Published online Mar 27, 2015. doi: 10.4254/wjh.v7.i3.362
Peer-review started: August 30, 2014
First decision: November 14, 2014
Revised: December 11, 2014
Accepted: December 29, 2014
Article in press: December 29, 2014
Published online: March 27, 2015
Hepatocellular carcinoma (HCC) is one of the most common malignancies leading to high mortality rates in the general population; in cirrhotic patients, it is the primary cause of death. The diagnosis is usually delayed in spite of at-risk population screening recommendations, i.e., patients infected with hepatitis B or C virus. Hepatocarcinogenesis hinges on a great number of genetic and molecular abnormalities that lead to tumor angiogenesis and foster their dissemination potential. The diagnosis is mainly based on imaging studies such as computed tomography and magnetic resonance, in which lesions present a characteristic classical pattern of early arterial enhancement followed by contrast medium “washout” in late venous phase. On occasion, when imaging studies are not conclusive, biopsy of the lesion must be performed to establish the diagnosis. The Barcelona Clinic Liver Cancer staging method is the most frequently used worldwide and recommended by the international guidelines of HCC management. Currently available treatments include tumor resection, liver transplant, sorafenib and loco-regional therapies (alcoholization, radiofrequency ablation, chemoembolization). The prognosis of hepatocarcinoma is determined according to the lesion’s stage and in cirrhotic patients, on residual liver function. Curative treatments, such as liver transplant, are sought in patients diagnosed in early stages; patients in more advanced stages, were not greatly benefitted by chemotherapy in terms of survival until the advent of target molecules such as sorafenib.
Core tip: This paper reviews the most recent evidence on hepatocarcinoma including its molecular pathogenesis and prognosis, with special emphasis on its diagnosis, staging and treatment. The most recent Easter and Western international guidelines are also reviewed.
- Citation: Tejeda-Maldonado J, García-Juárez I, Aguirre-Valadez J, González-Aguirre A, Vilatobá-Chapa M, Armengol-Alonso A, Escobar-Penagos F, Torre A, Sánchez-Ávila JF, Carrillo-Pérez DL. Diagnosis and treatment of hepatocellular carcinoma: An update. World J Hepatol 2015; 7(3): 362-376
- URL: https://www.wjgnet.com/1948-5182/full/v7/i3/362.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i3.362
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third cancer-related cause of death; it usually develops in patients with hepatic cirrhosis and is the primary cause of death in this patient group[1].
The prevalence of HCC varies worldwide, with a greater incidence in Asia (> 20 cases/100000) than in North America and Europe (< 5 cases/100000)[2]. Seventy to ninety percent (70%-90%) of patients with HCC also have cirrhosis although in Asia, there is a greater number of non-cirrhotic patients with HCC; their malignancy relates mostly to hepatitis B virus (HBV) and hepatitis C verus (HCV) infections[3].
There are several HCC staging systems but the most currently used is the Barcelona Clinic Liver Cancer (BCLC) staging system[4]. This system’s advantage relies on its inclusion of early-stage patients in the therapeutic decision-making schema. BCLC is the system recommended by the American Association for the Study of Liver Disease (AASLD) and the European Association for the Study of the Liver (EASL)[5,6]. The diagnostic methods of choice are magnetic resonance imaging and computed tomography in patients with the classical late washout pattern[6]. If not detected, a diagnostic biopsy must be obtained.
The mortality due to HCC is very high, particularly in patients diagnosed in late-stages and in correlation with the underlying liver disease; however, with the implementation of screening programs in high-risk populations[7], early-stage diagnoses have increased and opened the possibilities to curative therapy. These include surgical resection and the treatment of choice, orthotopic liver transplant. Patients outside the realm of curative therapy are managed loco-regionally (radiofrequency ablation, percutaneous ethanol injection and trans-catheter chemoembolization) or with systemic therapy (sorafenib, doxorubicin and bevacizumab) that have been proven to decrease mortality[8].
The molecular pathogenesis of HCC is a complex process involving numerous events and genetic abnormalities that provide oncogenic capacities to pre-neoplastic cells.
There are molecular abnormalities common to the various etiologies of hepatocarcinoma, the most relevant being mutations of the beta-catenin gene (CTNNB1 gene), the TP53 tumor suppressor gene and deletion of the Axin 1 and Axin 2 genes, both negative regulators of beta-catenin[9]. There is also VEGF gene overexpression (vascular endothelial growth factor) that correlates with the tumor’s angiogenic capacity[10] and has led to attempts to develop target therapies against VEGF[11]. Other oncogenic factors include the overexpression of extracellular matrix metalloprotease inducers (EMMPRIN or CD147) that have been associated to increased vascularization, invasion, metastases development and tumor recurrence[12]. Moreover, up-regulation of the JAK/STAT pathway that activates phosphorylation of the STAT3 transcription factor, found in 50%-100% of all HCC, is also related to angiogenesis and cellular differentiation; this has also recently become a therapeutic target[13,14]. Chromosomal instability is one of the most frequent abnormalities in hepatocarcinoma, whereby amplification of chromosome 1q is the most common followed by amplification of 8q and 5p[15]; HCC has also been associated to deletions of 4q, 8p, 13q, 16q, and 17p[16]. Micro RNA (miRNA) involvement has also been recently described in the development of malignancies since they can act like oncogenes or tumor suppressor genes; specifically in hepatocarcinoma, the relation between miRNA down-regulation (miR-122, miR-141), the up-regulation of others (mi-R21, miR-221), angiogenic capacity, metastases development and apoptosis has been well documented[17,18].
Furthermore, there are specific mechanisms involved in the different HCC etiologies such as hepatitis B infection (HBV), in which viral integration into the human genome leads to the production of truncated proteins such as HBx and pre S2/S that in turn, modulate signaling pathways and induce gene activation fostering oncogenesis[19,20]. Unlike HBV, in hepatitis C (HCV) infection there is no genomic integration and HCV-associated oncogenes have not been identified; hence, all pro-oncogenic abnormalities appear to be cytoplasmic and are conditioned by chronic inflammation, replicative senescence resulting from telomere shortening, oxidative stress, liver steatosis and miRNA overexpression, such as that of miR-155[21,22].
Most cases of HCC develop in patients with chronic liver disease (70%-90%)[23]. Risk factors depend on the region where the studies are conducted; for instance, HCV is a major factor in Europe, Japan and North America (50%-70%), HBV accounts for 10%-15%, alcohol 20% and others, 10%. In Asia and Africa, HBV is associated to 70% of cases and HCV to 20%[1,24] although the synergistic effect of non-alcoholic liver disease is becoming more relevant[25,26]. Diabetes mellitus is an independent risk factor in HCC[27]. Obesity is associated with an increased risk of HCC in both males and females[28]. Tobacco use also increases the risk while coffee intake decreases it[29,30].
The most frequent risk factor for HCC (50% of cases), is chronic HBV infection - including occult infection - secondary to exposure to aflatoxin B1[23,31]. Depending on the study, the relative risk of developing a tumor is close to 100-fold in HBV carriers vs non-carriers; in patients with associated cirrhosis, the risk is even greater[32] fostered by the viral load and the duration of infection[33]. HBV-related HCC may be prevented by vaccination and in patients with chronic infection and viral replication, treatment with antiviral agents may prevent progression of the liver disease and possibly, the long-term development of HCC, although recent evidence reveals that despite adequate viral suppression the risk remain high[34,35].
The incidence of HCC in individuals with cirrhosis due to HCV, is 3%-5% per year[36]. There is currently no available vaccine as in HBV, but preventing the progression of the acute infection to chronic hepatitis and finally cirrhosis with antiviral agents, prevents cancer development; however, the risk of HCC remain higher[37]. In randomized controlled trials, treatment has not been shown to modify disease progression rates or HCC development in patients with chronic HCV and advanced fibrosis[38,39]. There are recent studies showing that elimination of HCV in patients with compensated cirrhosis, decreases the risk of developing the tumor after 10 years[40]. Alcohol has an important influence on tumor development since it acts synergistically in individuals with chronic HBV and/or HCV infection[36]. HIV and HBV or HCV co-infection is an important risk factor, fostering faster liver disease progression than in individuals without HIV; if cirrhosis develops as a result, the risk for HCC is further increased[41].
Screening patients for HCC is recommended in high-risk populations in order to decrease associated mortality if detected in a curable stage[8]. Unfortunately, most detected cases are diagnosed in advanced stages since less than 20% of patients with cirrhosis are screened for HCC[42]; this is due, in great measure, to the first contact physicians’ lack of knowledge of the recommended clinical guidelines although they care for 60% of these patients[43].
The decision to begin screening depends on the individual’s risk and on whether they wish to be treated if diagnosed with HCC. Screening recommendations include: (1) patients with cirrhosis of any etiology, with conserved liver function (Child-Pugh A and B), lacking severe comorbidities; (2) decompensated cirrhosis (Child-Pugh C) on a transplant waiting list; (3) non-cirrhotic chronic HBV infection with active hepatitis or a family history of hepatocarcinoma; and (4) non-cirrhotic HCV infection and advanced liver fibrosis (F3)[5].
Liver ultrasound: Liver ultrasound twice a year is the screening procedure of choice since it is not an invasive method, it is easily available and its cost is moderate. Its sensitivity is 60%-80% and its specificity is above 90%[44]. A recent randomized prospective study revealed that its diagnostic yield was comparable to that of an annual triphasic computed tomography, and at a lower cost[45].
Serum alpha fetoprotein (AFP): Serologic tumor markers are of limited use: although more sensitive than other biomarkers with a cut-off point of 10.9 ng/mL[46], its diagnostic yield is inferior to ultrasound since its concentration depends on the tumor size and thus, preferentially detects tumors in advanced stages.
Ultrasound + alpha fetoprotein: If both strategies are combined, serum alpha fetoprotein levels only add 6%-8% to the number of cases undetected by hepatic ultrasound (HUS)[47]. The combination of these strategies increases the number of false positives as well as costs. There is currently insufficient evidence to support or refute the use of both methods in HCC screening/surveillance in the population with hepatitis B infection[44,48].
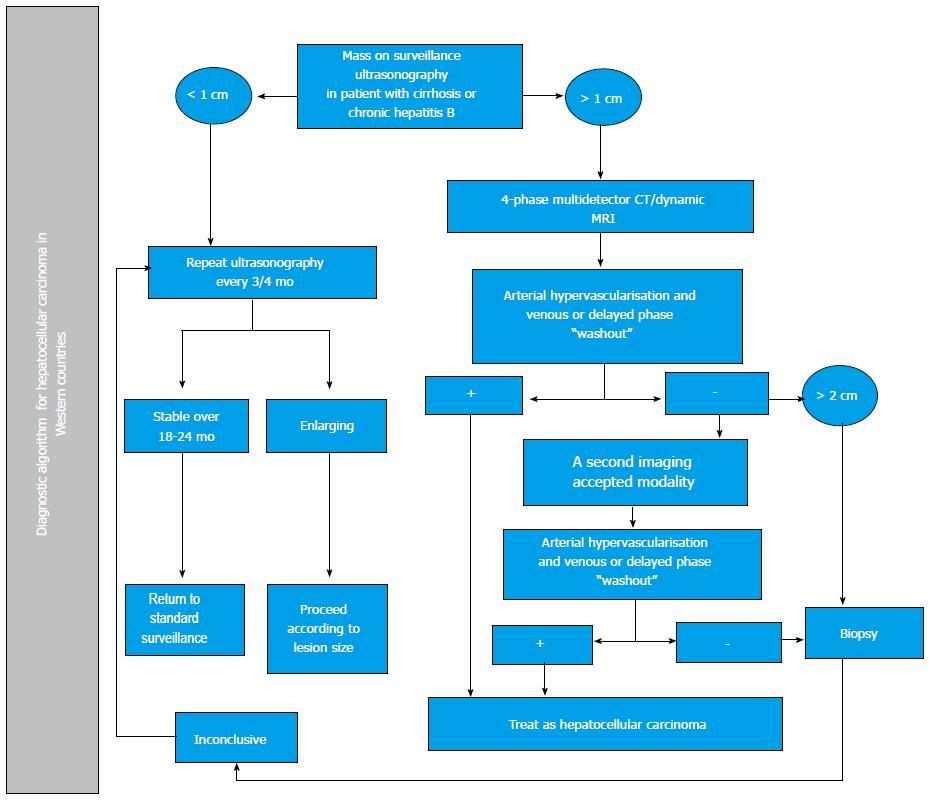
Pathology studies have revealed that most nodules < 1 cm detected in cirrhotic livers, are not HCC[49]. To date, HUS follow-up every 3-4 mo of lesions under 1 cm is recommended. If they grow, evaluation should be conducted according to the size of the lesion; if it remains stable, HUS is recommended every 4 mo[5,6]. In lesions greater than 1 cm, non-invasive diagnostic strategies should be followed with imaging methods; if a HCC diagnosis is not established, a liver biopsy is warranted. If this is inconclusive, the patient should be followed every 4 mo, but if the lesion grows or imaging patterns change, a second biopsy should be obtained[5].
The clinical and economic impact of using guidelines in the diagnosis of HCC, such as those proposed by the AASLD and EASLD, has been recently prospectively evaluated. The sequential approach to hepatic lesions leads to a decreased need for liver biopsies when evaluating nodules between 1 and 2 cm, and also reduces costs when compared with lesions > 2 cm[50].
There are some differences in terms of non-invasive diagnosis between Western and Eastern countries; these differences are reflected in different international guidelines pertaining to each geographical area: EASL[5], AASLD[6], Asian Pacific Association for the Study of the Liver (APASL)[51] and Japanese Society of Hepatology (JSH)[52].
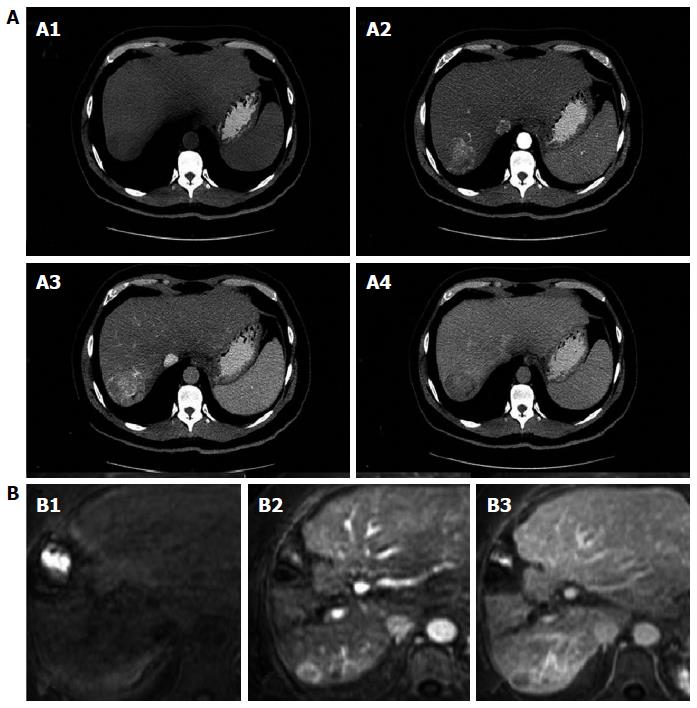
Imaging: Contrast - enhanced computed tomography and Dynamic contrast-enhanced magnetic resonance imaging: The diagnosis of HCC with non-invasive methods should be based on computed tomography (CT) and magnetic resonance imaging (MRI) results showing the characteristic pattern of early arterial enhancement followed by a contrast medium “washout” (Figure 1) phase in late venous phases; it is applicable to lesions > 1 cm[5,6].
Nodules between 1 and 2 cm have a malignancy rate of 14%-23%[53]. If this type of nodule has a characteristic contrast agent-mediated enhancement, the study’s positive predictive value is close to 100% and its sensitivity is 71%, as long as it was performed in a center with sophisticated equipment[6]. If not characteristic, continued evaluation will require the use of two accepted imaging modalities: four-phase CT with contrast medium or dynamic contrast MRI. If these two methods do not reveal the characteristic HCC pattern, the lesion must be biopsied (Figure 2)[54].
Western liver societies do not consider contrast-enhanced ultrasound (CEUS) an appropriate study in the diagnostic approach to HCC due to the theoretical qualm in differentiating HCC from cholangiocarcinoma[55].
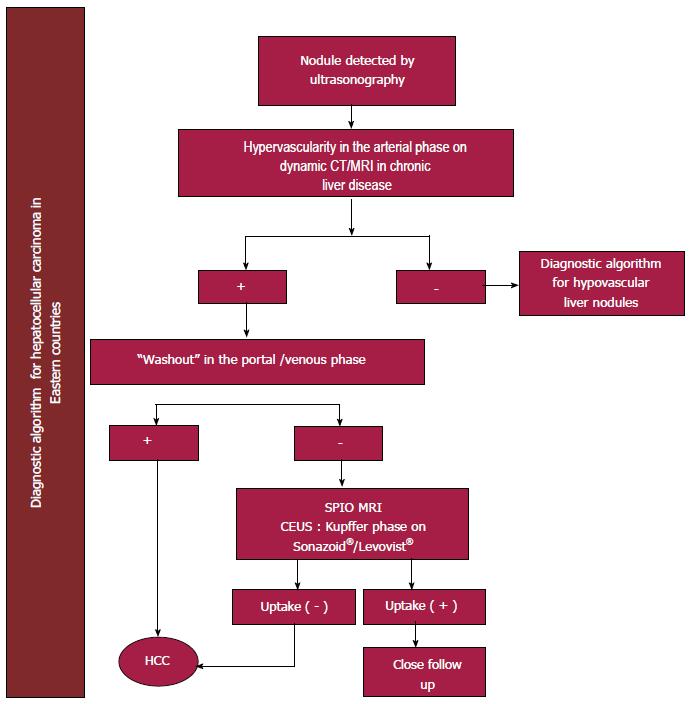
The guidelines proposed by the APASL and the JSH recommend following an algorithm that begins by evaluating the contrast medium pattern in the arterial phase of the imaging study and classifying it as hypervascular or hypovascular. Diagnostic tools include CT, dynamic contrast MRI and CEUS; hence, before the lesion can be classified as hypovascular, more than one study must be performed and should always include CEUS. Hypervascular lesions detected in the arterial phase as well as in the venous washout phase (classic pattern) or hypovascular lesions in the post-vascular phase of the CEUS with Sonazoid® as a contrast agent (in JHS guidelines), are diagnostic of HCC (Figure 3)[51,52]. None of the guidelines suggest that the use of positron-emission computed tomography (PET-CT) is pertinent in the diagnostic approach.
This imaging method is accepted as part of the diagnostic approach of patients with HCC[56-58] in Eastern countries[51,52] but not in the West. Some of its advantages when compared with other imaging methods, include the fact that the microbubbles make it amenable to imaging patients in renal failure and also captures the arterial enhancement phase in real time. Moreover, the washout period has apparently been reported more consistently than with CT or MRI[57,58].
Liver biopsy should only be considered when evaluating nodules greater than 2 cm, if radiological findings are not compatible with HCC, or if findings in any nodule are inconclusive after a thorough work-up. But biopsies can yield false negative results even with immunohistochemical techniques[59]. Alpha fetoprotein is not a useful tissue marker due to its low sensitivity (25%-30%)[60]. Some strategies such as biopsying nodules showing arterial hypervascularity in at least one imaging study or the presence of typical synchronic lesions, have proven to increase sensitivity (62%) and specificity (79%) in the diagnosis of malignancy in nodules between 1 and 2 cm and classified as indeterminate[53].
A histopathological diagnosis is established if the sample is positive for glypican 3, heat shock protein 70 (Hsp70) and glutamine synthetase. Positivity of at least two of these three markers has a diagnostic sensitivity of 72% and a specificity of 100%[60].
However, a negative biopsy does not preclude a HCC diagnosis since the rate of false negative results may reach 30%. This is due to sampling error or to the lack of specific histological findings[60].
The main international societies studying the liver (AASLD, EASL, APASL and JSH) have similarities and differences in terms of HCC screening and diagnosis. The most relevant differences in the HCC diagnostic guidelines[5,6,51,52] in the West and the East hinge on the non-invasive diagnostic algorithm. All four guidelines accept the contrast medium enhanced classic pattern as definitively diagnostic of HCC. Western guidelines (AASLD and EASL) only consider acceptable the following imaging studies: four-phase computed tomography and dynamic-contrast magnetic resonance. Eastern groups propose algorithms that begin by evaluating the size of the lesion. The APASL and JSH recommend initiating the evaluation by analyzing the lesion’s arterial vascularity (hyper or hypovascular). There are important differences between the Western and Eastern guidelines in terms of the non-invasive diagnosis of HCC.
Determining the prognosis of patients with HCC is a crucial step in the management of these patients. An early diagnosis and effective treatment is associated with survival beyond 5 years[5,6].
Several classifications have been proposed in order to stratify patients according to their expected outcomes[4]. Obviously, although there are established guidelines and recommendations, therapy decisions should be individualized taking into account the available scientific evidence and the patient’s personal profile.
Most cases of HCC develop in patients with cirrhosis so for now, determining the patient’s prognosis and therapy should consider the baseline degree of liver damage as well that due to HCC.
Several strategies have been proposed for prognostic staging and decision-making in patients with HCC: Child-Pugh[61], MELD[62], TNM classification[63], tumor volume estimation[64], evaluation of the patient’s performance status (ECOG)[65], all characterized and limited by their one-dimensional assessment.
The most used classification is that developed by the Barcelona Clinic Liver Cancer group[66], a multidimensional strategy. This strategy has been validated in different scenarios and has established recommendations for each stage of the disease[67,68].
For now, the BCLC system is the recommended staging system by international guidelines (Figure 4)[5,6], since it stages the disease and proposes treatment according to the stage:
Very early stage is patients with cirrhosis and compensated liver function (Child-Pugh A), with no signs of portal hypertension and with a single lesion ≤ 2 cm (carcinoma “in situ”). The performance status according to ECOG must be 0. If treated by resection, these patients’ 5-year survival is > 90%[69] and the tumor rarely recurs.
Early stage is patients with a single HCC lesion > 2 cm or three nodular lesions, each ≤ 3 cm in diameter. Liver function should be evaluated according to the Child-Pugh classification and should be limited to groups A and B. The lack of significant portal hypertension and normal serum bilirubin levels are survival predictors in patients with a single lesion that undergo resection[70]. The determined size of the tumor is a criterion when considering liver transplantation as established in the Milan criteria[71]. If these criteria are not fulfilled other therapies are less effective[72]. The risk of vascular invasion is directly proportional to the size of the tumor. Five-year survival in these patients is over 50% after curative transplant[73,74].
Intermediate stage includes patients with one, large HCC lesion as well as asymptomatic patients with multifocal disease and no vascular invasion or extrahepatic lesions. Their reported survival has been approximately 16 mo. Liver function must be preserved (Child-Pugh A and B). These patients may undergo trans-catheter arterial chemoembolization (TACE) which is associated with an increased survival[75]. A recent meta-analysis of randomized clinical trials, suggests that ascites (a contraindication to TACE), is the most important adverse prognostic factor in this sub-group of patients[76].
This stage includes patients who do not fulfill BCLC B criteria. They are symptomatic (pain, general malaise or ECOG 1-2), they have vascular invasion or extrahepatic HCC involvement. Their survival has recently increased (10.7 mo) with sorafenib, a tyrosine kinase inhibitor[67,77].
This stage includes patients with severe hepatic dysfunction (Child-Pugh C) that are not liver transplant candidates and those patients with an ECOG score greater than 2. They have a dire prognosis and a survival under 6 mo while benefitting from conservative therapy (no intervention)[6].
Evaluating a tumor’s molecular classification provides a biological sub-classification that can optimize molecular therapies. These biomarkers allow improved staging. Increased alpha fetoprotein levels are associated to a poor or dire prognosis. Although an optimal cutoff point has not been established, it appears that high alpha fetoprotein levels predict an increased risk of HCC progression while the patient is on the liver transplant waiting list[78].
HCC can be cured by surgical resection or liver transplant if it is diagnosed at an early stage; however, only 15% of cases are selected for management with these treatment modalities[79].
Deciding to perform a liver resection depends on three conditions: tumor size, tumor location and liver function. Resection is considered the treatment of choice in patients with solitary tumors limited to the liver, with no radiological evidence of vascular invasion and with normal liver function (normal total bilirubin, hepatic venous pressure gradient ≤ 10 mmHg, platelets > 100000 and no esophageal varices on endoscopy)[80]. The 5-year survival rate after tumor resection varies between 41% and 77%. Resection is also an option in multifocal HCC, fulfilling or not the Milan criteria or if the patient has mild portal hypertension and is not a liver transplant candidate[81,82]. Loco-regional therapy should be preferably considered in this group of patients, avoiding subsequent liver decompensation. The perioperative mortality after HCC resection in cirrhotic patients is approximately 2%-3%, greater than in patients with no cirrhosis. As a general rule, patients with some manifestation of decompensation (bleeding, ascites or portal hypertension), hepatic reserves are insufficient to consider surgical resection. Ideally, resection should only be considered in patients with tumors ≤ 5 cm in diameter[80,83], although there is consistently more evidence that size may not be a strict criterion in candidate selection; regardless, one must not ignore the fact that the greater the tumor mass, the greater the risk of vascular invasion or dissemination and the recurrence rate increases up to 70% at 5 years[79,84,85]. De novo tumor development may arise after primary resection, although most recurrences appear after 1 or 2 years as a result of dissemination of the primary tumor. The approach to post-resection has not been well studied yet, but repeating the resection is known to be of no value. Rescue liver transplantation or loco-regional therapies with or without multikinase inhibitors may be a viable alternative[86].
In patients with unresectable tumors, the most feasible surgical option is orthotopic liver transplant (OLT) in conjunction with adjuvant therapies such as TACE or percutaneous ablation[80,86]. However, OLT is not an optimal choice in all patients and in spite of a necessary and prudent evaluation, patients should be well selected when dealing with a scarce resource such as organ donation[80]. In 1996, Mazzaferro et al[87] published a prospective cohort study including 48 patients transplanted because of HCC and in accordance with the Milan criteria (a single lesion ≤ 5 cm or 3 lesions ≤ 3 cm each); their survival rate at 4 years was 75%. Therefore, deceased donor liver transplant is a real option in these patients. Over time, experience with this treatment modality has increased and current 5-year survival is above 70% with a 15% recurrence rate, a similar survival to OLT without HCC[5,6,88].
There are several studies investigating the expansion of the Milan criteria, so as to not restrict the tumor size. The University of California proposed the San Francisco criteria that include patients with a single nodule ≤ 6.5 cm or 3 nodules ≤ 4.5 cm and with a total volume no greater than 8 cm; there are also other retrospective and prospective studies with very similar results to the Milan criteria[89]. In spite of these results, international guidelines insist on adhering to the Milan criteria while awaiting more solid data[5,6,51,52].
Interest in down-staging has recently increased targeting patients with HCC exceeding the OLT criteria and that are treated with loco-regional therapy (TACE and/or ARF) in order to decrease the tumor’s size and then fulfill the OLT criteria[90,91]. Current data has led to conflicts, with some experts recommending OLT only in patients that are down-staged effectively while others favor liver transplant as rescue therapy in spite of not having achieved the desired response[92,93]. Yao et al[91] published a study on a down-staging protocol using TACE and/or radiofrequency ablation, and reported a 1-year survival of 96.2% and 92.1% at 4 years, in patients who underwent OLT; they were also recurrence-free after an average follow-up of 25 mo.
The down-staging approach is controversial: some experts believe that large or multifocal tumors have the same recurrence risk in spite of successful down-staging[92]. One of the main poor response and recurrence biomarkers after transplantation is AFP. With a cut-off limit above 1000 ng/mL it could indicate microvascular invasion although further studies are required for confirmation[94].
Upon HCC diagnosis, this group of patients usually has stable liver disease, a disadvantage when awaiting an OLT. In this context, the United Network for Organ Sharing determined that patients fulfilling the Milan criteria should have a MELD score of 22 when added to the transplant waiting list, and the score should increase every 3 mo (the equivalent to a 10% increase in mortality); this is established after computed tomography or magnetic resonance confirmation of Milan criteria fulfillment[95]. This is turn, depends on the study region and on the number of patients on the waiting list, since some remain with stable liver disease and on the list for up to two years. Hence, the Living Donor Living Transplant program is a viable alternative; the risk of donor death and developing complications is 0.3% and 2% respectively. This option is limited to centers of excellence. Whether this group of patients has the same long-term survival as recipients of deceased donor livers remains to be established with certainty.
Loco-regional treatments in patients with HCC are chosen based on their oncological stage, performance status and underlying liver disease(s).
Early stage (BCLC A): Currently, the most commonly used ablation methods are percutaneous ethanol injection (PEI) and radiofrequency ablation (RFA). PEI consists of the direct injection of ethanol into the HCC. This was the first treatment modality used before the development of RFA[96,97]. The curative capacity of PEI in tumors > 2 cm is limited and requires multiple injections over several sessions[98]. PEI can lead to complete tumor necrosis in 70% of nodules < 3 cm and in approximately 100% of nodules < 2 cm.
RFA is currently considered the safest ablation method and yields better results in BCLC A patients[97]. Complete response rates can reach 80% in patients with tumors < 3 cm, 50% in those with tumors between 3 and 5 cm and 25% in tumors > 5 cm. RFA is associated with a 5-year survival of 76%[98].
The available data is sufficient to conclude that RFA significantly improves survival and decreases local recurrence when compared to PEI. PEI use should be limited to circumstances when RFA is unavailable or technically not possible[99,100].
Intra-arterial chemoembolization is the main treatment modality in unresectable HCC. This procedure requires the endovascular placement of a catheter until it reaches the hepatic artery and a microcatheter is guided to the segmental and sub-segmental branches. The chemotherapeutic agents most commonly used are cis-platinum and doxorubicin mixed as an emulsion with lipiodol, an oily radio-opaque contrast agent concentrated in the tumor and that promotes the exposure of neoplastic cells to the drugs. This emulsion is distributed in the affected segments or lobes and selectively infused in the tumor[99]. Survival rates are 82%, 47% and 26% at 1, 3 and 5 years, respectively. Therapy leads to tumor necrosis in 30%-50% of patients but rarely leads to a complete response especially after only one session[98]. Embolizing agents are administered after the chemotherapy emulsion following the same procedure. The most commonly used are: Gelfoam®, polyvinyl alcohol microparticles and trisacryl gelatin microspheres. Vascular obstruction thus decreases the chemotherapeutic agents’ washout[98].
The soft embolization technique is very similar to TACE but without the administration of the chemotherapy emulsion with lipiodol. After diagnostic angiography, embolizing particles are injected directly into the tumor’s afferent artery in order to produce tumor ischemia and necrosis. This technique is useful in patients with a significant tumor load and in whom future progression may lead to no viable treatment options. It has also been associated with less adverse effects[101].
Most advantages of soft embolization are shared with TACE and the debate continues on which technique offers the greatest benefits. Among the few controlled trials comparing TACE/soft embolization vs conservative treatment, survival was the greatest at 1 and 2 years with chemoembolization, 82% and 63% vs 75% and 50% with soft embolization and 63% and 27% with conservative management. Currently, the most commonly used standard technique is TACE. There is recent evidence that TACE in combination with sorafenib may decrease by 35% the risk of death in patients with intermediate and advanced HCC[102].
Terminal stage (BCLC D): This stage includes patients with Child-Pugh C and some with high score B liver disease associated to other comorbidities and terminal stage oncological symptoms. They must be very carefully evaluated and in most cases, loco-regional therapies are not an option since they can lead to the development of severe and even fatal adverse effects[99].
Another application of intra-arterial embolization is in patients in an early HCC stage and in whom ablation therapy is precluded due to the tumor’s location (close to the gallbladder, main bile ducts or main portal vein branches) or other contraindications.
The combination of chemoembolization and radiofrequency ablation has proven to better control tumor growth in lesions between 3 and 5 cm.
The advantages of combined therapy include the fact that hypoxic aggression from embolization and the effects of the chemotherapy agents are synergistic in decreasing the tumor’s blood flow and impedance. Moreover, a disruption of the intra-tumoral septa after chemoembolization, may foster the distribution of heat within the tumor and decrease perfusion-mediated tissue cooling, resulting in a greater ablated area. The suggested protocol is to first perform the selective chemoembolization followed by radiofrequency ablation within the subsequent 14 d[103].
An increase in survival has been demonstrated with combined treatment vs RFA with rates of 92%, 66% and 61% vs 85%, 59% and 45% at 1, 3 and 4 years, respectively. Recurrence-free survival rates have been reported as 79%, 60% and 54% vs 66%, 44% and 38% throughout the same follow-up periods[104].
The molecular pathways involved in the pathogenesis of HCC are manifold but there are few therapeutic modalities specifically directed to these molecular targets that have yielded relevant results; the most studied and validated is the use of sorafenib. This molecule acts by inhibiting multiple kinases, including the Raf-1 and B-Raf serine-threonine kinases, VEGFR 1, 2 and 3 and PDGFR-β[105]. In the initial phaseIstudies, sorafenib led to partial responses in various solid tumors and among them, one hepatocarcinoma case[106].
The SHARP study focused on the Western population. They assigned 602 patients with Child A cirrhosis and good performance status (ECOG 0 - 1 in over 90%), that had never received systemic therapy; they were randomized into a group treated with sorafenib 400 mg bid and a placebo group. Their main outcome was overall survival (OS) and symptomatic progression-free survival. Overall survival was significantly greater in the sorafenib arm, with a survival rate of 10.7 mo vs 7.9 mo (HR = 0.69, 95%CI: 0.55-0.87; P < 0.001) and there was no difference in terms of symptomatic progression (4.9 mo vs 4.1 mo; P = 0.77) although radiological progression did decrease when evaluated by RECIST (5.5 mo vs 2.8 mo, HR = 0.58, 95%CI: 0.45-0.74, P < 0.001). No patient had a complete response, only 2% of the patients had partial response in the sorafenib group and 1%in the placebo group. Up to 80% developed an adverse event, almost all grade 1 or 2. Grade 3 events not found in the placebo group included diarrhea and hand-foot syndrome, each in 8% of cases; there were no grade 4 events[67]. In an Asian phase III study of 226 patients fulfilling similar selection criteria, results were very similar. The increase in OS was a little less marked, 6.5 mo vs 4.2 mo (HR = 0.68, 95%CI: 0.50-0.93; P = 0.014) and in terms of disease progression, 4.2 vs 2.8 mo (HR = 0.57, 95%CI: 0.42-0.79; P = 0.0005). Although survival in this study was not as good as in SHARP, this difference was attributed to the fact that they included patients with worse performance status and more advanced disease; HR were very similar[107]. A SHARP sub-analysis revealed that patients with HCV benefitted more from sorafenib (14 mo vs 7.4 mo, difference of 6.6 mo) when compared with patients with HBV (10.3 mo vs 8 mo, difference of 2.3 mo); one must emphasize that almost 75% of patients in the Asian study were infected with HBV while only 20% were so in the Western study, another possible explanation for the observed difference between studies.
In the United States, the Food and Drug Administration approved sorafenib without specifying the severity of liver disease, but in patients with Child B cirrhosis its benefits are much less evident. A retrospective analysis of 59 patients (26 Child A, 23 Child B, 10 Child C) revealed an OS of 8.3, 4.3 and 1.5 mo, respectively; grade 3-4 adverse events were present in 15% of Child A patients vs 30% in Child B[108].
In the GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafenib), there were also more reported G3-4 adverse events in Child B patients (67% vs 42%) and a greater possibility of abandoning treatment due to these adverse effects (40% vs 25%)[109].
Other strategies such as combining sorafenib with chemotherapy have been attempted. In a phase II study of 96 Child A patients, two groups were defined: sorafenib 400 mg bid and doxorubicin 60 mg/m2vs doxorubicin and placebo, OS was 13.7 mo vs 6.5 mo (P = 0.006) and progression-free survival was 6.0 mo vs 2.7 mo[110]. A phase II study is currently being conducted (CALGB 80802). Other studies of targeted therapy plus chemotherapy have yielded controversial results.
Other treatments such as sunitinib have been attempted but a phase III study revealed worse survival (7.9 mo vs 10.2 mo when compared with sorafenib) and more frequent and severe toxicity[111]. Other molecules such as cetuximab, erlotinib and everolimus have also not proven to be superior to sorafenib or have not been studied comparatively.
Sorafenib is currently considered first-line systemic therapy due its effectiveness and toxicity profile. Some clinical markers (rash, hypertension) as well as molecular markers (VEGF genotypes, VEGF polymorphisms, Mcl-1 expression, pERK) may reflect its efficacy, but none have been validated[109].
In spite of advances in treatment, mortality in HCC remains high. In untreated patients, 1-year survival is 17.5% and 7.3% at 2 years[76]. Due to patient heterogeneity, their clinical status, the available therapeutic options and particularly the presence or lack of liver disease, prognosis is difficult to establish unlike in other neoplasias in which prognostic factors are solely determined by the tumor.
There are currently numerous staging systems[4] and although there is no consensus, the AASLD and EASL guidelines recommend the use of the BCLC system; according to this classification, 5-year survival of stage A patients is 50%-70% after curative treatment, 16-20 mo in stage B, 6-10 mo in stage C and 3-4 mo in stage D[66,73,74,76]. However, several factors of great impact on mortality are not considered in this classification.
HCV and HBV infection also compromise survival in non-cirrhotic patients undergoing curative surgery by conferring an increased and earlier risk of recurrence. Persistent HBV viremia also fosters an increased recurrence risk[4,35,112-115].
In patients without liver disease, HCC tends to be diagnosed at a more advanced age than in patients with cirrhosis and it is usually detected in latter stages (BCLC D in 51.6% vs 42% in patients with cirrhosis) due to the lack of screening; however, mortality in patients in intermediate stages is lower than that in patients with cirrhosis. In this group of patients, the BCLC classification correlates best with survival than other staging systems and their survival rates are better due to the possibility of providing curative treatment of larger lesions in turn, leading to decreased recurrences (27% vs 73%) and greater survival (81% vs 23%)[116,117].
Upon recent inclusion of molecular markers such as wtER, IGF and VEGF-1 in prognostic scoring systems such as CLIP, their precision has been favorable although they are not currently routinely used[118-120].
P- Reviewer: Higuera-de la Tijera MF, Morales-Gonzalez JA, Pan WS S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11128] [Cited by in F6Publishing: 11614] [Article Influence: 893.4] [Reference Citation Analysis (4)] |
| 2. | Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15 Suppl 4:5-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 626] [Cited by in F6Publishing: 702] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 3. | Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1013] [Cited by in F6Publishing: 938] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 4. | Maida M, Orlando E, Cammà C, Cabibbo G. Staging systems of hepatocellular carcinoma: a review of literature. World J Gastroenterol. 2014;20:4141-4150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 80] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4059] [Cited by in F6Publishing: 4345] [Article Influence: 362.1] [Reference Citation Analysis (2)] |
| 6. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6338] [Article Influence: 487.5] [Reference Citation Analysis (1)] |
| 7. | Yeh YP, Hu TH, Cho PY, Chen HH, Yen AM, Chen SL, Chiu SY, Fann JC, Su WW, Fang YJ. Evaluation of abdominal ultrasonography mass screening for hepatocellular carcinoma in Taiwan. Hepatology. 2014;59:1840-1849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 929] [Cited by in F6Publishing: 1055] [Article Influence: 105.5] [Reference Citation Analysis (1)] |
| 9. | Kan Z, Zheng H, Liu X, Li S, Barber TD, Gong Z, Gao H, Hao K, Willard MD, Xu J. Whole-genome sequencing identifies recurrent mutations in hepatocellular carcinoma. Genome Res. 2013;23:1422-1433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 378] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 10. | Yu DC, Chen J, Ding YT. Hypoxic and highly angiogenic non-tumor tissues surrounding hepatocellular carcinoma: the ‘niche’ of endothelial progenitor cells. Int J Mol Sci. 2010;11:2901-2909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Zhu AX, Finn RS, Mulcahy M, Gurtler J, Sun W, Schwartz JD, Dalal RP, Joshi A, Hozak RR, Xu Y. A phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancer. Clin Cancer Res. 2013;19:6614-6623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Tang X, Guo N, Xu L, Gou X, Mi M. CD147/EMMPRIN: an effective therapeutic target for hepatocellular carcinoma. J Drug Target. 2012;Aug 29; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Yang SF, Wang SN, Wu CF, Yeh YT, Chai CY, Chunag SC, Sheen MC, Lee KT. Altered p-STAT3 (tyr705) expression is associated with histological grading and intratumour microvessel density in hepatocellular carcinoma. J Clin Pathol. 2007;60:642-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Mohan CD, Bharathkumar H, Bulusu KC, Pandey V, Rangappa S, Fuchs JE, Shanmugam MK, Dai X, Li F, Deivasigamani A. Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription (STAT) pathway in hepatocellular carcinoma in vitro and in vivo. J Biol Chem. 2014;289:34296-34307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 15. | Fernandez-Banet J, Lee NP, Chan KT, Gao H, Liu X, Sung WK, Tan W, Fan ST, Poon RT, Li S. Decoding complex patterns of genomic rearrangement in hepatocellular carcinoma. Genomics. 2014;103:189-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Homayounfar K, Schwarz A, Enders C, Cameron S, Baumhoer D, Ramadori G, Lorf T, Gunawan B, Sander B. Etiologic influence on chromosomal aberrations in European hepatocellular carcinoma identified by CGH. Pathol Res Pract. 2013;209:380-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ, Shen R, Huang Y, Chen HC, Lee CH, Tsai TF. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest. 2012;122:2884-2897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 612] [Cited by in F6Publishing: 631] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 18. | Xu J, Wu C, Che X, Wang L, Yu D, Zhang T, Huang L, Li H, Tan W, Wang C. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog. 2011;50:136-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 448] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 19. | Amaddeo G, Cao Q, Ladeiro Y, Imbeaud S, Nault JC, Jaoui D, Gaston Mathe Y, Laurent C, Laurent A, Bioulac-Sage P. Integration of tumour and viral genomic characterisations in HBV-related hepatocellular carcinomas. Gut. 2014;Jun 9; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Lin CM, Wang GM, Jow GM, Chen BF. Functional analysis of hepatitis B virus pre-s deletion variants associated with hepatocellular carcinoma. J Biomed Sci. 2012;19:17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Lin MV, King LY, Chung RT. Hepatitis C virus-associated cancer. Annu Rev Pathol. 2015;10:345-370. [PubMed] [Cited in This Article: ] |
| 22. | Zhang Y, Wei W, Cheng N, Wang K, Li B, Jiang X, Sun S. Hepatitis C virus-induced up-regulation of microRNA-155 promotes hepatocarcinogenesis by activating Wnt signaling. Hepatology. 2012;56:1631-1640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 241] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 23. | Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 24. | Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe, 1980-2004. Hepatology. 2008;48:137-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 321] [Article Influence: 20.1] [Reference Citation Analysis (1)] |
| 25. | Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972-1978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 893] [Cited by in F6Publishing: 883] [Article Influence: 63.1] [Reference Citation Analysis (1)] |
| 26. | Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513-521, 521.e1-e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 671] [Cited by in F6Publishing: 652] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 27. | Elkrief L, Chouinard P, Bendersky N, Hajage D, Larroque B, Babany G, Kutala B, Francoz C, Boyer N, Moreau R. Diabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis C. Hepatology. 2014;60:823-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 28. | Chen Y, Wang X, Wang J, Yan Z, Luo J. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. Eur J Cancer. 2012;48:2137-2145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 29. | Koh WP, Robien K, Wang R, Govindarajan S, Yuan JM, Yu MC. Smoking as an independent risk factor for hepatocellular carcinoma: the Singapore Chinese Health Study. Br J Cancer. 2011;105:1430-1435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Setiawan VW, Wilkens LR, Lu SC, Hernandez BY, Le Marchand L, Henderson BE. Association of coffee intake with reduced incidence of liver cancer and death from chronic liver disease in the US multiethnic cohort. Gastroenterology. 2015;148:118-125; quiz e15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 31. | Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, Su J, Sun CA, Liaw YF, Chen CJ. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138:1747-1754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 247] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 32. | Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1838] [Cited by in F6Publishing: 1721] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 33. | Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, Su J, Hsiao CK, Wang LY, You SL. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology. 2011;141:1240-1248, 1248.e1-e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 34. | Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53:348-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 35. | Cho JY, Paik YH, Sohn W, Cho HC, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut. 2014;63:1943-1950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 36. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1691] [Cited by in F6Publishing: 1665] [Article Influence: 83.3] [Reference Citation Analysis (2)] |
| 37. | Dohmen K, Kawano A, Takahashi K, Shigematsu H, Tanaka H, Haruno M, Yanagita K, Ichiki Y, Mori T, Hayashida K. The incidence and risk factors for the development of hepatocellular carcinoma after peginterferon plus ribavirin therapy for chronic hepatitis C. Hepatogastroenterology. 2013;60:2034-2038. [PubMed] [Cited in This Article: ] |
| 38. | Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, Lee WM, Lok AS, Bonkovsky HL, Morgan TR. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429-2441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 326] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 39. | Bruix J, Poynard T, Colombo M, Schiff E, Burak K, Heathcote EJ, Berg T, Poo JL, Mello CB, Guenther R. Maintenance therapy with peginterferon alfa-2b does not prevent hepatocellular carcinoma in cirrhotic patients with chronic hepatitis C. Gastroenterology. 2011;140:1990-1999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1165] [Cited by in F6Publishing: 1128] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 41. | Merchante N, Merino E, Rodríguez-Arrondo F, Tural C, Muñoz J, Delgado-Fernández M, Jover F, Galindo MJ, Rivero A, López-Aldeguer J. HIV/hepatitis C virus-coinfected patients who achieved sustained virological response are still at risk of developing hepatocellular carcinoma. AIDS. 2014;28:41-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, El-Serag HB. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154:85-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 43. | Dalton-Fitzgerald E, Tiro J, Kandunoori P, Halm EA, Yopp A, Singal AG. Practice Patterns and Attitudes of Primary Care Providers and Barriers to Surveillance of Hepatocellular Carcinoma in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2014;Jul 11; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 44. | Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 519] [Cited by in F6Publishing: 515] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 45. | Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography -- a randomised study. Aliment Pharmacol Ther. 2013;38:303-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 46. | Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, Reddy KR, Harnois D, Llovet JM, Normolle D. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 462] [Cited by in F6Publishing: 502] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 47. | Di Bisceglie AM, Sterling RK, Chung RT, Everhart JE, Dienstag JL, Bonkovsky HL, Wright EC, Everson GT, Lindsay KL, Lok AS. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43:434-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 48. | Aghoram R, Cai P, Dickinson JA. Alpha-foetoprotein and/or liver ultrasonography for screening of hepatocellular carcinoma in patients with chronic hepatitis B. Cochrane Database Syst Rev. 2012;9:CD002799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Bremner KE, Bayoumi AM, Sherman M, Krahn MD. Management of solitary 1 cm to 2 cm liver nodules in patients with compensated cirrhosis: a decision analysis. Can J Gastroenterol. 2007;21:491-500. [PubMed] [Cited in This Article: ] |
| 50. | Manini MA, Sangiovanni A, Fornari F, Piscaglia F, Biolato M, Fanigliulo L, Ravaldi E, Grieco A, Colombo M. Clinical and economical impact of 2010 AASLD guidelines for the diagnosis of hepatocellular carcinoma. J Hepatol. 2014;60:995-1001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 732] [Cited by in F6Publishing: 813] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 52. | Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 549] [Cited by in F6Publishing: 630] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 53. | Khalili K, Kim TK, Jang HJ, Yazdi LK, Guindi M, Sherman M. Indeterminate 1-2-cm nodules found on hepatocellular carcinoma surveillance: biopsy for all, some, or none? Hepatology. 2011;54:2048-2054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 724] [Cited by in F6Publishing: 691] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 55. | Vilana R, Forner A, Bianchi L, García-Criado A, Rimola J, de Lope CR, Reig M, Ayuso C, Brú C, Bruix J. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology. 2010;51:2020-2029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 56. | Ryu SW, Bok GH, Jang JY, Jeong SW, Ham NS, Kim JH, Park EJ, Kim JN, Lee WC, Shim KY. Clinically useful diagnostic tool of contrast enhanced ultrasonography for focal liver masses: comparison to computed tomography and magnetic resonance imaging. Gut Liver. 2014;8:292-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Takahashi M, Maruyama H, Shimada T, Kamezaki H, Sekimoto T, Kanai F, Yokosuka O. Characterization of hepatic lesions (≤ 30 mm) with liver-specific contrast agents: a comparison between ultrasound and magnetic resonance imaging. Eur J Radiol. 2013;82:75-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Quaia E, De Paoli L, Angileri R, Cabibbo B, Cova MA. Indeterminate solid hepatic lesions identified on non-diagnostic contrast-enhanced computed tomography: assessment of the additional diagnostic value of contrast-enhanced ultrasound in the non-cirrhotic liver. Eur J Radiol. 2014;83:456-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Caturelli E, Solmi L, Anti M, Fusilli S, Roselli P, Andriulli A, Fornari F, Del Vecchio Blanco C, de Sio I. Ultrasound guided fine needle biopsy of early hepatocellular carcinoma complicating liver cirrhosis: a multicentre study. Gut. 2004;53:1356-1362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 60. | International Consensus Group for Hepatocellular NeoplasiaThe International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 610] [Cited by in F6Publishing: 554] [Article Influence: 36.9] [Reference Citation Analysis (1)] |
| 61. | Reichman TW, Bahramipour P, Barone A, Koneru B, Fisher A, Contractor D, Wilson D, Dela Torre A, Cho KC, Samanta A. Hepatitis status, child-pugh classification, and serum AFP levels predict survival in patients treated with transarterial embolization for unresectable hepatocellular carcinoma. J Gastrointest Surg. 2005;9:638-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Huo TI, Lin HC, Hsia CY, Wu JC, Lee PC, Chi CW, Lee SD. The model for end-stage liver disease based cancer staging systems are better prognostic models for hepatocellular carcinoma: a prospective sequential survey. Am J Gastroenterol. 2007;102:1920-1930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Kee KM, Wang JH, Lin CY, Wang CC, Cheng YF, Lu SN. Validation of the 7th edition TNM staging system for hepatocellular carcinoma: an analysis of 8,828 patients in a single medical center. Dig Dis Sci. 2013;58:2721-2728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, Yamaoka Y, Belghiti J, Lauwers GY, Poon RT. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086-1092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 488] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 65. | Hsu CY, Lee YH, Hsia CY, Huang YH, Su CW, Lin HC, Lee RC, Chiou YY, Lee FY, Huo TI. Performance status in patients with hepatocellular carcinoma: determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology. 2013;57:112-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 66. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2645] [Cited by in F6Publishing: 2724] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 67. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9016] [Cited by in F6Publishing: 9507] [Article Influence: 594.2] [Reference Citation Analysis (1)] |
| 68. | Takaki H, Yamakado K, Tsurusaki M, Yasumoto T, Baba Y, Narimatsu Y, Shimohira M, Yamaguchi M, Matsuo K, Inaba Y. Hepatic arterial infusion chemotherapy with fine-powder cisplatin and iodized-oil suspension in patients with intermediate-stage and advanced-stage (Barcelona Clinic Liver Cancer stage-B or stage-C) hepatocellular carcinoma: multicenter phase-II clinical study. Int J Clin Oncol. 2014;Nov 29; Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 69. | Dai Y, Li C, Wen TF, Yan LN. Comparison of liver resection and transplantation for Child-pugh A cirrhotic patient with very early hepatocellular carcinoma and portal hypertension. Pak J Med Sci. 2014;30:996-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1331] [Cited by in F6Publishing: 1393] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 71. | Otto G, Schuchmann M, Hoppe-Lotichius M, Heise M, Weinmann A, Hansen T, Pitton MP. How to decide about liver transplantation in patients with hepatocellular carcinoma: size and number of lesions or response to TACE? J Hepatol. 2013;59:279-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 72. | Sala M, Llovet JM, Vilana R, Bianchi L, Solé M, Ayuso C, Brú C, Bruix J. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352-1360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 329] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 73. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1267] [Cited by in F6Publishing: 1421] [Article Influence: 88.8] [Reference Citation Analysis (1)] |
| 74. | Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 532] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 75. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2207] [Cited by in F6Publishing: 2190] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 76. | Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274-1283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 312] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 77. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 839] [Cited by in F6Publishing: 873] [Article Influence: 34.9] [Reference Citation Analysis (1)] |
| 78. | Llovet JM, Peña CE, Lathia CD, Shan M, Meinhardt G, Bruix J. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18:2290-2300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 406] [Cited by in F6Publishing: 436] [Article Influence: 36.3] [Reference Citation Analysis (2)] |
| 79. | Roxburgh P, Evans TR. Systemic therapy of hepatocellular carcinoma: are we making progress? Adv Ther. 2008;25:1089-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Wong R, Frenette C. Updates in the management of hepatocellular carcinoma. Gastroenterol Hepatol (N Y). 2011;7:16-24. [PubMed] [Cited in This Article: ] |
| 81. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 513] [Cited by in F6Publishing: 543] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 82. | Allemann P, Demartines N, Bouzourene H, Tempia A, Halkic N. Long-term outcome after liver resection for hepatocellular carcinoma larger than 10 cm. World J Surg. 2013;37:452-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Choi SH, Choi GH, Kim SU, Park JY, Joo DJ, Ju MK, Kim MS, Choi JS, Han KH, Kim SI. Role of surgical resection for multiple hepatocellular carcinomas. World J Gastroenterol. 2013;19:366-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 34] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 84. | Zhong JH, Xiang BD, Gong WF, Ke Y, Mo QG, Ma L, Liu X, Li LQ. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One. 2013;8:e68193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 85. | Liu PH, Lee YH, Hsu CY, Hsia CY, Huang YH, Chiou YY, Lin HC, Huo TI. Surgical resection is better than transarterial chemoembolization for hepatocellular carcinoma beyond Milan criteria independent of performance status. J Gastrointest Surg. 2014;18:1623-1631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 86. | Mancuso A. Management of hepatocellular carcinoma: Enlightening the gray zones. World J Hepatol. 2013;5:302-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5110] [Cited by in F6Publishing: 4991] [Article Influence: 178.3] [Reference Citation Analysis (0)] |
| 88. | Figueras J, Jaurrieta E, Valls C, Benasco C, Rafecas A, Xiol X, Fabregat J, Casanovas T, Torras J, Baliellas C. Survival after liver transplantation in cirrhotic patients with and without hepatocellular carcinoma: a comparative study. Hepatology. 1997;25:1485-1489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 215] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 89. | Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587-2596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 382] [Cited by in F6Publishing: 397] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 90. | Yao FY. Expanded criteria for hepatocellular carcinoma: down-staging with a view to liver transplantation--yes. Semin Liver Dis. 2006;26:239-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Yao FY, Kerlan RK, Hirose R, Davern TJ, Bass NM, Feng S, Peters M, Terrault N, Freise CE, Ascher NL. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 386] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 92. | Ahn CS, Moon DB, Lee SG, Hwang S, Kim KH, Ha TY, Song GW, Jung DH, Park GC, Park YH. Survival differences between Milan criteria after down-staging and De novo Milan in living donor liver transplantation for hepatocellular carcinoma. Hepatogastroenterology. 2014;61:187-191. [PubMed] [Cited in This Article: ] |
| 93. | De Carlis L, Di Sandro S, Giacomoni A, Slim A, Lauterio A, Mangoni I, Mihaylov P, Pirotta V, Aseni P, Rampoldi A. Beyond the Milan criteria: what risks for patients with hepatocellular carcinoma progression before liver transplantation? J Clin Gastroenterol. 2012;46:78-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 94. | Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level & gt; 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014;20:945-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 200] [Article Influence: 20.0] [Reference Citation Analysis (1)] |
| 95. | Crissien AM, Frenette C. Current management of hepatocellular carcinoma. Gastroenterol Hepatol (N Y). 2014;10:153-161. [PubMed] [Cited in This Article: ] |
| 96. | McGahan JP, Brock JM, Tesluk H, Gu WZ, Schneider P, Browning PD. Hepatic ablation with use of radio-frequency electrocautery in the animal model. J Vasc Interv Radiol. 1992;3:291-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 208] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 97. | Marrero JA. Multidisciplinary management of hepatocellular carcinoma: where are we today? Semin Liver Dis. 2013;33 Suppl 1:S3-S10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 98. | Genco C, Cabibbo G, Maida M, Brancatelli G, Galia M, Alessi N, Butera G, Genova C, Romano P, Raineri M. Treatment of hepatocellular carcinoma: present and future. Expert Rev Anticancer Ther. 2013;13:469-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 99. | Meza-Junco J, Montano-Loza AJ, Liu DM, Sawyer MB, Bain VG, Ma M, Owen R. Locoregional radiological treatment for hepatocellular carcinoma; Which, when and how? Cancer Treat Rev. 2012;38:54-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 100. | Seinstra BA, van Delden OM, van Erpecum KJ, van Hillegersberg R, Mali WP, van den Bosch MA. Minimally invasive image-guided therapy for inoperable hepatocellular carcinoma: What is the evidence today? Insights Imaging. 2010;1:167-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 101. | Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 666] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 102. | Zhang L, Hu P, Chen X, Bie P. Transarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: a meta-analysis. PLoS One. 2014;9:e100305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 103. | Min JH, Lee MW, Cha DI, Jeon YH, Shin SW, Cho SK, Rhim H, Lim HK. Radiofrequency ablation combined with chemoembolization for intermediate-sized (3-5 cm) hepatocellular carcinomas under dual guidance of biplane fluoroscopy and ultrasonography. Korean J Radiol. 2013;14:248-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 361] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 105. | Germano D, Daniele B. Systemic therapy of hepatocellular carcinoma: current status and future perspectives. World J Gastroenterol. 2014;20:3087-3099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 106. | Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, Faghih M, Brendel E, Voliotis D, Haase CG. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 678] [Cited by in F6Publishing: 648] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 107. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3854] [Cited by in F6Publishing: 4336] [Article Influence: 271.0] [Reference Citation Analysis (0)] |
| 108. | Pinter M, Sieghart W, Graziadei I, Vogel W, Maieron A, Königsberg R, Weissmann A, Kornek G, Plank C, Peck-Radosavljevic M. Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. Oncologist. 2009;14:70-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 109. | Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JF, Ladrón de Guevara L, Papandreou C. First interim analysis of the GIDEON (Global Investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafeNib) non-interventional study. Int J Clin Pract. 2012;66:675-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 110. | Abou-Alfa GK, Johnson P, Knox JJ, Capanu M, Davidenko I, Lacava J, Leung T, Gansukh B, Saltz LB. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154-2160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 327] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 111. | Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Chung HC, Song X, Xu J, Poggi G. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067-4075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 523] [Cited by in F6Publishing: 564] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 112. | Sasaki Y, Yamada T, Tanaka H, Ohigashi H, Eguchi H, Yano M, Ishikawa O, Imaoka S. Risk of recurrence in a long-term follow-up after surgery in 417 patients with hepatitis B- or hepatitis C-related hepatocellular carcinoma. Ann Surg. 2006;244:771-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 113. | Roayaie S, Haim MB, Emre S, Fishbein TM, Sheiner PA, Miller CM, Schwartz ME. Comparison of surgical outcomes for hepatocellular carcinoma in patients with hepatitis B versus hepatitis C: a western experience. Ann Surg Oncol. 2000;7:764-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 114. | Kim BK, Park JY, Kim do Y, Kim JK, Kim KS, Choi JS, Moon BS, Han KH, Chon CY, Moon YM. Persistent hepatitis B viral replication affects recurrence of hepatocellular carcinoma after curative resection. Liver Int. 2008;28:393-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 115. | Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, Huo TI, Sheen IJ, Lee SD, Lui WY. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51:890-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 322] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 116. | Schütte K, Schulz C, Poranzke J, Antweiler K, Bornschein J, Bretschneider T, Arend J, Ricke J, Malfertheiner P. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 117. | Wörns MA, Bosslet T, Victor A, Koch S, Hoppe-Lotichius M, Heise M, Hansen T, Pitton MB, Niederle IM, Schuchmann M. Prognostic factors and outcomes of patients with hepatocellular carcinoma in non-cirrhotic liver. Scand J Gastroenterol. 2012;47:718-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 118. | Shi L, Feng Y, Lin H, Ma R, Cai X. Role of estrogen in hepatocellular carcinoma: is inflammation the key? J Transl Med. 2014;12:93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 119. | Kaseb AO, Hassan MM, Lin E, Xiao L, Kumar V, Pathak P, Lozano R, Rashid A, Abbruzzese JL, Morris JS. V-CLIP: Integrating plasma vascular endothelial growth factor into a new scoring system to stratify patients with advanced hepatocellular carcinoma for clinical trials. Cancer. 2011;117:2478-2488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 120. | Kaseb AO, Abbruzzese JL, Vauthey JN, Aloia TA, Abdalla EK, Hassan MM, Lin E, Xiao L, El-Deeb AS, Rashid A. I-CLIP: improved stratification of advanced hepatocellular carcinoma patients by integrating plasma IGF-1 into CLIP score. Oncology. 2011;80:373-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |