Published online Jul 8, 2016. doi: 10.4254/wjh.v8.i19.785
Peer-review started: March 16, 2016
First decision: May 17, 2016
Revised: May 23, 2016
Accepted: June 14, 2016
Article in press: June 16, 2016
Published online: July 8, 2016
Hepatitis C virus (HCV) affects 3% of the world population. It represents the main cause of chronic liver disease and is responsible for extra-hepatic complications, such as type 2 diabetes and cardiovascular diseases. HCV includes 7 genotypes differing in the nucleotide sequence variability, the geographic distribution, the rates of viral clearance, the risk of progression to liver fibrosis and to hepatocellular carcinoma, and the response to therapy. Last years have seen remarkable advances in the field of HCV infection with the approval of direct antiviral agents (DAAs) targeting key viral proteins involved in the HCV replication. Several oral regimens combining DAAs from different families have been developed and these regimens showed increased and sustained virological response rates to above 90% reducing the treatment duration to 12 wk or less. In particular, sofosbuvir, a nucleotide analogue nonstructural (NS)5B polymerase inhibitor, and velpatasvir, a NS5A inhibitor, have been tested in two phase 3 trials, the ASTRAL-2 (against HCV genotype 2) and the ASTRAL-3 (against HCV genotype 3), demonstrating to be effective, safe, and well tolerated in patients who were 18 years of age or older and had at least a 6-mo history of HCV infection with a compensated liver disease.
Core tip: Hepatitis C virus (HCV) spread all over the world. In the last years, new therapies with direct antiviral agents draw a great revolution thanks to several oral regimens combining different drugs of this class. The present editorial provides a brief overview on the association between two direct antiviral agents, sofozsbuvir and velpatasvir, and their implication in the treatment of HCV infection.
- Citation: Bonaventura A, Montecucco F. Sofosbuvir/velpatasvir: A promising combination. World J Hepatol 2016; 8(19): 785-789
- URL: https://www.wjgnet.com/1948-5182/full/v8/i19/785.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i19.785
Hepatitis C virus (HCV) infects approximately 3% of the world population and leads to cirrhosis and hepatocellular carcinoma[1]. HCV is a member of the Flaviviridae family of RNA viruses and is classified into 7 genotypes; each genotype is different from the others in its nucleotide sequence. This remarkable genetic diversity represents a challenge for the development of new therapies[2]. HCV genotypes differ not only in the nucleotide sequence variability, but also in their geographic distribution, rates of viral clearance, risk of progression to liver fibrosis and to hepatocellular carcinoma, and response to therapy[3].
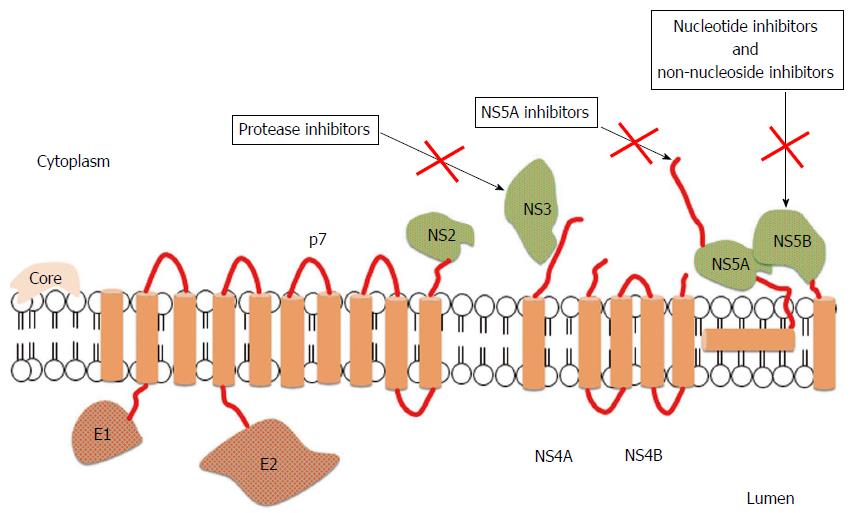
In last years, HCV treatment has undergone substantial advances. Direct antiviral agents (DAAs), when combined with pegylated interferon (PegIFN) and ribavirin (RVR), demonstrated increased rates of cure in chronic HCV infections when compared to PegIFN and RVR alone[4]. The first class of DAAs to be approved for treatment of HCV was that of protease inhibitors targeting the nonstructural protein (NS)3/4A serine protease, responsible for the processing of the nascent viral polyprotein[4]. The search for new key viral targets continued and compounds against two additional targets - NS5A replication scaffold and the NS5B RNA-dependent RNA polymerase (RdRp) - have been generated[5]. Drugs inhibiting NS5B include two subclasses: Nucleos(t)ide inhibitors (NIs) and non-nucleoside inhibitors (NNIs). NS5B is strictly required during the HCV replication both to copy the RNA genome and to transcribe messenger RNA: These steps are essential and the inhibition of NS5B is able to block viral propagation (Figure 1).
Many phase 2 trials have been conducted with promising results. Two phase 3 trials have done encouraging results with the combination of the nucleotide polymerase inhibitor sofosbuvir and the NS5A inhibitor velpatasvir in patients chronically infected with HCV genotype 2 and 3[6].
Sofosbuvir (SOF) (formerly known as GS-7977; Gilead Sciences, Foster City, CA, United States) is a NS5B NI[7]. It is converted into a pharmacologically active form (GS-461203) within hepatocytes, inhibits RdRp activity by competing with uridine, and blocks RNA synthesis by acting as “chain terminator”[8]. Given the high conservation of the catalytic site of the NS5B protein, this drug is believed to have pangenotypic activity[8]. The combination of SOF and RVR achieved sustained virologic response (SVR) rates of 100% for genotype 2 infection and 91% for genotype 3 infection[9]. On December 6, 2013, the United States Food and Drug Administration (FDA) approved SOF (Sovaldi™) for the treatment of chronic HCV, genotypes 1, 2, 3 and 4, in combination with Peg-IFN and RVR or with RVR alone. SOF is also highly effective in HCV patients who are co-infected with human immunodeficiency virus (HIV). It should not be administered with potent inducers of intestinal P-glycoprotein, such as rifampin and Saint John’s wort, resulting in reduced absorption[10]. Moreover, co-administration of SOF with select anticonvulsants, rifabutin, rifapentine, or tipranavir/ritonavir is not recommended[5]. SOF showed no clinically significant drug interactions with many of the common medications metabolized by CYP3A enzymes, such as tacrolimus, cyclosporine, and methadone, as well as with the common combination therapies for HIV[5].
Velpatasvir (VEL) (formerly GS-5816; Gilead Sciences; Foster City, CA, United States) is a new NS5A protein inhibitor with pan-genotypic activity in vitro[11]. In phase 2 trials, it demonstrated high rates of SVR in patients with HCV genotypes 2 and 3 in combination with sofosbuvir for a period of 12 wk of treatment[12,13]. Regimens including an NS5A inhibitor have demonstrated high tolerability and high antiviral efficacy in phase 3 studies[11].
In phase 2 studies, the SOF/VEL combination administered for 12 wk showed high rates of SVR and was well tolerated in all HCV genotype infections[12,13].
In the phase 2 study by Everson et al[12], treatment-naïve non-cirrhotic patients achieved high rates of SVR at 12 wk with SOF/VEL, independently of HCV genotypes. Virologic failure was rare; the nonresponse of 1 patient with HCV genotype 3 infection and the relapse in a patient with HCV genotype 1 infection, both receiving 25 mg of VEL, may suggest that the 100 mg dose could have a clinical advantage over the 25 mg one. In general, the therapy with SOF/VEL with or without RVR was well tolerated. In the other phase 2 study by Pianko et al[13], SOF/VEL demonstrated to be safe, effective, and well-tolerated for treatment-experienced patients with HCV infection genotype 1 or 3, including those with compensated cirrhosis, which are the most common genotypes accounting for approximately 46% and 22% of all global infections, respectively[14]. In particular, the SVR at 12 wk in patients with genotype 3 HCV infection under VEL 100 mg compared favorably with those previously reported for other regimens[15-17].
The ASTRAL program aimed to evaluate the safety and the efficacy of the association SOF/VEL in patients with HCV genotype 1-6 infection including patients with decompensated liver disease. ASTRAL-2 and ASTRAL-3 trials are two randomized, controlled, phase 3 trials, in which a fixed-dose combination tablet of SOF/VEL for 12 wk was compared to standard treatment with SOF plus RVR for 12 or 24 wk in patients already treated for HCV genotype 2 and 3 infection and in those who had not received this treatment, including the ones with decompensated cirrhosis[6].
The two studies shared the same eligibility criteria, except for HCV genotype. Patients who were 18 years of age or older and with at least a 6-mo history of HCV infection could enter the study, whilst patients with clinical evidence of hepatic decompensation were excluded. In the two trials, patients with chronic HCV infection were randomly assigned to receive a fixed-dose combination tablet containing 400 mg of SOF and 100 mg of VEL once daily for 12 wk or 400 mg of SOF plus RVR for 12 wk (for patients with HCV genotype 2) or 24 wk (for patients with HCV genotype 3). RVR was administered orally twice daily, with body weight-determined doses. The primary endpoint was a SVR, defined as an HCV RNA level of less than 15 IU per milliliter at 12 wk after the end of treatment.
In the ASTRAL-2 trial, patients who had received SOF/VEL met the primary endpoint with no virologic failures. In the ASTRAL-3 trial, patients who were administered with SOF/VEL gained a SVR, but 4% of them showed virologic failure after the end of treatment. Overall, the rate of SVR was higher among patients without cirrhosis and who did not receive any previous treatment.
In both trials, rates of adverse events (AEs) were lower among patients who received SOF/VEL than those who received SOF/RVR. In both trials, only 1 patient receiving SOF/VEL stopped the treatment prematurely because of an AE (anxiety, headache, and difficulty in concentrating). Common AEs, such as headache, fatigue, and nausea have been reported in 10%-38% of SOF/VEL patients, together with insomnia, irritability, pruritus, nasopharyngitis, cough, and dyspesia[6]. Serious AEs in the SOF/VEL group of the ASTRAL-2 included enteritis, pneumonia, and abdominal pain, whereas in the ASTRAL-3 acute myocardial infarction, acute cholecystitis, food poisoning, hematochezia, intracranial aneurysm, and rupture of ovarian cyst have been described[6]. In both trials, only 1 patient receiving SOF/VEL stopped the treatment prematurely because of an AE (anxiety, headache, and difficulty of concentration). Death after treatment occurred in two patients in the ASTRAL-2 (1 from cardiac arrest and one from metastatic lung cancer complications); in the ASTRAL-3, two deaths occurred during treatment (1 from unknown cause and 1 from gunshot wounds) and one in the post-treatment period (from unknown cause).
NIs inhibit the RdRp by mimicking NS5B protein substrate leading to the termination of the new viral RNA chain; they possess a high-resistance barrier, are highly effective, and own a pan-genotypic activity[18]. NNIs behave as allosteric inhibitors by binding to the RdRp blocking polymerase function through conformational change; this results in a lower barrier to resistance and lower anti-viral activity with respect to NIs[18].
SOF is the only NI approved and was associated with high SVR rates in all kind of patients. The addition of SOF to PegIFN and RVR demonstrated to be the most effective IFN-containing regimen in HCV patients with compensated cirrhosis. In the NEUTRINO and FISSION studies, patients receiving SOF had rapid and substantial decreases in serum HCV RNA levels. Moreover, AEs were uncommon among patients receiving SOF regimens as well as severe AEs were few in all study groups[19]. In IFN-free regimens, SOF was tested in combination with the first generation NS5A inhibitor ledispavir both in treatment-naïve and pre-treated patients and showed good response in terms of SVR[20,21]. Nonetheless, among all patients, the majority had at least one, mild-to-moderate AE (fatigue, headache, insomnia, and nausea), but also serious AEs occurred in 6%-8% of patients of the 24 wk-SOF regimens[20,21]. AEs were higher and more serious in the groups concomitantly treated with RVR. Finally, some mild modifications of the haemochrome were present, whilst the increase in bilirubin and transaminases were more frequently in the group treated with RVR.
Among NNI, dasabuvir is the only drug approved and is usually administered in combination with ritonavir/paritaprevir and ombitasvir. Dasabuvir is mainly effective against HCV genotype 1. When used in combination, these three drugs showed excellent SVR rates at 12 wk in patients with HCV-1-compensated cirrhosis. Mild AEs were recorded in nearly 80% of patients, especially when treated with RVR and included nausea, fatigue, pruritus, and headache. A modest decrease of hemoglobin was reported, sometimes reaching the lower limit of the normal range[22-26]. Serious AEs were rare. However, in the post-marketing surveillance many cirrhotic were found developing hepatic decompensation and/or liver failure. For this reason, the United States FDA issued a warning in which it was noted that this treatment could cause serious liver injury in cirrhotic patients[27].
The development of DAAs revolutionized the therapeutic weapons against HCV infection. These advances have been possible thanks to the increased knowledge of the structures of HCV protease and HCV polymerase, which permitted to design structure-based drug inhibiting key viral enzymes. Indeed, HCV drug development was fast for different reasons, such as a shorter treatment duration, no need for control arms, and the short period to evaluate the SVR (12-24 wk)[28].
Since 2014, many drugs were approved: The first one was SOF, followed by simeprevir and daclatasvir. In 2015, ledipasvir in combination with SOF, and paritaprevir in combination with ombitasvir and dasabuvir were approved in several countries. In 2016/2017, three other combinations could be approved, such as grazoprevir in combination with elbasvir, SOF with VEL, and ABT-493 plus ABT-530 combination therapy[28].
The combination SOF/VEL was extensively studied in phase 2 and 3 studies. In the ASTRAL-2 and ASTRAL-3 studies, SOF/VEL showed great efficacy against HCV infections genotype 2 and 3 (except for a virologic failure after the end of treatment for 11 patients in the ASTRAL-3) and a reasonable safety[6]. The treatment of patients with HCV genotype 3 is still a challenge, even in the era of DAAs and further researches are needed to increase the rate of SVR in this subtype of patients. As the same researchers of ASTRAL-2 and ASTRAL-3 state, the generalizability of the results are limited by the small number of black patients included in the trials, considering that genotype 2 is present in sub-Saharian Africa[14]. Moreover, the exclusion of patients with decompensated cirrhosis represents another pivotal point to cope with, given the huge importance of HCV in the natural history of cirrhosis and hepatocellular carcinoma, never forgiving the extrahepatic complications, such as type 2 diabetes mellitus and cardiovascular diseases.
Further trials evaluating new DAAs should focus on the prolongation of the SVR and the inclusion of patients such as those abovementioned, which can get a real benefit from these new therapies, if safe, effective, and well tolerated.
Manuscript source: Invited manuscript
P- Reviewer: Kim SR, Kuramitsu Y, Pellicano R S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Cholongitas E, Pipili C, Papatheodoridis G. Interferon-free regimens for the treatment of hepatitis C virus in liver transplant candidates or recipients. World J Gastroenterol. 2015;21:9526-9533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 965] [Cited by in F6Publishing: 924] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 3. | Eslam M, George J. Is hepatitis C subtyping still relevant in the era of direct-acting antiviral therapy? Hepatol Int. 2015;9:5-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Chae HB, Park SM, Youn SJ. Direct-acting antivirals for the treatment of chronic hepatitis C: open issues and future perspectives. ScientificWorldJournal. 2013;2013:704912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | McQuaid T, Savini C, Seyedkazemi S. Sofosbuvir, a Significant Paradigm Change in HCV Treatment. J Clin Transl Hepatol. 2015;3:27-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, Lawitz E, Thompson A, Shiffman ML, Cooper C. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med. 2015;373:2608-2617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 640] [Cited by in F6Publishing: 625] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 7. | Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, Hindes RG, Berrey MM. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368:34-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 603] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 8. | Zopf S, Kremer AE, Neurath MF, Siebler J. Advances in hepatitis C therapy: What is the current state - what come’s next? World J Hepatol. 2016;8:139-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Zhang X. Direct anti-HCV agents. Acta Pharm Sin B. 2016;6:26-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Rodríguez-Torres M. Sofosbuvir (GS-7977), a pan-genotype, direct-acting antiviral for hepatitis C virus infection. Expert Rev Anti Infect Ther. 2013;11:1269-1279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Lawitz E, Freilich B, Link J, German P, Mo H, Han L, Brainard DM, McNally J, Marbury T, Rodriguez-Torres M. A phase 1, randomized, dose-ranging study of GS-5816, a once-daily NS5A inhibitor, in patients with genotype 1-4 hepatitis C virus. J Viral Hepat. 2015;22:1011-1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Everson GT, Towner WJ, Davis MN, Wyles DL, Nahass RG, Thuluvath PJ, Etzkorn K, Hinestrosa F, Tong M, Rabinovitz M. Sofosbuvir With Velpatasvir in Treatment-Naive Noncirrhotic Patients With Genotype 1 to 6 Hepatitis C Virus Infection: A Randomized Trial. Ann Intern Med. 2015;163:818-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Pianko S, Flamm SL, Shiffman ML, Kumar S, Strasser SI, Dore GJ, McNally J, Brainard DM, Han L, Doehle B. Sofosbuvir Plus Velpatasvir Combination Therapy for Treatment-Experienced Patients With Genotype 1 or 3 Hepatitis C Virus Infection: A Randomized Trial. Ann Intern Med. 2015;163:809-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1325] [Cited by in F6Publishing: 1311] [Article Influence: 131.1] [Reference Citation Analysis (0)] |
| 15. | Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, Svarovskaia E, Brainard DM, Symonds WT. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993-2001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 638] [Cited by in F6Publishing: 629] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 16. | Foster GR, Pianko S, Brown A, Forton D, Nahass RG, George J, Barnes E, Brainard DM, Massetto B, Lin M. Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in patients with hepatitis C virus genotype 3 infection and treatment-experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology. 2015;149:1462-1470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 17. | Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, Freilich BF, Younes ZH, Harlan W, Ghalib R. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 504] [Cited by in F6Publishing: 500] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 18. | Majumdar A, Kitson MT, Roberts SK. Systematic review: current concepts and challenges for the direct-acting antiviral era in hepatitis C cirrhosis. Aliment Pharmacol Ther. 2016;43:1276-1292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1322] [Cited by in F6Publishing: 1287] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 20. | Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-1493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1065] [Cited by in F6Publishing: 1042] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 21. | Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1357] [Cited by in F6Publishing: 1329] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 22. | Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, Müllhaupt B, Horsmans Y, Weiland O, Reesink HW. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147:359-365.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 304] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 23. | Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594-1603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 681] [Cited by in F6Publishing: 650] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 24. | Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, Marinho RT, Tsai N, Nyberg A. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983-1992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 556] [Cited by in F6Publishing: 584] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 25. | Kowdley KV, Lawitz E, Poordad F, Cohen DE, Nelson DR, Zeuzem S, Everson GT, Kwo P, Foster GR, Sulkowski MS. Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. N Engl J Med. 2014;370:222-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 225] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 26. | Sulkowski MS, Eron JJ, Wyles D, Trinh R, Lalezari J, Wang C, Slim J, Bhatti L, Gathe J, Ruane PJ. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA. 2015;313:1223-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 270] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 27. | Food and Drug Administration. FDA Drug Safety Communication: FDA warns of serious liver injury risk with hepatitis C treatments Viekira Pak and Technivie, 2015. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm468634.. [Cited in This Article: ] |
| 28. | Asselah T, Boyer N, Saadoun D, Martinot-Peignoux M, Marcellin P. Direct-acting antivirals for the treatment of hepatitis C virus infection: optimizing current IFN-free treatment and future perspectives. Liver Int. 2016;36 Suppl 1:47-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 29. | Chevaliez S, Pawlotsky JM. HCV Genome and Life Cycle. Hepatitis C Viruses: Genomes and Molecular Biology. Chapter 1. Norfolk (UK): Horizon Bioscience; 2006; Available from: http://www.ncbi.nlm.nih.gov/books/NBK1630/. [Cited in This Article: ] |