Published online Apr 18, 2016. doi: 10.4254/wjh.v8.i11.530
Peer-review started: November 12, 2015
First decision: January 4, 2016
Revised: February 22, 2016
Accepted: March 9, 2016
Article in press: March 14, 2016
Published online: April 18, 2016
Crigler Najjar syndrome is associated with indirect hyperbilirubinemia due to a deficiency of enzyme Uridine Di Phospho Glucoronosyl Transferase (UDPGT). Presented here is a case of a female in the first trimester of pregnancy, who was diagnosed to have type 2 Crigler Najjar syndrome. We also discuss the management of this rare disease especially in pregnancy. Unconjugated bilirubin can cross the placental barrier causing neurological damage in the newborn. Patient was carefully monitored during pregnancy and treatment with phenobarbitone in low doses was adjusted such that the serum bilirubin levels were below 10 mg/dL. Crigler Najjar syndrome being rare needs to be diagnosed early in pregnancy to avoid adverse fetal outcomes. Phenobarbitone being an inducer of enzyme UDPGT is used as the first line of treatment and is not teratogenic in the low doses used. Treatment protocol followed was on the basis of previous reported cases and successful perinatal outcome was achieved.
Core tip: Crigler Najjar syndrome type 2 is a rare disorder causing indirect hyperbilirubinemia. In pregnancy placental crossing of unconjugated bilirubin can cause high bilirubin levels in the fetus with low Uridine Di Phospho Glucoronosyl Transferase activity causing permanent neurological impairment in the newborn. Hence timely diagnosis and treatment with low dose phenobarbitone is required.
- Citation: Chaubal AN, Patel R, Choksi D, Shah K, Ingle M, Sawant P. Management of pregnancy in Crigler Najjar syndrome type 2. World J Hepatol 2016; 8(11): 530-532
- URL: https://www.wjgnet.com/1948-5182/full/v8/i11/530.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i11.530
Crigler Najjar type 2 is a rare condition with an incidence of 1 per 1000000 births. There is no predilection to any race or sex. Being an autosomal recessive disorder consanguineous marriage is a risk factor. Uridine Di Phospho Glucoronosyl Transferase (UDPGT) level in the liver is less than 10% of normal. The serum bilirubin ranges from 3-20 mg/dL. Patients usually present with jaundice in the first year of life but can sometimes occur even in the third decade. Acute increase in bilirubin levels can occur during fasting or illness. DNA analysis of UDPGT gene shows mutation in exon 1 × 1-5. Expression analysis of the gene shows residual activity[1]. Greater than 25% fall in bilirubin levels after treatment with phenobarbitone distinguishes it from Crigler Najjar type 1.
Levels of bilirubin can be elevated due to the stress of pregnancy. The placenta is an ineffective barrier for unconjugated bilirubin and can result in high bilirubin levels in the neonate causing kernicterus and sometimes even death[2].
Proper identification of the condition and timely treatment with phenobarbitone can avoid morbidity and mortality in the neonate[3].
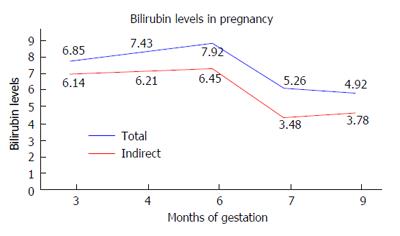
A female patient of age 24 years had a history of jaundice since childhood. She came to us in her first trimester of pregnancy because her jaundice had increased since the previous 2 wk. Patient had unconjugated hyperbilirubinemia with normal liver enzymes. Tests for viral hepatitis, autoimmune liver disease and Wilson’s disease were negative. Abdominal ultrasound including a selective hepatobiliary scan did not show any abnormalities. The UDT1A1 gene was studied for the TATA sequence. The result was negative; Gilbert’s syndrome was thus ruled out. Total bilirubin at 12 wk of gestation was 6.85 mg/dL with indirect bilirubin being 6.14 mg/dL and albumin of 4 g/dL. Her liver enzymes were normal. A dose of 30 mg/d of phenobarbitone was started for the patient. Her serum bilirubin and albumin levels were measured at weekly intervals for the first month and then monthly. Her liver enzymes were also measured simultaneously. We diagnosed the patient to be a case of Crigler Najjar type 2 based on: (1) history of hyperbilirubinemia since childhood; and (2) response to phenobarbitone. A congenital anomaly scan at 20 wk showed no fetal abnormalities. Through her pregnancy, bilirubin levels were maintained in the range of 4 to 8 mg/dL. Figure 1 shows readings of the patient’s bilirubin levels taken throughout pregnancy. Total bilirubin was measured at 4.92 mg/dL at time of delivery, which was completed at the normal full term. At the same time, indirect bilirubin was 3.78 mg/dL. Bilirubin levels in the neonate were normal. As a result, no treatment of any form was required.
Crigler Najjar syndrome is a rare autosomal recessive condition with an incidence of 1 in 1000000 births. Pregnancy in Crigler Najjar syndrome type 2 has been reported only in 6 cases so far (type 1-4 type 2-6 cases)[4].
Our patient was a case of Crigler Najjar type 2 where serum bilirubin usually does not exceed 10 mg/dL. However pregnancy being a stressful condition the bilirubin levels can increase to more than 10 mg/dL. We had monitored the patient’s bilirubin levels, serum albumin and liver enzymes at monthly intervals.
Crigler-Najjar disease type 2 seems to pose no unique maternal risk during pregnancy. The fetus seems to be resistant to elevated maternal unconjugated bilirubin, but the neonate may require therapy for hyperbilirubinemia[5]. Unconjugated bilirubin crosses the placental barrier to cause high levels of bilirubin in the fetus resulting in neurological damage or even death[6]. There is no fixed level of bilirubin at which neurological damage occurs but a proposed level above 10 mg/dL has been suggested[4]. In a study by Holstein et al[7], maternal bilirubin levels between 4.2 and 8.9 maintained by treatment with phototherapy/phenobarbitone resulted in a normal neonate.
Pinkee et al[8] observed that a maternal bilirubin of 10.8 mg/dL at delivery necessitated treatment with exchange transfusions and phototherapy. We had started our patient on low dose phenobarbitone (30 mg daily) and we were able to maintain bilirubin levels less than 10 mg/dL. Phenobarbitone is known to be teratogenic causing facial dysmorphism and mental retardation. However this is seen only at high doses of 750-1500 mg/d and has not been observed at enzyme inducing doses of 60 mg/d[3].
It has been recommended that an acute increase in bilirubin should be treated with phototherapy and albumin. If patient is already on phototherapy then duration of phototherapy should be increased to 24 h. If neurological toxicity develops then plasmapheresis should be done. However our patient had maintained bilirubin levels with phenobarbitone alone.
The newborn was born without jaundice and did not require any treatment. Bilirubin levels over 10 mg/dL is an indication for phototherapy in term infants without risk factors 4 mg/dL in infants with high risk for kernicterus (preterm, low birth weight)[9]. A follow-up of the infant is required for atl east 18 mo[10]. A study by Taylor et al[11] showed that an untreated maternal level of 20 mg/dL resulted in a normal infant at birth but the child developed quadriplegia at 18 mo of age. Hence we propose that the standard guidelines for the management of pregnancy in Crigler najjar syndrome type 2 should be followed[9]: (1) Genetic counselling before becoming pregnant; (2) Folic acid at a dose of 10 mg during pregnancy; (3) Maternal bilirubin serum levels should be below 10 mg/dL; (4) Phenobarbitone at low dose of 60 mg/d; (5) Avoid drugs that increase unbound, unconjugated bilirubin like sulfonamides, salicylates, furosemide, ampicillin, and ceftriaxone; and (6) Neurologic follow-up of the newborn including hearing disorders (brainstem evoked potentials).
Pregnant female with jaundice.
Icterus with negative abdominal findings.
Viral hepatitis, auto-immune hepatitis, Wilson’s disease, hyperbilirubinemias, biliary pathology.
Indirect hyperbilirubinemia with normal liver enzymes.
Normal hepatobiliary scan.
Phenobarbitone 30-60 mg once daily.
Suspicion for indirect hyperbilirubinemias for patients presenting with jaundice and management of Crigler Najjar syndrome in pregnancy with low dose Phenobarbitone.
Short, clear and well written manuscript. A rare disease that should be of interest to the Journal readers.
P- Reviewer: Morini S, Rovas L, Younis JS S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Kadakol A, Ghosh SS, Sappal BS, Sharma G, Chowdhury JR, Chowdhury NR. Genetic lesions of bilirubin uridine-diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler-Najjar and Gilbert syndromes: correlation of genotype to phenotype. Hum Mutat. 2000;16:297-306. [PubMed] [Cited in This Article: ] |
| 2. | Raimondi F, Capasso L, Migliaro F, Romano A, Paludetto R. Prenatal exposure to conjugated bilirubin. Pediatrics. 2006;118:2265. [PubMed] [Cited in This Article: ] |
| 3. | Kjaer D, Horvath-Puhó E, Christensen J, Vestergaard M, Czeizel AE, Sørensen HT, Olsen J. Use of phenytoin, phenobarbital, or diazepam during pregnancy and risk of congenital abnormalities: a case-time-control study. Pharmacoepidemiol Drug Saf. 2007;16:181-188. [PubMed] [Cited in This Article: ] |
| 4. | Passuello V, Puhl AG, Wirth S, Steiner E, Skala C, Koelbl H, Kohlschmidt N. Pregnancy outcome in maternal Crigler-Najjar syndrome type II: a case report and systematic review of the literature. Fetal Diagn Ther. 2009;26:121-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Smith JF, Baker JM. Crigler-Najjar disease in pregnancy. Obstet Gynecol. 1994;84:670-672. [PubMed] [Cited in This Article: ] |
| 6. | Serrano MA, Bayón JE, Pascolo L, Tiribelli C, Ostrow JD, Gonzalez-Gallego J, Marin JJ. Evidence for carrier-mediated transport of unconjugated bilirubin across plasma membrane vesicles from human placental trophoblast. Placenta. 2002;23:527-535. [PubMed] [Cited in This Article: ] |
| 7. | Holstein A, Plaschke A, Lohse P, Egberts EH. Successful photo-and phenobarbital therapy during pregnancy in a woman with Crigler-Najjar syndrome type II. Scand J Gastroenterol. 2005;40:1124-1126. [PubMed] [Cited in This Article: ] |
| 8. | Pinkee S, Renu A, Bharati M. Crigler-Najjar syndrome with pregnancy. J Obstet Gynecol India. 2005;55:270-271. [Cited in This Article: ] |
| 9. | Wilson JH, Sinaasappel M, Lotgering FK, Langendonk JG. Recommendations for pregnancies in patients with crigler-najjar syndrome. JIMD Rep. 2013;7:59-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Shapiro SM. Definition of the clinical spectrum of kernicterus and bilirubin-induced neurologic dysfunction (BIND). J Perinatol. 2005;25:54-59. [PubMed] [Cited in This Article: ] |
| 11. | Taylor WG, Walkinshaw SA, Farquharson RG, Fisken RA, Gilmore IT. Pregnancy in Crigler-Najjar syndrome. Case report. Br J Obstet Gynaecol. 1991;98:1290-1291. [PubMed] [Cited in This Article: ] |