Published online Sep 18, 2015. doi: 10.4254/wjh.v7.i20.2292
Peer-review started: May 19, 2015
First decision: July 6, 2015
Revised: August 4, 2015
Accepted: August 30, 2015
Article in press: August 31, 2015
Published online: September 18, 2015
The global distribution of hepatocellular carcinoma (HCC) varies markedly among regions, and patients in East Asia and Central Africa account for about 80% of all cases. The risk factors are hepatitis B, hepatitis C, alcohol, and etc. The risk of carcinogenesis further increases with progression to hepatic cirrhosis in all liver disorders. Radical treatment of HCC by liver resection without causing liver failure has been established as a safe approach through selection of an appropriate range of resection of the damaged liver. This background indicates that both evaluation of hepatic functional reserve and measures against concomitant diseases such as thrombocytopenia accompanying portal hypertension, prevention of rupture of esophageal varices, reliable control of ascites, and improvement of hypoalbuminemia are important issues in liver resection in patients with hepatic cirrhosis. We review the latest information on perioperative management of liver resection in HCC patients with hepatic cirrhosis.
Core tip: Radical treatment of hepatocellular carcinoma (HCC) by liver resection without causing liver failure has been established as a safe approach through selection of an appropriate range of resection of the damaged liver. This background indicates that both evaluation of hepatic functional reserve and measures against concomitant diseases such as thrombocytopenia accompanying portal hypertension, prevention of rupture of esophageal varices, reliable control of ascites, and improvement of hypoalbuminemia are important issues in liver resection in patients with hepatic cirrhosis. The latest information on perioperative management of liver resection in HCC patients with hepatic cirrhosis was reviewed.
- Citation: Nakayama H, Takayama T. Management before hepatectomy for hepatocellular carcinoma with cirrhosis. World J Hepatol 2015; 7(20): 2292-2302
- URL: https://www.wjgnet.com/1948-5182/full/v7/i20/2292.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i20.2292
The global distribution of hepatocellular carcinoma (HCC) varies markedly among regions, and patients in East Asia and Central Africa account for about 80% of all cases[1,2]. The risk factors are hepatitis B and aflatoxin in these regions[3], whereas hepatitis C and alcohol are risk factors in North America, Europe and Japan[4,5]. The risk of carcinogenesis further increases with progression to hepatic cirrhosis in all liver disorders. Hepatic cirrhosis is an irreversible pathological change and inhibition of disease progression has previously been considered difficult. However, advances in antiviral therapy now permit eradication or inhibition of replication of viruses[6].
Radical treatment of HCC by liver resection without causing liver failure has been established as a safe approach through selection of an appropriate range of resection of the damaged liver[7,8]. In the HCC practice guidelines of the Barcelona Clinic Liver Cancer (BCLC) staging system, liver resection is not recommended for patients with portal hypertension[9], and radiofrequency ablation (RFA) and transcatheter arterial chemoembolization are selected in many countries. In Japan, liver resection using appropriate preoperative management has been found to be safe and to improve the prognosis for patients with portal hypertension[10].
This background indicates that both evaluation of hepatic functional reserve[11] and measures against concomitant diseases such as thrombocytopenia accompanying portal hypertension, prevention of rupture of esophageal varices, reliable control of ascites, and improvement of hypoalbuminemia are important issues in liver resection in patients with hepatic cirrhosis[12]. In this report, we review the latest information on perioperative management of liver resection in HCC patients with hepatic cirrhosis.
Hepatic cirrhosis is the terminal stage of chronic liver disease, in which fibrous tissue accumulation due to necrotizing inflammatory reactions makes the liver surface rough and irregular[12]. Histologically, lobular structure remodeling and pseudolobule formation are observed; i.e., hepatic cirrhosis is a morphologically defined disease[13].
Hepatic cirrhosis is classified based on: (1) cause; (2) function and clinical stage; and (3) node size-based morphology (World Health Organization classification). In (2), hepatic cirrhosis is classified into compensated and decompensated phases, and by the Child-Pugh classification, as described below. In (3), hepatic cirrhosis is classified into three types: micro-nodular type, with nodes < 3 mm, macro-nodular type, with nodes ≥ 3 mm, and mixed nodular type, in which both nodules are mixed.
Persistent hepatitis virus B and C infections and excessive alcohol intake are the causes in many patients. The specific types are primary biliary hepatic cirrhosis; autoimmune hepatitis; non-alcoholic steatohepatitis; and metabolic (Wilson disease, hemochromatosis), congestive (Budd-Chiari syndrome), parasitic, and drug-induced types[12].
Hepatic cirrhosis is definitively diagnosed by histological confirmation of lobular structure remodeling and pseudolobule formation on liver biopsy. However, liver biopsy is not optimal because performance of this procedure before liver resection has a risk of complications. Thus, it is desirable to evaluate the presence of hepatic cirrhosis based on blood chemistry and diagnostic imaging. Several formulas for this purpose using blood tests have been reported[14-17] (Table 1). The aspartate aminotransferase (AST) to platelet ratio index (APRI index) is based on the AST level and platelet count. The diagnostic performance for hepatic cirrhosis C using a cut-off of 1.0 is about 77% sensitivity and 75% specificity[16].
| Year | Ref. | Formula |
| 2000 | Ikeda et al[14] | Z = (0.124) × [γ globulin (%)] + (0.001) × hyaluronic acid (ng/mL) + (0.075) × platelet count (104/μL) + (-0.413) × gender (male = 1, female = 2) + (-2.005) |
| The condition is hepatic cirrhosis when Z is positive, and chronic hepatitis when Z is negative | ||
| 2007 | Koda et al[15] | Fibroindex = (1.738) + (-0.064) × platelet count (104/μL) + (0.005) × AST (IU/L) + (0.463) × [γ globulin (g/dL)] |
| The fibroindex value corresponds to fibrosis stage | ||
| 2003 | Wai et al[16] | APRI = 100 × [AST level/(upper limit of normal AST)/platelet count (× 109/L)] |
| 2006 | Sterling et al[17] | FIB-4 = [age × AST (U/L)]/[platelet count (× 109/L) × ALT (U/L)1/2] |
In imaging diagnosis, transient elastography (FibroScan™) can be used for noninvasive measurement of liver stiffness (stiffness), in which liver elasticity is determined by measuring the velocity of transmission in the liver of a single shear wave emitted from a specific probe of an ultrasonic diagnostic device[18,19]. A strong correlation between liver elasticity and fibrosis stage has been reported[20].
The most common hepatic cirrhosis classification is the Child-Pugh classification, in which 5 factors are scored: encephalopathy, ascites, serum bilirubin level, serum albumin level, and prothrombin activity[21,22]. However, diagnoses of encephalopathy and ascites are subjective, and evaluation of liver function is determined specifically at the time of the test, which are disadvantages in evaluation of hepatic functional reserve for liver resection. In planning for liver resection, the liver damage classification is more appropriate, particularly for HCC[23]. This classification uses the indocyanine green retention rate at 15 min (ICG-R15), instead of encephalopathy in the Child-Pugh classification, and stricter measurements of serum albumin and prothrombin levels. This classification is particularly useful for preoperative selection of patients with favorable hepatic functional reserve[24].
The prognosis of HCC depends on the hepatic functional reserve and tumor stage. These variables are integrated in staging systems including the model for end-stage liver disease[25], OKUDA[26], cancer of the liver Italian program (CLIP) (Table 2)[27], Japan integrated staging (JIS) score (Table 3)[28], modified-JIS score[29], and the Tokyo score[30], all of which are useful predictors of outcomes. Kudo et al[28] proposed the JIS score, in which the TNM stage and Child-Pugh classification are integrated. This score has advantages over the CLIP score (integration of the Child-Pugh classification, tumor morphology, alpha-fetoprotein, and portal vein tumor thrombosis) because (1) stratification of scores is distinct; (2) the prognosis of score-0 liver cancer is favorable; and (3) there is a definitive JIS score for cases with a poor prognosis. Integrated staging is useful for prediction of outcomes, but inappropriate for selection and comparison of treatment methods[31].
| Variable | Score | ||
| 0 | 1 | 2 | |
| Child-Pugh stage | A | B | C |
| Tumor morphology | Uninodular and extension ≤ 50% | Multinodular and extension ≤ 50% | Massive or extension > 50% |
| AFP (ng/mL) | < 400 | ≥ 400 | |
| Portal vein thrombosis | No | Yes | |
A reduced platelet count is an indicator of hepatic cirrhosis, and liver resection requires measures against thrombocytopenia to reduce the risk of hemorrhage[12]. Low preoperative platelet count is independently associated with increased major complications, postoperative liver insufficiency, and mortality after resection of HCC[32].
Partial splenic embolization is performed to improve hypersplenism through partially necrotizing the spleen by embolization of the splenic artery with a gelatin sponge or metal coil[33,34]. Long-term maintenance of the increased platelet count requires extensive splenic embolization of about 80% (splenic volumes ≤ 700 mL)[35], but this treatment is accompanied by risks of complications such as abdominal pain (82.4%), fever (94.1%), and splenic abscess (1.2%)[36]. A short-term minimum effect of embolization is believed to be sufficient to prevent hemorrhage after liver resection[37].
Splenectomy reliably improves portal hypertension and hypersplenism. In HCC accompanied by hepatic cirrhosis, splenectomy improves the serum bilirubin, albumin, and prothrombin levels, and splenectomy performed before liver resection has a significant benefit[38]. In contrast, splenectomy before brain dead liver transplantation causes an increase in infection, decrease in survival rate, and high mortality[39,40]. Thus, it has been suggested that cases should be carefully selected for splenectomy. Also, since immune function is reduced in patients with hepatic cirrhosis, overwhelming post-splenectomy infection syndrome (OPSI) is a concern[41]. OPSI is a complication that develops rapidly regardless of the time after surgery and has a poor prognosis and high mortality (50%-70%)[42-44]. Pneumococcus is the causative bacteria in 80% of cases and a pneumococcus vaccine is recommended for splenectomized patients. Interferon administration following splenectomy may also induce OPSI; thus, antiviral therapy should be performed carefully. The incidences of portal vein thrombosis after splenectomy are 9%-29% and 1.6%-8.0% in patients with and without concomitant spleen enlargement, respectively[45-48]. Doppler ultrasonography and contrast CT are useful for early diagnosis of portal vein thrombosis following splenectomy. The timing of splenectomy varies among institutions (Table 4). Sugawara et al[49] recommended simultaneous splenectomy for readily resectable HCC in cases with favorable liver function and general conditions, and earlier splenectomy if these criteria are not met.
| Year | Ref. | Simultaneous splenectomy, 2-stage (No. of patients) | Platelet count (×104/μL) | Child-Pugh (A/B/C) | Mortality | Morbidity | Survival rate | Effect |
| 1989 | Takayama et al[38] | Simultaneous (12), 2-stage (8) | 4.6 | N | N | N | N | Expansion of indication of liver resection |
| 1999 | Lin et al[94] | Simultaneous (11) | 5.2 ± 1.5 | 5/6/0 | 9.1% | 27.3% | 5 yr recurrence-free 66.7% | Improvement of serum bilirubin level |
| 2000 | Sugawara et al[49] | Simultaneous (35), 2-stage (13) | 4.7 ± 0.3 | N | 0 | 47.9 | 3/5 yr survival rate: 72.3%/38.9% | Improvement of safety |
| 2000 | Shimada et al[95] | 2-stage (6) | 5.2 ± 1.5 | 1/4/1 | 0 | 17 | N | Improvement of platelet count, albumin level, and Child classification |
| 2003 | Oh et al[96] | Simultaneous (12), no sp (6) | 5.5 ± 1.5 | 10/8/0 | 11.1 | 66.7 | N | Expansion of indication of liver resection |
| 2004 | Wu et al[97] | Simultaneous (41), no sp (485) | 3.8 ± 2.1 | 419/85/23 | 1.5 | 20.5 | N | Improvement of recurrence-free survival rate |
| 2005 | Chen et al[98] | Simultaneous (94), no sp (110) | 6.2 | 125/79/0 | N | 15.2 | 5 yr survival 56%, recurrence-free survival 35% | Improvement of recurrence-free survival rate |
| 2008 | Sugimachi et al[99] | Simultaneous (4), no sp (11) | 4.2 ± 0.8 | 9/6/0 | 6.7 | 47 | N | 3-yr survival rate equivalent to that after conventional liver resection |
| 2015 | Zhang et al[100] | Simultaneous (84), no sp (84) | 6.1 ± 4.2 | 84/0/0 | 0 | 39.3 | 1/3/5 yr survival: 90%/78%/66% | Improvement of recurrence-free survival rate |
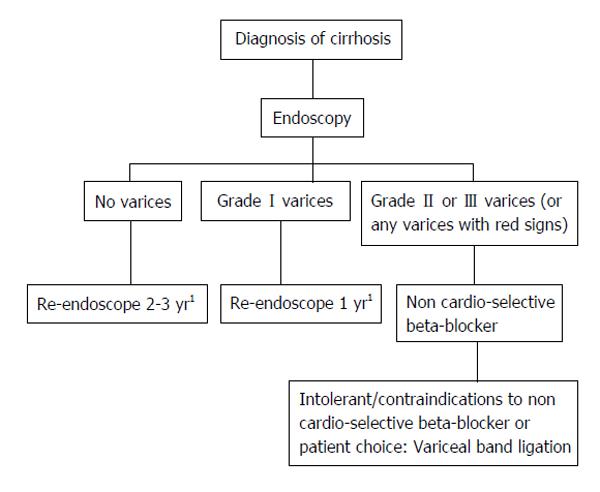
The prognosis for patients with cirrhosis primarily depends on the occurrence of hemorrhage from esophageal varices and gastropathy. General rules for recording endoscopic findings of esophagogastric varices is formatted with location, form, color, red color signs, bleeding sings and mucosal findings (Table 5)[50]. According to the United Kingdom guide lines, esophageal varices are classified into 3 grades based on the size of varices[51]. Grade II/III varices (large) are indicated to beta-blocker or variceal ligation (Figure 1). McCormack classification is useful to definite of portal hypertensive gastropathy (Table 6)[52]. Thus, endoscopy should be performed before liver resection to avoid overlooking esophageal varices because the portal blood pressure rises after liver resection and this may aggravate varices. For patients with a history of hemorrhage from a varix, treatment of the varix before liver resection is required. For patients with a large (F2 or larger) varix accompanied by red color sign based on above general rules, preventive treatment is indicated[53].
| Category | Code subcategory |
| Location (L) | Ls: Locus superior |
| Lm: Locus medialis | |
| Li: Locus inferior | |
| Lg-c: Adjacent to the cardiac orifice | |
| Lg-cf: Extension from the cardiac orifice to the fornix | |
| Lg-f: Isolated in the fornix | |
| Lg-b: Located in the gastric body | |
| Lg-a: Located in the gastric antrum | |
| Form (F) | F0: No varicose appearance |
| F1: Straight, small-caliber varices | |
| F2: Moderately enlarged, beady varices | |
| F3: Markedly enlarged, nodular or tumor-shaped varices | |
| Color (C) | Cw: White varices |
| Cb: Blue varices | |
| Cw-Th: Thrombosed white varices | |
| Cb-Th: Thrombosed blue varices | |
| Red color signs (RC) | RWM: Red wale markings |
| CRS: Cherry red spots | |
| HCS: Hematocystic spots | |
| Esophageal varices: RC0, RC1, RC2, RC3 | |
| Gastric varices: RC0, RC1 | |
| Te: Telangiectasia | |
| Bleeding signs | Gushing bleeding |
| Spurting bleeding | |
| Oozing bleeding | |
| Red plug | |
| White plug | |
| Mucosal findings | E: Erosion |
| Ul: Ulcer | |
| S: Scar |
| Mild gastropathy | Fine pink speckling |
| Superficial reddening | |
| Snakeskin (Mosaic-like) appearance | |
| Severe gastropathy | Cherry-red spots |
| Diffuse hemorrhagic lesion |
Currently, endoscopic treatment is the standard for esophageal varices, using endoscopic injection sclerotherapy and endoscopic variceal ligation[54]. Balloon-occluded retrograde transvenous obliteration (BRTO) improves the varix and ICG-R15 value in patients with a gastric varix[55], but there is no evidence that BRTO improves the safety of liver resection. The endoscopic F factor (large varices) rating of bleeding esophageal varices can be a significant predictive factor for HCC[56]. So the screening of HCC is required after the treatment of large varices.
Ascites accompanying hepatic cirrhosis involves interactions among various factors, including enhancement of liver lymph production with elevation of the portal blood pressure, enhancement of intra-abdominal portal permeability, reduction of the effective circulating blood volume, and enhancement of the sympathetic nervous system[12]. Treatment of fluid retention, which manifests as ascites, includes restriction of salts and water, administration of diuretics, and transfusion of an albumin preparation. If no effect is obtained in a short time, the patient is at high risk of liver or multiple organ failure, and liver resection should be avoided[57]. Selection of the smallest possible range of resection in patients with relatively favorable liver function and early resolution of ascites is the key to safe and successful liver resection[11].
The association between preoperative sarcopenia and postoperative morbidity/mortality has been reported for various types of surgeries. Preoperative sarcopenia increased the morbidity rate including the rate of liver failure, in patients who underwent major hepatectomy with extrahepatic bile duct resection[58]. European Society for Parenteral and Enteral Nutrition guidelines recommended an energy intake of 35-40 kcal/kgBW per day (147-168 kJ/kgBW per day) and a protein intake of 1.2-1.5 g/kgBW per day for cirrhotic patients perioperatively[59].
Protein-energy malnutrition (PEM) occurs in 27%-87% of patients with hepatic cirrhosis, and the level of branched-chain amino acids (BCAAs) is markedly reduced[60]. In PEM, hypoalbuminemia is observed and BCAAs are used for processing of ammonia and as an energy source for gluconeogenesis in skeletal muscle. In hepatic cirrhosis, serum albumin and plasma BCAA levels are positively correlated, and the prognosis is significantly poorer when serum albumin is < 3.5 g/dL[61,62]. Oral administration of BCAAs is of interest as a pharmacological and nutritional approach for improvement of hypoalbuminemia and insulin resistance, inhibition of angiogenesis, and activation of immune function[63]. In a randomized controlled trial (RCT) in 646 patients with decompensated hepatic cirrhosis who were divided into groups with and without treatment with oral BCAAs for 2 years, the incidences of death, liver cancer, rupture of esophageal varix, and liver failure were lower in the BCAA group and the prognosis was improved[64].
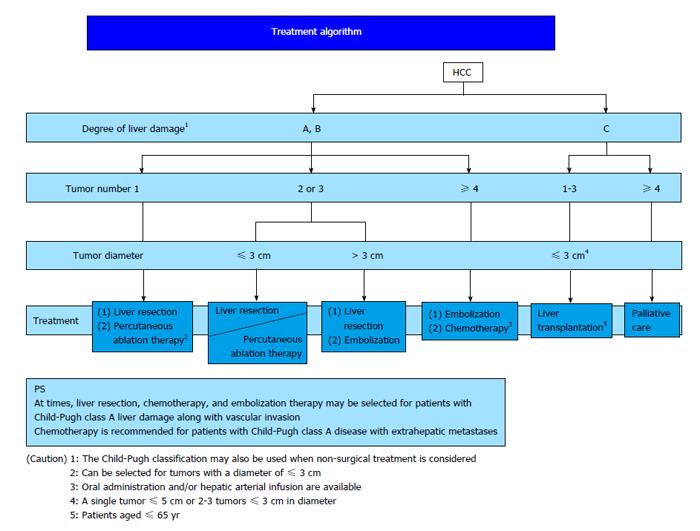
In Japan, East Asia and some European countries, ICG-R15 is used as an index of hepatic functional reserve. ICG-R15 is also a predictor of morbidity and mortality after surgery[65,66] and can be used to determine the acceptable liver resection range. In the therapeutic strategy for HCC, the BCLC staging system recommended by AASLD and EASL is used worldwide[9]. In Japan, the “treatment algorithm” described in the Clinical Guidelines for HCC is widely used to select the optimum treatment based on the liver function and tumor status (Figure 2)[67]. The Japanese treatment algorithm differs markedly from the BCLC system with regard to HCC with concomitant portal hypertension[68]. In the BCLC system, liver resection is not indicated if portal hypertension is present, and liver transplantation and RFA are recommended. In contrast, liver resection is recommended based on the ICG-R15 level in the Japanese treatment algorithm, and favorable outcomes have been reported[10].
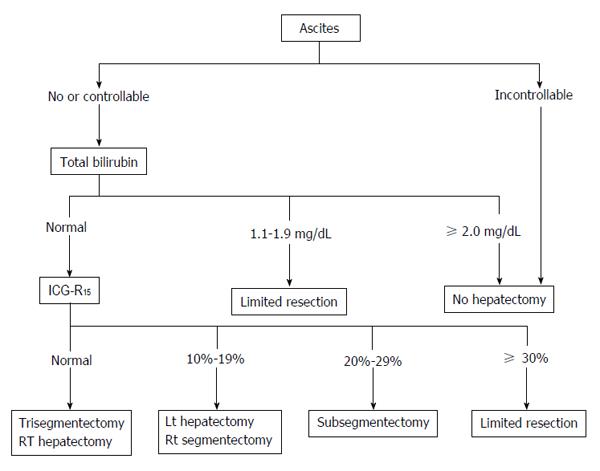
Liver resection for HCC is chosen based on the balance between tumor status and liver function. Resection exceeding the hepatic functional reserve with the goal of cancer cure may lead to liver failure, whereas insufficient resection of the cancer due to excessive safety concerns may have a high risk of early recurrence. Therefore, it is important to select the optimum surgical procedure based on the tumor advancement and the acceptable liver resection range. Preoperative liver function can be evaluated using a galactose tolerance test, 99mTc-GSA liver scintigraphy, and an ICG tolerance test. The Makuuchi criteria are particularly useful for chronic hepatitis and hepatic cirrhosis cases (Figure 3)[11]. These criteria use the presence or absence of ascites, serum bilirubin level, and ICG-R15 as evaluation items. Surgery is not indicated for cases with persistent ascites despite treatment with diuretics or if the serum bilirubin level is consistently > 2.0 mg/dL. The range of resection is determined based on ICG-R15 in patients with a normal bilirubin level of ≤ 1.0 mg/dL, i.e., procedures can be selected for resection of up to 2/3 of the total liver volume (such as right lobectomy) in patients with normal ICG-R15 (< 10%), up to 1/3 of the total liver volume (such as left lobectomy) for patients with ICG-R15 of 10%-19%, and up to 1/6 of the total liver volume (Couinaud’s segmentectomy) for patients with ICG-R15 of 20%-29%. When ICG-R15 exceeds 30%, surgery is limited to partial resection or enucleation. In a study in 1056 patients who underwent liver resection based on these criteria, the surgical mortality was 0%[8].
Systematic resection of cancer-containing regions perfused by branches of the portal vein should be performed within the range allowed by the liver function and with consideration of HCC invasion of the portal vein. Systematic subsegmentectomy of the liver was developed to overcome two contradictory goals: cancer curability and conservation of liver function[69]. Since HCC develops in a liver damaged by chronic hepatitis and hepatic cirrhosis in many cases, an insufficient volume of residual liver after major hepatectomy, such as lobectomy, may result in liver failure. To prevent liver failure, portal vein embolization (PE) is applied to the branch of the portal vein perfusing the planned region for resection to induce compensatory hypertrophy of the region remaining after liver resection[70]. PE is indicated for cases with ICG-R15 < 10% and a ratio of the non-tumorous parenchymal volume of the resected liver to that of the whole liver (R2) of ≥ 60%, and for cases with ICG-R15≥ 10% to < 20% and R2 of 40%-60%[71]. Both degree of liver hypertrophy and growth rate after PE are strong predictors of post-hepatectomy liver failure[72]. Recent introduction of 3-dimensional computed tomography (CT) has enabled simple and accurate determination of the positional relationship between the main vessels and the tumor, the range of resection, and measurement of the residual liver volume[73].
Since 1990, liver resection for HCC has been performed with acceptable blood loss at high-volume medical centers, and centers performing surgery with blood loss of about 500 mL have increased[74-77]. Blood transfusion may promote cancer recurrence and is likely to induce hyperbilirubinemia and liver failure[78]. Since a low hematocrit value is preferable for microcirculation of the liver, perioperative allogeneic transfusion should be avoided as much as possible in liver resection. Autologous blood transfusion is safe and useful for avoidance of allogeneic transfusion without increasing the risk of cancer recurrence[79]. Administration of fresh frozen plasma is recommended to supplement coagulation factors and maintain the effective plasma volume[80], but administration of fresh frozen plasma does not influence the course after liver resection and is not necessary if the serum albumin level 2 d after surgery is ≥ 2.4 g/dL in Child-Pugh class A cases with intraoperative blood loss of < 1000 mL[81].
The immunosuppressed state after liver resection may lead to progression of liver failure and disseminated intravascular coagulation. In a RCT of steroid administration after liver resection, postoperative liver function was compared between groups treated with and without 500 mg/body hydrocortisone before liver resection. Serum bilirubin significantly decreased 2 d after surgery in the steroid group and there were significant differences in the time-courses of the bilirubin level and the prothrombin activity for 7 d after surgery. These results show the efficacy of steroid administration for liver resection[82].
HCC often recurs even after curative liver resection or RFA. It has been believed that controlling hepatitis and ameliorating the symptoms of cirrhosis prevent the recurrence of HCC. Several studies have examined the adjuvant therapies for their ability to prevent recurrence[83]. Eight RCTs were carried out to verify the efficacy of adjuvant interferon therapy for postoperative HCC[84-91]. It is suggested that adjuvant interferon-α reduced HCC recurrence and improved overall survival in patients with hepatitis C virus-infected HCC following curative treatment. The available evidence suggests that antivirus therapy with nucleoside analogs (lamivudine) should be recommended a postoperative preventive therapy for patients with hepatitis B virus-related HCC (> 500 copies of hepatitis B virus DNA/mL)[92,93].
Perioperative management is important in liver resection for patients with HCC and hepatic cirrhosis. New methods for evaluation and improvement of liver function are likely to facilitate expansion of the indication for liver resection.
P- Reviewer: Wang K S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Tejeda-Maldonado J, García-Juárez I, Aguirre-Valadez J, González-Aguirre A, Vilatobá-Chapa M, Armengol-Alonso A, Escobar-Penagos F, Torre A, Sánchez-Ávila JF, Carrillo-Pérez DL. Diagnosis and treatment of hepatocellular carcinoma: An update. World J Hepatol. 2015;7:362-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3249] [Cited by in F6Publishing: 3477] [Article Influence: 289.8] [Reference Citation Analysis (3)] |
| 3. | Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053-2063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1004] [Cited by in F6Publishing: 1041] [Article Influence: 104.1] [Reference Citation Analysis (0)] |
| 4. | Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet. 2015;385:1124-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 382] [Cited by in F6Publishing: 306] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 5. | Echeverría N, Moratorio G, Cristina J, Moreno P. Hepatitis C virus genetic variability and evolution. World J Hepatol. 2015;7:831-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 68] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 6. | Muir AJ, Poordad F, Lalezari J, Everson G, Dore GJ, Herring R, Sheikh A, Kwo P, Hézode C, Pockros PJ. Daclatasvir in combination with asunaprevir and beclabuvir for hepatitis C virus genotype 1 infection with compensated cirrhosis. JAMA. 2015;313:1736-1744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 7. | Zhou Y, Lei X, Wu L, Wu X, Xu D, Li B. Outcomes of hepatectomy for noncirrhotic hepatocellular carcinoma: a systematic review. Surg Oncol. 2014;23:236-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, Takayama T, Makuuchi M. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198-1206; discussion 1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 597] [Cited by in F6Publishing: 585] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 9. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6340] [Article Influence: 487.7] [Reference Citation Analysis (1)] |
| 10. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 513] [Cited by in F6Publishing: 543] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 11. | Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298-304. [PubMed] [Cited in This Article: ] |
| 12. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1139] [Cited by in F6Publishing: 1188] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 13. | Hytiroglou P, Snover DC, Alves V, Balabaud C, Bhathal PS, Bioulac-Sage P, Crawford JM, Dhillon AP, Ferrell L, Guido M. Beyond “cirrhosis”: a proposal from the International Liver Pathology Study Group. Am J Clin Pathol. 2012;137:5-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Ikeda K, Saitoh S, Kobayashi M, Suzuki Y, Tsubota A, Suzuki F, Arase Y, Murashima N, Chayama K, Kumada H. Distinction between chronic hepatitis and liver cirrhosis in patients with hepatitis C virus infection. Practical discriminant function using common laboratory data. Hepatol Res. 2000;18:252-266. [PubMed] [Cited in This Article: ] |
| 15. | Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology. 2007;45:297-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2762] [Cited by in F6Publishing: 2990] [Article Influence: 142.4] [Reference Citation Analysis (0)] |
| 17. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2633] [Cited by in F6Publishing: 2998] [Article Influence: 166.6] [Reference Citation Analysis (0)] |
| 18. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [PubMed] [Cited in This Article: ] |
| 19. | Lucidarme D, Foucher J, Le Bail B, Vergniol J, Castera L, Duburque C, Forzy G, Filoche B, Couzigou P, de Lédinghen V. Factors of accuracy of transient elastography (fibroscan) for the diagnosis of liver fibrosis in chronic hepatitis C. Hepatology. 2009;49:1083-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] [Cited in This Article: ] |
| 21. | Child CG. The liver and portal hypertension. 3rd ed. Philadelphia: WB Saunders 1964; . [Cited in This Article: ] |
| 22. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] [Cited in This Article: ] |
| 23. | The general rules for the clinical and pathological study of primary liver cancer. Liver Cancer Study Group of Japan. Jpn J Surg. 1989;19:98-129. [PubMed] [Cited in This Article: ] |
| 24. | Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245:909-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 263] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 25. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3462] [Cited by in F6Publishing: 3432] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 26. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [PubMed] [Cited in This Article: ] |
| 27. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 908] [Cited by in F6Publishing: 933] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 28. | Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40:1396-1405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 258] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 29. | Ikai I, Takayasu K, Omata M, Okita K, Nakanuma Y, Matsuyama Y, Makuuchi M, Kojiro M, Ichida T, Arii S. A modified Japan Integrated Stage score for prognostic assessment in patients with hepatocellular carcinoma. J Gastroenterol. 2006;41:884-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, Obi S, Sato S, Koike Y, Fujishima T. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 31. | Duseja A. Staging of hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4:S74-S79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Maithel SK, Kneuertz PJ, Kooby DA, Scoggins CR, Weber SM, Martin RC, McMasters KM, Cho CS, Winslow ER, Wood WC. Importance of low preoperative platelet count in selecting patients for resection of hepatocellular carcinoma: a multi-institutional analysis. J Am Coll Surg. 2011;212:638-648; discussion 648-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Yoshida H, Mamada Y, Taniai N, Tajiri T. Partial splenic embolization. Hepatol Res. 2008;38:225-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Hayashi H, Beppu T, Shirabe K, Maehara Y, Baba H. Management of thrombocytopenia due to liver cirrhosis: a review. World J Gastroenterol. 2014;20:2595-2605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 82] [Cited by in F6Publishing: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 35. | Hayashi H, Beppu T, Okabe K, Masuda T, Okabe H, Ishiko T, Baba H. Therapeutic factors considered according to the preoperative splenic volume for a prolonged increase in platelet count after partial splenic embolization for liver cirrhosis. J Gastroenterol. 2010;45:554-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Sakai T, Shiraki K, Inoue H, Sugimoto K, Ohmori S, Murata K, Takase K, Nakano T. Complications of partial splenic embolization in cirrhotic patients. Dig Dis Sci. 2002;47:388-391. [PubMed] [Cited in This Article: ] |
| 37. | Hadduck TA, McWilliams JP. Partial splenic artery embolization in cirrhotic patients. World J Radiol. 2014;6:160-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 61] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Takayama T, Makuuchi M, Yamazaki S, Hasegawa H. [The role of splenectomy in patients with hepatocellular carcinoma and hypersplenism as an aid to hepatectomy]. Nihon Geka Gakkai Zasshi. 1989;90:1043-1048. [PubMed] [Cited in This Article: ] |
| 39. | Lüsebrink R, Blumhardt G, Lohmann R, Bachmann S, Knoop M, Lemmens HP, Neuhaus P. Does concommitant splenectomy raise the mortality of liver transplant recipients? Transpl Int. 1994;7 Suppl 1:S634-S636. [PubMed] [Cited in This Article: ] |
| 40. | Samimi F, Irish WD, Eghtesad B, Demetris AJ, Starzl TE, Fung JJ. Role of splenectomy in human liver transplantation under modern-day immunosuppression. Dig Dis Sci. 1998;43:1931-1937. [PubMed] [Cited in This Article: ] |
| 41. | Okabayashi T, Hanazaki K. Overwhelming postsplenectomy infection syndrome in adults - a clinically preventable disease. World J Gastroenterol. 2008;14:176-179. [PubMed] [Cited in This Article: ] |
| 42. | Waghorn DJ, Mayon-White RT. A study of 42 episodes of overwhelming post-splenectomy infection: is current guidance for asplenic individuals being followed? J Infect. 1997;35:289-294. [PubMed] [Cited in This Article: ] |
| 43. | Waghorn DJ. Overwhelming infection in asplenic patients: current best practice preventive measures are not being followed. J Clin Pathol. 2001;54:214-218. [PubMed] [Cited in This Article: ] |
| 44. | Sinwar PD. Overwhelming post splenectomy infection syndrome - review study. Int J Surg. 2014;12:1314-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 45. | Kercher KW, Carbonell AM, Heniford BT, Matthews BD, Cunningham DM, Reindollar RW. Laparoscopic splenectomy reverses thrombocytopenia in patients with hepatitis C cirrhosis and portal hypertension. J Gastrointest Surg. 2004;8:120-126. [PubMed] [Cited in This Article: ] |
| 46. | Yoshida M, Watanabe Y, Horiuchi A, Yamamoto Y, Sugishita H, Kawachi K. Portal and splenic venous thrombosis after splenectomy in patients with hypersplenism. Hepatogastroenterology. 2009;56:538-541. [PubMed] [Cited in This Article: ] |
| 47. | Stamou KM, Toutouzas KG, Kekis PB, Nakos S, Gafou A, Manouras A, Krespis E, Katsaragakis S, Bramis J. Prospective study of the incidence and risk factors of postsplenectomy thrombosis of the portal, mesenteric, and splenic veins. Arch Surg. 2006;141:663-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 48. | Winslow ER, Brunt LM, Drebin JA, Soper NJ, Klingensmith ME. Portal vein thrombosis after splenectomy. Am J Surg. 2002;184:631-635; discussion 635-636. [PubMed] [Cited in This Article: ] |
| 49. | Sugawara Y, Yamamoto J, Shimada K, Yamasaki S, Kosuge T, Takayama T, Makuuchi M. Splenectomy in patients with hepatocellular carcinoma and hypersplenism. J Am Coll Surg. 2000;190:446-450. [PubMed] [Cited in This Article: ] |
| 50. | Tajiri T, Yoshida H, Obara K, Onji M, Kage M, Kitano S, Kokudo N, Kokubu S, Sakaida I, Sata M. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc. 2010;22:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 51. | Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, Austin A, Ferguson JW, Olliff SP, Hudson M. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 351] [Article Influence: 39.0] [Reference Citation Analysis (2)] |
| 52. | McCormack TT, Sims J, Eyre-Brook I, Kennedy H, Goepel J, Johnson AG, Triger DR. Gastric lesions in portal hypertension: inflammatory gastritis or congestive gastropathy? Gut. 1985;26:1226-1232. [PubMed] [Cited in This Article: ] |
| 53. | Liu HT, Cheng SB, Wu CC, Yeh HZ, Chang CS, Wang J. Impact of severe oesophagogastric varices on liver resection for hepatocellular carcinoma in cirrhotic patients. World J Surg. 2015;39:461-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Sarin SK, Govil A, Jain AK, Guptan RC, Issar SK, Jain M, Murthy NS. Prospective randomized trial of endoscopic sclerotherapy versus variceal band ligation for esophageal varices: influence on gastropathy, gastric varices and variceal recurrence. J Hepatol. 1997;26:826-832. [PubMed] [Cited in This Article: ] |
| 55. | Akahane T, Iwasaki T, Kobayashi N, Tanabe N, Takahashi N, Gama H, Ishii M, Toyota T. Changes in liver function parameters after occlusion of gastrorenal shunts with balloon-occluded retrograde transvenous obliteration. Am J Gastroenterol. 1997;92:1026-1030. [PubMed] [Cited in This Article: ] |
| 56. | Nakayama H, Masuda H, Miyake H, Takayama T, Yokoyama E. Endoscopic prediction of hepatocellular carcinoma by evaluation of bleeding esophageal varices. Digestion. 2004;70:233-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 57. | Donadon M, Costa G, Cimino M, Procopio F, Fabbro DD, Palmisano A, Torzilli G. Safe hepatectomy selection criteria for hepatocellular carcinoma patients: a validation of 336 consecutive hepatectomies. The BILCHE score. World J Surg. 2015;39:237-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Otsuji H, Yokoyama Y, Ebata T, Igami T, Sugawara G, Mizuno T, Nagino M. Preoperative sarcopenia negatively impacts postoperative outcomes following major hepatectomy with extrahepatic bile duct resection. World J Surg. 2015;39:1494-1500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 59. | Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J, Ferenci P, Holm E, Vom Dahl S, Müller MJ. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr. 2006;25:285-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 465] [Cited by in F6Publishing: 388] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 60. | McCullough AJ, Bugianesi E. Protein-calorie malnutrition and the etiology of cirrhosis. Am J Gastroenterol. 1997;92:734-738. [PubMed] [Cited in This Article: ] |
| 61. | Plauth M, Cabré E, Campillo B, Kondrup J, Marchesini G, Schütz T, Shenkin A, Wendon J. ESPEN Guidelines on Parenteral Nutrition: hepatology. Clin Nutr. 2009;28:436-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 62. | Nishiguchi S, Habu D. Effect of oral supplementation with branched-chain amino acid granules in the early stage of cirrhosis. Hepatol Res. 2004;30S:36-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Suzuki K, Endo R, Kohgo Y, Ohtake T, Ueno Y, Kato A, Suzuki K, Shiraki R, Moriwaki H, Habu D. Guidelines on nutritional management in Japanese patients with liver cirrhosis from the perspective of preventing hepatocellular carcinoma. Hepatol Res. 2012;42:621-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 64. | Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705-713. [PubMed] [Cited in This Article: ] |
| 65. | Lau H, Man K, Fan ST, Yu WC, Lo CM, Wong J. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255-1259. [PubMed] [Cited in This Article: ] |
| 66. | Nakayama H, Takayama T, Okubo T, Higaki T, Midorikawa Y, Moriguchi M, Aramaki O, Yamazaki S. Subcutaneous drainage to prevent wound infection in liver resection: a randomized controlled trial. J Hepatobiliary Pancreat Sci. 2014;21:509-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res. 2015;45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 302] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 68. | Nakayama H, Takayama T. Role of surgical resection for hepatocellular carcinoma based on Japanese clinical guidelines for hepatocellular carcinoma. World J Hepatol. 2015;7:261-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161:346-350. [PubMed] [Cited in This Article: ] |
| 70. | Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521-527. [PubMed] [Cited in This Article: ] |
| 71. | Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 198] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 72. | Leung U, Simpson AL, Araujo RL, Gönen M, McAuliffe C, Miga MI, Parada EP, Allen PJ, D’Angelica MI, Kingham TP. Remnant growth rate after portal vein embolization is a good early predictor of post-hepatectomy liver failure. J Am Coll Surg. 2014;219:620-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 73. | Saito S, Yamanaka J, Miura K, Nakao N, Nagao T, Sugimoto T, Hirano T, Kuroda N, Iimuro Y, Fujimoto J. A novel 3D hepatectomy simulation based on liver circulation: application to liver resection and transplantation. Hepatology. 2005;41:1297-1304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 74. | Grazi GL, Ercolani G, Pierangeli F, Del Gaudio M, Cescon M, Cavallari A, Mazziotti A. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2001;234:71-78. [PubMed] [Cited in This Article: ] |
| 75. | Jarnagin WR, Schwartz LH, Gultekin DH, Gönen M, Haviland D, Shia J, D’Angelica M, Fong Y, Dematteo R, Tse A. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20:1589-1595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 76. | Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698-708; discussion 708-710. [PubMed] [Cited in This Article: ] |
| 77. | Aramaki O, Takayama T, Higaki T, Nakayama H, Ohkubo T, Midorikawa Y, Moriguchi M, Matsuyama Y. Decreased blood loss reduces postoperative complications in resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2014;21:585-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 78. | Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, Fong Y, D’Angelica MI, Blumgart LH, Dematteo RP. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 307] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 79. | Ishizawa T, Hasegawa K, Tsuno NH, Tanaka M, Mise Y, Aoki T, Imamura H, Beck Y, Sugawara Y, Makuuchi M. Predeposit autologous plasma donation in liver resection for hepatocellular carcinoma: toward allogenic blood-free operations. J Am Coll Surg. 2009;209:206-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 80. | Makuuchi M, Takayama T, Gunvén P, Kosuge T, Yamazaki S, Hasegawa H. Restrictive versus liberal blood transfusion policy for hepatectomies in cirrhotic patients. World J Surg. 1989;13:644-648. [PubMed] [Cited in This Article: ] |
| 81. | Yamazaki S, Takayama T, Kimura Y, Moriguchi M, Higaki T, Nakayama H, Fujii M, Makuuchi M. Transfusion criteria for fresh frozen plasma in liver resection: a 3 + 3 cohort expansion study. Arch Surg. 2011;146:1293-1299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 82. | Hayashi Y, Takayama T, Yamazaki S, Moriguchi M, Ohkubo T, Nakayama H, Higaki T. Validation of perioperative steroids administration in liver resection: a randomized controlled trial. Ann Surg. 2011;253:50-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 83. | Zhong JH, Ma L, Li LQ. Postoperative therapy options for hepatocellular carcinoma. Scand J Gastroenterol. 2014;49:649-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 84. | Chen LT, Chen MF, Li LA, Lee PH, Jeng LB, Lin DY, Wu CC, Mok KT, Chen CL, Lee WC. Long-term results of a randomized, observation-controlled, phase III trial of adjuvant interferon Alfa-2b in hepatocellular carcinoma after curative resection. Ann Surg. 2012;255:8-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 85. | Ikeda K, Arase Y, Saitoh S, Kobayashi M, Suzuki Y, Suzuki F, Tsubota A, Chayama K, Murashima N, Kumada H. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor-A prospective randomized study of hepatitis C virus-related liver cancer. Hepatology. 2000;32:228-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 288] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 86. | Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Shuto T, Kinoshita H. Randomized clinical trial of long-term outcome after resection of hepatitis C virus-related hepatocellular carcinoma by postoperative interferon therapy. Br J Surg. 2002;89:418-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 87. | Lin SM, Lin CJ, Hsu CW, Tai DI, Sheen IS, Lin DY, Liaw YF. Prospective randomized controlled study of interferon-alpha in preventing hepatocellular carcinoma recurrence after medical ablation therapy for primary tumors. Cancer. 2004;100:376-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 88. | Lo CM, Liu CL, Chan SC, Lam CM, Poon RT, Ng IO, Fan ST, Wong J. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg. 2007;245:831-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 89. | Shiratori Y, Shiina S, Teratani T, Imamura M, Obi S, Sato S, Koike Y, Yoshida H, Omata M. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann Intern Med. 2003;138:299-306. [PubMed] [Cited in This Article: ] |
| 90. | Sun HC, Tang ZY, Wang L, Qin LX, Ma ZC, Ye QH, Zhang BH, Qian YB, Wu ZQ, Fan J. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: a randomized clinical trial. J Cancer Res Clin Oncol. 2006;132:458-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 91. | Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, Capussotti L, Calise F, Pellicci R, Belli G. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44:1543-1554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 279] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 92. | Yin J, Li N, Han Y, Xue J, Deng Y, Shi J, Guo W, Zhang H, Wang H, Cheng S. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol. 2013;31:3647-3655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 93. | Wong JS, Wong GL, Tsoi KK, Wong VW, Cheung SY, Chong CN, Wong J, Lee KF, Lai PB, Chan HL. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;33:1104-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 94. | Lin MC, Wu CC, Ho WL, Yeh DC, Liu TJ, P’eng FK. Concomitant splenectomy for hypersplenic thrombocytopenia in hepatic resection for hepatocellular carcinoma. Hepatogastroenterology. 1999;46:630-634. [PubMed] [Cited in This Article: ] |
| 95. | Shimada M, Hashizume M, Shirabe K, Takenaka K, Sugimachi K. A new surgical strategy for cirrhotic patients with hepatocellular carcinoma and hypersplenism. Performing a hepatectomy after a laparoscopic splenectomy. Surg Endosc. 2000;14:127-130. [PubMed] [Cited in This Article: ] |
| 96. | Oh JW, Ahn SM, Kim KS, Choi JS, Lee WJ, Kim BR. The role of splenectomy in patients with hepatocellular carcinoma and secondary hypersplenism. Yonsei Med J. 2003;44:1053-1058. [PubMed] [Cited in This Article: ] |
| 97. | Wu CC, Cheng SB, Ho WM, Chen JT, Yeh DC, Liu TJ, P’eng FK. Appraisal of concomitant splenectomy in liver resection for hepatocellular carcinoma in cirrhotic patients with hypersplenic thrombocytopenia. Surgery. 2004;136:660-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 98. | Chen XP, Wu ZD, Huang ZY, Qiu FZ. Use of hepatectomy and splenectomy to treat hepatocellular carcinoma with cirrhotic hypersplenism. Br J Surg. 2005;92:334-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 99. | Sugimachi K, Ikeda Y, Tomikawa M, Taketomi A, Tsukamoto S, Kawasaki K, Yamamura S, Korenaga D, Maehara Y, Takenaka K. Appraisal of hepatic resection in the treatment of hepatocellular carcinoma with severe thrombocytopenia. World J Surg. 2008;32:1077-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 100. | Zhang XY, Li C, Wen TF, Yan LN, Li B, Yang JY, Wang WT, Jiang L. Synchronous splenectomy and hepatectomy for patients with hepatocellular carcinoma and hypersplenism: A case-control study. World J Gastroenterol. 2015;21:2358-2366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 22] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |