24. | Miszczuk G, Mediavilla MG, Pizarro MD, Tiribelli C, Rodríguez J, Mamprin ME. Expression and distribution of aquaporin 8 in rat hepatocytes cold stored 72 hours in modified University of Wisconsin and bes-gluconate-sucrose solutions. Study of their correlation with water content. Cryo Letters. 2012;33:75-85. [PubMed] [Cited in This Article: ] |
| 25. | Calligaris SD, Almada LL, Guibert EE, Tiribelli C, Rodriguez JV. Ammonium detoxifying activity is maintained after 72 hours of cold preservation of rat hepatocytes in University of Wisconsin (UW) solution. Cryo Letters. 2002;23:245-254. [PubMed] [Cited in This Article: ] |
| 26. | Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 986] [Cited by in F6Publishing: 932] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 27. | Southard JH, Belzer FO. Control of canine kidney cortex slice volume and ion distribution at hypothermia by impermeable anions. Cryobiology. 1980;17:540-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Hoekstra R, Nibourg GA, van der Hoeven TV, Plomer G, Seppen J, Ackermans MT, Camus S, Kulik W, van Gulik TM, Elferink RP. Phase 1 and phase 2 drug metabolism and bile acid production of HepaRG cells in a bioartificial liver in absence of dimethyl sulfoxide. Drug Metab Dispos. 2013;41:562-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Hochleitner B, Hengster P, Bucher H, Ladurner R, Schneeberger S, Krismer A, Kleinsasser A, Barnas U, Klima G, Margreiter R. Significant survival prolongation in pigs with fulminant hepatic failure treated with a novel microgravity-based bioartificial liver. Artif Organs. 2006;30:906-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Selden C, Spearman CW, Kahn D, Miller M, Figaji A, Erro E, Bundy J, Massie I, Chalmers SA, Arendse H. Evaluation of encapsulated liver cell spheroids in a fluidised-bed bioartificial liver for treatment of ischaemic acute liver failure in pigs in a translational setting. PLoS One. 2013;8:e82312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |