Published online Aug 27, 2010. doi: 10.4254/wjh.v2.i8.302
Revised: June 30, 2010
Accepted: July 7, 2010
Published online: August 27, 2010
The regulation of iron metabolism involves multiple organs including the duodenum, liver and bone marrow. The recent discoveries of novel iron-regulatory proteins have brought the liver to the forefront of iron homeostasis. The iron overload disorder, genetic hemochromatosis, is one of the most prevalent genetic diseases in individuals of Caucasian origin. Furthermore, patients with non-hemochromatotic liver diseases, such as alcoholic liver disease, chronic hepatitis C or nonalcoholic steatohepatitis, often exhibit elevated serum iron indices (ferritin, transferrin saturation) and mild to moderate hepatic iron overload. Clinical data indicate significant differences between men and women regarding liver injury in patients with alcoholic liver disease, chronic hepatitis C or nonalcoholic steatohepatitis. The penetrance of genetic hemochromatosis also varies between men and women. Hepcidin has been suggested to act as a modifier gene in genetic hemochromatosis. Hepcidin is a circulatory antimicrobial peptide synthesized by the liver. It plays a pivotal role in the regulation of iron homeostasis. Hepcidin has been shown to be regulated by iron, inflammation, oxidative stress, hypoxia, alcohol, hepatitis C and obesity. Sex and genetic background have also been shown to modulate hepcidin expression in mice. The role of gender in the regulation of human hepcidin gene expression in the liver is unknown. However, hepcidin may play a role in gender-based differences in iron metabolism and liver diseases. Better understanding of the mechanisms associated with gender-related differences in iron metabolism and chronic liver diseases may enable the development of new treatment strategies.
- Citation: Harrison-Findik DD. Gender-related variations in iron metabolism and liver diseases. World J Hepatol 2010; 2(8): 302-310
- URL: https://www.wjgnet.com/1948-5182/full/v2/i8/302.htm
- DOI: https://dx.doi.org/10.4254/wjh.v2.i8.302
Clinical data suggest that men and women exhibit differences regarding the progression of certain liver diseases such as alcoholic liver disease, chronic hepatitis C and non-alcoholic steatohepatitis. Sex hormones and their effect on metabolic processes and oxidative stress have been suggested to play a role in this process. Interestingly, patients with alcoholic liver disease, chronic hepatitis C or non-alcoholic steatohepatitis often display elevated serum iron and mild to moderate hepatic iron overload. Recently, alcohol, hepatitis C viral proteins and obesity have all been shown to affect the expression of the key iron regulatory protein, hepcidin. Oxidative stress and sex-specific differences have also been postulated to be involved in the regulation of hepcidin expression by alcohol in the liver. However, it is unclear whether the pathophysiological differences observed between men and women with chronic liver disease are associated with gender-based variances in iron metabolism. This review will highlight gender-related differences in liver diseases and iron metabolism including the role of the key iron-regulatory hormone, hepcidin.
Iron is essential for an array of key biological processes including erythrocyte production, DNA synthesis and cellular respiration[1-3]. The normal iron content of the body in an adult male is 35 to 45 mg of iron per kilogram of body weight. The majority of the iron is bound to hemoglobin in erythrocytes. Macrophages of the reticuloendothelial system supply the iron to the plasma transferrin pool to be delivered to bone marrow (~24 mg/d) for hemoglobin synthesis in red blood cell precursors[4-6]. About 20% of women, 50% of pregnant women and 3% of men do not have adequate iron stores. Based on the differences between the amount of iron available for absorption and the increased requirement for iron, most females of reproductive age, especially in the developing world, exhibit iron deficiency anemia[7]. Pregnant women require more iron due to the increasing iron demands of the growing fetus, the placenta and the elevated red cell mass of the mother[8]. However, it must also be noted that there is no regulated pathway for the excretion of iron in the body except by blood loss or desquamated intestinal cells. Parenchymal cells of the liver and reticuloendothelial macrophages serve as depots for excess iron storage. Liver not only carries the main burden of iron overload but also acts as the central organ in the regulation of body iron stores[9].
Hepatic iron overload is common in many liver diseases where iron is a risk factor in disease progression[10-16]. Genetic hemochromatosis (GH) is a prevalent iron overload disorder among the Caucasian population. Mutations in the Hfe gene are the main cause of primary iron overload observed in GH[14]. Patients with genetic hemochromatosis absorb more than the normal amount of iron through the intestine. Iron accumulation subsequently results in organ damage including liver injury[17,18]. GH is not a gender-specific disease. However, more males than females present with symptoms of hemochromatosis. Men accumulate more iron and have a higher incidence of liver injury. Iron overload also affects the hypothalamic-pituitary axis eventually leading to hypogonadism, exposure of sperm to oxidative injury and infertility[19]. The clinical symptoms of GH usually start later with women, possibly due to blood loss experienced with menstruation and childbirth. The majority of patients exhibiting the clinical symptoms of GH are homozygous for a Cys282-Tyr (C282Y) mutation in GH gene, Hfe[20]. Of note, a male-specific association of C282Y mutation with childhood acute lymphoblastic leukemia has also been reported[21]. The C282Y mutation inhibits the heterodimer formation of Hfe with the beta2-microglobulin (β2M) light chain and its delivery to the plasma membrane[22]. Interestingly, female mice deficient in β2M expression have been shown to exhibit more hepatic iron loading than male β2M-deficient mice which is in contrast to that observed with genetic hemochromatosis patients[23]. However, it should be noted that unlike humans, female laboratory mice do not experience menstrual bleeding and live in a controlled environment. The observed sex differences in β2M-deficient mice may be due to a possible protective effect of the Y chromosome or to hormonal differences[23].
β-thalassemia is a genetic hematological disorder whereby repeated blood transfusions and dysregulated iron homeostasis lead to secondary iron overload[24,25]. Distinct from GH, patients with β-thalassemia also exhibit iron deposition in the pituitary gland and hypothalamus[26,27]. Thalassemic males develop hypogonadotropic hypogonadism whereas females have amenorrhea due to pituitary and gonadal damage caused by iron overload[26,28]. However, paternity has been shown to be less common in males including those with normal sperm counts[27-29].
Patients with non-hemochromatotic liver diseases such as chronic hepatitis C, alcoholic liver disease (ALD) and non-alcoholic fatty liver disease (NAFLD) frequently display an increase in serum iron values and mild to moderate elevation of hepatic iron concentration[11-13,15,30-37]. Studies with HCV-infected chimpanzees also demonstrate that the viral infection leads to an increase in body iron levels[38]. Furthermore, as shown by in vitro studies, iron alters HCV replication[39,40]. In male patients with chronic hepatitis C over 50 years of age, iron has been implicated to be a fibrinogenetic factor in comparison to female patients of the same age[41]. Menstruating or iron deficient women with chronic hepatitis C have been reported to have a slower rate of disease compared to men of comparable age and women with normal iron status[42]. Population-based studies indicate differences in HCV clearance rates and the severity of disease between men and women[43-45]. IL-10 promoter polymorphisms have also been postulated to be associated with gender susceptibility to HCV infection[46].
In vivo whole-body retention studies have demonstrated a two-fold increase in intestinal iron absorption in chronic alcoholics[13]. Recently, even mild to moderate alcohol consumption has been shown to elevate the indices of iron stores[12]. Experimental animal models of ALD have also been reported to exhibit increased iron content in Kupffer cells which leads to the activation of the transcription factor, nuclear factor-kappa (NF-κB), and increased expression of the proinflammatory cytokine, tumor necrosis factor-alpha (TNF-α)[47,48]. These effects are abolished by iron chelation, thereby indicating a role for iron-mediated cell signaling in the pathogenesis of experimental alcoholic liver disease[49]. There are sex-specific differences in the metabolism and elimination of ethanol both in humans and rodents[50,51]. The rates of ethanol elimination are higher in women[50]. The activity of the alcohol metabolizing enzyme, alcohol dehydrogenase (ADH) in rodent livers is elevated in females and castration of males increases ADH activity[52]. Moreover, men with prostatic metastatic carcinoma who have undergone therapeutic orchiectomy have been shown to exhibit an increase in ethanol elimination[53]. Clinical studies demonstrate that females exhibit a greater susceptibility to alcohol-induced liver injury than men[54]. Estrogens, endotoxin and inflammatory processes have been suspected to play a role. However, it is unknown whether there is any association between iron and the gender-related differences observed in alcohol-induced liver injury. Alcohol suppresses the expression of the key iron regulatory molecule, hepcidin in the liver, which leads to an increase in duodenal iron transport[55-58]. Interestingly, male mice have been reported to display significantly lower hepcidin expression compared to female mice following acute alcohol exposure[55].
NAFLD is the hepatic manifestation of metabolic syndrome[59-61]. NAFLD ranges from benign steatosis to nonalcoholic steatohepatitis (NASH) which is differentiated by histopathologic evaluation[62]. NASH is the severe manifestation of disease which can lead to liver fibrosis and hepatocellular carcinoma[63,64]. Increased iron stores have been reported in NAFLD/NASH[15,36,37,65,66]. However, the relevance of iron accumulation in disease progression is unclear[15,36,37,65,66]. Excess hepatic iron is postulated to cause insulin resistance[16,67]. Interestingly, iron depletion via phlebotomy in patients with NAFLD has been shown to have a positive effect on insulin resistance and to reduce serum TNF-α levels[68,69]. Serum ferritin levels are also positively associated with BMI and serum glucose levels[70-73]. However, it should be noted that ferritin is an acute phase protein and may not accurately reflect the extent of iron overload in NAFLD. There is a relationship between gender and NAFLD. However, the data from several studies are conflicting regarding the prevalence of NAFLD among men and women[74-79]. Population-based studies suggest a protective role for endogenous estrogens in non-alcoholic hepatic steatosis[80]. The prevalence of NAFLD increases in women over 50 years of age[81]. Interestingly, the deletion of histone variant macroH2A1 which is enriched on the inactive X-chromosome in females has been postulated to cause female-specific steatosis in mice[82].
Since there is no physiological pathway of excretion for excess iron in the body, the uptake, transport and storage of iron must be tightly regulated. Divalent metal transporter 1 (DMT1), a multi-transmembrane protein, is responsible for importing dietary non-heme iron through the apical site of absorptive enterocytes in the duodenum[83,84]. Conversely, the iron transporter ferroportin is responsible for exporting iron into the circulation[85]. The ferroportin Q248H polymorphism is associated with increased serum ferritin levels in Sub-Saharan Africans and African Americans[86]. The frequency of ferroportin Q248H polymorphism has been reported to be higher in African American males with elevated serum ferritin levels compared to those with normal serum ferritin. However, these differences were not observed among African American women. Furthermore, men with elevated serum ferritin were three times more likely to have Q248H polymorphism than women with elevated serum ferritin[86].
In the duodenum, the basolateral transport of iron from the enterocytes into the bloodstream also requires hephaestin, a transmembrane-bound multicopper ferroxidase[87,88]. Like its homolog ceruloplasmin in the liver, hephaestin also links copper and iron metabolism[89]. Sex-linked anemia is an X-linked inherited iron deficiency anemia, first observed in the male descendants of an irradiated mouse[90]. Sex linked anemia (sla) mice are impaired in intestinal iron transport and contain a deletion in Heph gene yielding a truncated hephaestin protein[87].
In the plasma, iron circulates by binding to the glycoprotein, transferrin[91]. There are different glycosylated forms of transferrin which are different in the number of N-linked oligosaccharide chains[92,93]. Heavy alcohol drinkers display abnormal serum transferrin profile[94,95]. Males with high alcohol intake have been shown to display higher amounts of disialotransferrin in the serum when compared to females. There are no gender-related differences in serum disialotransferrin levels between nondrinker males and females[96]. Iron-bound transferrin is taken up into the cell by transferrin receptors 1 and 2 (TrfR1, TrfR2)[97,98]. TrfR1 is ubiquitously expressed whereas TrfR2 is mainly expressed in the liver[98]. The regulation of iron metabolism involves multiple organs including the duodenum, liver and bone marrow. Hepcidin is the iron-regulatory hormone which mediates iron homeostasis between these distant organs[2,99].
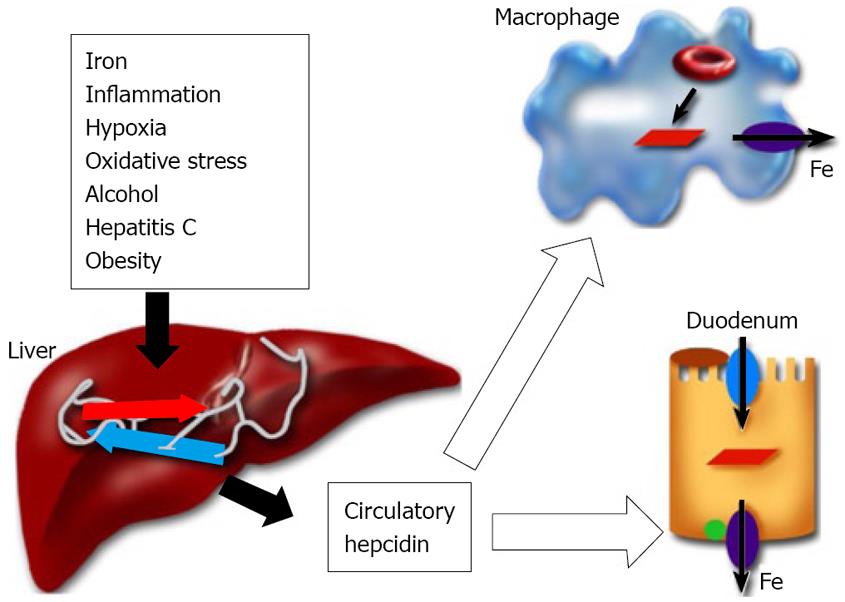
Hepcidin is a circulatory antimicrobial peptide, synthesized in the hepatocytes of the liver as an 84 amino acid precursor protein[100,101]. It is subsequently cleaved into the 25 amino acid cysteine-rich mature (biologically active) peptide form[102,103]. Hepcidin achieves the regulation of iron homeostasis by binding to the iron exporter ferroportin and thereby inhibiting the iron transport in the duodenum and the release of iron from reticuloendothelial macrophages (Figure 1)[104]. During pregnancy, iron is transferred from the mother to the fetus and hepcidin regulates maternofetal iron transport across the placenta[105]. Transgenic mice studies have confirmed the role of hepcidin in the regulation of iron metabolism[106,107]. Hepcidin synthesis in the liver is sensitive to body iron levels; increasing with iron overload and decreasing in the case of iron deficiency[2]. Hepcidin levels in humans have been reported to correlate with the liver iron concentration and the parameters of hepatic function (e.g. serum albumin)[108]. Furthermore, inflammatory signals and the inflammatory cytokines IL-1 and IL-6 elevate hepcidin expression in the liver[109,110]. Conversely, hypoxia and anemia down-regulate hepcidin expression[111]. The decrease in hepcidin expression in the liver leads to increased iron absorption through the duodenum and the mobilization of iron from reticuloendothelial stores to meet the demands of erythrocyte production[2]. The synthesis of hepcidin in the liver is modulated by upstream regulators. Transferrin receptor2, Hfe, the juvenile hemochromatosis gene product, Hjv, and bone morphogenetic protein 6 are positive regulators of hepcidin expression[112-118]. On the other hand, TMPRSS6 (matriptase 2), a transmembrane serine protease, is the negative regulator of liver hepcidin expression[119,120]. Patients expressing TMPRSS6 mutations exhibit iron-refractory iron deficiency anemia due to elevated hepcidin production[119].
Human hepcidin gene (HAMP, HEPC, OMIM 606464) is located on the long arm of chromosome 19 at position 13.1[2,100]. Unlike humans or rats, mice have 2 hepcidin genes, hepc1 and hepc2, and both genes are located on mouse chromosome 7[106,121]. Hepcidin expression in the liver has been reported to differ by gender[122]. Female mice express significantly higher hepcidin levels in the liver than males[122,123]. Both hepcidin1 and hepcidin2 respond to iron. The higher level of hepcidin expression in female mice is also associated with elevated liver and spleen iron concentrations[122,123]. However, it is unclear whether the elevated expression of hepcidin in female mice is due to the increase in iron stores. It is also not known whether women and men differ in the level of hepcidin expression in the liver. Women usually have lower iron stores than men mainly due to the physiological loss of blood. A study utilizing enzyme-linked immunoabsorbent assay reported lower serum hepcidin levels in healthy female volunteers compared to those measured in males[124]. The level of serum hepcidin has been postulated to correlate with that of serum ferritin levels[124]. However, it should be noted that besides iron, hepcidin is also regulated by other stimuli which may also play a role in sex-specific expression of hepcidin in the liver.
Accumulating evidence suggests hepcidin as the modifier gene in genetic hemochromatosis. Hepcidin mRNA expression is reduced in patients with GH and in Hfe knockout mice[125,126]. Some patients with Hfe C282Y homozygosity have been reported to carry additional mutations in hepcidin gene (HAMP)[127-129]. GH patients subjected to acute oral iron challenge have been shown to display a blunted hepcidin response compared to healthy control subjects[130]. Constitutive expression of hepcidin has been shown to prevent iron overload in Hfe knockout mice[131]. Hepcidin is also altered in other non-Hfe-related forms of hemochromatosis. Hemochromatosis patients harboring mutations in transferrin receptor 2 gene have lower urinary hepcidin levels[112]. Mutations in the hepcidin gene and the juvenile hemochromatosis gene, hemojuvelin (Hjv), have been identified in juvenile hemochromatosis patients[114,132]. In contrast to hepcidin, Hjv does not respond to iron levels but its inactivation results in hepcidin deficiency[114,132-134]. Hjv acts as a bone morphogenic protein (BMP) co-receptor[118]. Furthermore, BMP6 regulates hepcidin expression[115,116].
Hepcidin expression is also altered in other liver diseases. Patients with alcoholic liver disease or chronic hepatitis C and animal models of alcohol and HCV display reduced hepcidin expression[35,56-58,135,136]. Hepcidin has been reported to be expressed in adipose tissue and the expression was increased in obese patients; correlating with the body mass index (BMI)[137]. The pathogenesis of nonalcoholic steatohepatitis is associated with insulin resistance and metabolic syndrome[59,60,79]. However, hepcidin expression in the livers of these patients was unchanged[137]. High levels of leptin accompany insulin resistance which is suggested to play a role in the progression of NAFLD to NASH[138-140]. Interestingly, an in vitro study performed with Huh7 human hepatoma cells showed that the adipokine, leptin, increased the expression of hepcidin through the Jak2/Stat3 signaling pathway[141].
The liver is sensitive to the action of sex hormones including estrogens[142-144]. There is some evidence that estrogens can increase the production of reactive oxygen species in the liver[145]. Recently, oxidative stress has been reported to regulate hepcidin transcription in the liver[55]. It is therefore possible that estrogens may play a role in sex-specific regulation of hepcidin expression in the liver. A study of patients with chronic hepatitis reported higher c-myc expression in the livers of patients in which the liver expressed a variant form of the estrogen receptor that exhibits constitutive transcriptional activity compared to patients in whom the liver expressed wild type estrogen receptor[145,146]. Estrogen has also been reported to cause c-myc overexpression in hamster kidneys[147]. c-myc belongs to the basic helix-loop-helix/leucine zipper (bHLH/zip) family of transcription factors which also includes upstream stimulatory factor (USF) and transcription factor E (TFE)[148]. These transcription factors bind to E-Box motifs in the regulatory elements of promoter sequences of target genes[148]. Hepcidin genes are located directly downstream of the Usf2 gene. An involvement of USF1, USF2 and c-myc in the transcriptional regulation of human and mouse hepcidin genes has been postulated[106,107,149]. Moreover, the mutation of E-box motifs in the human hepcidin gene promoter has been shown to abolish the transcriptional regulation by USF1, USF2 or c-myc[149]. However, it remains to be seen whether estrogen plays a role in the transcriptional regulation of hepcidin and in sex-based differences observed regarding the expression of hepcidin in the liver.
Iron is essential for many biological processes. However, excess iron is harmful and can lead to tissue injury. The liver acts as a storage depot for iron and plays a central role in the regulation of iron metabolism. The key iron regulatory hormone, hepcidin, is synthesized in the liver. Genetic hemochromatosis (GH) is a prevalent iron overload disorder in the Caucasian population. Patients with non-hemochromatotic liver diseases such as alcoholic liver disease, chronic hepatitis C and non-alcoholic steatohepatitis also frequently exhibit evidence of iron overload. Hepcidin is suggested to play a role in GH and has been shown to be modulated by alcohol, hepatitis C viral proteins and obesity. Genotypic and sex differences have been shown to be involved in the regulation of liver hepcidin expression in mice. Men and women exhibit clinical differences in the severity of various liver diseases. Women of childbearing age usually have lower iron stores compared to men mainly due to the physiological loss of blood. However, an association between body iron levels and the gender-specific differences observed in the progression of chronic liver diseases has yet to be established. Gender-specific regulation of hepcidin synthesis in the liver may play a role in this process. Further understanding of the mechanisms underlying the gender-based differences in the pathophysiology of chronic liver diseases may lead to the development of novel diagnostic markers and treatment strategies.
Peer reviewer: Ajith TA, PhD, Assistant Professor of Biochemistry, Department of Biochemistry, Amala Institute of Medical Sciences, Amala Nagar, Thrissur 680555, India
| 1. | Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112:219-230. [Cited in This Article: ] |
| 2. | Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323-342. [Cited in This Article: ] |
| 3. | Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol. 2001;33:940-959. [Cited in This Article: ] |
| 4. | Wood R, Ronnenberg A. Minerals. Modern nutrition in health and disease. 10 ed: Lippincott Williams & Wilkins 2006; 248-271. [Cited in This Article: ] |
| 5. | Knutson M, Wessling-Resnick M. Iron metabolism in the reticuloendothelial system. Crit Rev Biochem Mol Biol. 2003;38:61-88. [Cited in This Article: ] |
| 6. | Andrews NC, Fleming MD, Gunshin H. Iron transport across biologic membranes. Nutr Rev. 1999;57:114-123. [Cited in This Article: ] |
| 7. | Beard JL. Iron requirements in adolescent females. J Nutr. 2000;130:440S-442S. [Cited in This Article: ] |
| 8. | Beard JL. Effectiveness and strategies of iron supplementation during pregnancy. Am J Clin Nutr. 2000;71:1288S-1294S. [Cited in This Article: ] |
| 9. | Ganz T. Molecular control of iron transport. J Am Soc Nephrol. 2007;18:394-400. [Cited in This Article: ] |
| 10. | Bonkovsky HL. Iron as a comorbid factor in chronic viral hepatitis. Am J Gastroenterol. 2002;97:1-4. [Cited in This Article: ] |
| 11. | Ioannou GN, Dominitz JA, Weiss NS, Heagerty PJ, Kowdley KV. Racial differences in the relationship between hepatitis C infection and iron stores. Hepatology. 2003;37:795-801. [Cited in This Article: ] |
| 12. | Ioannou GN, Dominitz JA, Weiss NS, Heagerty PJ, Kowdley KV. The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia. Gastroenterology. 2004;126:1293-1301. [Cited in This Article: ] |
| 13. | Duane P, Raja KB, Simpson RJ, Peters TJ. Intestinal iron absorption in chronic alcoholics. Alcohol Alcohol. 1992;27:539-544. [Cited in This Article: ] |
| 14. | Pietrangelo A. Hemochromatosis: an endocrine liver disease. Hepatology. 2007;46:1291-1301. [Cited in This Article: ] |
| 15. | Sumida Y, Yoshikawa T, Okanoue T. Role of hepatic iron in non-alcoholic steatohepatitis. Hepatol Res. 2009;39:213-222. [Cited in This Article: ] |
| 16. | Lecube A, Hernández C, Simó R. Glucose abnormalities in non-alcoholic fatty liver disease and chronic hepatitis C virus infection: the role of iron overload. Diabetes Metab Res Rev. 2009;25:403-410. [Cited in This Article: ] |
| 17. | Fleming RE, Britton RS, Waheed A, Sly WS, Bacon BR. Pathogenesis of hereditary hemochromatosis. Clin Liver Dis. 2004;8:755-773, vii. [Cited in This Article: ] |
| 18. | Andrews NC. Inherited iron overload disorders. Curr Opin Pediatr. 2000;12:596-602. [Cited in This Article: ] |
| 19. | Siemons LJ, Mahler CH. Hypogonadotropic hypogonadism in hemochromatosis: recovery of reproductive function after iron depletion. J Clin Endocrinol Metab. 1987;65:585-587. [Cited in This Article: ] |
| 20. | Eijkelkamp EJ, Yapp TR, Powell LW. HFE-associated hereditary hemochromatosis. Can J Gastroenterol. 2000;14:121-125. [Cited in This Article: ] |
| 21. | Dorak MT, Burnett AK, Worwood M. HFE gene mutations in susceptibility to childhood leukemia: HuGE review. Genet Med. 2005;7:159-168. [Cited in This Article: ] |
| 22. | Feder JN, Tsuchihashi Z, Irrinki A, Lee VK, Mapa FA, Morikang E, Prass CE, Starnes SM, Wolff RK, Parkkila S. The hemochromatosis founder mutation in HLA-H disrupts beta2-microglobulin interaction and cell surface expression. J Biol Chem. 1997;272:14025-14028. [Cited in This Article: ] |
| 23. | Sproule TJ, Jazwinska EC, Britton RS, Bacon BR, Fleming RE, Sly WS, Roopenian DC. Naturally variant autosomal and sex-linked loci determine the severity of iron overload in beta 2-microglobulin-deficient mice. Proc Natl Acad Sci USA. 2001;98:5170-5174. [Cited in This Article: ] |
| 25. | Abramson SD, Abramson N. ‘Common’ uncommon anemias. Am Fam Physician. 1999;59:851-858. [Cited in This Article: ] |
| 26. | Chatterjee R, Katz M. Reversible hypogonadotrophic hypogonadism in sexually infantile male thalassaemic patients with transfusional iron overload. Clin Endocrinol (Oxf). 2000;53:33-42. [Cited in This Article: ] |
| 27. | Chatterjee R, Katz M, Cox TF, Porter JB. Prospective study of the hypothalamic-pituitary axis in thalassaemic patients who developed secondary amenorrhoea. Clin Endocrinol (Oxf). 1993;39:287-296. [Cited in This Article: ] |
| 28. | Perera D, Pizzey A, Campbell A, Katz M, Porter J, Petrou M, Irvine DS, Chatterjee R. Sperm DNA damage in potentially fertile homozygous beta-thalassaemia patients with iron overload. Hum Reprod. 2002;17:1820-1825. [Cited in This Article: ] |
| 29. | Aessopos A, Karabatsos F, Farmakis D, Katsantoni A, Hatziliami A, Youssef J, Karagiorga M. Pregnancy in patients with well-treated beta-thalassemia: outcome for mothers and newborn infants. Am J Obstet Gynecol. 1999;180:360-365. [Cited in This Article: ] |
| 30. | Di Bisceglie AM, Axiotis CA, Hoofnagle JH, Bacon BR. Measurements of iron status in patients with chronic hepatitis. Gastroenterology. 1992;102:2108-2113. [Cited in This Article: ] |
| 31. | Bonkovsky HL, Banner BF, Rothman AL. Iron and chronic viral hepatitis. Hepatology. 1997;25:759-768. [Cited in This Article: ] |
| 32. | Ioannou GN, Tung BY, Kowdley KV. Iron in hepatitis C: villain or innocent bystander? Semin Gastrointest Dis. 2002;13:95-108. [Cited in This Article: ] |
| 33. | Chapman RW, Morgan MY, Laulicht M, Hoffbrand AV, Sherlock S. Hepatic iron stores and markers of iron overload in alcoholics and patients with idiopathic hemochromatosis. Dig Dis Sci. 1982;27:909-916. [Cited in This Article: ] |
| 34. | Tavill AS, Qadri AM. Alcohol and iron. Semin Liver Dis. 2004;24:317-325. [Cited in This Article: ] |
| 35. | Harrison-Findik DD. Role of alcohol in the regulation of iron metabolism. World J Gastroenterol. 2007;13:4925-4930. [Cited in This Article: ] |
| 36. | Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103-1109. [Cited in This Article: ] |
| 37. | Pietrangelo A. Iron in NASH, chronic liver diseases and HCC: how much iron is too much? J Hepatol. 2009;50:249-251. [Cited in This Article: ] |
| 38. | Bassett SE, Di Bisceglie AM, Bacon BR, Sharp RM, Govindarajan S, Hubbard GB, Brasky KM, Lanford RE. Effects of iron loading on pathogenicity in hepatitis C virus-infected chimpanzees. Hepatology. 1999;29:1884-1892. [Cited in This Article: ] |
| 39. | Kakizaki S, Takagi H, Horiguchi N, Toyoda M, Takayama H, Nagamine T, Mori M, Kato N. Iron enhances hepatitis C virus replication in cultured human hepatocytes. Liver. 2000;20:125-128. [Cited in This Article: ] |
| 40. | Fillebeen C, Rivas-Estilla AM, Bisaillon M, Ponka P, Muckenthaler M, Hentze MW, Koromilas AE, Pantopoulos K. Iron inactivates the RNA polymerase NS5B and suppresses subgenomic replication of hepatitis C Virus. J Biol Chem. 2005;280:9049-9057. [Cited in This Article: ] |
| 41. | Rigamonti C, Andorno S, Maduli E, Capelli F, Boldorini R, Sartori M. Gender and liver fibrosis in chronic hepatitis: the role of iron status. Aliment Pharmacol Ther. 2005;21:1445-1451. [Cited in This Article: ] |
| 42. | Sartori M, Andorno S, Rigamonti C, Grossini E, Nicosia G, Boldorini R. Chronic hepatitis C is mild in menstruating women. J Gastroenterol Hepatol. 2000;15:1411-1417. [Cited in This Article: ] |
| 43. | Yamakawa Y, Sata M, Suzuki H, Noguchi S, Tanikawa K. Higher elimination rate of hepatitis C virus among women. J Viral Hepat. 1996;3:317-321. [Cited in This Article: ] |
| 44. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The obsvirc, metavir, clinivir, and dosvirc groups. Lancet. 1997;349:825-832. [Cited in This Article: ] |
| 45. | Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis c. J Hepatol. 2001;34:730-739. [Cited in This Article: ] |
| 46. | Paladino N, Fainboim H, Theiler G, Schroder T, Muñoz AE, Flores AC, Galdame O, Fainboim L. Gender susceptibility to chronic hepatitis C virus infection associated with interleukin 10 promoter polymorphism. J Virol. 2006;80:9144-9150. [Cited in This Article: ] |
| 47. | Tsukamoto H, Horne W, Kamimura S, Niemelä O, Parkkila S, Ylä-Herttuala S, Brittenham GM. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest. 1995;96:620-630. [Cited in This Article: ] |
| 48. | She H, Xiong S, Lin M, Zandi E, Giulivi C, Tsukamoto H. Iron activates NF-kappaB in Kupffer cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G719-G726. [Cited in This Article: ] |
| 49. | Lin M, Rippe RA, Niemelä O, Brittenham G, Tsukamoto H. Role of iron in NF-kappa B activation and cytokine gene expression by rat hepatic macrophages. Am J Physiol. 1997;272:G1355-G1364. [Cited in This Article: ] |
| 50. | Mishra L, Sharma S, Potter JJ, Mezey E. More rapid elimination of alcohol in women as compared to their male siblings. Alcohol Clin Exp Res. 1989;13:752-754. [Cited in This Article: ] |
| 51. | Rachamin G, MacDonald JA, Wahid S, Clapp JJ, Khanna JM, Israel Y. Modulation of alcohol dehydrogenase and ethanol metabolism by sex hormones in the spontaneously hypertensive rat. Effect of chronic ethanol administration. Biochem J. 1980;186:483-490. [Cited in This Article: ] |
| 52. | Mezey E. Influence of sex hormones on alcohol metabolism. Alcohol Clin Exp Res. 2000;24:421. [Cited in This Article: ] |
| 53. | Mezey E, Oesterling JE, Potter JJ. Influence of male hormones on rates of ethanol elimination in man. Hepatology. 1988;8:742-744. [Cited in This Article: ] |
| 54. | Sato N, Lindros KO, Baraona E, Ikejima K, Mezey E, Järveläinen HA, Ramchandani VA. Sex difference in alcohol-related organ injury. Alcohol Clin Exp Res. 2001;25:40S-45S. [Cited in This Article: ] |
| 55. | Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, Clemens D, Fein E, Andriopoulos B, Pantopoulos K, Gollan J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J Biol Chem. 2006;281:22974-22982. [Cited in This Article: ] |
| 56. | Bridle K, Cheung TK, Murphy T, Walters M, Anderson G, Crawford DG, Fletcher LM. Hepcidin is down-regulated in alcoholic liver injury: implications for the pathogenesis of alcoholic liver disease. Alcohol Clin Exp Res. 2006;30:106-112. [Cited in This Article: ] |
| 57. | Ohtake T, Saito H, Hosoki Y, Inoue M, Miyoshi S, Suzuki Y, Fujimoto Y, Kohgo Y. Hepcidin is down-regulated in alcohol loading. Alcohol Clin Exp Res. 2007;31:S2-S8. [Cited in This Article: ] |
| 58. | Harrison-Findik DD. Is the iron regulatory hormone hepcidin a risk factor for alcoholic liver disease? World J Gastroenterol. 2009;15:1186-1193. [Cited in This Article: ] |
| 59. | Moseley RH. Progress in understanding the pathogenesis of nonalcoholic fatty liver disease. Hepatology. 2005;41:204-206. [Cited in This Article: ] |
| 60. | Cortez-Pinto H, de Moura MC, Day CP. Non-alcoholic steatohepatitis: from cell biology to clinical practice. J Hepatol. 2006;44:197-208. [Cited in This Article: ] |
| 61. | Diehl AM. Nonalcoholic steatohepatitis. Semin Liver Dis. 1999;19:221-229. [Cited in This Article: ] |
| 62. | Yeh MM, Brunt EM. Pathology of nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;128:837-847. [Cited in This Article: ] |
| 63. | Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99-S112. [Cited in This Article: ] |
| 64. | Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820-1832. [Cited in This Article: ] |
| 65. | Younossi ZM, Gramlich T, Bacon BR, Matteoni CA, Boparai N, O’Neill R, McCullough AJ. Hepatic iron and nonalcoholic fatty liver disease. Hepatology. 1999;30:847-850. [Cited in This Article: ] |
| 66. | Bugianesi E, Manzini P, D’Antico S, Vanni E, Longo F, Leone N, Massarenti P, Piga A, Marchesini G, Rizzetto M. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179-187. [Cited in This Article: ] |
| 67. | Mendler MH, Turlin B, Moirand R, Jouanolle AM, Sapey T, Guyader D, Le Gall JY, Brissot P, David V, Deugnier Y. Insulin resistance-associated hepatic iron overload. Gastroenterology. 1999;117:1155-1163. [Cited in This Article: ] |
| 68. | Guillygomarc’h A, Mendler MH, Moirand R, Lainé F, Quentin V, David V, Brissot P, Deugnier Y. Venesection therapy of insulin resistance-associated hepatic iron overload. J Hepatol. 2001;35:344-349. [Cited in This Article: ] |
| 69. | Aigner E, Theurl I, Theurl M, Lederer D, Haufe H, Dietze O, Strasser M, Datz C, Weiss G. Pathways underlying iron accumulation in human nonalcoholic fatty liver disease. Am J Clin Nutr. 2008;87:1374-1383. [Cited in This Article: ] |
| 70. | Gillum RF. Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men--the Third National Health and Nutrition Examination Survey. Int J Obes Relat Metab Disord. 2001;25:639-645. [Cited in This Article: ] |
| 71. | Fernández-Real JM, López-Bermejo A, Ricart W. Iron stores, blood donation, and insulin sensitivity and secretion. Clin Chem. 2005;51:1201-1205. [Cited in This Article: ] |
| 72. | Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. NAFLD and hyperinsulinemia are major determinants of serum ferritin levels. J Hepatol. 2007;46:700-707. [Cited in This Article: ] |
| 73. | Tanaka N, Tanaka E, Sheena Y, Komatsu M, Okiyama W, Misawa N, Muto H, Umemura T, Ichijo T, Matsumoto A. Useful parameters for distinguishing nonalcoholic steatohepatitis with mild steatosis from cryptogenic chronic hepatitis in the Japanese population. Liver Int. 2006;26:956-963. [Cited in This Article: ] |
| 74. | James O, Day C. Non-alcoholic steatohepatitis: another disease of affluence. Lancet. 1999;353:1634-1636. [Cited in This Article: ] |
| 75. | Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561-e565. [Cited in This Article: ] |
| 76. | O’Connor BJ, Kathamna B, Tavill AS. Nonalcoholic fatty liver (NASH syndrome). Gastroenterologist. 1997;5:316-329. [Cited in This Article: ] |
| 77. | Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74-80. [Cited in This Article: ] |
| 78. | Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960-967. [Cited in This Article: ] |
| 79. | Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649-1657. [Cited in This Article: ] |
| 80. | Völzke H, Schwarz S, Baumeister SE, Wallaschofski H, Schwahn C, Grabe HJ, Kohlmann T, John U, Dören M. Menopausal status and hepatic steatosis in a general female population. Gut. 2007;56:594-595. [Cited in This Article: ] |
| 81. | Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, Sung IK, Sohn CI, Keum DK, Kim BI. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21:138-143. [Cited in This Article: ] |
| 82. | Boulard M, Storck S, Cong R, Pinto R, Delage H, Bouvet P. Histone variant macroH2A1 deletion in mice causes female-specific steatosis. Epigenetics Chromatin. 2010;3:8. [Cited in This Article: ] |
| 83. | Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482-488. [Cited in This Article: ] |
| 84. | Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115:1258-1266. [Cited in This Article: ] |
| 85. | Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776-781. [Cited in This Article: ] |
| 86. | Rivers CA, Barton JC, Gordeuk VR, Acton RT, Speechley MR, Snively BM, Leiendecker-Foster C, Press RD, Adams PC, McLaren GD. Association of ferroportin Q248H polymorphism with elevated levels of serum ferritin in African Americans in the Hemochromatosis and Iron Overload Screening (HEIRS) Study. Blood Cells Mol Dis. 2007;38:247-252. [Cited in This Article: ] |
| 87. | Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet. 1999;21:195-199. [Cited in This Article: ] |
| 88. | Frazer DM, Anderson GJ. The orchestration of body iron intake: how and where do enterocytes receive their cues? Blood Cells Mol Dis. 2003;30:288-297. [Cited in This Article: ] |
| 89. | Syed BA, Beaumont NJ, Patel A, Naylor CE, Bayele HK, Joannou CL, Rowe PS, Evans RW, Srai SK. Analysis of the human hephaestin gene and protein: comparative modelling of the N-terminus ecto-domain based upon ceruloplasmin. Protein Eng. 2002;15:205-214. [Cited in This Article: ] |
| 90. | Pinkerton PH, Bannerman RM. Hereditary defect in iron absorption in mice. Nature. 1967;216:482-483. [Cited in This Article: ] |
| 91. | Aisen P, Wessling-Resnick M, Leibold EA. Iron metabolism. Curr Opin Chem Biol. 1999;3:200-206. [Cited in This Article: ] |
| 92. | Peter J, Unverzagt C, Engel WD, Renauer D, Seidel C, Hösel W. Identification of carbohydrate deficient transferrin forms by MALDI-TOF mass spectrometry and lectin ELISA. Biochim Biophys Acta. 1998;1380:93-101. [Cited in This Article: ] |
| 93. | März L, Hatton MW, Berry LR, Regoeczi E. The structural heterogeneity of the carbohydrate moiety of desialylated human transferrin. Can J Biochem. 1982;60:624-630. [Cited in This Article: ] |
| 94. | Flahaut C, Michalski JC, Danel T, Humbert MH, Klein A. The effects of ethanol on the glycosylation of human transferrin. Glycobiology. 2003;13:191-198. [Cited in This Article: ] |
| 95. | Stibler H, Borg S. Glycoprotein glycosyltransferase activities in serum in alcohol-abusing patients and healthy controls. Scand J Clin Lab Invest. 1991;51:43-51. [Cited in This Article: ] |
| 96. | Bergström JP, Helander A. Clinical characteristics of carbohydrate-deficient transferrin (%disialotransferrin) measured by HPLC: sensitivity, specificity, gender effects, and relationship with other alcohol biomarkers. Alcohol Alcohol. 2008;43:436-441. [Cited in This Article: ] |
| 97. | Ponka P, Lok CN. The transferrin receptor: role in health and disease. Int J Biochem Cell Biol. 1999;31:1111-1137. [Cited in This Article: ] |
| 98. | Kawabata H, Yang R, Hirama T, Vuong PT, Kawano S, Gombart AF, Koeffler HP. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999;274:20826-20832. [Cited in This Article: ] |
| 99. | Nicolas G, Viatte L, Bennoun M, Beaumont C, Kahn A, Vaulont S. Hepcidin, a new iron regulatory peptide. Blood Cells Mol Dis. 2002;29:327-335. [Cited in This Article: ] |
| 100. | Hunter HN, Fulton DB, Ganz T, Vogel HJ. The solution structure of human hepcidin, a peptide hormone with antimicrobial activity that is involved in iron uptake and hereditary hemochromatosis. J Biol Chem. 2002;277:37597-37603. [Cited in This Article: ] |
| 101. | Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loréal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811-7819. [Cited in This Article: ] |
| 102. | Valore EV, Ganz T. Posttranslational processing of hepcidin in human hepatocytes is mediated by the prohormone convertase furin. Blood Cells Mol Dis. 2008;40:132-138. [Cited in This Article: ] |
| 103. | Lee P. Commentary to: “Post-translational processing of hepcidin in human hepatocytes is mediated by the prohormone convertase furin,” by Erika Valore and Tomas Ganz. Blood Cells Mol Dis. 2008;40:139-140. [Cited in This Article: ] |
| 104. | Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090-2093. [Cited in This Article: ] |
| 105. | Ganz T. Hepcidin in iron metabolism. Curr Opin Hematol. 2004;11:251-254. [Cited in This Article: ] |
| 106. | Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA. 2001;98:8780-8785. [Cited in This Article: ] |
| 107. | Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, Sirito M, Sawadogo M, Kahn A, Vaulont S. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci USA. 2002;99:4596-4601. [Cited in This Article: ] |
| 108. | Détivaud L, Nemeth E, Boudjema K, Turlin B, Troadec MB, Leroyer P, Ropert M, Jacquelinet S, Courselaud B, Ganz T. Hepcidin levels in humans are correlated with hepatic iron stores, hemoglobin levels, and hepatic function. Blood. 2005;106:746-748. [Cited in This Article: ] |
| 109. | Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271-1276. [Cited in This Article: ] |
| 110. | Lee P, Peng H, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci USA. 2005;102:1906-1910. [Cited in This Article: ] |
| 111. | Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037-1044. [Cited in This Article: ] |
| 112. | Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2 hemochromatosis. Blood. 2005;105:1803-1806. [Cited in This Article: ] |
| 113. | Wallace DF, Summerville L, Subramaniam VN. Targeted disruption of the hepatic transferrin receptor 2 gene in mice leads to iron overload. Gastroenterology. 2007;132:301-310. [Cited in This Article: ] |
| 114. | Papanikolaou G, Samuels ME, Ludwig EH, MacDonald ML, Franchini PL, Dubé MP, Andres L, MacFarlane J, Sakellaropoulos N, Politou M. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet. 2004;36:77-82. [Cited in This Article: ] |
| 115. | Andriopoulos B Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482-487. [Cited in This Article: ] |
| 116. | Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41:478-481. [Cited in This Article: ] |
| 117. | Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, Cooperman S, Eckhaus M, Rouault T, Mishra L. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399-409. [Cited in This Article: ] |
| 118. | Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531-539. [Cited in This Article: ] |
| 119. | Finberg KE, Heeney MM, Campagna DR, Aydinok Y, Pearson HA, Hartman KR, Mayo MM, Samuel SM, Strouse JJ, Markianos K. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40:569-571. [Cited in This Article: ] |
| 120. | Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, Khovananth K, Mudd S, Mann N, Moresco EM. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088-1092. [Cited in This Article: ] |
| 121. | Lou DQ, Nicolas G, Lesbordes JC, Viatte L, Grimber G, Szajnert MF, Kahn A, Vaulont S. Functional differences between hepcidin 1 and 2 in transgenic mice. Blood. 2004;103:2816-2821. [Cited in This Article: ] |
| 122. | Courselaud B, Troadec MB, Fruchon S, Ilyin G, Borot N, Leroyer P, Coppin H, Brissot P, Roth MP, Loréal O. Strain and gender modulate hepatic hepcidin 1 and 2 mRNA expression in mice. Blood Cells Mol Dis. 2004;32:283-289. [Cited in This Article: ] |
| 123. | Krijt J, Cmejla R, Sýkora V, Vokurka M, Vyoral D, Necas E. Different expression pattern of hepcidin genes in the liver and pancreas of C57BL/6N and DBA/2N mice. J Hepatol. 2004;40:891-896. [Cited in This Article: ] |
| 124. | Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292-4297. [Cited in This Article: ] |
| 125. | Bridle KR, Frazer DM, Wilkins SJ, Dixon JL, Purdie DM, Crawford DH, Subramaniam VN, Powell LW, Anderson GJ, Ramm GA. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet. 2003;361:669-673. [Cited in This Article: ] |
| 126. | Muckenthaler M, Roy CN, Custodio AO, Miñana B, deGraaf J, Montross LK, Andrews NC, Hentze MW. Regulatory defects in liver and intestine implicate abnormal hepcidin and Cybrd1 expression in mouse hemochromatosis. Nat Genet. 2003;34:102-107. [Cited in This Article: ] |
| 127. | Biasiotto G, Belloli S, Ruggeri G, Zanella I, Gerardi G, Corrado M, Gobbi E, Albertini A, Arosio P. Identification of new mutations of the HFE, hepcidin, and transferrin receptor 2 genes by denaturing HPLC analysis of individuals with biochemical indications of iron overload. Clin Chem. 2003;49:1981-1988. [Cited in This Article: ] |
| 128. | Biasiotto G, Roetto A, Daraio F, Polotti A, Gerardi GM, Girelli D, Cremonesi L, Arosio P, Camaschella C. Identification of new mutations of hepcidin and hemojuvelin in patients with HFE C282Y allele. Blood Cells Mol Dis. 2004;33:338-343. [Cited in This Article: ] |
| 129. | Jacolot S, Le Gac G, Scotet V, Quere I, Mura C, Ferec C. HAMP as a modifier gene that increases the phenotypic expression of the HFE pC282Y homozygous genotype. Blood. 2004;103:2835-2840. [Cited in This Article: ] |
| 130. | Piperno A, Girelli D, Nemeth E, Trombini P, Bozzini C, Poggiali E, Phung Y, Ganz T, Camaschella C. Blunted hepcidin response to oral iron challenge in HFE-related hemochromatosis. Blood. 2007;110:4096-4100. [Cited in This Article: ] |
| 131. | Nicolas G, Viatte L, Lou DQ, Bennoun M, Beaumont C, Kahn A, Andrews NC, Vaulont S. Constitutive hepcidin expression prevents iron overload in a mouse model of hemochromatosis. Nat Genet. 2003;34:97-101. [Cited in This Article: ] |
| 132. | Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, Christakis J, Loukopoulos D, Camaschella C. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33:21-22. [Cited in This Article: ] |
| 133. | Papanikolaou G, Tzilianos M, Christakis JI, Bogdanos D, Tsimirika K, MacFarlane J, Goldberg YP, Sakellaropoulos N, Ganz T, Nemeth E. Hepcidin in iron overload disorders. Blood. 2005;105:4103-4105. [Cited in This Article: ] |
| 134. | Krijt J, Vokurka M, Chang KT, Necas E. Expression of Rgmc, the murine ortholog of hemojuvelin gene, is modulated by development and inflammation, but not by iron status or erythropoietin. Blood. 2004;104:4308-4310. [Cited in This Article: ] |
| 135. | Fujita N, Sugimoto R, Takeo M, Urawa N, Mifuji R, Tanaka H, Kobayashi Y, Iwasa M, Watanabe S, Adachi Y. Hepcidin expression in the liver: relatively low level in patients with chronic hepatitis C. Mol Med. 2007;13:97-104. [Cited in This Article: ] |
| 136. | Nishina S, Hino K, Korenaga M, Vecchi C, Pietrangelo A, Mizukami Y, Furutani T, Sakai A, Okuda M, Hidaka I. Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology. 2008;134:226-238. [Cited in This Article: ] |
| 137. | Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, Ben Amor I. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788-796. [Cited in This Article: ] |
| 138. | Poordad FF. The role of leptin in NAFLD: contender or pretender? J Clin Gastroenterol. 2004;38:841-843. [Cited in This Article: ] |
| 139. | Chitturi S, Farrell G, Frost L, Kriketos A, Lin R, Fung C, Liddle C, Samarasinghe D, George J. Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: a manifestation of lipotoxicity? Hepatology. 2002;36:403-409. [Cited in This Article: ] |
| 140. | Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35:762-771. [Cited in This Article: ] |
| 141. | Chung B, Matak P, McKie AT, Sharp P. Leptin increases the expression of the iron regulatory hormone hepcidin in HuH7 human hepatoma cells. J Nutr. 2007;137:2366-2370. [Cited in This Article: ] |
| 142. | Bannister P, Oakes J, Sheridan P, Losowsky MS. Sex hormone changes in chronic liver disease: a matched study of alcoholic versus non-alcoholic liver disease. Q J Med. 1987;63:305-313. [Cited in This Article: ] |
| 143. | De Maria N, Manno M, Villa E. Sex hormones and liver cancer. Mol Cell Endocrinol. 2002;193:59-63. [Cited in This Article: ] |
| 144. | Villa E, Colantoni A, Grottola A, Ferretti I, Buttafoco P, Bertani H, De Maria N, Manenti F. Variant estrogen receptors and their role in liver disease. Mol Cell Endocrinol. 2002;193:65-69. [Cited in This Article: ] |
| 145. | Farinati F, Cardin R, Bortolami M, Grottola A, Manno M, Colantoni A, Villa E. Estrogens receptors and oxidative damage in the liver. Mol Cell Endocrinol. 2002;193:85-88. [Cited in This Article: ] |
| 146. | Fuqua SA, Fitzgerald SD, Chamness GC, Tandon AK, McDonnell DP, Nawaz Z, O'Malley BW, McGuire WL. Variant human breast tumor estrogen receptor with constitutive transcriptional activity. Cancer Res. 1991;51:105-109. [Cited in This Article: ] |
| 147. | Li JJ, Hou X, Banerjee SK, Liao DZ, Maggouta F, Norris JS, Li SA. Overexpression and amplification of c-myc in the Syrian hamster kidney during estrogen carcinogenesis: a probable critical role in neoplastic transformation. Cancer Res. 1999;59:2340-2346. [Cited in This Article: ] |