Published online Jun 27, 2014. doi: 10.4254/wjh.v6.i6.370
Revised: January 23, 2014
Accepted: March 13, 2014
Published online: June 27, 2014
Cytomegalovirus (CMV) is one of the most common viral pathogens causing clinical disease in liver transplant recipients, and contributing to substantial morbidity and occasional mortality. CMV causes febrile illness often accompanied by bone marrow suppression, and in some cases, invades tissues including the transplanted liver allograft. In addition, CMV has been significantly associated with an increased predisposition to acute and chronic allograft rejection, accelerated hepatitis C recurrence, and other opportunistic infections, as well as reduced overall patient and allograft survival. To negate the adverse effects of CMV infection on transplant outcome, its prevention, whether through antiviral prophylaxis or preemptive therapy, is an essential component to the management of liver transplant recipients. Two recently updated guidelines have suggested that antiviral prophylaxis or preemptive therapy are similarly effective in preventing CMV disease in modest-risk CMV-seropositive liver transplant recipients, while antiviral prophylaxis is the preferred strategy over preemptive therapy for the prevention of CMV disease in high-risk recipients [CMV-seronegative recipients of liver allografts from CMV-seropositive donors (D+/R-)]. However, antiviral prophylaxis has only delayed the onset of CMV disease in many CMV D+/R- liver transplant recipients, and such occurrence of late-onset CMV disease was significantly associated with increased all-cause and infection-related mortality after liver transplantation. Therefore, a search for better strategies for prevention, such as prolonged duration of antiviral prophylaxis, a hybrid approach (antiviral prophylaxis followed by preemptive therapy), or the use of immunologic measures to guide antiviral prophylaxis has been suggested to prevent late-onset CMV disease. The standard treatment of CMV disease consists of intravenous ganciclovir or oral valganciclovir, and if feasible, reduction in pharmacologic immunosuppression. In one clinical trial, oral valganciclovir was as effective as intravenous ganciclovir for the treatment of mild to moderate CMV disease in solid organ (including liver) transplant recipients. The aim of this article is to provide a state-of-the art review of the epidemiology, diagnosis, prevention, and treatment of CMV infection and disease after liver transplantation.
Core tip: This paper summarizes the current state in the management of cytomegalovirus disease after liver transplantation, including a review of recently updated guidelines for diagnosis, prevention and treatment.
- Citation: Bruminhent J, Razonable RR. Management of cytomegalovirus infection and disease in liver transplant recipients. World J Hepatol 2014; 6(6): 370-383
- URL: https://www.wjgnet.com/1948-5182/full/v6/i6/370.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i6.370
Cytomegalovirus (CMV) is the single most common viral pathogen that influences the outcome of liver transplantation[1,2]. CMV is a ubiquitous herpes virus that, depending on the population studied, infects 50%-100% of humans[1,2]. Primary CMV infection in immune competent individuals presents most commonly as an asymptomatic illness or less commonly as a benign infectious mononucleosis-like syndrome. When CMV infection occurs in individuals with compromised immunity, such as liver transplant recipients, clinical disease with high morbidity may develop and, occasionally, may lead to death if untreated[1,2].
Primary infection results in viral latency in various cells, and ensures the persistence of the virus throughout the life of the host[1,2]. Such characteristic plays an important role in how liver recipients develop CMV infection. First, cellular sites of viral latency become reservoirs for reactivation during periods of inflammation (such as allograft rejection and critical illness). And second, cellular sites of viral latency serve as vehicles for transmission to susceptible hosts (i.e., during blood transfusions and transplantation of liver allografts latently infected with CMV)[1-5].
The classic illness caused by CMV after liver transplantation is manifested most commonly as fever and bone marrow suppression (most commonly, leukopenia and neutropenia, termed CMV syndrome). CMV syndrome accounts for over 60% of CMV diseases after liver transplantation. Less commonly, CMV infection may clinically manifest as tissue-invasive disease (which may involve any organ system) (Table 1)[1]. The most common organ system involved is the gastrointestinal tract (in the form of CMV gastritis, esophagitis, enteritis, and colitis). Gastrointestinal CMV disease accounts for over 70% of tissue-invasive CMV disease cases in liver and other solid organ transplant recipients[6]. The transplanted liver allograft is also predisposed to develop tissue-invasion by CMV (i.e., CMV hepatitis), and this is often manifested with symptoms that may be clinically indistinguishable from acute rejection[7].
| Direct effects | Indirect effects |
| CMV syndrome | Acute allograft rejection |
| Fever | Chronic allograft rejection |
| Myelosuppression | Vanishing bile duct syndrome |
| Malaise | Chronic ductopenic rejection |
| Tissue-invasive CMV disease1 | Hepatitis C virus recurrence |
| Gastrointestinal disease | Allograft hepatitis, fibrosis |
| (colitis, esophagitis, gastritis, | Allograft failure |
| enteritis) | Opportunistic and other infections |
| Hepatitis | Fungal superinfection |
| Pneumonitis | Nocardiosis |
| CNS disease | Bacterial superinfection |
| Retinitis | Epstein-Barr virus and PTLD |
| Mortality | HHV-6 and HHV-7 infections |
| Vascular thrombosis | |
| New onset diabetes mellitus | |
| Mortality |
CMV disease among liver recipients who are not receiving antiviral prophylaxis occur most commonly during the first 3 mo after transplantation[8]. Overall, it is estimated that 18%-29% of all liver transplant recipients will develop CMV disease in the absence of prevention strategy (Table 2)[4,5,9-11]. However, this incidence varies depending upon donor and recipient CMV serologic status; it may be as high as 44%-65% in CMV D+/R-, or as low as 1%-2% among CMV D-/R- patients (who may still acquire the virus from natural transmission or through blood transfusion). The incidence is between 8%-19% among CMV-seropositive (CMV R+) liver transplant recipients[4,9,11].
| Use of anti-CMV prophylaxis for 3-6 mo | ||
| Yes1 | No | |
| CMV D+/R- | 12%-30% | 44%-65% |
| CMV D+/R+ | 2.70% | 18.20% |
| CMV D-/R+ | 3.90% | 7.90% |
| CMV D-/R- | 0% | 1%-2% |
| All patients | 4.80% | 18%-29% |
The incidence of CMV disease is markedly reduced in liver transplant recipients who received 3 mo of valganciclovir or oral ganciclovir prophylaxis. The CMV disease incidence rates are 12%-30% in CMV D+/R-, and < 10% of CMV R+ liver transplant recipients who received 3 mo of antiviral prophylaxis[3,4,9,11-13]. The onset of disease in these patients occurs during first 3-6 mo after completing antiviral prophylaxis; hence, the term late-onset CMV disease[3]. To reduce the incidence of late onset CMV disease, there have been efforts to prolong prophylaxis to 6 mo in CMV D+/R- liver recipients. There is limited data available on the incidence of late-onset CMV disease after 6 mo of prophylaxis, although this is estimated to be further reduced by half (e.g., about 15% of CMV D+/R- liver recipients).
CMV has a variety of indirect effects that are believed to be mediated by the ability of the virus to modulate the immune system (Table 1)[1,2]. CMV is a potent up-regulator of alloantigens, which increases the risk of acute rejection and chronic allograft dysfunction[14]. CMV has been associated with vanishing bile duct syndrome and ductopenic rejection that leads to chronic cholestasis and allograft failure[15-17]. A higher incidence of vascular and hepatic artery thrombosis has been reported in liver recipients with CMV disease, and this effect is postulated to result from infection of the vascular endothelial cells[18,19].
The immunomodulatory effects of CMV may account for a higher predisposition to develop opportunistic infections due to fungi, other viruses, and bacteria[20,21]. CMV-infected transplant recipients are more likely to develop Epstein-Barr virus-associated post-transplant lymphoproliferative disorders, or develop co-infections with other viruses such as human herpesvirus (HHV)-6 and HHV-7[20-22]. Co-infection with HHV-6 and HHV-7 is significantly associated with an increased predisposition to CMV disease[23-25]. Similarly, there is a significant association between CMV and hepatitis C virus (HCV) recurrence after liver transplantation[26-31], and this is clinically manifested as a more accelerated clinical course of HCV recurrence[29,31]. A recent retrospective study of 347 HCV-infected liver recipients observed that CMV infection increased by 1.5 times the risk of allograft fibrosis, while CMV disease increased by 3.4 times the risk of allograft inflammation[32]. A significant association between CMV infection and metabolic disease such as post-transplant diabetes mellitus has been reported. In a recent study of 169 non-diabetic liver recipients, CMV infection was a significant risk factor for development of new-onset diabetes after transplantation[33].
Through direct, indirect and possibly immunomodulatory mechanisms, CMV is associated with higher risk of death after liver transplantation[20,34,35]. The use of intravenous (IV) and oral ganciclovir has reduced the incidence of CMV disease and the risk of death due to CMV[20,36-38]. Despite these improvements in CMV prevention with use of antiviral drugs, late-onset CMV disease continues to occur, particularly among CMV D+/R- liver transplant recipients. Notably, late-onset CMV disease remains significantly associated with increased risk of mortality after liver transplantation[35]. In an analysis of 437 liver transplant recipients, CMV disease occurred in 37 patients (8.5%) and its occurrence was independently associated with a 5-fold increased risk of all-cause mortality, and 11-fold increased risk of infection-related mortality[35].
The most important risk factor for CMV disease after liver transplantation is a lack of effective CMV-specific immunity. In the clinical setting, this is best measured by serology to detect immunoglobulin G against CMV. Specifically, CMV D+/R- patients are at highest risk of CMV disease[4,20], while CMV R+ patients have modest and CMV D-/R- have the lowest risk of CMV disease after liver transplantation (Table 3).
| CMV D+/R- > CMV R+ |
| Allograft rejection |
| High viral replication |
| Mycophenolate mofetil |
| Anti-thymocyte globulin |
| Alemtuzumab |
| Human herpesvirus-6 |
| Human herpesvirus-7 |
| Renal insufficiency |
| Deficiency in CMV-specific CD4+ T cells |
| Deficiency in CMV-specific CD8+ T cells |
| Toll-like receptor gene polymorphism |
| Mannose binding lectin deficiency |
| Chemokine and cytokine defects (IL-10, MCP-1, CCR5) |
| Expression of immune evasion genes |
| Programmed cell death 1 expression |
| Others1 |
Drug-induced immunosuppression impairs the ability of liver recipients to mount an effective immune response against CMV, thereby predisposing to higher risk of CMV disease[4,20]. Immune dysfunction is particularly intense with the use of lymphocyte-depleting drugs, as either induction or rejection therapy[39,40]. When alemtuzumab, an anti-CD52 lymphocytic antibody, is used for short-course induction therapy, the risk of CMV disease is not significantly increased[41,42]. However, when alemtuzumab is used as treatment for rejection, the risk of CMV disease is higher suggesting that rejection per se also increases the risk[42]. Basiliximab and daclizumab are associated with lower risk of CMV disease compared to anti-thymocyte globulin[43].
The combined effects of drugs for maintenance immunosuppression have been associated with CMV disease[1,2,20], although specific agents such as mycophenolate mofetil, when used at high doses has also been implicated to increase the risk[44,45]. In contrast, some of the newer immunosuppressive drugs such as sirolimus and everolimus [mammalian target of rapamycin (mTOR) inhibitor] have been associated with lower risk of CMV disease[46,47]. These observations have generated special interest in the use of the mTOR agents for patients at high risk of CMV disease.
Inherent defects in innate immunity, such as mutations in innate immunity-associated genes, increase the risk of CMV disease (Table 3). In a pilot study in 92 liver recipients with chronic HCV, the R753Q single nucleotide polymorphism (SNP) in the Toll-like receptor 2 (TLR2) gene was associated with a higher CMV replication and higher incidence of CMV disease. TLR2 is a pattern recognition receptor that senses the presence of CMV and signals the immune cells to produce antiviral peptides and cytokines; the R753Q SNP impairs this immunologic cascade[48]. A larger study of 737 liver recipients confirmed that TLR2 R753Q SNP was significantly and independently associated with CMV disease after liver transplantation, especially for tissue-invasive disease[49].
The lectin pathway of complement activation is also important in the innate immune response to CMV. Mannose binding lectin levels or mutation in its gene has been assessed as prognostic indicators of CMV disease after transplantation[50]. In a study of 295 liver recipients, whose donors were also genotyped for SNPs in mannose-binding lectin (MBL2), Ficolin-2 (FCN2) and MBL-associated serine protease genes, the risk of CMV infection was 2.77 fold higher with the gene profile of the donor and 4.57 fold higher for the combined MBL2 and FCN2 donor-recipient mismatch profile. These results were independent from donor-recipient CMV serostatus[51].
Other immune measures, such as programmed death-1 expression[52] have also been assessed for their association with CMV infection. In one study, programmed death-1 receptor up-regulation was significantly associated with incipient and overt CMV disease and with CMV viremia[52].
Cell-mediated immunity are the most essential components to the control of CMV after liver transplantation[40]. Hence, measuring CMV-specific cell-mediated immunity is a promising strategy in CMV management after transplantation[53]. In one study, secretion of interferon-γ by CD8+ T cells during in vitro stimulation with CMV peptides was associated with a lower incidence of CMV disease in solid organ transplant recipients (including liver recipients)[54]. A variety of CMV-specific T-cell assays are currently being developed including QuantiFERON-CMV assay, ELISpot assay, and intracellular cytokine staining for IFN-γ using flow cytometry. The principle of these assays relies on the detection of cytokine (most commonly interferon-γ) production following in vitro stimulation with CMV antigens[55]. Recently, QuantiFERON-CMV assay was studied in a multi-center study that enrolled 124 high-risk (D+/R-) solid-organ transplant (including liver) recipients. Twenty five percent of patients had positive result, 65.3% had a negative result, and 9.7% had an indeterminate result. At 12 mo follow-up, patients with a positive QuantiFERON-CMV assay had a significantly lower risk of CMV disease (6.4%) compared to those with negative (22.2%) and indeterminate result (58.3%). The assay provides a positive and negative predictive values for protection from CMV disease of 0.90 (95%CI: 0.74-0.98) and 0.27 (95%CI: 0.18-0.37), respectively[53,56]. Collectively, these studies indicate that immune monitoring of CMV-specific T-cell responses may have a potential to predict individuals at increased risk of CMV disease, and may be useful in guiding the use of prophylaxis.
Allograft rejection can trigger CMV reactivation after transplantation[13]. The cytokines released during acute rejection, particularly tumor necrosis factor-α[57], could transactivate CMV from latency[58,59]. Subsequent therapy for allograft rejection (intensified immunosuppression with the use of high doses of steroids or lymphocyte-depleting drugs) enhances viral replication by impairing the generation of an effective CMV-specific cell-mediated immunity[60]. In a bidirectional relationship, CMV increases the risk of allograft rejection[61].
Interactions among reactivated viruses have been proposed to enhance the risk of CMV disease after liver transplantation[22,23,27-31]. HHV-6 increases the risk of CMV disease after liver transplantation[22,23,25]. Likewise, HCV-infected liver transplant patients have a higher incidence of CMV disease[62], although the data in the era of valganciclovir prophylaxis has refuted this observation[26].
The risk of CMV disease after liver transplantation is associated, in direct proportion, with viral burden and the degree of CMV replication[9,24,63,64]. Other factors associated with CMV disease after liver transplantation include cold ischemia time, bacterial and fungal infections and sepsis, the amount of blood loss, fulminant hepatic failure as the indication for liver transplantation, age, female gender, and renal insufficiency[2,3,20,65].
There are two major strategies for CMV disease prevention after liver transplantation: (1) preemptive therapy; and (2) antiviral prophylaxis. For preemptive therapy, patients are monitored for evidence of CMV replication by sensitive assays, most commonly using quantitative nucleic acid amplification tests by PCR and less commonly by detection of pp65 antigenemia, and upon the detection of asymptomatic CMV replication, antiviral therapy is administered preemptively to prevent progression to symptomatic clinical disease. In contrast, antiviral prophylaxis entails the administration of antiviral drugs such as valganciclovir to all patients at risk of CMV disease after liver transplantation[20]. Both of these strategies are similarly effective in preventing CMV disease after liver transplantation[4,5,66-69]. However, there has not been a large prospective well-controlled randomized trial directly comparing preemptive therapy and prophylaxis in liver transplant recipients. In a retrospective study comparing the two approaches in liver transplant recipients, antiviral prophylaxis was more effective in prevention of CMV disease in high risk D+/R-, but there were no differences in acute rejection, opportunistic infections, or rate of mortality[40,70]. Another retrospective study reported the incidence of CMV viremia was 4.9% and 50.0% (P < 0.001) at 3 mo in the antiviral prophylaxis and preemptive therapy groups, respectively, but the rates were expectedly reversed, at 24.6% and 8.3% (P = 0.026), respectively at 6 mo; the reversal of the rates during the latter period accounts for the higher rates of late onset CMV disease with antiviral prophylaxis[71]. An NIH-sponsored prospective study is being conducted in six transplant centers in the United States to compare the efficacy and safety of antiviral prophylaxis vs preemptive therapy in CMV D+/R- liver transplant recipients.
According to the recently updated American Society of Transplantation (AST) and The Transplantation Society (TTS) guidelines, preemptive therapy may be an option in CMV D+/R- liver transplant recipients, however, many authorities prefer to use antiviral prophylaxis in this high-risk population and reserve preemptive therapy for lower-risk populations[39,72]. The main reason for this preference for antiviral prophylaxis is the rapidity of CMV replication in CMV D+/R- liver recipients, which may escape detection with once weekly CMV surveillance. Indeed, antiviral prophylaxis has been used by the majority of American and European transplant centers in preventing primary CMV disease in high-risk CMV D+/R- liver transplant recipients[73,74]. Moreover, primary antiviral prophylaxis has the added benefit of reduction in bacterial and fungal opportunistic infections and mortality[34,35,37,75].
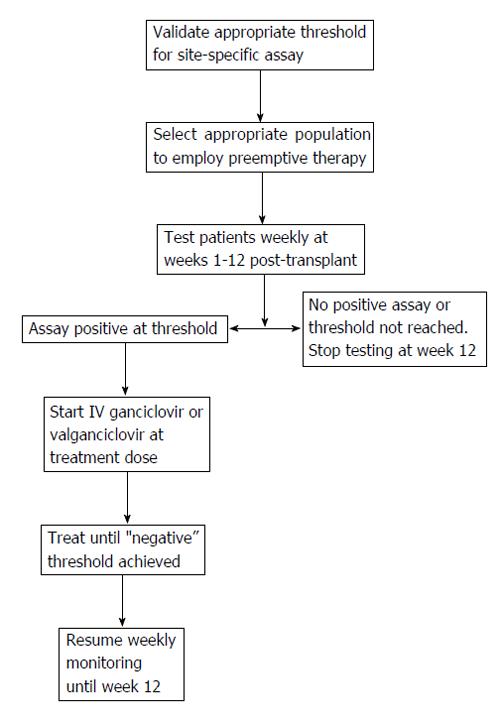
The basic principle of preemptive therapy is to detect the presence of early CMV replication prior to the onset of clinical symptoms, so that antiviral therapy is administered early in order to prevent the progression of asymptomatic infection to clinical disease[64,66,67,69,76]. An example of a preemptive algorithm is shown in Figure 1. Preemptive therapy has the potential advantage of targeting therapy to the highest risk patients and thereby decreasing drug costs and toxicity. The success of this approach relies on several aspects including: (1) the optimal laboratory test and frequency and duration of monitoring; (2) selection of the appropriate population for preemptive therapy; and (3) choosing the type, dose and duration of an antiviral drug.
The two laboratory methods used for CMV surveillance for preemptive therapy are pp65 antigenemia assay and nucleic acid testing (NAT). During the past decade, clinical laboratories have been moving towards preference for NAT over antigenemia, mainly for assay sensitivities, performance and logistics. The pp65 antigenemia assay, a semi-quantitative assay based on detection of CMV pp65 antigen in infected leukocytes, has comparable sensitivity to CMV NAT[77], but it needs to be processed within 6-8 h of blood collection, it requires a large sample volume, it has subjective interpretation of results, and is labor-intensive. Accordingly, quantitative NAT is now the preferred method for detecting CMV after transplantation[78]. The assay has a better precision and faster turnaround time[79]. Because of its quantitative ability, the assay can distinguish between active viral replication (typically with high-level viremia) from latent virus (low-level viremia if using highly sensitive tests)[78]. In the past, NAT lacked standardization, and this prevented the generation of widely applicable viral load thresholds for various clinical applications. In 2011, CMV viral load standardization was made possible with the release of the World Health Organization (WHO) calibrator standard. A recent study applied this assay in the plasma samples of 267 solid organ (including liver) transplant recipients. This study demonstrated that patients with pretreatment CMV DNA of less than 18200 [4.3 log (10)] IU/mL have 1.5 fold higher chance for CMV disease resolution. Likewise, CMV suppression to less than 137 [2.1 log (10)] IU/mL is predictive of clinical response to antiviral treatment[80].
The optimal interval and duration of monitoring for preemptive therapy is still unknown, but guidelines recommend once weekly CMV NAT for 12 wk after liver transplantation. If a patient shows viremia above a defined threshold during the surveillance period, antiviral therapy (with oral valganciclovir or intravenous ganciclovir) should be initiated and continued until CMV viremia is no longer detectable[55,72]. Several studies have reported the success of IV ganciclovir or oral valganciclovir for preemptive treatment of CMV infection in liver transplant recipients, including high-risk CMV D+/R- patients[68,76]. However, some studies have indicated that preemptive therapy may not be completely effective in CMV D+/R- liver recipients since the replication kinetics of CMV in immune-deficient individuals is so rapid[63] that it may escape detection with once weekly surveillance[9,58,66]. Indeed, in our clinical experience, nearly 25% of CMV D+/R- liver recipients who developed CMV disease were not identified early despite weekly CMV PCR assay[9,58,66]. Accordingly, the recently updated AST and TTS guidelines prefer antiviral prophylaxis in CMV D+/R- liver recipients. In contrast, preemptive therapy is recommended for preventing CMV disease in CMV-seropositive liver recipients[55,72].
Clinical trials have demonstrated the efficacy of preemptive therapy in CMV disease prevention[66-68,76]. Three meta-analyses that collectively analyzed data from prospective clinical trials demonstrated the benefits of preemptive therapy in preventing CMV disease[35,36,68]. When conducted properly, preemptive therapy, with the use of IV ganciclovir or oral valganciclovir resulted in the reduction of CMV disease by about 70%[37,38,75]. Moreover, preemptive therapy is much less likely associated with late onset CMV disease (unlike in antiviral prophylaxis, as discussed below)[66,67]. Currently, valganciclovir is the most commonly used drug for preemptive therapy[73], and in one non-controlled study, it was demonstrated to be as effective in terms of clinical and virologic response, when compared to IV ganciclovir)[66,67]. In addition, preemptive therapy may be beneficial in reducing the indirect effects of CMV, although to a much lesser degree compared to antiviral prophylaxis. In one study, the incidence of major opportunistic infections, bacteremia, bacterial infection, HCV recurrence, and rejection were not significantly different between liver transplant patients who received preemptive therapy and those who did not have CMV reactivation[81].
Antiviral prophylaxis is highly effective in preventing the direct effects, and there is increasing evidence that it reduces the indirect effects of CMV after liver transplantation[4,5,37,38,75]. Compared to placebo or no treatment, patients who received antiviral prophylaxis had lower incidence of CMV disease (58%-80% reduction) and CMV infection (about 40% reduction)[75]. In one meta-analysis, a 25% reduction in the incidence of acute allograft rejection was observed[37]. In two studies, a reduction in all-cause mortality was observed[37,75], mainly due to a decline in CMV-related death[75]. A reduction in the incidence of other herpes viruses, bacterial, and protozoan infections were also observed[37]. Because of these additional benefits, liver transplant centers prefer the use of antiviral prophylaxis over preemptive therapy in the prevention of CMV disease, particularly in CMV D+/R- liver transplant recipients[73]. Table 4 shows the currently available antiviral drugs for CMV prophylaxis and treatment in liver transplant recipients.
| Drug | Route | Usual adult prophylaxis dose | Usual adult treatment dose | Comments on use and major toxicity |
| Ganciclovir | Intravenous | 5 mg/kg once daily | 5 mg/kg twice daily | Intravenous access; leukopenia |
| Ganciclovir | Oral | 1 g three times daily | Not applicable | Low oral bioavailability; high pill burden |
| Valganciclovir | Oral | 900 mg once daily | 900 mg twice daily | Ease of administration; leukopenia |
| Foscarnet | Intravenous | Not recommended | 60 mg/kg every 8 h (or 90 mg/kg every 12 h) | Second-line drug Intravenous access; nephrotoxicity |
| Cidofovir | Intravenous | Not recommended | 5 mg/kg once weekly × 2 then every 2 wk thereafter | Third-line drug Intravenous access; nephrotoxicity |
Ganciclovir-based regimen is more effective than acyclovir or immunoglobulins in reducing the incidence of CMV disease after liver transplantation. In one study, the administration of IV ganciclovir for 90-100 d reduced the incidence of CMV disease in CMV D+/R- liver transplant recipients to 5.4% (compared to 40% in patients who received less than 7 wk of prophylaxis)[44]. Oral ganciclovir, administered at 1000 mg PO three times daily, was compared to placebo, and there was a significant reduction in the 6-mo incidence of CMV infection (51.5% vs 24.5%, P < 0.001), and CMV disease (19% vs 5%, P < 0.001) in liver transplant recipients[4], including CMV D+/R- patients (44% vs 15%, P = 0.02) and patients who received antilymphocyte antibodies (33% vs 5%; P = 0.002)[4]. Among CMV R+ liver transplant recipients, oral ganciclovir for 12 wk reduced the incidence of CMV disease to 1% (compared to 7% in patients who received acyclovir)[82]. Oral ganciclovir, however, is poorly absorbed, and its oral administration results in low systemic ganciclovir levels[83].
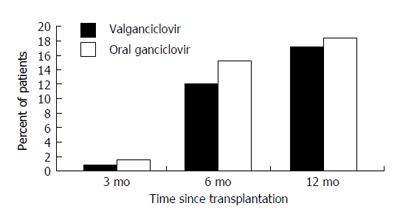
Valganciclovir provides systemic ganciclovir levels that are comparable to IV ganciclovir[83,84]. Pharmacokinetic studies indicate that a 900 mg dose of valganciclovir achieves a similar daily area under the concentration time curve (AUC24) as an IV dose of 5 mg/kg of ganciclovir[83]. The role of valganciclovir in the prevention of CMV disease after liver transplantation was evaluated in a multicenter randomized non-inferiority clinical trial that compared it with oral ganciclovir in a cohort of 364 CMV D+/R- solid organ (including liver) transplant recipients (Figure 2). Among all solid organ transplant recipients, the 6-mo incidence of CMV disease was 12% and 15% in the valganciclovir and oral ganciclovir groups, respectively. Follow-up at one year, demonstrated that the incidence of protocol-defined CMV disease in all patients was 17% and 18% with valganciclovir and oral ganciclovir, respectively[5].
However, in a subgroup analysis of the 177 liver transplant recipients, the incidence of CMV disease was 19% in the valganciclovir group as opposed to only 12% in the ganciclovir group. There was also a higher incidence of tissue-invasive CMV disease in the valganciclovir group[5]. As a result of these findings, valganciclovir did not gain approval from the United States food and drug administration (US-FDA) for prophylaxis against CMV disease after liver transplantation. A recent meta-analysis of 5 controlled clinical studies, including 380 liver transplant recipients who received valganciclovir (450 or 900 mg daily) prophylaxis, showed the overall CMV disease rate was 12%, and the rate among D+/R- patients was 20%. The risk of CMV disease with valganciclovir was 1.8-fold higher than oral ganciclovir. For CMV D+/R- patients, the risk of CMV disease was 2-fold higher than oral ganciclovir. The risk of CMV disease remained significant with valganciclovir 900-mg daily dose, but not with the 450 mg dose. The risk of leukopenia with valganciclovir was 1.9-fold higher than those using oral ganciclovir[85]. Despite these findings, and even if not FDA-approved for this indication, valganciclovir remains as the most widely used drug for CMV prophylaxis after liver transplantation[73].
Maribavir, an investigational oral benzimidazole riboside with in vitro activity against CMV, was compared to oral ganciclovir, for prophylaxis in 303 high-risk liver transplant recipients. In this randomized, double blind, multicenter controlled trial, maribavir was less effective than oral ganciclovir for the prevention of CMV disease. Significantly fewer patients who received oral ganciclovir prophylaxis had confirmed CMV disease or CMV infection compared to maribavir at 100 d (20% vs 60%, P < 0.0001) and at 6 mo (53% vs 72%, P = 0.0053) after liver transplantation. Because of this finding (and the results of the bone marrow transplant trial), the clinical development of maribavir for CMV management is on hold[86].
A combination of anti-CMV drugs and CMV immunoglobulin has been used in a clinical practice for prophylaxis. A pooled analysis of previous studies revealed a combination regimen may reduce severe CMV disease and mortality in solid organ transplant recipients; however the finding has been debated[87,88].
In many high-risk CMV D+/R- individuals, the use of antiviral prophylaxis for 100 d has only delayed the onset of CMV disease to 3-6 mo after liver transplantation[3,5,13]. In our analysis of 67 CMV D+/R- liver transplant recipients who received 3 mo of oral ganciclovir and valganciclovir prophylaxis, the two-year incidence of CMV disease was 29%, and was similar between the two drugs (22% vs 28%, P = 0.63)[3]. The most common presentation of late-onset CMV disease was CMV syndrome, with fever and bone marrow suppression[3]. In less than half of the patients, CMV manifested as tissue-invasive disease, and frequently affected the gastrointestinal tract[3]. Factors such as age[3], female gender[3,89], renal dysfunction[77], and allograft rejection[13] predisposed to the development of late-onset primary CMV disease. Late-onset CMV disease appears to be clinically less severe, although it is associated with significant mortality after liver transplantation[35].
Because of the negative effect of late-onset CMV disease on overall outcome, a better method for CMV prevention is needed among CMV D+/R- liver transplant recipients. The recently updated AST and TTS guidelines suggest that the duration of antiviral prophylaxis may be prolonged from the standard 3 mo to 6 mo in CMV D+/R- liver transplant recipients[41,81]. This recommendation is based on the trial that investigated the approach in CMV high-risk D+/R- “kidney” transplant recipients. In the Improved Protection Against Cytomegalovirus in Transplantation study, the incidence of CMV disease was significantly lower in the 200 d vs 100 d of prophylaxis at the end of 1 year (16.1% vs 36.8%, P < 0.0001) and the result was persistent up to 2 years after transplantation (21.3% vs 38.7%, P < 0.001)[90,91]. In a retrospective study on 203 liver transplant recipients who received valganciclovir 900 mg daily for 3 to 6 mo, the overall incidence of CMV disease was 14%. The incidence was highest in D+/R- (26%) compared to 16% in D+/R+ group and 7% in D-/R+ group[92]. However, it is emphasized that 6 mo of antiviral prophylaxis has not yet been studied prospectively in the liver transplant recipients, and that valganciclovir is not FDA-approved for the prevention of CMV disease after liver transplantation. In addition, there are theoretical concerns about ganciclovir resistance and drug toxicity particularly with leukopenia with prolonged prophylaxis, although these were not demonstrated in the clinical trial. The cost of prophylaxis will need to be evaluated with the use of prolonged prophylaxis.
In summary, the duration of prophylaxis in D+/R- liver transplant recipients should generally be between 3 and 6 mo. For seropositive patients with either donor seropositive or seronegative, a majority of the experts suggested that 3 mo of prophylaxis is sufficient[55].
A new strategy has been utilized in some transplant centers to prevent late-onset CMV disease is hybrid strategy in which preemptive monitoring is initiated after completing prophylaxis. A retrospective study of 199 liver transplant recipients [including 23 (11%) high-risk D+/R- patients] who received 3 mo of valganciclovir prophylaxis and were monitored by CMV antigenemia after prophylaxis (twice a month up to month 6, and monthly until one year). The results were modest at best[93], possibly due to difficult and non-standardized logistics of this approach[94].
The first line treatment of CMV disease after liver transplantation is IV ganciclovir or valganciclovir[62,76,93]. In contrast, oral ganciclovir should not be used for the treatment of CMV disease because of its poor bioavailability[20]. In addition, the degree of pharmacologic immunosuppression should be reduced if possible[20].
In a multi-center non-inferiority trial, 321 solid organ (including liver) transplant recipients with non-severe CMV disease were randomized to valganciclovir (900 mg twice daily) or IV ganciclovir (5 mg/kg twice daily) for a fixed 21-d course, followed by valganciclovir (900 mg once daily) maintenance treatment for 4 wk; the proportion of patients with viral eradication at 21 and 49 d were comparable in the IV ganciclovir and valganciclovir groups[93]. The overall time to viral eradication was 21 d with valganciclovir and 19 d with IV ganciclovir. The calculated viral decay was 11.5 d with valganciclovir and 10.4 d with IV ganciclovir. Likewise, clinical resolution was not different between the two groups. It was noted that patients enrolled into this trial were mostly CMV-seropositive, the majority were kidney recipients (although there were good number of liver transplant recipients), and patients with severe CMV disease were excluded. Despite these limitations, this pivotal trial now supports the use of valganciclovir for oral treatment of CMV disease, at least in selected transplant patients[93]. IV ganciclovir is preferable to valganciclovir in patients with severe or life-threatening disease, or in patients who may have a problem with gastrointestinal absorption of oral drug. In many instances, valganciclovir is used as a step-down treatment when the clinical symptoms have resolved after an initial induction treatment with IV ganciclovir.
The duration of treatment of CMV disease should be individualized[62,77]. The persistence of the virus at the end of therapy (by PCR or pp65 antigenemia) is associated with a higher risk of clinical relapse[78]. In the recent study that evaluated the role of viral load using a WHO standard calibrated assay, the degree of viral load at the time of CMV disease diagnosis and the presence or absence of viral load at the end of treatment were significantly associated with CMV disease resolution. It is now generally accepted that multiple (at least two) weekly negative CMV PCR results should be obtained before antiviral therapy is discontinued. Although this may be true for non-tissue invasive CMV syndromes, the utility of such an approach may not necessarily apply to some tissue-invasive disease, which may manifest as “compartmentalized disease”[20].
Compartmentalized CMV disease refers to clinical syndromes wherein the virus is detected in the affected tissues but is minimally detectable or undetectable in the blood[20]. In the current era, gastrointestinal CMV disease constitutes the vast majority of tissue-invasive cases[3,8,20], and in a number of cases, especially in CMV R+ patients, this type of CMV disease is “compartmentalized”. In a retrospective study, the sensitivity of pp65 antigenemia assay (defined as detection of ≥ 1 positive cells/2 × 105 leukocytes) for diagnosis of CMV gastrointestinal disease was only 54%[79]. Such a clinical presentation is reminiscent of CMV retinitis, a very rare manifestation of tissue-invasive CMV disease after transplantation, that is often not accompanied by viremia[75,80]. This dilemma brings to the forefront the limitation of viral load monitoring in assessing duration of treatment. In our clinical practice, it is not uncommon to have negative blood PCR assay even when there remains histologic evidence of tissue invasion. Accordingly, it has been suggested to perform colonoscopy or upper endoscopy to document clearance of gastrointestinal CMV disease prior to discontinuation of therapy. However, our retrospective review of this practice suggests that this should not be generalized to all patients with gastrointestinal CMV disease. We observed that relapse of gastrointestinal CMV disease was significantly associated with extensive involvement of gastrointestinal tract at the time of diagnosis[81]. In contrast, CMV serologic conversion, degree of viral load, treatment duration, maintenance therapy, and endoscopic findings at the end of therapy were not significantly predictive of CMV relapse. Our experience indicates that endoscopic evidence of resolution of gastrointestinal disease may not be necessary in mild to moderate disease as long as sufficient therapy is provided[81].
Ganciclovir-resistant CMV is now emerging as an important complication of prolonged antiviral drug use after transplantation[2,20,44]. Currently, ganciclovir-resistant CMV is very rarely seen in liver transplant recipients (while it is relatively more common after kidney-pancreas and lung transplantation). The estimated incidence of ganciclovir-resistant CMV after liver transplantation is < 0.5%[95,96]. Several studies have identified risk factors for ganciclovir-resistant CMV[2,20,44], including CMV D+/R- status, high levels of viral replication, potent immunosuppressive therapy, and suboptimal ganciclovir levels. The vast majority of drug-resistant cases involve the selection of viral strains with UL97 (kinase) mutation[2,20,44,83,84]. UL97 mutation generally confers resistance to ganciclovir, although in some cases, a concomitant UL54 mutation (CMV DNA polymerase) is also observed, in which case, cross-resistance with cidofovir and/or foscarnet is likely.
Drug-resistant CMV is associated with significant morbidity and mortality, and there is a very limited number of antiviral drugs (which are often toxic) available for treatment[82]. Drug-resistant CMV should be suspected when viral load or antigenemia rises or does not decline to undetectable levels despite IV ganciclovir treatment. In our retrospective study of 225 CMV D+/R- solid organ transplant recipients who received 3 mo of valganciclovir prophylaxis, CMV disease occurred in 65 patients (29%), including four (8%) caused by drug-resistant CMV, judged by the failure of the viral load to decline to undetectable levels while on IV ganciclovir treatment. The diagnosis is confirmed by genetic analysis to demonstrate mutational changes in UL97 and UL54 genes encoding for kinase and polymerase, respectively[40,82]. In patients where foscarnet or cidofovir was used, nephrotoxicity was a major and common adverse effect[85].
Other potential drugs for treatment of multi-drug resistant CMV include the off-label use of CMV Immunoglobulin (Cytogam®), adoptive infusions of CMV-specific T cells, leflunomide (an immunosuppressive drug), and artesunate (anti-malaria drug), although data supporting their use are only anecdotal[20,86]. Leflunomide acts at the stage of viral capsid assembly, not DNA replication, and therefore there is a potential use against ganciclovir-resistant strains. A single center retrospective study including 15 solid organ transplant recipients (but not including liver recipients) with drug-resistant[20,86], CMV infection treated with leflunomide monotherapy or in combination with other drugs showed some potential utility. At least half of patients (53%) had long-term responses in terms of control of CMV viremia and recurrences. The common side effects from this medication included diarrhea, anemia, and hepatic dysfunction[97].
Maribavir has also been used for treatment of drug-resistant CMV[98]. Anecdotal use in a small case series of 9 solid organ transplant recipients infected with resistant CMV showed the individual changes varied from a rapid decrease in viral load (n = 4) to no response (n = 3) with some late response slowly decreasing CMV viremia (n = 3)[99]. It has been used as salvage therapy at a higher dose (400 mg twice daily) for drug-resistant CMV infection, with mixed results including success in treating lower initial viral loads[97]. A new phase II trial of maribavir for salvage treatment of refractory and resistant CMV infection was launched in 2012 (ClinicalTrials.gov ID: NCT01611974).
Other investigational drugs being developed for CMV management are CMX001 and AIC246 (Letermovir). CMX001 is an orally bioavailable derivative of cidofovir with lipid acyclic nucleotide converted intracellularly to the active antiviral to avoid the high renal concentrations and nephrotoxicity[100]. It has demonstrated in vitro activity against CMV. It has successfully completed phase II clinical development for the prevention of CMV infection. There is an ongoing open-label, expanded-access study, CMX001-350 (ClinicalTrials.gov ID: NCT01143181), to provide access to CMX001 for patients who had no other treatment options[101]. Optimal dosing has yet to be determined, and diarrhea is a dose-limiting adverse effect. Letermovir (AIC246) is a small-molecular-weight compound with both in vitro and in vivo anti-CMV activity. It has distinct mechanism which acts late in the CMV replication cycle via a mechanism by not involving polymerase. Due to a lack of a human counterpart of the viral terminase complex, target-related toxicities are not expected. It also does not affect human blood precursor cells, and thus may allow the generation and expansion of CMV specific immunity during treatment. Theoretically, this may result in a lower rate of relapse after treatment of CMV infection or disease. Antiviral efficacy of letermovir was reported in phase II prophylaxis studies in HSCT recipients[102]. The successful use of letermovir in decreasing viral load has been reported in one case report of lung transplant recipient with drug-resistant CMV disease[103].
Remarkable advances in molecular diagnostics and therapeutics led to marked reduction in the incidence and severity of CMV disease after liver transplantation, and a parallel decline in associated morbidity and mortality. However, despite these improvements, CMV remains a common infectious complication and continues to negatively influence the outcome of liver transplantation. In addition to viral factors and pharmacologic immunosuppression, the role of innate and adaptive immune deficiencies is being recognized in the pathogenesis of CMV disease after liver transplantation. Such novel findings should provide additional avenues and opportunities for improving our management strategies. Indeed, there have been increasing evidence to support the use of immunodiagnostics, by measuring CMV-specific T cells, as a tool to predict the risk of CMV disease. Prevention of CMV with antiviral prophylaxis and preemptive therapy is effective, and a clinical trial assessing and comparing these two strategies in a head-to-head comparison in liver transplant recipients is currently being performed in the United States. The international standard for CMV viral load testing has allowed for standardization of viral load reporting, hence permitting the derivation of thresholds for preemptive and diagnostic protocols. Currently, valganciclovir prophylaxis is the most common approach for the prevention of CMV disease in CMV D+/R- and R+ liver transplant recipients. Hybrid approach of prevention (antiviral prophylaxis followed by preemptive therapy) has been utilized in some institutions among high-risk D+/R- liver transplant patients, but the efficacy is debatable due to inconsistency in the monitoring logistics. The practice of prolonging antiviral prophylaxis in D+/R- liver transplant recipients from 3 to 6 mo has been extrapolated from studies in kidney transplant recipients. IV ganciclovir and oral valganciclovir are the standard drugs for treatment of established CMV disease, although valganciclovir should be limited to patients with mild to moderate CMV disease. Oral valganciclovir should be avoided as initial therapy for patients with severe CMV disease and those with questionable gastrointestinal absorption. The duration of treatment should be individualized, depending upon clinical and laboratory parameters such as the decline of CMV load in the blood as measured by rapid and sensitive molecular standardized testing. In this context, it is generally recommended that treatment be continued until all evidence of active infection, such as positive CMV viral load, has resolved. Ganciclovir-resistant CMV and compartmentalized tissue-invasive disease (most commonly with gastrointestinal CMV disease) are emerging challenges to the management of CMV after liver transplantation. These, together with the common occurrence of late-onset CMV disease in high-risk patients, should serve as catalysts to the ongoing search for the optimal management strategy for CMV disease after liver transplantation.
P- Reviewers: Sugawara Y, Tanaka K, Yan LN S- Editor: Gou SX L- Editor: A E- Editor: Liu SQ
| 1. | Razonable RR, Emery VC. Management of CMV infection and disease in transplant patients. 27-29 February 2004. Herpes. 2004;11:77-86. [PubMed] [Cited in This Article: ] |
| 2. | Razonable RR, Paya CV. Herpesvirus infections in transplant recipients: current challenges in the clinical management of cytomegalovirus and Epstein-Barr virus infections. Herpes. 2003;10:60-65. [PubMed] [Cited in This Article: ] |
| 3. | Arthurs SK, Eid AJ, Pedersen RA, Dierkhising RA, Kremers WK, Patel R, Razonable RR. Delayed-onset primary cytomegalovirus disease after liver transplantation. Liver Transpl. 2007;13:1703-1709. [Cited in This Article: ] |
| 4. | Gane E, Saliba F, Valdecasas GJ, O’Grady J, Pescovitz MD, Lyman S, Robinson CA. Randomised trial of efficacy and safety of oral ganciclovir in the prevention of cytomegalovirus disease in liver-transplant recipients. The Oral Ganciclovir International Transplantation Study Group [corrected]. Lancet. 1997;350:1729-1733. [PubMed] [Cited in This Article: ] |
| 5. | Paya C, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, Freeman R, Heaton N, Pescovitz MD. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4:611-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 678] [Cited by in F6Publishing: 603] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 6. | Fica A, Cervera C, Pérez N, Marcos MA, Ramírez J, Linares L, Soto G, Navasa M, Cofan F, Ricart MJ. Immunohistochemically proven cytomegalovirus end-organ disease in solid organ transplant patients: clinical features and usefulness of conventional diagnostic tests. Transpl Infect Dis. 2007;9:203-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Paya CV, Hermans PE, Wiesner RH, Ludwig J, Smith TF, Rakela J, Krom RA. Cytomegalovirus hepatitis in liver transplantation: prospective analysis of 93 consecutive orthotopic liver transplantations. J Infect Dis. 1989;160:752-758. [PubMed] [Cited in This Article: ] |
| 8. | Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094-1097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 941] [Cited by in F6Publishing: 902] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 9. | Razonable RR, van Cruijsen H, Brown RA, Wilson JA, Harmsen WS, Wiesner RH, Smith TF, Paya CV. Dynamics of cytomegalovirus replication during preemptive therapy with oral ganciclovir. J Infect Dis. 2003;187:1801-1808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Singh N, Wagener MM. Strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med. 2006;144:456-457; author reply 457. [PubMed] [Cited in This Article: ] |
| 11. | Singh N, Wannstedt C, Keyes L, Wagener MM, Cacciarelli TV. Who among cytomegalovirus-seropositive liver transplant recipients is at risk for cytomegalovirus infection. Liver Transpl. 2005;11:700-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Razonable RR. Epidemiology of cytomegalovirus disease in solid organ and hematopoietic stem cell transplant recipients. Am J Health Syst Pharm. 2005;62:S7-S13. [PubMed] [Cited in This Article: ] |
| 13. | Razonable RR, Rivero A, Rodriguez A, Wilson J, Daniels J, Jenkins G, Larson T, Hellinger WC, Spivey JR, Paya CV. Allograft rejection predicts the occurrence of late-onset cytomegalovirus (CMV) disease among CMV-mismatched solid organ transplant patients receiving prophylaxis with oral ganciclovir. J Infect Dis. 2001;184:1461-1464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Razonable RR, Paya CV. Infections and allograft rejection - intertwined complications of organ transplantation. Swiss Med Wkly. 2005;135:571-573. [PubMed] [Cited in This Article: ] |
| 15. | O’Grady JG, Alexander GJ, Sutherland S, Donaldson PT, Harvey F, Portmann B, Calne RY, Williams R. Cytomegalovirus infection and donor/recipient HLA antigens: interdependent co-factors in pathogenesis of vanishing bile-duct syndrome after liver transplantation. Lancet. 1988;2:302-305. [PubMed] [Cited in This Article: ] |
| 16. | Noack KB, Wiesner RH, Batts K, van Hoek B, Ludwig J. Severe ductopenic rejection with features of vanishing bile duct syndrome: clinical, biochemical, and histologic evidence for spontaneous resolution. Transplant Proc. 1991;23:1448-1451. [PubMed] [Cited in This Article: ] |
| 17. | Ludwig J, Wiesner RH, Batts KP, Perkins JD, Krom RA. The acute vanishing bile duct syndrome (acute irreversible rejection) after orthotopic liver transplantation. Hepatology. 1987;7:476-483. [PubMed] [Cited in This Article: ] |
| 18. | Pastacaldi S, Teixeira R, Montalto P, Rolles K, Burroughs AK. Hepatic artery thrombosis after orthotopic liver transplantation: a review of nonsurgical causes. Liver Transpl. 2001;7:75-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Madalosso C, de Souza NF, Ilstrup DM, Wiesner RH, Krom RA. Cytomegalovirus and its association with hepatic artery thrombosis after liver transplantation. Transplantation. 1998;66:294-297. [PubMed] [Cited in This Article: ] |
| 20. | Eid AJRR. Cytomegalovirus disease in solid organ transplant recipients: advances lead to new challenges and opportunities. Curr Opin Organ Tran. 2007;12:610-617. [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Peleg AY, Husain S, Qureshi ZA, Silveira FP, Sarumi M, Shutt KA, Kwak EJ, Paterson DL. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: a matched case-control study. Clin Infect Dis. 2007;44:1307-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 251] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 22. | Mendez JC, Dockrell DH, Espy MJ, Smith TF, Wilson JA, Harmsen WS, Ilstrup D, Paya CV. Human beta-herpesvirus interactions in solid organ transplant recipients. J Infect Dis. 2001;183:179-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Dockrell DH, Prada J, Jones MF, Patel R, Badley AD, Harmsen WS, Ilstrup DM, Wiesner RH, Krom RA, Smith TF. Seroconversion to human herpesvirus 6 following liver transplantation is a marker of cytomegalovirus disease. J Infect Dis. 1997;176:1135-1140. [PubMed] [Cited in This Article: ] |
| 24. | Mendez J, Espy M, Smith TF, Wilson J, Wiesner R, Paya CV. Clinical significance of viral load in the diagnosis of cytomegalovirus disease after liver transplantation. Transplantation. 1998;65:1477-1481. [PubMed] [Cited in This Article: ] |
| 25. | Razonable RR, Rivero A, Brown RA, Hart GD, Espy MJ, van Cruijsen H, Wilson J, Groettum C, Kremers W, Smith TF. Detection of simultaneous beta-herpesvirus infections in clinical syndromes due to defined cytomegalovirus infection. Clin Transplant. 2003;17:114-120. [PubMed] [Cited in This Article: ] |
| 26. | Humar A, Washburn K, Freeman R, Paya CV, Mouas H, Alecock E, Razonable RR. An assessment of interactions between hepatitis C virus and herpesvirus reactivation in liver transplant recipients using molecular surveillance. Liver Transpl. 2007;13:1422-1427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Humar A, Kumar D, Raboud J, Caliendo AM, Moussa G, Levy G, Mazzulli T. Interactions between cytomegalovirus, human herpesvirus-6, and the recurrence of hepatitis C after liver transplantation. Am J Transplant. 2002;2:461-466. [PubMed] [Cited in This Article: ] |
| 28. | Rosen HR, Chou S, Corless CL, Gretch DR, Flora KD, Boudousquie A, Orloff SL, Rabkin JM, Benner KG. Cytomegalovirus viremia: risk factor for allograft cirrhosis after liver transplantation for hepatitis C. Transplantation. 1997;64:721-726. [PubMed] [Cited in This Article: ] |
| 29. | Razonable RR, Burak KW, van Cruijsen H, Brown RA, Charlton MR, Smith TF, Espy MJ, Kremers W, Wilson JA, Groettum C. The pathogenesis of hepatitis C virus is influenced by cytomegalovirus. Clin Infect Dis. 2002;35:974-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Singh N, Husain S, Carrigan DR, Knox KK, Weck KE, Wagener MM, Gayowski T. Impact of human herpesvirus-6 on the frequency and severity of recurrent hepatitis C virus hepatitis in liver transplant recipients. Clin Transplant. 2002;16:92-96. [PubMed] [Cited in This Article: ] |
| 31. | Burak KW, Kremers WK, Batts KP, Wiesner RH, Rosen CB, Razonable RR, Paya CV, Charlton MR. Impact of cytomegalovirus infection, year of transplantation, and donor age on outcomes after liver transplantation for hepatitis C. Liver Transpl. 2002;8:362-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 201] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 32. | Bosch W, Heckman MG, Pungpapong S, Diehl NN, Shalev JA, Hellinger WC. Association of cytomegalovirus infection and disease with recurrent hepatitis C after liver transplantation. Transplantation. 2012;93:723-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Van Laecke S, Desideri F, Geerts A, Van Vlierberghe H, Berrevoet F, Rogiers X, Troisi R, de Hemptinne B, Vanholder R, Colle I. Hypomagnesemia and the risk of new-onset diabetes after liver transplantation. Liver Transpl. 2010;16:1278-1287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Arthurs SK, Eid AJ, Pedersen RA, Kremers WK, Cosio FG, Patel R, Razonable RR. Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis. 2008;46:840-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 35. | Limaye AP, Bakthavatsalam R, Kim HW, Randolph SE, Halldorson JB, Healey PJ, Kuhr CS, Levy AE, Perkins JD, Reyes JD. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. 2006;81:1645-1652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Hodson EM, Barclay PG, Craig JC, Jones C, Kable K, Strippoli GF, Vimalachandra D, Webster AC. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2005;CD003774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Kalil AC, Levitsky J, Lyden E, Stoner J, Freifeld AG. Meta-analysis: the efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med. 2005;143:870-880. [PubMed] [Cited in This Article: ] |
| 38. | Small LN, Lau J, Snydman DR. Preventing post-organ transplantation cytomegalovirus disease with ganciclovir: a meta-analysis comparing prophylactic and preemptive therapies. Clin Infect Dis. 2006;43:869-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 39. | Portela D, Patel R, Larson-Keller JJ, Ilstrup DM, Wiesner RH, Steers JL, Krom RA, Paya CV. OKT3 treatment for allograft rejection is a risk factor for cytomegalovirus disease in liver transplantation. J Infect Dis. 1995;171:1014-1018. [PubMed] [Cited in This Article: ] |
| 40. | Winston DJ, Imagawa DK, Holt CD, Kaldas F, Shaked A, Busuttil RW. Long-term ganciclovir prophylaxis eliminates serious cytomegalovirus disease in liver transplant recipients receiving OKT3 therapy for rejection. Transplantation. 1995;60:1357-1360. [PubMed] [Cited in This Article: ] |
| 41. | Malek SK, Obmann MA, Gotoff RA, Foltzer MA, Hartle JE, Potdar S. Campath-1H induction and the incidence of infectious complications in adult renal transplantation. Transplantation. 2006;81:17-20. [PubMed] [Cited in This Article: ] |
| 42. | Peleg AY, Husain S, Kwak EJ, Silveira FP, Ndirangu M, Tran J, Shutt KA, Shapiro R, Thai N, Abu-Elmagd K. Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clin Infect Dis. 2007;44:204-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 43. | Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967-1977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 573] [Cited by in F6Publishing: 526] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 44. | Winston DJ, Wirin D, Shaked A, Busuttil RW. Randomised comparison of ganciclovir and high-dose acyclovir for long-term cytomegalovirus prophylaxis in liver-transplant recipients. Lancet. 1995;346:69-74. [PubMed] [Cited in This Article: ] |
| 45. | Sarmiento JM, Dockrell DH, Schwab TR, Munn SR, Paya CV. Mycophenolate mofetil increases cytomegalovirus invasive organ disease in renal transplant patients. Clin Transplant. 2000;14:136-138. [PubMed] [Cited in This Article: ] |
| 46. | Demopoulos L, Polinsky M, Steele G, Mines D, Blum M, Caulfield M, Adamkovic A, Liu Q, Harler MB, Hahn C. Reduced risk of cytomegalovirus infection in solid organ transplant recipients treated with sirolimus: a pooled analysis of clinical trials. Transplant Proc. 2008;40:1407-1410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 47. | Vítko S, Margreiter R, Weimar W, Dantal J, Kuypers D, Winkler M, Øyen O, Viljoen HG, Filiptsev P, Sadek S. Three-year efficacy and safety results from a study of everolimus versus mycophenolate mofetil in de novo renal transplant patients. Am J Transplant. 2005;5:2521-2530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 180] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 48. | Kijpittayarit S, Eid AJ, Brown RA, Paya CV, Razonable RR. Relationship between Toll-like receptor 2 polymorphism and cytomegalovirus disease after liver transplantation. Clin Infect Dis. 2007;44:1315-1320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 49. | Kang SH, Abdel-Massih RC, Brown RA, Dierkhising RA, Kremers WK, Razonable RR. Homozygosity for the toll-like receptor 2 R753Q single-nucleotide polymorphism is a risk factor for cytomegalovirus disease after liver transplantation. J Infect Dis. 2012;205:639-646. [Cited in This Article: ] |
| 50. | Humar A, Mazzulli T, Moussa G, Razonable RR, Paya CV, Pescovitz MD, Covington E, Alecock E. Clinical utility of cytomegalovirus (CMV) serology testing in high-risk CMV D+/R- transplant recipients. Am J Transplant. 2005;5:1065-1070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 51. | de Rooij BJ, van der Beek MT, van Hoek B, Vossen AC, Rogier Ten Hove W, Roos A, Schaapherder AF, Porte RJ, van der Reijden JJ, Coenraad MJ. Mannose-binding lectin and ficolin-2 gene polymorphisms predispose to cytomegalovirus (re)infection after orthotopic liver transplantation. J Hepatol. 2011;55:800-807. [Cited in This Article: ] |
| 52. | La Rosa C, Krishnan A, Longmate J, Martinez J, Manchanda P, Lacey SF, Limaye AP, Diamond DJ. Programmed death-1 expression in liver transplant recipients as a prognostic indicator of cytomegalovirus disease. J Infect Dis. 2008;197:25-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Giulieri S, Manuel O. QuantiFERON(R)-CMV assay for the assessment of cytomegalovirus cell-mediated immunity. Expert Rev Mol Diagn. 2011;11:17-25. [Cited in This Article: ] |
| 54. | Kumar D, Chernenko S, Moussa G, Cobos I, Manuel O, Preiksaitis J, Venkataraman S, Humar A. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant. 2009;9:1214-1222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 55. | Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, Humar A. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 604] [Cited by in F6Publishing: 549] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 56. | Manuel O, Husain S, Kumar D, Zayas C, Mawhorter S, Levi ME, Kalpoe J, Lisboa L, Ely L, Kaul DR. Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clin Infect Dis. 2013;56:817-824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 211] [Article Influence: 17.6] [Reference Citation Analysis (1)] |
| 57. | Warlé MC, Farhan A, Metselaar HJ, Hop WC, van der Plas AJ, Kap M, de Rave S, Kwekkeboom J, Zondervan PE, IJzermans JN. In vitro cytokine production of TNFalpha and IL-13 correlates with acute liver transplant rejection. Hum Immunol. 2001;62:1258-1265. [PubMed] [Cited in This Article: ] |
| 58. | Fietze E, Prösch S, Reinke P, Stein J, Döcke WD, Staffa G, Löning S, Devaux S, Emmrich F, von Baehr R. Cytomegalovirus infection in transplant recipients. The role of tumor necrosis factor. Transplantation. 1994;58:675-680. [PubMed] [Cited in This Article: ] |
| 59. | Cook CH, Trgovcich J, Zimmerman PD, Zhang Y, Sedmak DD. Lipopolysaccharide, tumor necrosis factor alpha, or interleukin-1beta triggers reactivation of latent cytomegalovirus in immunocompetent mice. J Virol. 2006;80:9151-9158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 60. | Hooks MA, Perlino CA, Henderson JM, Millikan WJ, Kutner MH. Prevalence of invasive cytomegalovirus disease with administration of muromonab CD-3 in patients undergoing orthotopic liver transplantation. Ann Pharmacother. 1992;26:617-620. [PubMed] [Cited in This Article: ] |
| 61. | Bosch W, Heckman MG, Diehl NN, Shalev JA, Pungpapong S, Hellinger WC. Association of cytomegalovirus infection and disease with death and graft loss after liver transplant in high-risk recipients. Am J Transplant. 2011;11:2181-2189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 62. | Singh N, Gayowski T, Wagener MM, Marino IR. Increased infections in liver transplant recipients with recurrent hepatitis C virus hepatitis. Transplantation. 1996;61:402-406. [PubMed] [Cited in This Article: ] |
| 63. | Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet. 2000;355:2032-2036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 415] [Cited by in F6Publishing: 419] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 64. | Mattes FM, Hainsworth EG, Hassan-Walker AF, Burroughs AK, Sweny P, Griffiths PD, Emery VC. Kinetics of cytomegalovirus load decrease in solid-organ transplant recipients after preemptive therapy with valganciclovir. J Infect Dis. 2005;191:89-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 65. | Singh N. Cytomegalovirus infection in solid organ transplant recipients: new challenges and their implications for preventive strategies. J Clin Virol. 2006;35:474-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 66. | Paya CV, Wilson JA, Espy MJ, Sia IG, DeBernardi MJ, Smith TF, Patel R, Jenkins G, Harmsen WS, Vanness DJ. Preemptive use of oral ganciclovir to prevent cytomegalovirus infection in liver transplant patients: a randomized, placebo-controlled trial. J Infect Dis. 2002;185:854-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 67. | Singh N, Wannstedt C, Keyes L, Gayowski T, Wagener MM, Cacciarelli TV. Efficacy of valganciclovir administered as preemptive therapy for cytomegalovirus disease in liver transplant recipients: impact on viral load and late-onset cytomegalovirus disease. Transplantation. 2005;79:85-90. [PubMed] [Cited in This Article: ] |
| 68. | Singh N, Paterson DL, Gayowski T, Wagener MM, Marino IR. Cytomegalovirus antigenemia directed pre-emptive prophylaxis with oral versus I.V. ganciclovir for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, controlled trial. Transplantation. 2000;70:717-722. [PubMed] [Cited in This Article: ] |
| 69. | Singh N, Yu VL. Preemptive therapy for cytomegalovirus. Liver Transpl. 2006;12:327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 70. | Bodro M, Sabé N, Lladó L, Baliellas C, Niubó J, Castellote J, Fabregat J, Rafecas A, Carratalà J. Prophylaxis versus preemptive therapy for cytomegalovirus disease in high-risk liver transplant recipients. Liver Transpl. 2012;18:1093-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 71. | Onor IO, Todd SB, Meredith E, Perez SD, Mehta AK, Marshall Lyon G, Knechtle SJ, Hanish SI. Evaluation of clinical outcomes of prophylactic versus preemptive cytomegalovirus strategy in liver transplant recipients. Transpl Int. 2013;26:592-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Razonable RR, Humar A. Cytomegalovirus in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:93-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 353] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 73. | Levitsky J, Singh N, Wagener MM, Stosor V, Abecassis M, Ison MG. A survey of CMV prevention strategies after liver transplantation. Am J Transplant. 2008;8:158-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Vandecasteele E, De Waele J, Vandijck D, Blot S, Vogelaers D, Rogiers X, Van Vlierberghe H, Decruyenaere J, Hoste E. Antimicrobial prophylaxis in liver transplant patients--a multicenter survey endorsed by the European Liver and Intestine Transplant Association. Transpl Int. 2010;23:182-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Hodson EM, Jones CA, Webster AC, Strippoli GF, Barclay PG, Kable K, Vimalachandra D, Craig JC. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet. 2005;365:2105-2115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 76. | Singh N, Wannstedt C, Keyes L, Mayher D, Tickerhoof L, Akoad M, Wagener MM, Cacciarelli TV. Valganciclovir as preemptive therapy for cytomegalovirus in cytomegalovirus-seronegative liver transplant recipients of cytomegalovirus-seropositive donor allografts. Liver Transpl. 2008;14:240-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Caliendo AM, St George K, Kao SY, Allega J, Tan BH, LaFontaine R, Bui L, Rinaldo CR. Comparison of quantitative cytomegalovirus (CMV) PCR in plasma and CMV antigenemia assay: clinical utility of the prototype AMPLICOR CMV MONITOR test in transplant recipients. J Clin Microbiol. 2000;38:2122-2127. [PubMed] [Cited in This Article: ] |
| 78. | Razonable RR, Paya CV, Smith TF. Role of the laboratory in diagnosis and management of cytomegalovirus infection in hematopoietic stem cell and solid-organ transplant recipients. J Clin Microbiol. 2002;40:746-752. [PubMed] [Cited in This Article: ] |
| 79. | Piiparinen H, Höckerstedt K, Grönhagen-Riska C, Lautenschlager I. Comparison of two quantitative CMV PCR tests, Cobas Amplicor CMV Monitor and TaqMan assay, and pp65-antigenemia assay in the determination of viral loads from peripheral blood of organ transplant patients. J Clin Virol. 2004;30:258-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 80. | Razonable RR, Åsberg A, Rollag H, Duncan J, Boisvert D, Yao JD, Caliendo AM, Humar A, Do TD. Virologic suppression measured by a cytomegalovirus (CMV) DNA test calibrated to the World Health Organization international standard is predictive of CMV disease resolution in transplant recipients. Clin Infect Dis. 2013;56:1546-1553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 81. | Singh N, Wannstedt C, Keyes L, Wagener MM, Gayowski T, Cacciarelli TV. Indirect outcomes associated with cytomegalovirus (opportunistic infections, hepatitis C virus sequelae, and mortality) in liver-transplant recipients with the use of preemptive therapy for 13 years. Transplantation. 2005;79:1428-1434. [PubMed] [Cited in This Article: ] |
| 82. | Winston DJ, Busuttil RW. Randomized controlled trial of oral ganciclovir versus oral acyclovir after induction with intravenous ganciclovir for long-term prophylaxis of cytomegalovirus disease in cytomegalovirus-seropositive liver transplant recipients. Transplantation. 2003;75:229-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 83. | Razonable RR, Paya CV. Valganciclovir for the prevention and treatment of cytomegalovirus disease in immunocompromised hosts. Expert Rev Anti Infect Ther. 2004;2:27-41. [PubMed] [Cited in This Article: ] |
| 84. | Pescovitz MD, Rabkin J, Merion RM, Paya CV, Pirsch J, Freeman RB, O’Grady J, Robinson C, To Z, Wren K. Valganciclovir results in improved oral absorption of ganciclovir in liver transplant recipients. Antimicrob Agents Chemother. 2000;44:2811-2815. [PubMed] [Cited in This Article: ] |
| 85. | Kalil AC, Mindru C, Botha JF, Grant WJ, Mercer DF, Olivera MA, McCartan MA, McCashland TM, Langnas AN, Florescu DF. Risk of cytomegalovirus disease in high-risk liver transplant recipients on valganciclovir prophylaxis: a systematic review and meta-analysis. Liver Transpl. 2012;18:1440-1447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 86. | Winston DJ, Saliba F, Blumberg E, Abouljoud M, Garcia-Diaz JB, Goss JA, Clough L, Avery R, Limaye AP, Ericzon BG. Efficacy and safety of maribavir dosed at 100 mg orally twice daily for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, double-blind, multicenter controlled trial. Am J Transplant. 2012;12:3021-3030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 87. | Hodson EM, Jones CA, Strippoli GF, Webster AC, Craig JC. Immunoglobulins, vaccines or interferon for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2007;CD005129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 88. | Humar A, Paya C, Pescovitz MD, Dominguez E, Washburn K, Blumberg E, Alexander B, Freeman R, Heaton N, Mueller B. Clinical utility of cytomegalovirus viral load testing for predicting CMV disease in D+/R- solid organ transplant recipients. Am J Transplant. 2004;4:644-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 89. | Freeman RB, Paya C, Pescovitz MD, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, Heaton N. Risk factors for cytomegalovirus viremia and disease developing after prophylaxis in high-risk solid-organ transplant recipients. Transplantation. 2004;78:1765-1773. [Cited in This Article: ] |
| 90. | Humar A, Lebranchu Y, Vincenti F, Blumberg EA, Punch JD, Limaye AP, Abramowicz D, Jardine AG, Voulgari AT, Ives J. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant. 2010;10:1228-1237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 377] [Cited by in F6Publishing: 356] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 91. | Humar A, Limaye AP, Blumberg EA, Hauser IA, Vincenti F, Jardine AG, Abramowicz D, Ives JA, Farhan M, Peeters P. Extended valganciclovir prophylaxis in D+/R- kidney transplant recipients is associated with long-term reduction in cytomegalovirus disease: two-year results of the IMPACT study. Transplantation. 2010;90:1427-1431. [PubMed] [Cited in This Article: ] |
| 92. | Jain A, Orloff M, Kashyap R, Lansing K, Betts R, Mohanka R, Menegus M, Ryan C, Bozorgzadeh A. Does valganciclovir hydrochloride (valcyte) provide effective prophylaxis against cytomegalovirus infection in liver transplant recipients. Transplant Proc. 2005;37:3182-3186. [Cited in This Article: ] |
| 93. | Montejo M, Montejo E, Gastaca M, Valdivieso A, Fernandez JR, Testillano M, Gonzalez J, Bustamante J, Ruiz P, Suarez MJ. Prophylactic therapy with valgancyclovir in high-risk (cytomegalovirus D+/R-) liver transplant recipients: a single-center experience. Transplant Proc. 2009;41:2189-2191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Boillat Blanco N, Pascual M, Venetz JP, Nseir G, Meylan PR, Manuel O. Impact of a preemptive strategy after 3 months of valganciclovir cytomegalovirus prophylaxis in kidney transplant recipients. Transplantation. 2011;91:251-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 95. | Limaye AP. Ganciclovir-resistant cytomegalovirus in organ transplant recipients. Clin Infect Dis. 2002;35:866-872. [Cited in This Article: ] |
| 96. | Limaye AP. Antiviral resistance in cytomegalovirus: an emerging problem in organ transplant recipients. Semin Respir Infect. 2002;17:265-273. [Cited in This Article: ] |
| 97. | Avery RK, Mossad SB, Poggio E, Lard M, Budev M, Bolwell B, Waldman WJ, Braun W, Mawhorter SD, Fatica R. Utility of leflunomide in the treatment of complex cytomegalovirus syndromes. Transplantation. 2010;90:419-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 98. | Marty FM, Ljungman P, Papanicolaou GA, Winston DJ, Chemaly RF, Strasfeld L, Young JA, Rodriguez T, Maertens J, Schmitt M. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis. 2011;11:284-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 256] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 99. | Alain S, Revest M, Veyer D, Essig M, Rerolles JP, Rawlinson W, Mengelle C, Huynh A, Kamar N, Garrigue I. Maribavir use in practice for cytomegalovirus infection in French transplantation centers. Transplant Proc. 2013;45:1603-1607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 100. | Painter W, Robertson A, Trost LC, Godkin S, Lampert B, Painter G. First pharmacokinetic and safety study in humans of the novel lipid antiviral conjugate CMX001, a broad-spectrum oral drug active against double-stranded DNA viruses. Antimicrob Agents Chemother. 2012;56:2726-2734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 101. | Mommeja-Marin HBT, Chittick G. Demographic/Baseline Characteristics of Patients Treated with CMX001 for Serious or Life-threatening Double-stranded DNA (dsDNA) Virus Infections: Predictors of Multiple dsDNA virus Infection. Europeon group for blood and marrow transplantation meeting. London: UK 2013; . [Cited in This Article: ] |
| 102. | Chemaly RFEG, Champlin R, Richard M, Zimmermann H, Lischka P, Stoelben S, McCormick D, Ruebsamen-Schaeff H. Letermovir (AIC246) for the Prevention of CMV Infections Meets Primary Endpoint in Phase 2b Trial in Human Blood Precursor Cell Transplant Recipients. 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy. San Francisco, CA: United States 2012; . [Cited in This Article: ] |
| 103. | Kaul DR, Stoelben S, Cober E, Ojo T, Sandusky E, Lischka P, Zimmermann H, Rubsamen-Schaeff H. First report of successful treatment of multidrug-resistant cytomegalovirus disease with the novel anti-CMV compound AIC246. Am J Transplant. 2011;11:1079-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 104. | Razonable RR. Cytomegalovirus infection after liver transplantation: current concepts and challenges. World J Gastroenterol. 2008;14:4849-4860. [PubMed] [Cited in This Article: ] |