Published online Jun 8, 2016. doi: 10.4254/wjh.v8.i16.665
Peer-review started: March 13, 2016
First decision: April 18, 2016
Revised: April 25, 2016
Accepted: May 17, 2016
Article in press: May 27, 2016
Published online: June 8, 2016
PubMed, EMBASE, Orphanet, MIDLINE, Google Scholar and Cochrane Library were searched for articles published between 1983 and 2015. Relevant articles were selected by using the following terms: “Liver cirrhosis”, “Endothelial dysfunction”, “Sinusoidal remodeling”, “Intrahepatic angiogenesis” and “Pathogenesis of portal hypertension”. Then the reference lists of identified articles were searched for other relevant publications as well. Besides gross hepatic structural disorders related to diffuse fibrosis and formation of regenerative nodules, the complex morphofunctional rearrangement of the hepatic microvascular bed and intrahepatic angiogenesis also play important roles in hemodynamic disturbances in liver cirrhosis. It is characterized by endothelial dysfunction and impaired paracrine interaction between activated stellate hepatocytes and sinusoidal endotheliocytes, sinusoidal remodeling and capillarization, as well as development of the collateral microcirculation. In spite of the fact that complex morphofunctional rearrangement of the hepatic microvascular bed and intrahepatic angiogenesis in liver cirrhosis are the compensatory-adaptive reaction to the deteriorating conditions of blood circulation, they contribute to progression of disease and development of serious complications, in particular, related to portal hypertension.
Core tip: Besides gross hepatic structural disorders related to diffuse fibrosis and formation of regenerative nodules, the complex morphofunctional rearrangement of the hepatic microvascular bed and intrahepatic angiogenesis play important roles in hemodynamic disturbances in liver cirrhosis. In spite of the fact that these changes of the hepatic vasculature are the compensatory-adaptive reaction to the deteriorating conditions of blood circulation, they contribute to the progression of disease and development of serious complications, in particular, related to portal hypertension.
- Citation: Garbuzenko DV, Arefyev NO, Belov DV. Mechanisms of adaptation of the hepatic vasculature to the deteriorating conditions of blood circulation in liver cirrhosis. World J Hepatol 2016; 8(16): 665-672
- URL: https://www.wjgnet.com/1948-5182/full/v8/i16/665.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i16.665
Besides gross hepatic structural disorders related to diffuse fibrosis and formation of regenerative nodules, the complex morphofunctional rearrangement of the hepatic microvascular bed in liver cirrhosis also contributes to the development of severe complications, in particular, associated with portal hypertension[1]. In this situation, the main place of resistance to portal blood flow is in pathologically modified sinusoids. Sinusoidal endothelial cells (SEC) become dysfunctional and among other features acquire a vasoconstrictor phenotype. It leads to increasing of SEC sensitivity to endogenous vasoconstrictors, such as endothelin, norepinephrine, angiotensin II, vasopressin, leukotrienes, thromboxane A2. In contrast, the production of nitric oxide (the most studied vasodilator involved in the regulation of hepatic vascular tone) is reduced. The reason for this may be insufficient activity of endothelial nitric oxide synthase (eNOS) due to its increased interaction with caveolin-1. Furthermore, endotelin-1 activates G-protein-coupled receptor kinase-2 which directly interacts with and inhibits protein kinase B (Akt) phosphorylation and decreases the production of nitric oxide (NO)[2].
One of the main factors of sinusoidal endothelial dysfunction in cirrhosis is intrahepatic oxidative stress, which is associated with a decrease of eNOS expression and NO bioavailability. For example, cyclooxygenase attenuates Akt-eNOS signalization by stimulating thromboxane A2, which inhibits Akt phosphorylation in endothelial cells, as well as excessive activation of Rho-kinase. Asymmetric dimethylarginine, an endogenous inhibitor of NOS, causes uncoupling of NOS leading to generation of reactive nitrogen species such as peroxynitrite, and down-regulated tetrahydrobiopterin expression promotes that the eNOS cannot generate NO but instead produces O2-, thereby leading to further decreases in NO production. In addition, it was reported that a possible reason for the insufficient bioavailability of nitric oxide might be a reduction of superoxide dismutase (“an enzyme that saves NO”) and increase of homocysteine level in the serum due to reduced expression of cystathionine-γ-lyase and cystathionine-β-syntase[3].
Activated hepatic stellate cells (HSCs) and its paracrine interaction with SEC play very important roles in the sinusoidal microcirculation in liver cirrhosis. In pathological conditions violation of the structure and function of HSCs accompanied by a loss of retinoids reserve and HSCs transformation into myofibroblasts. Activated HSCs start to perform the functions of pericytes. This is confirmed by the expression of its phenotypic markers such as α-smooth muscle actin, desmin, NG2, glial fibrillary acidic protein, as well as emergence or increase of receptors for growth factors, cytokines and endothelin, and a number of cell adhesion molecules on its surface[4].
HSCs, located in the subendothelial Disse spaces between the SEC and hepatocytes, are contacted because of the long branching cytoplasmic processes with nerve endings, which contains various neurotransmitters such as substance P, vasoactive intestinal peptide, somatostatin, cholecystokinin, neurotensin, NO, calcitonin gene-related peptide, and neuropeptide Y. Some vasoactive substances are able to regulate the tone of HSCs. Substances for instance endothelin-1, substance P, angiotensin II, norepinephrine, prostaglandin F2, thromboxane A2, platelet activating factor (PAF) and thrombin can trigger HSC contractility. In contrast, vasoactive substances such as acetylcholine, vasoactive intestinal peptide, NO, carbon monoxide, hydrogen sulfide, prostaglandin E2, and adrenomedullin are known for the ability to relax HSCs[5].
Myosin II is involved in the HSCs contraction, and Ca2+-dependent and Ca2+-independent pathways mediate this process. In a Ca2+-dependent pathway, myosin light chains are phosphorylated by activated myosin light chain kinase, whose activation is induced in response to an increase in intracellular Ca2+ concentration ([Ca2+]i) and subsequent formation of the Ca2+/calmodulin complex. In a Ca2+-independent pathway, Rho kinase and protein kinase C inhibit the activity of myosin light chain phosphatase, an enzyme that dephosphorylates phosphorylated myosin light chains and induces relaxation[6].
Endothelin (ET) as a powerful endogenous vasoconstrictor modulates the tone of the HSCs. ET has three kinds of isoform, ET-1, 2, and 3, which are synthesized from ET by endothelin-converting enzymes. They interact with conjugated protein G receptors A and B types, which are well expressed in the HSCs. ET-1 is the most studied. The main site of its synthesis in liver cirrhosis is activated HSCs. Stimulation of endothelin A receptors leads to its proliferation[7]. Angiotensin II has a similar effect. In liver cirrhosis, HSCs increase its synthesis because of increased expression of angiotensin-converting enzyme. HSCs constriction may also be caused by decreased NO production and/or bioavailability in cirrhotic liver[8]. In contrast, carbon monoxide overproduction by Kupffer cells causes a dilation of the sinusoids and a decrease of hepatic vascular resistance (HVR) because of paracrine impact on HSCs and SEC[9].
Increased HSCs mobility and migration in liver cirrhosis are required to promote enhanced coverage of HSCs around an EC-lined sinusoid, contributing to the process of sinusoidal remodeling[10]. Changes in the structure of the HSCs membrane plays an important role in this process. Cellular locomotion requires dynamic but regulated actin remodeling to form membrane structures that facilitate cell extension. These include lamellipodia, which are membrane protrusions that form the leading edge toward directed cell migration, and filopodia, which are thin, actin filament-structured spikes emanating from the plasma membrane. Small guanosine triphosphatases from the Rho family including RhoA (Rho), Rac1 (Rac), and Cdc42 in turn, closely regulate formation of actin-based structures. Proved that if Rac contributes to HSC migration due to formation of filopodia, the Rho causes a resistant to the inhibitory action of NO and restores the chemotactic response to platelet-derived growth factor (PDGF) in the absence of a functional Rac[11].
A key molecule responsible for proliferation, migration, mobility and recruitment of HSCs is PDGF, which is secreted by endothelial cells and binds to its cognate PDGF receptor (PDGFR-β) on pericytes, in particular due to an ephrin-B2/EphB4 signaling pathway[12]. Moreover, activation of the PDGFR-β causes to stimulation of Raf-1 kinase, MEK kinase and extracellular-signal regulated kinase (ERK), which leads to the proliferation of the HSCs. Phosphatidylinositol 3-kinase activation is also necessary for both mitogenesis and chemotaxis induced by PDGF[13]. In addition, it is shown that the axonal guidance molecule neuropilin-1 contributes to the chemotactic response to PDGF too[14].
Activated HSCs are a rich source of polypeptides, eicosanoids and various other molecules with paracrine, juxtacrine, and autocrine signalization or chemoattractant activity, which include: (1) polypeptides which enhance cells proliferation in an autocrine and paracrine manner: Hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), endothelin-1, insulin-like growth factor, transforming growth factor (TGF)-α, epidermal growth factor (EGF) and acidic fibroblast growth factor (aFGF); (2) members of the TGF-β family; (3) neurotrophins; and (4) hematopoietic growth factors such as erythropoietin[15].
When the liver is damaged, activated HSC proliferate and migrate to areas of inflammation and necrosis of hepatocytes, producing excessive amounts of extracellular matrix components. TGF-β1, PDGF, connective tissue growth factor and FGF regulate this process[16].
Overall there are three general sources of fibrogenic cells in the liver: (1) endogenous (resident) fibroblast or myofibroblast-like cells, mainly represented by HSCs, but also by portal fibroblast, vascular smooth muscle cells and pericytes; (2) the epithelial-mesenchymal transition that may occur in the liver as well as in other organs and lead to transdifferentiation of parenchymal cells; and (3) recruitment of fibrocytes from the bone marrow[17].
In 1983, Rappaport et al[18] were among the first who had described the collateral microcirculation in cirrhotic liver. Nowadays, pathological angiogenesis well characterized in experimental liver fibrosis, as well as in patients with chronic viral and autoimmune liver diseases and nonalcoholic steatohepatitis[19].
Angiogenesis is the complicated physiological process through which new blood vessels form from pre-existing vessels. It is accomplished by the activation of endothelial cells, expression in it proteases, destruction of the extracellular matrix, proliferation, migration of the endothelial cells and formation of high permeability primary vascular structures[20].
The primary inducer of angiogenesis in physiological and pathological conditions is hypoxia. Cells respond to hypoxic stress through multiple mechanisms, including the stabilization of hypoxia-inducible factors (HIFs), which directly regulate the expression of angiogenic growth factors. The family of HIFs includes three α-subunits, which are associated with a common β-subunit (HIF-1β). HIF-1α appears to be ubiquitously expressed, whereas HIF-2α is detected in a more restricted set of cell types, including vascular endothelial cells, hepatocytes, type II pneumocytes, and macrophages. A third mammalian HIF-α subunit, HIF-3α, has also been described, although its role in hypoxic responses is less well understood[21].
NADPH oxidase is an important mediator of angiogenic signaling pathways. It was noted that the increased NADPH oxidase expression because of NADPH oxidase subunit p 47phox phosphorylation leads to an increase in the reactive oxygen species (ROS) levels, contributing to HIF-1α induction, VEGF-receptors (VEGFR) activation and EGF-receptors transactivation[22].
The important role of miRNA has been shown recently in the regulation of cellular response to hypoxia. In particular, Let-7 and miR-103/107 favor the VEGF induction by targeting argonaute 1 protein[23].
The most studied angiogenic growth factors include VEGF family consisting of five homologs: VEGF-A, B, C, D and placental growth factor (PlGF). VEGF stimulates both physiological and pathological angiogenesis. All members of this family are connected to different homologous receptors: VEGFR-1 (Flt-1), VEGFR-2 (KDR/Flk-1), VEGFR-3 (Flt-4), of which only the first and second responsible for angiogenic signals transmitting. Besides that, the binding of VEGA-A to VEGFR-2 and increasing vascular permeability through the nitric oxide are the mechanisms triggering angiogenesis and vasculogenesis.
PlGF, a homolog of VEGF binding VEGFR-1, enhances angiogenesis, but only in pathological conditions affecting, directly and indirectly, multiple cell types, including endothelial cells. In addition, it is assumed that PlGF, breaking the binding of VEGF with VEGFR-1, makes the binding of VEGF with VEGFR-2 more probable. Mass spectrometry studies showed that PlGF and VEGF each induce the phosphorylation of distinct tyrosine residues in VEGFR-1, further indicating that PlGF and VEGF transmit distinct angiogenic signals through VEGFR-1.
Different mechanisms are the basis of synergism between PlGF and VEGF. By activating VEGFR-1, PlGF induces an intermolecular cross talk between VEGFR-1 and VEGFR-2, which thereby is more response to VEGF. PlGF, as a subunit of PlGF/VEGF heterodimer, induces the formation of VEGFR-1/2 heterodimers, which transphosphorylate each other in an intramolecular reaction. By producing PlGF, endothelial cells are thus capable of enhancing their own responsiveness to VEGF but adjacent stromal or inflammatory cells may also release PlGF.
PlGF directly affects smooth muscle cells and fibroblasts, which express VEGFR-1, but may also indirectly influence its proliferation and migration through cytokine release from activated endothelial cells. Through these effects, PlGF recruits smooth muscle cells around nascent vessels, thereby stabilizing them into mature, durable and non-leaky vessels.
PlGF also mobilizes VEGFR-1 positive hematopoietic progenitor cells from the bone marrow and recruits, indirectly via upregulation of VEGF expression, VEGFR-2-positive endothelial progenitor cells to the ischemic tissue. PlGF is also chemoattractive for monocytes and macrophages, which express VEGFR-1[24].
FGF family members are also able to stimulate angiogenesis. Cellular response to FGFs occurs through specific binding FGF-receptor (FGFR), which has internal tyrosine kinase activity. FGFR dimerization is a prerequisite for phosphorylation and activation of signaling molecules with the participation of heparin-binding proteins. This causes migration, proliferation, cell differentiation and destruction of extracellular matrix. It should be noted that while VEGF family members are involved mainly in the formation of the capillaries, FGFs primarily involved in arteriogenesis[25].
Although the angiogenic effect of PDGF is not so expressed as in VEGF, PIGF and FGF, studies in vivo have shown that it may induce the formation of blood vessels and regulate their tone[26].
Tie-2 (Tek), an endothelial-specific receptor tyrosine kinase, and its ligands, the angiopoietins, have been identified as critical mediators of vascular development. Angiopoetin-1 induces endothelial cells migration, inhibits endothelial cells apoptosis and stimulates its formation, promoting stabilization of vessels. At the same time, NADPH oxidase is involved in the ang-1-mediated activation of Akt and mitogen-activated protein kinase (p42/p44 MAPK, or ERK2 and ERK1) and subsequent modulation of endothelial cell migration and angiogenesis[27]. In contrast, angiopoietin-2 causes vascular destabilization by shifting the endothelial cells from the stable state to the proliferative phenotype. However, it may also stimulate angiogenesis in the presence of VEGF[28].
Integrin αVβ3 and αVβ5 are adhesion receptors promoting angiogenesis by mediating migration and proliferation of endothelial cells and the formation of new blood vessels[29].
Endothelial-specific adhesion molecule vascular endothelial cadherin contributes to cell-cell junctions during neovascularization and controls the passage of molecules through the endothelial lining[30].
Thrombospondin-1 - one of the five known thrombospondins - is an adhesive protein that regulates the interaction of cells with each other and with the extracellular matrix. Its expression increases with the progression of liver cirrhosis and strongly correlated with the severity of fibrosis and angiogenesis. However, the precise role of thrombospondin-1 is not defined in this process. It may function as a promoter or inhibitor of angiogenesis that may depend on its concentration, the type of domain being activated and the type of receptors present on endothelial cells[31].
Angiostatin - a fragment of plasminogen, and endostatin - a fragment of the C-terminal part of the collagen XVIII α1-chain, inhibits the migration of human endothelial cells stimulated with FGF and VEGF and do not affect intracellular signaling pathways stimulated by FGF and VEGF[32].
Toll-like receptor 4, which recognizes bacterial lipopolysaccharide, is expressed by SECs involved in fibrosis-associated angiogenesis in cirrhotic liver. These properties are related through the cytosolic adapter protein MyD88, which is involved in the production of extracellular protease regulating the invasive ability of SECs[33].
Hepatic apelin system (apelin/APJ-receptor) - the connecting link between chronic inflammation and subsequent fibrogenic and angiogenic processes in liver cirrhosis. On the one hand, hypoxia and inflammation initiate expression of APJ, on the other profibrogenic activation of APJ mediates the induction of profibrogenic genes, HSCs proliferation and secretion of pro-angiogenic factors[34].
Aquaporin-1 is an integral membrane channel protein, overexpressed in cirrhosis, that promotes angiogenesis by enhancing endothelial invasion[35].
It is known that chemokines from CXC family are involved in angiogenesis. ELR-positive chemokines stimulate this process, and ELR-negative suppress it[36].
Neuropilin-1 and neuropilin-2 are transmembrane glycoproteins with large extracellular domains that interact with both class 3 semaphorins, VEGF and the classical receptors for VEGF, VEGFR-1 and -2, mediating signal transduction. Neuropilin-1 expressed mainly by arterial endothelium, whereas neuropilin-2 is only expressed by venous and lymphatic endothelium. Both neuropilins are commonly over-expressed in regions of physiological and pathological angiogenesis, but the definitive role of neuropilins in angiogenic processes are not fully characterized[37].
Hepatic angiogenesis may substantially differ from homologous processes in other organs or tissue on the basis of: (1) the rather unique phenotypic profile and functional role of activated HSCs and of other liver myofibroblasts; (2) the presence of two different microvascular structures described (i.e., sinusoids lined by fenestrated endothelium vs large vessels lined by a continuous one); and (3) the existence of ANGPTL3, a liver-specific angiogenic factor.
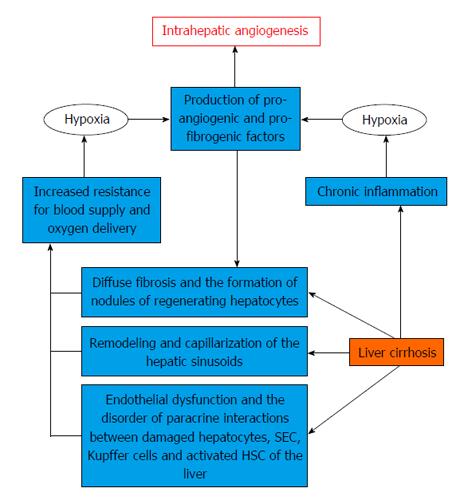
There are two main ways of forming new blood vessels in liver cirrhosis[38] (Figure 1). One of them is associated with increased expression of pro-angiogenic growth factors, cytokines and matrix metalloproteinases on the background of chronic inflammation[39]. Proinflammatory mediators produced by Kupffer cells, mast cells and leukocytes may produce angiogenic response due to induction and increased transcriptional activity of HIF-1α[40].
It is believed that macrophages in the normal state are not directly involved in angiogenesis. In contrast, activated Kupffer cells contribute to the formation of new blood vessels through the production of cytokines, ROS and PAF in liver cirrhosis[41].
Kupffer cells also produce tumor necrosis factor-α (TNF-α), which induces the migration of cells and regulates apoptosis and angiogenesis[42]. The increase of ROS in the liver stimulates angiogenesis due to enhanced expression of TNF-α, NO, HIF-1 and VEGF[43]. PAF promotes the development of VEGF by the activation of nuclear transcription factor nuclear factor κB (NF-κB)[44]. Mast cells involved in the formation of new blood vessels through the production of heparin, histamine, tryptase, cytokines (TGF-β1, TNF-α, interleukins) and VEGF. They can also increase the number of SECs in vitro[45]. Soluble mediators, in particular, pro-inflammatory cytokines, growth factors, proteases, and products of oxidative stress regulate increased expression of chemokines in chronic inflammation of the liver. Leukocytes can thereby penetrate into the liver tissue where they produce angiogenic factors such as VEGF, PIGF, PDGF, FGF, TGF-β1, EGF, angiopoietin-2 and different interleukins[46].
On the one hand, hypoxia, caused by HIF-1α stimulation, activates the HSCs and leads to the production of various angiogenic and fibrogenic factors (PIGF, VEGF, NO, HGF, PDGF)[47], promoting angiogenesis and progression of hepatic fibrosis[48]. On the other hand, diffuse fibrosis, regenerative liver nodules and forming of sinusoidal capillarization cause an increase of HVR and impair the oxygen supply to the liver cells[49]. Accumulation of HIFs, in particular, HIF-1α, increases the VEGF, angiopoietin-1 and their receptors expression on activated HSCs. This leads to involvement and stimulation of SECs, stabilizing the newly formed vessels and providing them with strength[50]. In turn, SECs generate PDGF and TGF-β, helping to attract and migration of HSCs. This process includes ROS-mediated activation of ERK and c-Jun-NH2-terminal kinase (JNK) followed by a delayed- and HIF-1α-dependent up-regulation and release of VEGF[51].
Respectively there are two different phases of an angiogenic process occurring in the liver cirrhosis. Initially, the formation of blood vessels occurs in developing incomplete septa in which concomitant expression of VEGF, Flk-1, and Tie-2 is restricted by HSCs. In a later phase, angiogenesis occurs in large bridging septa and the expression of this proangiogenic panel is limited to endothelial cells and aims to stabilize the newly formed blood vessels[52]. Some of them occur mainly along areas of active inflammation and fibrous septa, probably favors inflammation, tissue repair, and gives rise to intrahepatic shunts. Some of them probably needed for compensation of insufficient intrahepatic blood flow. Other forms intrahepatic shunts bypassing sinusoids and draining blood from the portal to the central venule. Although they perform the decompression role, they can lead to liver dysfunction due to declining oxygen delivery and nutrients to the liver tissues and limiting the free exchange between hepatocytes and sinusoids[53].
During last years it has been found that endothelial progenitor cells produced by stem cells of the bone marrow are capable of causing in situ neovascularization in both physiological and pathological conditions (postnatal vasculogenesis). In particular, they may play an important paracrine role in liver angiogenesis by stimulating resident SECs through as factors as PDGF and VEGF in liver cirrhosis[54]. However, their angiogenic ability is significantly reduced in patients in this category, especially with severe hepatic dysfunction. Perhaps this is because chronic inflammation stimulates the release of angiogenic factors by resident HSCs and SECs, and inhibits the endothelial progenitor cells mobilization into the bloodstream[55].
Endothelial dysfunction and impaired paracrine interaction between activated HSCs and SECs, as well as sinusoidal remodeling and capillarization play an important role in improving the HVR to portal blood flow, adding structural changes associated with diffuse fibrosis and regenerative nodules in liver cirrhosis. The development of intrahepatic angiogenesis can be regarded as a compensatory mechanism that is aimed at decompression of the portal system. However, the newly formed vessels carrying blood to bypass the sinusoids are unable to provide oxygen and nutrients to the liver tissue which leads to progression of the disease. A comprehensive assessment of morphological and functional changes of hepatic vessels in the liver cirrhosis might allow to develop some new correction methods of its specific hemodynamic disorders. Moreover, it could help to enhance the effectiveness of therapeutic interventions aimed at the prevention of portal hypertension complications.
P- Reviewer: Abdel-Razik A, Sharma M S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | Fernandez M. Molecular pathophysiology of portal hypertension. Hepatology. 2015;61:1406-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | García-Pagán JC, Gracia-Sancho J, Bosch J. Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J Hepatol. 2012;57:458-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 3. | Hu LS, George J, Wang JH. Current concepts on the role of nitric oxide in portal hypertension. World J Gastroenterol. 2013;19:1707-1717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 49] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Hellerbrand C. Hepatic stellate cells--the pericytes in the liver. Pflugers Arch. 2013;465:775-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Ueno T, Bioulac-Sage P, Balabaud C, Rosenbaum J. Innervation of the sinusoidal wall: regulation of the sinusoidal diameter. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:868-873. [PubMed] [Cited in This Article: ] |
| 6. | Iizuka M, Murata T, Hori M, Ozaki H. Increased contractility of hepatic stellate cells in cirrhosis is mediated by enhanced Ca2+-dependent and Ca2+-sensitization pathways. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1010-G1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Takashimizu S, Kojima S, Nishizaki Y, Kagawa T, Shiraishi K, Mine T, Watanabe N. Effect of endothelin A receptor antagonist on hepatic hemodynamics in cirrhotic rats. Implications for endothelin-1 in portal hypertension. Tokai J Exp Clin Med. 2011;36:37-43. [PubMed] [Cited in This Article: ] |
| 8. | Lugo-Baruqui A, Muñoz-Valle JF, Arévalo-Gallegos S, Armendáriz-Borunda J. Role of angiotensin II in liver fibrosis-induced portal hypertension and therapeutic implications. Hepatol Res. 2010;40:95-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Reynaert H, Urbain D, Geerts A. Regulation of sinusoidal perfusion in portal hypertension. Anat Rec (Hoboken). 2008;291:693-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Lee JS, Semela D, Iredale J, Shah VH. Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte? Hepatology. 2007;45:817-825. [PubMed] [Cited in This Article: ] |
| 11. | Lee JS, Kang Decker N, Chatterjee S, Yao J, Friedman S, Shah V. Mechanisms of nitric oxide interplay with Rho GTPase family members in modulation of actin membrane dynamics in pericytes and fibroblasts. Am J Pathol. 2005;166:1861-1870. [PubMed] [Cited in This Article: ] |
| 12. | Semela D, Das A, Langer D, Kang N, Leof E, Shah V. Platelet-derived growth factor signaling through ephrin-b2 regulates hepatic vascular structure and function. Gastroenterology. 2008;135:671-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Pinzani M. PDGF and signal transduction in hepatic stellate cells. Front Biosci. 2002;7:d1720-d1726. [PubMed] [Cited in This Article: ] |
| 14. | Cao S, Yaqoob U, Das A, Shergill U, Jagavelu K, Huebert RC, Routray C, Abdelmoneim S, Vasdev M, Leof E. Neuropilin-1 promotes cirrhosis of the rodent and human liver by enhancing PDGF/TGF-beta signaling in hepatic stellate cells. J Clin Invest. 2010;120:2379-2394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2084] [Cited by in F6Publishing: 2041] [Article Influence: 127.6] [Reference Citation Analysis (0)] |
| 16. | Seki E, Brenner DA. Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci. 2015;22:512-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 17. | Svegliati-Baroni G, De Minicis S, Marzioni M. Hepatic fibrogenesis in response to chronic liver injury: novel insights on the role of cell-to-cell interaction and transition. Liver Int. 2008;28:1052-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Rappaport AM, MacPhee PJ, Fisher MM, Phillips MJ. The scarring of the liver acini (Cirrhosis). Tridimensional and microcirculatory considerations. Virchows Arch A Pathol Anat Histopathol. 1983;402:107-137. [PubMed] [Cited in This Article: ] |
| 19. | Elpek GÖ. Angiogenesis and liver fibrosis. World J Hepatol. 2015;7:377-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273-286. [PubMed] [Cited in This Article: ] |
| 21. | Skuli N, Majmundar AJ, Krock BL, Mesquita RC, Mathew LK, Quinn ZL, Runge A, Liu L, Kim MN, Liang J. Endothelial HIF-2α regulates murine pathological angiogenesis and revascularization processes. J Clin Invest. 2012;122:1427-1443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Brandes RP, Miller FJ, Beer S, Haendeler J, Hoffmann J, Ha T, Holland SM, Görlach A, Busse R. The vascular NADPH oxidase subunit p47phox is involved in redox-mediated gene expression. Free Radic Biol Med. 2002;32:1116-1122. [PubMed] [Cited in This Article: ] |
| 23. | Chen Z, Lai TC, Jan YH, Lin FM, Wang WC, Xiao H, Wang YT, Sun W, Cui X, Li YS. Hypoxia-responsive miRNAs target argonaute 1 to promote angiogenesis. J Clin Invest. 2013;123:1057-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Carmeliet P. Manipulating angiogenesis in medicine. J Intern Med. 2004;255:538-561. [PubMed] [Cited in This Article: ] |
| 25. | Klein S, Roghani M, Rifkin DB. Fibroblast growth factors as angiogenesis factors: new insights into their mechanism of action. EXS. 1997;79:159-192. [PubMed] [Cited in This Article: ] |
| 26. | Hellberg C, Ostman A, Heldin CH. PDGF and vessel maturation. Recent Results Cancer Res. 2010;180:103-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 27. | Chen JX, Zeng H, Lawrence ML, Blackwell TS, Meyrick B. Angiopoietin-1-induced angiogenesis is modulated by endothelial NADPH oxidase. Am J Physiol Heart Circ Physiol. 2006;291:H1563-H1572. [PubMed] [Cited in This Article: ] |
| 28. | Pauta M, Ribera J, Melgar-Lesmes P, Casals G, Rodríguez-Vita J, Reichenbach V, Fernandez-Varo G, Morales-Romero B, Bataller R, Michelena J. Overexpression of angiopoietin-2 in rats and patients with liver fibrosis. Therapeutic consequences of its inhibition. Liver Int. 2015;35:1383-1392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Patsenker E, Popov Y, Stickel F, Schneider V, Ledermann M, Sägesser H, Niedobitek G, Goodman SL, Schuppan D. Pharmacological inhibition of integrin alphavbeta3 aggravates experimental liver fibrosis and suppresses hepatic angiogenesis. Hepatology. 2009;50:1501-1511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 30. | Kevil CG, Payne DK, Mire E, Alexander JS. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J Biol Chem. 1998;273:15099-15103. [PubMed] [Cited in This Article: ] |
| 31. | Elpek GO, Gokhan GA, Bozova S. Thrombospondin-1 expression correlates with angiogenesis in experimental cirrhosis. World J Gastroenterol. 2008;14:2213-2217. [PubMed] [Cited in This Article: ] |
| 32. | Eriksson K, Magnusson P, Dixelius J, Claesson-Welsh L, Cross MJ. Angiostatin and endostatin inhibit endothelial cell migration in response to FGF and VEGF without interfering with specific intracellular signal transduction pathways. FEBS Lett. 2003;536:19-24. [PubMed] [Cited in This Article: ] |
| 33. | Jagavelu K, Routray C, Shergill U, O’Hara SP, Faubion W, Shah VH. Endothelial cell toll-like receptor 4 regulates fibrosis-associated angiogenesis in the liver. Hepatology. 2010;52:590-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Melgar-Lesmes P, Pauta M, Reichenbach V, Casals G, Ros J, Bataller R, Morales-Ruiz M, Jiménez W. Hypoxia and proinflammatory factors upregulate apelin receptor expression in human stellate cells and hepatocytes. Gut. 2011;60:1404-1411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Huebert RC, Jagavelu K, Hendrickson HI, Vasdev MM, Arab JP, Splinter PL, Trussoni CE, Larusso NF, Shah VH. Aquaporin-1 promotes angiogenesis, fibrosis, and portal hypertension through mechanisms dependent on osmotically sensitive microRNAs. Am J Pathol. 2011;179:1851-1860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Sahin H, Borkham-Kamphorst E, Kuppe C, Zaldivar MM, Grouls C, Al-samman M, Nellen A, Schmitz P, Heinrichs D, Berres ML. Chemokine Cxcl9 attenuates liver fibrosis-associated angiogenesis in mice. Hepatology. 2012;55:1610-1619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 37. | Staton CA, Kumar I, Reed MW, Brown NJ. Neuropilins in physiological and pathological angiogenesis. J Pathol. 2007;212:237-248. [PubMed] [Cited in This Article: ] |
| 38. | Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50:604-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 413] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 39. | Ehling J, Bartneck M, Wei X, Gremse F, Fech V, Möckel D, Baeck C, Hittatiya K, Eulberg D, Luedde T. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut. 2014;63:1960-1971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 199] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 40. | Chaparro M, Sanz-Cameno P, Trapero-Marugan M, Garcia-Buey L, Moreno-Otero R. Mechanisms of angiogenesis in chronic inflammatory liver disease. Ann Hepatol. 2007;6:208-213. [PubMed] [Cited in This Article: ] |
| 41. | Steib CJ. Kupffer cell activation and portal hypertension. Gut. 2011;60:1307-1308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Lochhead PA, Gilley R, Cook SJ. ERK5 and its role in tumour development. Biochem Soc Trans. 2012;40:251-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8:425-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 814] [Cited by in F6Publishing: 747] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 44. | Ko HM, Seo KH, Han SJ, Ahn KY, Choi IH, Koh GY, Lee HK, Ra MS, Im SY. Nuclear factor kappaB dependency of platelet-activating factor-induced angiogenesis. Cancer Res. 2002;62:1809-1814. [PubMed] [Cited in This Article: ] |
| 45. | Franceschini B, Ceva-Grimaldi G, Russo C, Dioguardi N, Grizzi F. The complex functions of mast cells in chronic human liver diseases. Dig Dis Sci. 2006;51:2248-2256. [PubMed] [Cited in This Article: ] |
| 46. | Marra F. Chemokines in liver inflammation and fibrosis. Front Biosci. 2002;7:d1899-d1914. [PubMed] [Cited in This Article: ] |
| 47. | Copple BL, Bai S, Burgoon LD, Moon JO. Hypoxia-inducible factor-1α regulates the expression of genes in hypoxic hepatic stellate cells important for collagen deposition and angiogenesis. Liver Int. 2011;31:230-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 48. | Lemoinne S, Cadoret A, El Mourabit H, Thabut D, Housset C. Origins and functions of liver myofibroblasts. Biochim Biophys Acta. 2013;1832:948-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 49. | Yokomori H, Oda M, Yoshimura K, Hibi T. Enhanced expressions of apelin on proliferative hepatic arterial capillaries in human cirrhotic liver. Hepatol Res. 2012;42:508-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Coulon S, Heindryckx F, Geerts A, Van Steenkiste C, Colle I, Van Vlierberghe H. Angiogenesis in chronic liver disease and its complications. Liver Int. 2011;31:146-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 51. | Novo E, Povero D, Busletta C, Paternostro C, di Bonzo LV, Cannito S, Compagnone A, Bandino A, Marra F, Colombatto S. The biphasic nature of hypoxia-induced directional migration of activated human hepatic stellate cells. J Pathol. 2012;226:588-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 52. | Novo E, Cannito S, Zamara E, Valfrè di Bonzo L, Caligiuri A, Cravanzola C, Compagnone A, Colombatto S, Marra F, Pinzani M. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Am J Pathol. 2007;170:1942-1953. [PubMed] [Cited in This Article: ] |
| 53. | Vanheule E, Geerts AM, Van Huysse J, Schelfhout D, Praet M, Van Vlierberghe H, De Vos M, Colle I. An intravital microscopic study of the hepatic microcirculation in cirrhotic mice models: relationship between fibrosis and angiogenesis. Int J Exp Pathol. 2008;89:419-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Kaur S, Tripathi D, Dongre K, Garg V, Rooge S, Mukopadhyay A, Sakhuja P, Sarin SK. Increased number and function of endothelial progenitor cells stimulate angiogenesis by resident liver sinusoidal endothelial cells (SECs) in cirrhosis through paracrine factors. J Hepatol. 2012;57:1193-1198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Chen CH, Chang LT, Tung WC, Chen YL, Chang CL, Leu S, Sun CK, Tsai TH, Tsai IT, Chang HW. Levels and values of circulating endothelial progenitor cells, soluble angiogenic factors, and mononuclear cell apoptosis in liver cirrhosis patients. J Biomed Sci. 2012;19:66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |