Published online May 27, 2010. doi: 10.4254/wjh.v2.i5.185
Revised: April 7, 2010
Accepted: April 14, 2010
Published online: May 27, 2010
AIM: To create new diabodies with improved binding activity to antigen of the variable light - variable heavy (VH-VL) oriented single-chain Fv dimers genes (scFv).
METHODS: The linker between VH and VL genes was shortened to 3-5 amino acid residues and cloned into the vector pCANTAB5E. The recombinant plasmids were transformed into TG1 cells and sequenced. The positive transformed cells were infected by M13K07 helper phage to form human recombinant phage antibodies. Expressed products were identified by SDS-PAGE, Western blotting, size exclusion gel chromatography (SEC), ELISA and immunohistochemistry.
RESULTS: Three scFv (scFv-3, scFv-4, scFv-5) were constructed successfully with binding ability to hepatocellular carcinoma 3.5-6 fold greater than their parental scFv. The single-chain Fv dimer (scFv-5, termed BDM3) with the best binding ability was successfully expressed in Yeast pichlia, as shown by. SDS-PAGE and Western blotting. SEC results suggested the molecular weight of the expressed products was about 61 kDa. Expressed products showed significantly stronger binding to hepatocellular carcinoma cells than scFv, still having 50% binding activity even after 16 h incubation as 37°C. The purified dimers were bound specifically to the tumor antigen of HCC.
CONCLUSION: we have generated scFv dimers by shortening a series of linkers to 3-5 amino acid residues in VH-linker-VL orientation, resulting in highly stable and affinity-improved dimeric molecules. These will become an attractive targeting moiety in immunotherapeutic and diagnostic applications for HCC.
- Citation: Bie CQ, Yang DH, Liang XJ, Tang SH. Construction of non-covalent single-chain Fv dimers for hepatocellular carcinoma and their biological functions. World J Hepatol 2010; 2(5): 185-191
- URL: https://www.wjgnet.com/1948-5182/full/v2/i5/185.htm
- DOI: https://dx.doi.org/10.4254/wjh.v2.i5.185
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third most common cause of cancer-related death. The incidence of HCC is rising around the world. The diagnosis and treatment of HCC have undergone major changes over the past decade. Unfortunately, there is no completely safe and effective treatment available for those who have developed HCC. Antibodies are a powerful tool for use in diagnosis and therapy of the tumor, because they bind with high specificity and high affinity to a variety of molecules, notably proteins and peptides. Whole antibodies provide high target binding specificity but their use in rapid tumor targeting and in vivo imaging is limited by slow tissue penetration, long circulating half-lives and often undesirable functions[1]. Small monovalent antibody fragments (scFv and Fab) exhibit good tissue penetration but their lack of avidity results in faster off-rates and rapid clearance. Diabodies are noncovalent single-chain Fv (scFv) dimers formed by producing scFv with short (3-5 AA) linkers between their variable light (VL) and variable heavy (VH) chains. This prevents the VH and VL chains in a single molecule from associating with each other in a scFv orientation. Instead, the VL from one molecule associates with the VH from a second molecule, to form a dimer that binds antigen divalently[2]. Several studies have demonstrated that genetically engineered antitumor diabody molecules can be used as effective vehicles for radioimmunotherapy, or can effectively reduce the growth rate of human tumor xenografts[3-8]. Diabodies thus represent an improved strategy for selective tumor targeting as compared with scFv, Fab, or monoclonal antibody molecules.
In previous studies we have described the construction, screening and humanization of the scFv (termed HDM) against human hepatocellular carcinoma using phage display technology[9-11]. HDM has binding specificity to hepatocellular carcinoma and is potentially effective in tumor imaging and therapeutics. To improve its antigen-binding avidity, we constructed the scFv dimers by shorting the linkers to 3-5 amino acid residues in same orientation as their parental scFv and assayed their biological functions, anticipating good behavior in antibody-targeted immunotherapeutic and diagnostic applications.
The pCANAB5E-HDM encoding the anti-HCC scFv HDM was previously constructed in our laboratory. This scFv, which has specificity for human hepatocellular carcinoma, is in the VH-linker-VL format, where the linker consists of the sequence (Gly4Ser)3. The construct is cloned into the pCANAB 5E phagemid vector immediately downstream of the pIII leader sequence, which directs expression to the periplasm. The gene encoding the scFv is fused, via an amber codon, to the pIII gene of a filamentous phage. In supE strains of E. coli (TG1), this allows expression of the scFv on the surface of phage, as a fusion with the minor coat protein pIII, while in supE- strains (HB2151) translation is terminated at the amber codon, producing soluble scFv. A series of bivalent dimers (termed BDMs) was constructed by shortening the 15 amino acid linker between the VH and VL domain to 3-5 (Gly2Ser, Gly3Ser, Gly4Ser) residues. To obtain scFv dimers, BDM, the VH domain of scFv HDM, was PCR amplified with flanking restriction sites Sft I and the partial linker sequence. The VL domain of scFv HBM was PCR amplified with flanking restriction sites Not I and the partial linker sequence. Gene splicing by overlap extension was then performed using the VH sense and VL antisense primers. All primer sequences are showed in Table 1.
| scFv-5 residues | |
| VH | Sense: 5’-TATGGCCCAGCCGGCCATGG-3’ |
| Antisense: 5’-AGAACCACCACCACCTGAGGAGACGGTGACCGT-3’ | |
| VL | Sense: 5’-TCAGGTGGTGGTGGTTCTGACATTGAGCTCACCCAGTCTCCA-3’ |
| Antisense: 5’-TATGCGGCCGCCCGTTTCA-3’ | |
| scFv-4 residues | |
| VH | Sense: 5’-TATGGCCCAGCCGGCCATGG-3’ |
| Antisense: 5’-AGAACCACCACCTGAGGAGACGGTGACCGT-3’ | |
| VL | Sense: 5’-TCCTCAGGTGGTGGTTCTGACATTGAGCTCACCCAGTCTCCA-3’ |
| Antisense: 5’-TATGCGGCCGCCCGTTTCA-3’ | |
| scFv-3 residues | |
| VH | Sense: 5’-TATGGCCCAGCCGGCCATGG-3’ |
| Antisense: 5’-AGAACCACCTGAGGAGACGGTGACCGT-3’ | |
| VL | Sense: 5’-GTCTCCTCAGGTGGTTCTGACATTGAGCTCACCCAGTCTCCA-3’ |
| Antisense: 5’-TATGCGGCCGCCCGTTTCA-3’ | |
| Sequencing primers | |
| Sense: 5’-CAA CGT GAA AAA ATTATT CGC-3’ | |
| Antisense: 5’-GTA AAT GAA TTT TCT GTA TGA GG-3’ |
The PCR reactions were performed using standard conditions with 100 ng of template DNA, 200 mmol/L dNTP (TaKaRa, Japan) and 2.5 U PrimerStar polymerase (TaKaRa, Japan) with 35 cycles at 94°C for 30 sec, 64°C for 30 sec and 72°C for 1 min, using an Eppendorf Thermal Cycler (Eppendorf, Germany). The amplified products were then separated by agarose gel electrophoresis and gel purified using a Gel Extraction Kit (Omega, USA). Equimolar amounts of the two products were then mixed and used in a secondary SOE PCR. For the first five cycles no primers were added, and then the two ‘external’ primers (VH sense and VL antisense) were added for a further 30 cycles. The resulting full length product was then gel purified as described above, cut with the restriction enzymes SfiI and NotI (TaKaRa, Japan.) and ligated onto the pCANAB5E vector molecules at their C-terminal ends with a hexahistidine epitope tag for detection and purification purposes by overnight reaction at 16°C using 0.1 unit T4 ligase (TaKaRa, Japan). The products were transformed intoTG1 cells. 7-8 single colonies were picked from the each agar plate and grown overnight at 37°C in shaking culture in 2 × YT media containing 2% glucose and 100 μg/mL ampicillin. The sequence of positive colonies was determined with the ABI Perkin Elmer 373A auto-mated DNA sequencer (Applied Biosystems, Forster City, CA), using the sequencing primers. Each sequence was determined at least 2 times.
The positive colonies were grown at 37°C in shaking culture in 2 × YT media containing 2% glucose and 100 μg/mL ampicillin to an OD 600 of ~0.5 and 1 × 1010/mL M13K07 helper phage (Pharmacia, Amersham.) was added. The bacteria were incubated for 1 h at 37ºC before being centrifuged at 1500 g for 20 min and resuspended in 2 × YT containing 100 μg/mL ampicillin and 50 μg/mL kanamycin. Following overnight culture at 30°C the cultures were clarified by centrifugation and the phage precipitated using 2.5 mol/L NaCl/20% PEG 8000.
Cultures of E. coli HB2151(supE-) were infected with phage containing the relevant constructs. They were then diluted 1/100 and grown at 37°C in 2 × YT medium containing 2% glucose and 100 μg/mL ampicillin to an OD 600 of ~0.5. The bacteria were pelleted and resuspended in 2 × YT medium containing 100 μg/mL ampicillin and 1 mmol/L isopropyl-β-D-thiogalactopyranoside (IPTG). The cells were then grown for 12 h at 30°C[9,10], centrifuged at 1500 g for 20 min, extracted and harvested for soluble diabodies from the periplasm. The soluble diabodies was purified with a HiTrap Anti-E Tag antibody column (Amersham Pharmacia), utilizing the hexahistidine epitope tag on their C-terminal ends.
The relative molecular mass of each affinity purified scFv dimer was compared by size exclusion gel chromatography on a Superdex 200 HR10/30 column (Amersham Pharmacia) run in PBS at a flow rate of 0.5 mL/min, calibrated with Biorad Gel Filtration Standard proteins[12,13]. The purity of size-fractionated antibodies was monitored by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS–PAGE) under reducing conditions after staining with Simply Blue Safe Stain (Invitrogen, Carlsbad, CA). The specificity of eluted fractions was determined by Western blot analysis using an anti His-Tag-peroxidase conjugated mAb (Amersham Pharmacia) followed by chemiluminescent detection (ECL Plus, Amersham Pharmacia).
The binding of soluble scFv dimers molecules was determined by ELISA. The mixing suspension of Bel-7402, SMMC-7721 and HepG-2 cells (Cell Bank of Wuhan University, China) were harvested and coated onto microtitre plates. Unoccupied sites on the plates were blocked using PBS-milk and the samples, diluted in PBS-milk from 1:1 to 1:256, were added and incubated for 2 h at room temperature. The plates were washed 5 times with PBS-milk and 5 times with PBS. The soluble scFv dimers were detected using the anti His-Tag monoclonal antibody, labeled with horseradish peroxidase (HRP) conjugate (Pharmacia). The assays were developed using o-phenylenediamine (Dako) and absorbance was read at 490 nm wavelength in Model 680 Microplate Reader (Biorad, USA) with the parental scFv fragment HBM as control.
To determine the antigen-binding specificity of scFv dimers, immunohistochemistry was performed. Human hepatocellular carcinoma tissue and non-hepatocellular carcinoma tissue (donated by Professor Zhong, China) sections were heated at 56°C for 2 h, washed successively with dimethylbenzene twice for 20 min, 95% alcohol twice for 2 min, 80% alcohol once for 1 min, distilled water once for 1 min, PBS twice for 1 min. After washing, tissue sections were incubated with 3% H2O2 for 5 min in room temperature and the above washing steps were repeated once. Subsequently, sera (1:10) from BALB/c mice were added on the surfaces of tissue sections in a humidified atmosphere at room temperature for 10 min and the surplus sera were discarded. Purified scFv dimers were then added to the tissue sections. The tissue sections were kept in a humidified atmosphere at 4°C overnight and then washed with PBS for 5 min. The HRP-Anti-E Tag Conjugate was added and reacted under the above conditions overnight followed by washing with PBS for 3 min. Finally, 0.05% H2O2/DAB substrate was added to the tissue sections for 30 min. The specimens were photographed using the Leica Photo System (Qwin).
At a concentration of 10 μg/mL, soluble scFv dimers were incubated at 37°C for up to 7 d. Samples were taken at different time points (at intervals of 12 h) and frozen at -20°C until the end of the experiment. Samples were subsequently analyzed for binding activity to human hepatocellular carcinoma cells.
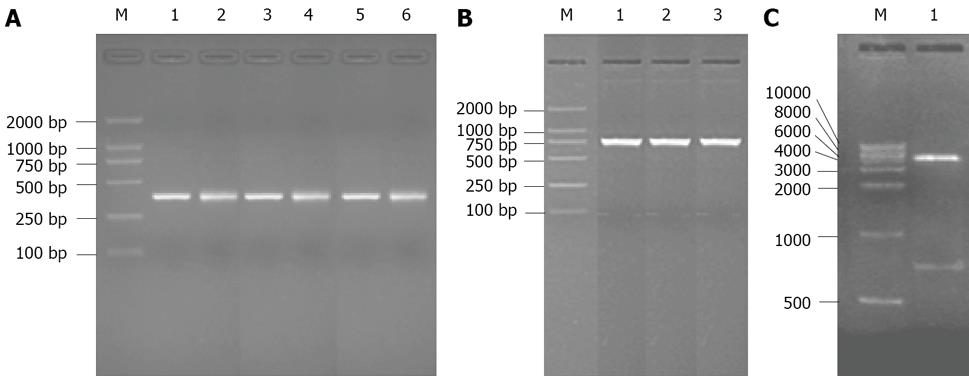
Three BDMs with short linkers, scFv-3, scFv-4 and scFv-5, were constructed in the pCANAB5E vector as described in Materials and Methods. The pictures of PCR reaction products and digested vector on agarose gel electrophoresis are shown in Figure 1. The DNA sequence of each scFv dimmer construct was confirmed in both orientations to ensure that the correct sequence had been inserted between the VH and VL domains(gene sequences of BDMs not shown).
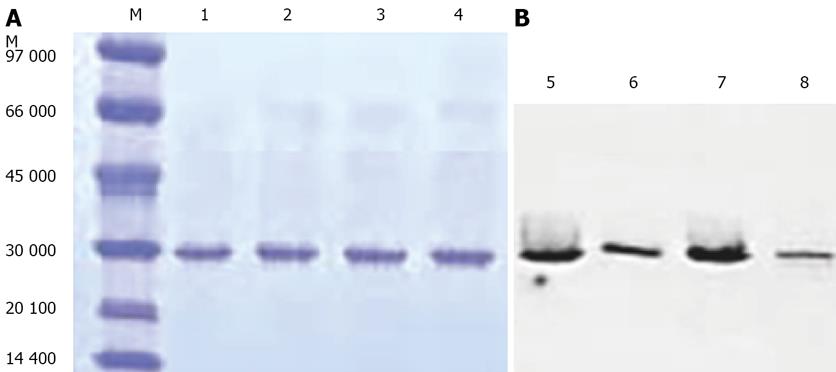
The constructs were transferred to the HB2151 strain of E.coli. The bacteria were induced for 12 h at 30°C with 1 mmol/L IPTG. The soluble scFv fragments was extracted and purified as described above. Affinity purified scFv fragments and parental scFv HBM migrated as single bands with the expected molecular mass (30 kDa) on a reducing SDS-PAGE gel (Figure 2A). Western blot analysis (Figure 2B) was used to detect the C-terminal his-tag with an scFv-specific probe. The result indicated that the scFv dimers had been expressed successfully, the bands of dimers and monovalent scFv were all at 30 kDa because of the reducing conditions.
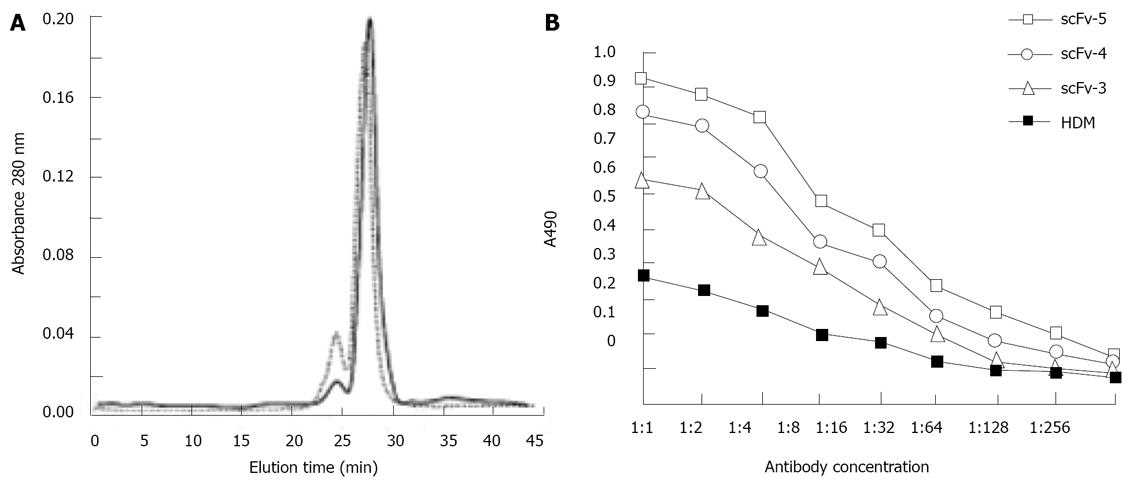
To examine the dimerization behavior of the scFv fragments, samples of scFv-3, scFv-4, scFv-5 were individually subjected to analysis by size exclusion gel chromatography on a calibrated Superdex 200 HR 10/30 column (Figure 3A). The major peaks for each of the scFv fragments showed the following distribution: ScFv-3 eluted in two peaks (25 and 28 min) corresponding to trimers (92 kDa) and dimers (61 kDa), respectively (Figure 3); ScFv-4 showed a predominant peak at an elution time of 28 min corresponding to a dimer (61 kDa) and an additional small peak indicating trimers (Figure 3); ScFv-5 was similar to ScFv-4 (not shown).
Affinity constants of the scFv dimers for binding to human hepatocellular carcinoma cell lines were determined using a Model 680 Microplate Reader (Biorad) at 490 nm wavelength. All three scFv dimers showed improved binding activity compared to parental HDM (Figure 3B). Fitting the data from the equilibrium-binding curves into the non-linear regression model according to the Levenberg-Marquard method revealed a 3.5, 5.0 and 6.0-fold higher apparent affinity compared to the patental HDM for scFv-3, scFv-4 and scFv-5 respectively. The result also showed binding affinity of three dimers in a rank of scFv-5, scFv-4, scFv-3, with the binding activity decreasing according to diluted concentration.
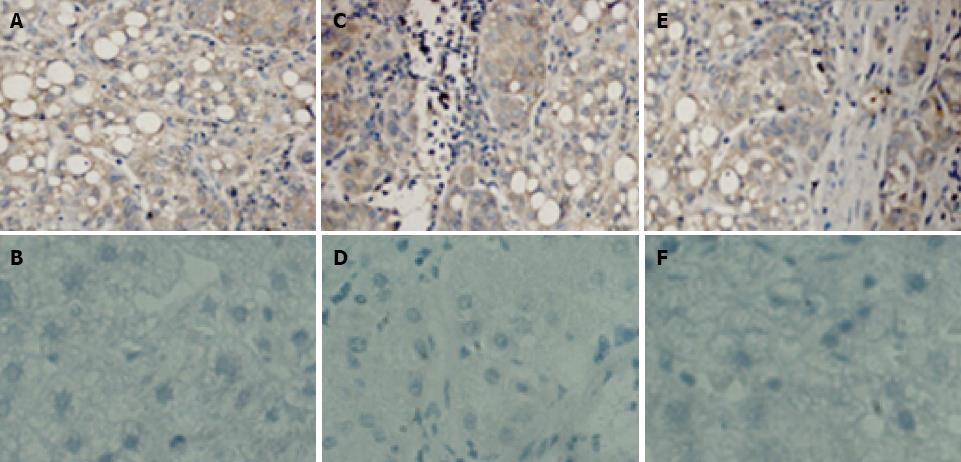
The results of Immunohistochemistry were showed in Figure 4. As expected, scFv dimers react with human hepatocellular carcinoma tissue but not with non-hepatocellular carcinoma tissue. This suggests that scFv dimers have ideal specificity for binding to human hepatocellular carcinoma tissue. Therefore, the potential application of scFv dimers in clinical antibody-targeted diagnosis and therapy for HCC appears highly promising.
The thermostability of the dimeric molecules was analyzed. scFv-3 lost 50% of its initial binding activity after only 18 hrs incubation at 37°C and completely lost binding activity after 70 hrs incubation. In contrast. scFv-4 was similar to scFv-5, with a 16 hr half life and totally lost binding activity after 60 hrs incubation.
Engineered antibodies possess considerable potential for immunotherapeutic and diagnostic applications and some engineered antibodies have already been approved by FDA in the USA for clinical uses. Antitumor antibodies must bind to tumor antigens with high affinity to achieve durable tumor retention. This has spurred efforts to generate high affinity antibodies for use in cancer therapy. In our previous work, we constructed and biopanned a recombinant phage scFv library to obtain a scFv which has specificity for human hepatocellular carcinoma. This was in the VH-linker-VL format, with the linker consisting of the sequence (Gly4Ser)3. We then mutated and humanized the scFv to get scFv dimers (BDMs), which possess reduced immunogenicity[9-11]. The purpose of this study was to generate and characterize bivalent scFv antibody derivatives from HDMs. The most straightforward method to generate a bivalent scFv is to shorten the variable domain connecting linker peptide, thereby allowing for the non-covalent association of multiple polypeptide chains to a dimeric molecule[2,12-17], and to form a ‘double-headed’ fragment with two antigen binding sites that point away from each other[18]. This compactness contributes not only to low immunogenicity and high tumor penetration but also to rapid clearance from the circulation[15,19,20]. In addition to the linker length, the orientation of the variable domains was shown to impact the multimerization behavior and affinity of the polypeptide chains[13,14,21,22]. The distance between the carboxyl terminus end of VL and the amino terminus of VH is greater than that for the opposite orientation. It has, therefore, been suggested that VL-VH orientated scFvs are more constrained than VH-VL oriented fragments when connected by the same linker and therefore tend to exhibit a higher tendency to form higher molecular weight oligomers[13,14,23].
In this regard, we made several scFv dimers variants differing in linker length with a VH-linker-VL orientation. The sequence analysis showed that the sequences of VH-Gly2Ser-VL, VH- Gly3Ser-VL, VH-Gly4Ser-VL were exactly correct. Based upon size exclusion chromatographic profiles on Superdex 200, we conclude that the scFv-3, scFv-4 and scFv-5 predominantly formed stable dimers. This result is consistent with reports of other scFvs generated in the same domain orientation and with the same 3-5 amino acid linker[12,21].
The most important advantage of multivalent scFvs over monovalent scFv and Fab fragments is the gain in functional binding affinity (avidity) to target antigens. High avidity requires that scFv multimers are capable of binding simultaneously to target antigens[24]. The functional affinity of the BDMs was 3.5-6 fold that determined for the parent HDM, reflecting the gain in avidity due to dimerization and the capability of the molecule for simultaneous binding to two epitopes. The findings in immunohistochemistry and thermostability analysis have indicated that scFv dimers have improved specificity and stability and will become the paradigm for high-affinity antibody-based therapeutic and diagnostic reagents for HCC.
In conclusion, we have generated scFv dimers by shortening a series linkers to 3-5 amino acid residues in VH-linker-VL orientation,resulting in a set of highly stable and affinity-improved dimeric molecules. This work is crucial in making the antibody an attractive targeting moiety for HCC.
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the second most common cause of cancer-related death in our country. Traditional therapies, such as resection, chemotherapy and radiotherapy do not have satisfactory efficacy. Single chain variable fragments (scFv) can be a good vector in targeted therapy for HCC as a result of their low molecular weight, strong tumor tissue penetration, weak immunogenicity and short half-lives in blood. They also possess the same binding ability and specificity as the whole antibody.
This team previously obtained a scFv against hepatocellular carcinoma (scFv 4-16, GenBank: DQ640759) through the phage display antibody library technology. After reconstruction of affinity maturation and humanization of scFv, we attained a scFv with high affinity to HCC (scFvDM) and a humanized scFv (scFvHDM).
scFv is a monovalent antibody with the shortcoming of low affinity, poor stability, too rapid clearance from the circulation and low-level expression in bacterial expression systems. This study set out to improve its binding activity to antigen by shortening the linker of the VH-VL oriented scFv to 3-5 residues. We examined the expression and biological functions of the resulting diabodies
These diabodies may become an attractive targeting moiety in immunotherapeutic and diagnostic applications for HCC.
The authors described an incremental improvement on the genetic engineering of HDM for targeting HCC, which remains to be one of most difficult solid tumor to manage. I recommend accepting this manuscript.
Peer reviewer: Lang Zhuo, PhD, Team Leader and Principal Research Scientist, Institute of Bioengineering and Nanotechnology, 31 Biopolis Way, The Nanos #04-16, 138669, Singapore
| 1. | Carter P. Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer. 2001;1:118-129. [Cited in This Article: ] |
| 2. | Holliger P, Prospero T, Winter G. "Diabodies": small bivalent and bispecific antibody fragments. Proc Natl Acad Sci USA. 1993;90:6444-6448. [Cited in This Article: ] |
| 3. | Adams GP, Shaller CC, Dadachova E, Simmons HH, Horak EM, Tesfaye A, Klein-Szanto AJ, Marks JD, Brechbiel MW, Weiner LM. A single treatment of yttrium-90-labeled CHX-A-C6.5 diabody inhibits the growth of established human tumor xenografts in immunodeficient mice. Cancer Res. 2004;64:6200-6206. [Cited in This Article: ] |
| 4. | Takemura S, Asano R, Tsumoto K, Ebara S, Sakurai N, Katayose Y, Kodama H, Yoshida H, Suzuki M, Imai K. Construction of a diabody (small recombinant bispecific antibody) using a refolding system. Protein Eng. 2000;13:583-588. [Cited in This Article: ] |
| 5. | Takemura S, Kudo T, Asano R, Suzuki M, Tsumoto K, Sakurai N, Katayose Y, Kodama H, Yoshida H, Ebara S. A mutated superantigen SEA D227A fusion diabody specific to MUC1 and CD3 in targeted cancer immunotherapy for bile duct carcinoma. Cancer Immunol Immunother. 2002;51:33-44. [Cited in This Article: ] |
| 6. | Asano R, Kudo T, Nishimura Y, Makabe K, Hayashi H, Suzuki M, Tsumoto K, Kumagai I. Efficient construction of a diabody using a refolding system: anti-carcinoembryonic antigen recombinant antibody fragment. J Biochem. 2002;132:903-909. [Cited in This Article: ] |
| 7. | Casey JL, Napier MP, King DJ, Pedley RB, Chaplin LC, Weir N, Skelton L, Green AJ, Hope-Stone LD, Yarranton GT. Tumour targeting of humanised cross-linked divalent-Fab antibody fragments: a clinical phase I/II study. Br J Cancer. 2002;86:1401-1410. [Cited in This Article: ] |
| 8. | Asano R, Sone Y, Makabe K, Tsumoto K, Hayashi H, Katayose Y, Unno M, Kudo T, Kumagai I. Humanization of the bispecific epidermal growth factor receptor x CD3 diabody and its efficacy as a potential clinical reagent. Clin Cancer Res. 2006;12:4036-4042. [Cited in This Article: ] |
| 9. | Zhou M, Yang DH, Tang SH, Lu XH, Wang W, Li K. Construction, screening and characterization of the single chain variable fragment antibody against human hepatocellular carcinoma. Zhong Guo Bing Li Sheng Li Zha Zhi. 2005;21:1246-1248. [Cited in This Article: ] |
| 10. | Lu XH, Yang DH, Zhou M, Tang SH. [Affinity maturation of a single-chain antibody against hepatocellular carcinoma]. Zhonghua Gan Zang Bing Za Zhi. 2006;14:19219-19225. [Cited in This Article: ] |
| 11. | Ye G, Yang DH, Lu XH, Zhou M, Tang SH, Wang W. Humanization of a single-chain antibody against hepatocellular carcinoma. Zhong Guo Bing Li Sheng Li Zha Zhi. 2008;11:1144-1150. [Cited in This Article: ] |
| 12. | Atwell JL, Breheney KA, Lawrence LJ, McCoy AJ, Kortt AA, Hudson PJ. scFv multimers of the anti-neuraminidase antibody NC10: length of the linker between VH and VL domains dictates precisely the transition between diabodies and triabodies. Protein Eng. 1999;12:597-604. [Cited in This Article: ] |
| 13. | Dolezal O, Pearce LA, Lawrence LJ, McCoy AJ, Hudson PJ, Kortt AA. ScFv multimers of the anti-neuraminidase antibody NC10: shortening of the linker in single-chain Fv fragment assembled in V(L) to V(H) orientation drives the formation of dimers, trimers, tetramers and higher molecular mass multimers. Protein Eng. 2000;13:565-574. [Cited in This Article: ] |
| 14. | Kortt AA, Lah M, Oddie GW, Gruen CL, Burns JE, Pearce LA, Atwell JL, McCoy AJ, Howlett GJ, Metzger DW. Single-chain Fv fragments of anti-neuraminidase antibody NC10 containing five- and ten-residue linkers form dimers and with zero-residue linker a trimer. Protein Eng. 1997;10:423-433. [Cited in This Article: ] |
| 15. | Adams GP, Schier R, McCall AM, Crawford RS, Wolf EJ, Weiner LM, Marks JD. Prolonged in vivo tumour retention of a human diabody targeting the extracellular domain of human HER2/neu. Br J Cancer. 1998;77:1405-1412. [Cited in This Article: ] |
| 16. | Le Gall F, Reusch U, Moldenhauer G, Little M, Kipriyanov SM. Immunosuppressive properties of anti-CD3 single-chain Fv and diabody. J Immunol Methods. 2004;285:111-127. [Cited in This Article: ] |
| 17. | Pei XY, Holliger P, Murzin AG, Williams RL. The 2.0-A resolution crystal structure of a trimeric antibody fragment with noncognate VH-VL domain pairs shows a rearrangement of VH CDR3. Proc Natl Acad Sci USA. 1997;94:9637-9642. [Cited in This Article: ] |
| 18. | Perisic O, Webb PA, Holliger P, Winter G, Williams RL. Crystal structure of a diabody, a bivalent antibody fragment. Structure. 1994;2:1217-1226. [Cited in This Article: ] |
| 19. | Wu AM, Chen W, Raubitschek A, Williams LE, Neumaier M, Fischer R, Hu SZ, Odom-Maryon T, Wong JY, Shively JE. Tumor localization of anti-CEA single-chain Fvs: improved targeting by non-covalent dimers. Immunotechnology. 1996;2:21-36. [Cited in This Article: ] |
| 20. | Adams GP, Tai MS, McCartney JE, Marks JD, Stafford WF 3rd, Houston LL, Huston JS, Weiner LM. Avidity-mediated enhancement of in vivo tumor targeting by single-chain Fv dimers. Clin Cancer Res. 2006;12:1599-1605. [Cited in This Article: ] |
| 21. | Arndt MA, Krauss J, Rybak SM. Antigen binding and stability properties of non-covalently linked anti-CD22 single-chain Fv dimers. FEBS Lett. 2004;578:257-261. [Cited in This Article: ] |
| 22. | Carmichael JA, Power BE, Garrett TP, Yazaki PJ, Shively JE, Raubischek AA, Wu AM, Hudson PJ. The crystal structure of an anti-CEA scFv diabody assembled from T84.66 scFvs in V(L)-to-V(H) orientation: implications for diabody flexibility. J Mol Biol. 2003;326:341-351. [Cited in This Article: ] |
| 23. | Plückthun A, Pack P. New protein engineering approaches to multivalent and bispecific antibody fragments. Immunotechnology. 1997;3:83-105. [Cited in This Article: ] |
| 24. | Todorovska A, Roovers RC, Dolezal O, Kortt AA, Hoogenboom HR, Hudson PJ. Design and application of diabodies, triabodies and tetrabodies for cancer targeting. J Immunol Methods. 2001;248:47-66. [Cited in This Article: ] |