Published online Jun 27, 2010. doi: 10.4254/wjh.v2.i6.243
Revised: June 6, 2010
Accepted: June 13, 2010
Published online: June 27, 2010
During liver resection clamping of the hepato-duodenal ligament (the Pringle maneuver) is performed to reduce intraoperative blood-loss. During this maneuver acute portal hypertension may lead to spontaneous splenic rupture requiring rapid splenectomy in order to control blood loss. We present 2 case of patients with hemorrhage from the spleen during clamping for liver surgery. A review of the literature with an emphasis on the pathophysiology of splenic hemorrhage is presented.
- Citation: Buijtenen JMV, Lamme B, Hesselink EJ. Spontaneous splenic rupture during Pringle maneuver in liver surgery. World J Hepatol 2010; 2(6): 243-245
- URL: https://www.wjgnet.com/1948-5182/full/v2/i6/243.htm
- DOI: https://dx.doi.org/10.4254/wjh.v2.i6.243
Previously, only a few cases of spontaneous splenic rupture during liver surgery have been described in the literature[1-4]. This rare complication may lead to acute life-threatening situations requiring immediate splenectomy in order to control massive blood loss. We present 2 cases of patients with splenic hemorrhage during portal vein and hepatic artery clamping (Pringle maneuver) for liver surgery as well as a review of the relevant literature.
A 68-year-old man with a previous history of diabetes mellitus and a coronary artery bypass graft presented with ongoing abdominal complaints after a sigmoid-resection for colon carcinoma (pT3N0Mx) 2 mo previously. His family history was negative for coagulopathic disorders. Computed tomography (CT) and positron emission tomography (PET) scans revealed 3 liver metastases although no other abnormalities to the internal organs were detected.
After cessation of calcium carbasalate (100 mg/d) treatment 7 d preoperatively, the patient underwent a laparotomy with intraoperative ultrasound of the liver. This revealed 3 liver metastases in the liver, one located close to the left hepatic vein and two located on the right side of the liver. Metastasectomy with an ultrasonic dissector (Ethicon Inc., Johnson and Johnson Medical) was performed. The two right-sided metastases were resected easily. In order to resect the left-sided metastasis a Pringle maneuver was performed by clamping of the hepatoduodenal ligament. Initially during this third metastasectomy the patient remained stable, however after only 20 min of clamping the operation field was rapidly filled with blood pouring from the left upper quadrant. Upon close inspection, a splenic rupture was detected and an emergency splenectomy was performed by quickly ligating the splenic artery and vein as well as the short gastric arteries. After the splenectomy the patient remained hemodynamically stable and the metastasectomy of the liver could be continued without any other complications. To compensate for the intraoperative blood-loss, 2 liters of packed-cells were administered.
On pathological examination the removed spleen showed a ruptured capsule and partial ablation with hematoma. The spleen weighed 95 grams and had a maximum diameter of nine centimeters. Microscopically, the radically removed liver metastases showed moderate differentiated adenocarcinoma of the intestinal type surrounded by normal liver parenchyma.
Postoperatively, the patient developed pneumonia which was treated successfully with antibiotics and was discharged after Pneumovax vaccination 14 d postoperatively. At 4 mo follow-up, multiple, diffuse liver metastases and a lung metastasis were detected on CT-scan and chemotherapy was initiated.
A 61-year-old-female treated for osteoporosis and rheumatoid arthritis was presented with rectal blood-loss. She underwent colonoscopy which revealed a poorly differentiated colonic carcinoma 25 cm from the anal rim. The carcinoembryonic antigen (CEA) level was 3.5 ng/mL. An uncomplicated sigmoid resection was performed with radical resection of the tumor (pT2N0). After 4 years of uneventful follow up, serum CEA-markers increased to 5.3 ng/mL and CT-scan disclosed an intrahepatic lesion of 3 cm diameter highly suspicious for a metastasis. Furthermore, this scan showed no other metastases and no signs of portal hypertension. The patient underwent a laparotomy for metastasectomy via bilateral subcostal incisions. After opening of the abdomen the retractors were closely positioned. After dissection of the liver hilum, the hepatoduodenal ligament was clamped according to the Pringle maneuver, as well as the right hepatic vein. The metastasis located at the right top of the liver was resected by ultracision. Sudden major blood-loss originating from the spleen was noted only 15 min after initiating the Pringle maneuver. An immediate splenectomy was performed. After the splenectomy blood-loss stopped and the metastectomy was continued uneventful. The tumor was resected with a margin of 1-2 centimeters. Total blood-loss was approximately 2.5 liters and the patient was therefore given 6 Units of packed-cells.
On pathological examination the removed spleen measured 10 cm × 8 cm × 2 cm and weighed 90 grams. A ruptured splenic capsule was noted. The contra-lateral side showed an isolated hematoma and ablation of the capsule. The resected metastasis was identified histologically as poorly differentiated adenocarcinoma and the encircling liver parenchyma appeared normal. The patient recovered uneventfully and was released from hospital 8 d postoperatively. To date, no evidence for recurrent disease was found during follow-up.
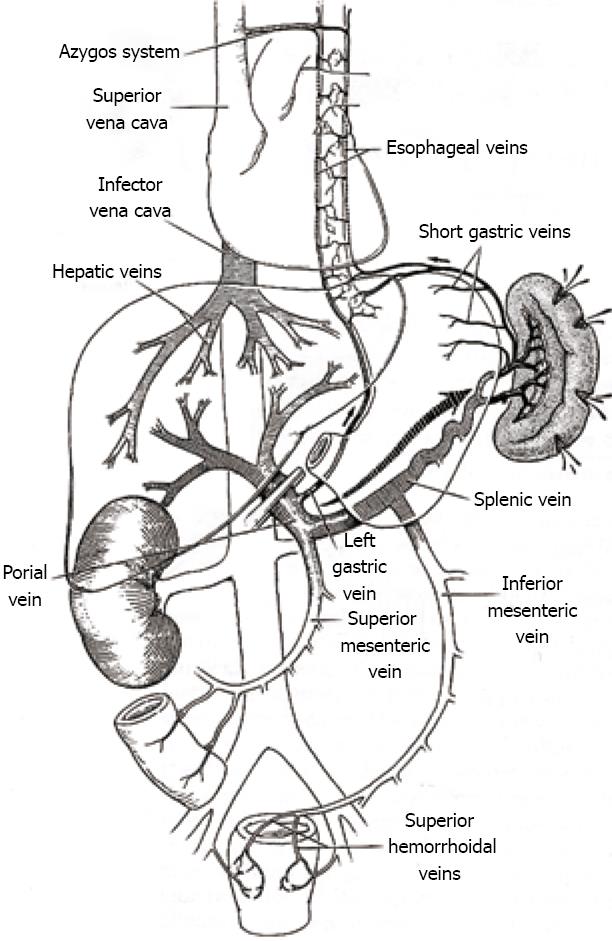
Only 5 cases of spontaneous splenic rupture during Pringle maneuver in liver surgery have been described previously[2-4]. Spontaneous splenic rupture can result from either intrasplenic or extrasplenic causes. Intrasplenic causes are of infectious, neoplastic, vascular or congenital etiology, whereas extrasplenic causes are altered blood pressure in the portal system, coagulopathy disorders, pregnancy or auto-immune anomalies (Figure 1). Spontaneous splenic rupture caused by malignancy, infection, congenital disorders or auto-immune disorders, has been documented frequently in case reports.
The Pringle maneuver can be employed during hepatic resections and consists of clamping of the portal vein, hepatic artery and common bile duct. This ‘inflow occlusion’ technique significantly reduces intraoperative blood loss and improves both short- and long-term outcomes[5,6]. Although used by surgeons for decennia, it was not until 1997 that a randomized trial proved that the Pringle maneuver resulted in less blood loss and in preservation of liver function in the early postoperative period due to retrograde flow from the hepatic veins[6]. Described adverse effects of the Pringle maneuver are ischaemic-reperfusion injury, leading to suppressed DNA synthesis, hepatocyte proliferation and delayed hepatic regeneration[7,8]. In 1999 a prospective randomized study concluded that intermittent clamping was favorable over continuous clamping, especially in patients with abnormal liver parenchyma such as in steatosis and cirrhosis and in patients particularly sensitive to ischaemia[8].
However, a recent randomized trial showed that hepatic resection without any form of hepatic pedicle clamping can be performed safely through novel surgical devices used for transection of the liver parenchyma and anaesthesiological techniques for controlling parenchymal bleeding[9]. In this way ischaemic-reperfusion injuries are avoided.
In performing the Pringle maneuver, intermittent clamping is carried out, at present only in case of excessive blood loss during liver transection. Portosystemic collaterals, which are collapsed normal under physiological circumstances, can be opened by a temporary increase in portal pressure and thus assist in lowering portal hypertension. Contrary to the situation in our patients, patients with cirrhosis and portal hypertension have well-developed portosystemic collateral channels that compensate for the sudden increase in portal venous pressure[3].
The Pringle maneuver leads to a small decrease in cardiac output, a decrease in ventricular filling pressure, an increase in arterial pressure and a marked increase in systemic vascular resistance. The latter counteracts the decrease in cardiac output and thus arterial pressures tend to remain at or higher than preclamping levels. Activation of baroreceptors in the portal venous system and spleen may elicit a reflex increase in systemic arterial pressures. Clamping of the portal vein leads to a moderate increase in portal venous pressure by shunting through portosystemic collateral channels. Delayed adaption in splangic bed capacity contributes to further pooling of venous blood[3,10]. The combination of these factors can ultimately lead to a spontaneous splenic rupture, as happened in our patients (Figure 1).
In conclusion, resection without any form of hepatic pedicle clamping can be performed safely and avoids ischemic injury as well as portal hypertension. In case of excessive blood-loss, the Pringle maneuver can be performed, preferably intermittently. However, using the Pringle maneuver, the risk of a spontaneous splenic rupture should be taken into account.
Peer reviewers: Paolo Feltracco, MD, Assistant Professor of Anaesthesia and Intensive Care, Istituto di Anestesiologia e Rianimazione, Università di Padova, Via Cesare Battisti 267, Padova 35100, Italy; Ruben Ciria, PhD, Department of Hepatobiliary Surgery and Liver Transplantation, Uniersity Hospital Reina Sofia, Avennida Menendez PidalI s/n, Cordoba 14004, Spain
| 1. | Giagounidis AA, Burk M, Meckenstock G, Koch AJ, Schneider W. Pathologic rupture of the spleen in hematologic malignancies: two additional cases. Ann Hematol. 1996;73:297-302. [Cited in This Article: ] |
| 2. | Baniel J, Bihrle R, Wahle GR, Foster RS. Splenic rupture during occlusion of the porta hepatis in resection of tumors with vena caval extension. J Urol. 1994;151:992-994. [Cited in This Article: ] |
| 3. | Douzdjian V, Broughan TA. Spontaneous splenic rupture during total vascular occlusion of the liver. Br J Surg. 1995;82:406-407. [Cited in This Article: ] |
| 4. | Baradaran S, Mischinger HJ, Bacher H, Werkgartner G, Karpf E, Linck FG. [Spontaneous splenic rupture during portal triad clamping]. Langenbecks Arch Chir. 1995;380:266-268. [Cited in This Article: ] |
| 5. | Dixon E, Vollmer CM Jr, Bathe OF, Sutherland F. Vascular occlusion to decrease blood loss during hepatic resection. Am J Surg. 2005;190:75-86. [Cited in This Article: ] |
| 6. | Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704-711; discussion 711-713. [Cited in This Article: ] |
| 7. | Melendez J, Ferri E, Zwillman M, Fischer M, DeMatteo R, Leung D. Extended hepatic resection: a 6-year retrospective study of risk factors for perioperative mortality. J Am Coll Surg. 2000;192:47-53. [Cited in This Article: ] |
| 8. | Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38-46. [Cited in This Article: ] |
| 9. | Capussotti L, Muratore A, Ferrero A, Massucco P, Ribero D, Polastri R. Randomized clinical trial of liver resection with and without hepatic pedicle clamping. Br J Surg. 2006;93:685-689. [Cited in This Article: ] |
| 10. | Delva E, Camus Y, Paugam C, Parc R, Huguet C, Lienhart A. Hemodynamic effects of portal triad clamping in humans. Anesth Analg. 1987;66:864-868. [Cited in This Article: ] |