Published online Jan 26, 2017. doi: 10.4252/wjsc.v9.i1.1
Peer-review started: July 5, 2016
First decision: August 11, 2016
Revised: August 22, 2016
Accepted: October 1, 2016
Article in press: October 9, 2016
Published online: January 26, 2017
Characterized by dysfunction of tissues, organs, organ systems and the whole organism, aging results from the reduced function of effective stem cell populations. Recent advances in aging research have demonstrated that old tissue stem cells can be rejuvenated for the purpose of maintaining the old-organ function by youthful re-calibration of the environment where stem cells reside. Biochemical cues regulating tissue stem cell function include molecular signaling pathways that interact between stem cells themselves and their niches. Historically, plasma fractions have been shown to contain factors capable of controlling age phenotypes; subsequently, signaling pathways involved in the aging process have been identified. Consequently, modulation of signaling pathways such as Notch/Delta, Wnt, transforming growth factor-β, JAK/STAT, mammalian target of rapamycin and p38 mitogen-activated protein kinase has demonstrated potential to rejuvenate stem cell function leading to organismic rejuvenation. Several synthetic agents and natural sources, such as phytochemicals and flavonoids, have been proposed to rejuvenate old stem cells by targeting these pathways. However, several concerns still remain to achieve effective organismic rejuvenation in clinical settings, such as possible carcinogenic actions; thus, further research is still required.
Core tip: Functional loss of stem cells plays an important role in organismic aging processes. Recent advances in aging research have uncovered the molecular mechanisms of aging, specifically signaling pathways involved in interactions between stem cells and their environment, the so-called “stem cell niche”. Investigating plasma fraction factors has revealed several key pathways involved in this process, including Notch/Delta, Wnt, transforming growth factor-β, JAK/STAT, mammalian target of rapamycin and p38 mitogen-activated protein kinase signaling. Stem cell rejuvenation has the potential to lead organismic rejuvenation by modulating these pathways, hopefully by synthetic or natural agents such as phytochemicals and flavonoids.
- Citation: Honoki K. Preventing aging with stem cell rejuvenation: Feasible or infeasible? World J Stem Cells 2017; 9(1): 1-8
- URL: https://www.wjgnet.com/1948-0210/full/v9/i1/1.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v9.i1.1
Preventing pathological conditions caused by aging, including cancer, osteoporosis, sarcopenia, and cognitive disorders, is one of the most important issues for human health, especially in societies with large aging populations. Although aging, defined by functional decline of cells/organs or accumulation of cell/organ damage, is one of the most recognizable biological characteristics in all creatures, our understanding of mechanisms underlying the aging process remains incomplete. The primary cause of functional declines occurring along with aging is considered to be the exhaustion of stem cell functions in their corresponding tissues. Stem cell exhaustion is induced by several mechanisms, including accumulation of DNA damage and increased expression of cell cycle inhibitory factors, such as p16 and p21[1].
Meanwhile, aging at cellular, tissue, organ and organismic levels has been reversed by exposing tissues from old animals to a young environment. Recent studies have suggested that stem cell rejuvenation could reverse organismal aging phenotypes, and that this could be achieved by inhibiting fibroblast growth factor 2[2], mammalian target of rapamycin (mTOR) complex 1[3], guanosine triphosphatase and cell division control protein 42[4]. Several additional experiments, such as cross-age transplantation and heterochronic parabiosis, have revealed that some factors in the young systemic milieu can rejuvenate declined thymus gland function, as well as neural and muscle stem cell functions, in samples derived from elderly donors[5,6]. Furthermore, heterochronic parabiosis experiments have also shown strong inhibition of young tissue stem cells by the aged systemic milieu or old serum[6].
Although cumulative cellular “intrinsic changes”, such as DNA damage, oxidative damage, increased expression of cell cycle inhibitors and mitochondria dysfunction, have been considered likely culprits for the tissue decline observed with aging, cellular rejuvenation induced by young systemic milieu would have been impossible if “intrinsic changes” were the only cause of cellular aging. Therefore, these so-called “causes of aging” should be more properly regarded as effects of aging (i.e., these processes are not causes, but rather consequences of aging), the result of cellular decisions often defined by responses to “extrinsic stimuli”.
Here some questions arise: If aging at the cellular level were reversed, would it lead to the rejuvenation of the animal at an organismic level? Would it result in prevention of aging and, eventually, life extension? In this editorial, the feasibility of stem cell rejuvenation will be discussed with specific focus on attenuation or reversal of tissue aging.
Numerous studies have shown experimental rejuvenation at cellular, tissue, organ and organismic levels. The first experiment to investigate the possibility of animal rejuvenation, performed by Mccay et al[7] used the uncommon technique of “parabiosis”, or surgically joining the circulatory systems of two animals. They observed old rats that had been sutured to young rats to establish heterochronic parabiosis appeared younger by visual appearance of tissues (mostly non-cellular cartilage). In 1972, Ludwig et al[8] performed similar, but more quantitative experiments demonstrating life extension in older animals, who benefitted from sharing the blood supply of younger animals.
Cross-age transplantation studies have also indicated the rejuvenation potential of tissue and organs. The first cross-age transplantation study of muscle, conducted by Carlson et al[5], showed that the mass and maximum force of old muscle grafted into young hosts were not significantly different from those of young muscle grafted into the same young hosts. Conversely, young muscle grafted into old hosts regenerated no better than old muscle grafted into the same old hosts. Hence, they concluded that chronological age alone is not a limiting factor for the intrinsic ability of muscle to regenerate. Further, poor regeneration of muscle in old animals is a function of the regenerative environment provided by the old host[5]. A thymus transplantation study also showed that senescent, involute thymus glands became fully functional upon transplantation into young animals[9].
Experiments undertaken by Lanza et al[10] demonstrated that nuclei of senescent cells are repairable, as evidenced by the productivity of normal offspring from bovine ova containing nuclei transplanted from senescent cells.
In 2005, Conboy et al[11] showed that stem cell tissues of older rats became phenotypically younger than age-matched controls when these animals were exposed to a young systemic environment. Further, differentiated cells can be reprogrammed to an embryonic-like state by transfer of nuclear contents into oocytes or by fusion with embryonic stem cells (ESCs). Next, Takahashi et al[12] demonstrated induction of pluripotent stem cells (iPSCs) from mouse embryonic cells or adult fibroblasts by introducing four factors (Oct3/4, Sox2, c-Myc, and Klf4) under ESC culture conditions in 2006. Lapasset et al[13] further demonstrated that iPSCs derived from centenarians were rejuvenated such as to be indistinguishable from those derived from youthful cells.
At the organismic level, recent heterochronic parabiotic experiments pairing young and old rats resulted in increased neurogenesis and functional improvement of cognitive ability in the older parabiotic partner, whereas the younger partner exhibited decreased neurogenesis and cognitive abilities, consistent with that of an older rat[14].
All of these studies raised the question as to whether cellular rejuvenation would have been possible if aging at the cellular level only resulted from accumulation of damage and/or toxic metabolic byproducts. The answer appears to be “no”, as aging seems to be controlled in a more “extrinsic manner”.
Signaling pathways involving Notch, transforming growth factor-β (TGF-β), JAK/STAT, p38 mitogen-activated protein kinase (MAPK), oxytocin/MAOI and mTOR regulate tissue stem cell functions, and their changes with age could affect tissue maintenance and repair systems. Proper modulation of these pathways enhanced the tissue regenerative capacity of experimental animals.
Based on the fact that broad rejuvenation of aging by young systemic milieu has been shown in derivatives from all three germ layers, i.e., muscle[15], liver[6] and brain[16], as well as pancreas[17] and heart[18], it can be speculated that young blood serum and its chemical components may contain molecules involved in signals controlling the aging phenotype. However, only a few potential systemic factors responsible for this phenomenon have been identified, as the positive effects of young systemic milieu on old age are very limited[11]. Further, aged systemic milieu or old serum can inhibit young tissue stem cell function[15], suggesting inhibitory components may also exist in the aged circulatory system. Thus, removal or neutralization of these inhibitory systemic components would be necessary to rejuvenate tissue or cellular function.
In this regard, circulating factors that are increased or decreased in old animals represent potential targetable signals and pathways against aging. For instance, several TGF-β and Wnt signaling pathway effectors increased in older animals have been identified as pro-aging circulatory factors capable of deteriorating muscle regeneration[15,19]. TGF-β and bone morphological protein pathways increase with age, activate p38 MAPK, and also act through SMADs. Inhibition of p38 MAPK and SMADs has been found to relieve some of the negative effects of pathogenic activation of these pathways occurring with age[20].
JAK/STAT is a cytokine receptor pathway that increases with age. Many inflammatory cytokines act through this pathway and its inhibition has been shown to restore stem cell symmetric expansion in muscle satellite stem cells[21]. C-C motif chemokine 11 (CCL11) is also increased in elderly individuals, whereby it impairs neurogenesis and decreases cognitive capacity[14].
Activation of Sirtuin family members is also related to rejuvenation, especially Sirtuin 6 (SIRT6), which is an important anti-aging factor in various cells. Downregulation of SIRT6 in bone marrow mesenchymal stem cells (BM-MSCs) impaired the proliferatory, migratory and oxidative stress resistance potentials of these cells. SIRT6 downregulation also enabled cellular senescence through increased senescence-associated β-galactosidase activity and p16 expression; although, SIRT6 is compensatorily overexpressed in aged BM-MSCs[22].
In contrast, Delta/Notch signaling decreases with age, and activation of this pathway restores regenerative potential in old muscle[23]. Oxytocin signaling also decreases with age, and restoring this signaling pathway has been shown to improve aged stem cell function in mesenchymal and muscle satellite stem cells through activation of the MAPK/extracellular signal-regulated kinase (ERK) signaling pathway[24,25].
However, therapeutic modulation of these key pathways is not easy. For instance, long-term activation of the Notch signaling pathway or downregulation of TGF-β/SMAD and Wnt signaling pathways has been shown to be successful in rejuvenation; however, several side effects also occurred including oncogenic transformation, inadequate hematopoiesis and immune deregulation[26]. Administration of oxytocin has also shown potential to stimulate malignant cell proliferation[27].
One thing that must be emphasized is that these signaling pathways are highly interactive with each other. TGF-β acts through SMADs to influence downstream cytokine production that acts on the JAK/STAT pathway. SMAD3 and the Notch intracellular domain directly interact to form a nuclear complex capable of binding specific DNA sequences[28]. The MAPK/ERK pathway is activated by oxytocin, as previously described, and the MAPK pathway is known to activate Notch signaling[29]. Raf/MAPK/ERK and phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling cascades also interact with several pathways through various mechanisms, such as crosstalk with TGF-β, Notch and Wnt pathways through Groucho/transducin-like enhancer of split or SMADs[30,31]. PI3K/Akt signaling interacts with the Wnt pathway via the signaling molecule 14-3-3η, which facilitates β-catenin activation by Akt and stabilizes the β-catenin complex to promote its nuclear translocation[32]. This crosstalk of pathways with each other is largely mediated through mTOR signaling. Given that targeting these pathways is very complicated, further studies will be required to confirm the absence of safety issues prior to clinical applications.
Regarding effects of the systemic environment on aging, as previously described, aged stem cells were rejuvenated by young plasma and young stem cells were aged by exposure to plasma from old animals[23]. Thus, the presence of positive factors that promote young phenotypes in young plasma or negative factors that promote aging phenotypes in old plasma is speculated. Another possibility could be the presence of factors in young plasma that inhibit or neutralize “negative” or “aging” factors. For instance, naturally decreasing levels of interleukin 15 cause aging symptoms such as sarcopenia and obesity, suggesting this cytokine could be a “positive” factor for young phenotypes[33]. In contrast, injection of CCL11/eotaxin, which is reduced by interleukin 15, into the systemic circulation of young animals caused a dysfunction in neurogenesis that resulted in brain aging and loss of cognitive function, suggesting this cytokine possesses pro-aging effects[14].
Additional examples of aging factors include oxytocin and lamin A (specifically, progerin, a truncated form of lamin A). As previously described, oxytocin signaling decreases with age; however, restoring this signaling pathway improved the function of aged mesenchymal and muscle satellite stem cells through activation of the MAPK/ERK signaling pathway, suggesting oxytocin could be a systemically acting anti-aging molecule[24,25]. Whereas, experimental induction of progerin reduced the regenerative capacity of cells by significantly disrupting the expression and localization of self-renewal markers, in part by deregulating Oct1, which perturbs both mTOR and autophagy pathways[34,35].
Another example of concern is the case of growth differentiation factor 11 (GDF11); however, age-related levels of GDF11 and its function have generated an apparent controversy. Sinha et al[36] argued that systemic GDF11 levels normally decline with age, and supplementation of GDF11 reversed functional impairments and restored genomic integrity in aged muscle satellite stem cells. Increased GDF11 levels in aged mice also improved muscle structural and functional features, and increased strength and endurance exercise capacity. However, Egerman et al[37] claimed that there was a trend toward increased GDF11 levels in the sera of aged rats and humans, and GDF11 mRNA also increased in rat muscle with age. They argued that GDF11 and myostatin both mechanistically induce SMAD2/3 phosphorylation, inhibit myoblast differentiation, and regulate identical downstream signaling. GDF11 significantly inhibited muscle regeneration and decreased muscle satellite stem cell expansion in mice. Thus, they concluded GDF11 could be a target for pharmacologic blockade to treat age-related sarcopenia.
One conclusion from these observations is that rejuvenation might require the presence, absence or a required concentration of a number of different factors, such that a cell placed in a young or old environment could assume the age phenotype appropriate to that environment. However, conclusions regarding whether an increase in positive factors, decrease in negative factors (possibly by dilution in young plasma), or their combination results in rejuvenation is still under investigation. Hopefully, further elucidation of the molecular mechanisms underlying aging and rejuvenation will narrow the search so researchers can focus on not only investigating serum or plasma fractionation, but also molecules and agents that affect the aging/rejuvenation process.
As previously described, cross-age transplantation studies and parabiosis experiments revealed that the environment provided by young blood or plasma is capable of rejuvenating aged cells in vivo, and young plasma is sufficient to rejuvenate old stem cells in vitro or vice versa, i.e., old plasma accelerates aging of young cells. Therefore, stem cells assumed the age phenotype of the “age environment” they are in, i.e., either young or old. As such, aging could be caused by an accumulation of negative factors (aging factors) or by a decrease of positive factors (youth phenotype-promoting factors). Examples of negative (pro-aging) factors are p16INK4a, TGF-β and TNF-α, and positive (anti-aging) factors are Notch/Delta and Wnt pathways. We will discuss about the details later in this section.
In this context, organismic rejuvenation is potentially achieved by either removing deleterious substances from old plasma and stem cell niches, or by providing factors that promote young phenotypes in old plasma; indeed, both might be beneficial. In cross-age organ transplantation, the recipient organ experiences an environment that is entirely young or old, although, this method might not be clinically feasible.
If aging is a programmed process coordinated by plasma-borne factors, then exposing cells to the plasma of a particular age should make those cells exhibit a corresponding age phenotype in terms of gene expression profiles. Thus, it is considered that organismic rejuvenation could be achieved by exchange of as much blood or plasma as possible to reduce the effects of original blood or plasma, i.e., heterochronic plasma exchange. Experimentally, the presence of positive, youth-promoting factors in young plasma has been demonstrated to rejuvenate neurogenesis and cognitive function by mere injection of young plasma into old mice[38]. This suggests plasma replacement could be a treatment option for age-related diseases including dementia. The problem is that it is necessary to neutralize or remove inhibitory components occurring within the aged circulation in order for small volumes of young plasma to effectively enhance tissue regeneration in the elderly. Additionally, it is unclear which levels of circulatory molecules are necessary and sufficient for pro-regenerative activity to occur in old stem cells. However, plasma from young animals should have sufficient factors/molecules for all signaling pathways described above to rejuvenate all stem cell types. Another potential issue could be that most or many of the important signaling molecules associated with age phenotype determination may have short half-lives, and would therefore be easily replaced by molecules from the recipient’s body.
An issue of greater concern is the stem cell niches, as the age of stem cells appears to be determined by the age of their niche or environment, rather than the age of the stem cell for many tissues, such that young local and/or systemic environments promoted effective regeneration of old stem cells[11]. Additionally, homeostasis of stem cell tissue maintenance and repair mechanisms would be regulated by the differentiated niches emanating signals, a feature that changes with age, such that niche cells also experience intrinsic aging, resulting in changed extrinsic influences on tissue stem cells. Considering these points, organismic rejuvenation must be performed by rejuvenating both stem cells and their niches. Most likely, rejuvenated niches could rejuvenate the stem cells already residing within them.
In that sense, plasma exchange, a replacement of plasma in an old body with plasma from a younger body, would be a potential means to rejuvenate old stem cells in vivo, although proper volumes and scheduling must be sufficient to allow cleansing of stem cell niches. The risks and costs of this process should also be weighed against potential benefits, as the effectiveness as well as safety issue of plasma exchange in rejuvenating stem cells has not yet been examined in humans. Thus, there remains a major hurdle to applying this technique in clinical settings.
Molecularly, activation of Notch/Delta and Wnt pathways, and inhibition of TGF-β and TNF-α are all restored to the aged muscle niche to rejuvenate muscle satellite stem cells[11], and down-modulation of mTOR rejuvenates hematopoietic stem cells[3]. Exposure to youthful circulation, especially CD45+ hematopoietic cells, modulates Wnt/β-catenin signaling to rejuvenate bone repair capacity[39]. Insulin/insulin-like growth factor 1 (IGF-1) signaling molecules that have been linked to longevity in mammals include daf-2, InR and their homologues. Inactivation of these corresponding genes has been shown to increase the life span of nematodes, fruit flies and mice[40]. If it is possible to target these molecular pathways by synthetic agents or natural sources, stem cell rejuvenation will be more feasible than plasma exchange; indeed, some agonists or antagonists of specific signaling pathways have already been developed and approved by the United States Food and Drug Administration. For instance, with the synthetic agents, activation of Notch and MAPK by attenuation of JAK/STAT signaling rejuvenates myogenesis[21]; whereas, a TGF-β inhibitor simultaneously rejuvenates myogenesis and hippocampal neurogenesis[41,42]. Attenuation of mTOR with rapamycin could also be a multi-faceted anti-aging strategy against senescence-associated cell cycle arrest to enhance tissue regeneration[43,44]. Attenuation of the IGF-1 pathway with metformin has shown life-extending potential[39]. Varieties of natural products such as phytochemicals, flavonoids or other plant extracts have also shown anti-aging effects by targeting various pathways including NF-κB, mTOR, IGF-1 and PI3K/Akt pathways. For instance, the polyphenols resveratrol and curcumin and the flavonoid genistein could be potential therapeutic agents to target signaling pathways involved in aging. Curcumin targets NF-κB, STAT3, PI3K/Akt[45] and mTOR[46] signaling; whereas, resveratrol targets PI3K/Akt signaling by downregulating cyclin-dependent kinase 2, cyclin D1, proliferative cell nuclear antigen, and Akt-ERK signaling[47]. Effective inhibition of multiple pathways involved in the aging process, including NF-κB, mTOR, IGF-1 and PI3K/Akt, can possibly be achieved by appropriately combining these chemicals to rejuvenate stem cell populations.
Although all stem and progenitor cell populations (not to mention their niches) might not be rejuvenated, the rejuvenation of some cell populations including muscle satellite cells, bone marrow stromal cells and hematopoietic stem cells, will benefit the elderly population by increasing their quality of life. Eventually, it may also result in the prevention of aging by increasing the duration of youthful health.
The population of elderly individuals is dramatically increasing worldwide; thus, the importance of extending healthy life expectancy has been emphasized, especially in the rapidly aging societies of many developed countries. Aging affects multiple signaling pathways and their crosstalk, and changes the interaction between stem cells and their niches. As described, recent advances in aging research have indicated the possibility of rejuvenation at cellular, tissue and organismic levels, and suggested that rejuvenation of tissue stem cells through modulation of specific pathways plays an important role in this phenomenon.
To make organismic rejuvenation effective, we must see which cell types (i.e., stem, progenitor, cycling and/or senescent) are capable of being rejuvenated and what modulation of signaling pathways can produce such cellular rejuvenation. Among multicellular organisms with reparable or regenerative tissues, aging entails another feature that causes a gain of function that allows cells to inappropriately proliferate and subsequently acquire phenotypes with increased ability to proliferate, migrate, colonize and survive in ectopic sites, as well as evade attacks by host immune surveillance systems. Thus, aging is one of the major drivers of malignant transformation. In contrast, in aging and cancer development processes, a stress response termed “cellular senescence” may be linked to multiple pathogeneses of both degenerative and hyperplastic diseases. In this regard, cellular senescence is generally considered to be a potent anti-carcinogenic program, and hyperplastic or neoplastic transformation possibly involves a series of events that bypass the senescence process[48].
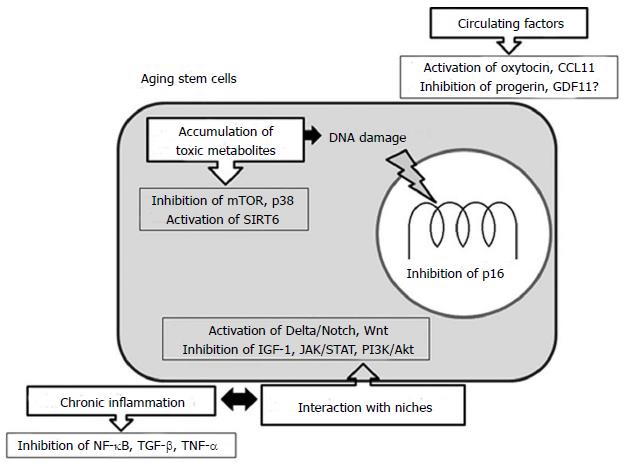
Figure 1 illustrates the possible targets for rejuvenating strategy in aging stem cells. To make stem cell rejuvenation more feasible and achieve the prevention or delay of aging, a better understanding of aging in terms of molecular signaling networks for cellular communication involved in tissue homeostasis, maintenance and repair mechanisms is still required.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: de la Serna I, Goebel WS, Scarfì S, Tanabe S, Vladimir H, Yao CL S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Honoki K, Fujii H, Tsukamoto S, Kishi S, Tsujiuchi T, Tanaka Y. Crossroads of hallmarks in aging and cancer: Anti-aging and anti-cancer target pathways can be shared? Tre Can Res. 2016;11:39-59. [Cited in This Article: ] |
| 2. | Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 555] [Cited by in F6Publishing: 574] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 3. | Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 400] [Cited by in F6Publishing: 470] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 4. | Florian MC, Dörr K, Niebel A, Daria D, Schrezenmeier H, Rojewski M, Filippi MD, Hasenberg A, Gunzer M, Scharffetter-Kochanek K. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell. 2012;10:520-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 351] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 5. | Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 1989;256:C1262-C1266. [PubMed] [Cited in This Article: ] |
| 6. | Conboy IM, Rando TA. Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches. Cell Cycle. 2012;11:2260-2267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 7. | Mccay CM, Pope F, Lunsford W, Sperling G, Sambhavaphol P. Parabiosis between old and young rats. Gerontologia. 1957;1:7-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 41] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Ludwig FC, Elashoff RM. Mortality in syngeneic rat parabionts of different chronological age. Trans N Y Acad Sci. 1972;34:582-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Song Z, Wang J, Guachalla LM, Terszowski G, Rodewald HR, Ju Z, Rudolph KL. Alterations of the systemic environment are the primary cause of impaired B and T lymphopoiesis in telomere-dysfunctional mice. Blood. 2010;115:1481-1489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Lanza RP, Cibelli JB, Blackwell C, Cristofalo VJ, Francis MK, Baerlocher GM, Mak J, Schertzer M, Chavez EA, Sawyer N. Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science. 2000;288:665-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 373] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 11. | Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1544] [Cited by in F6Publishing: 1534] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 12. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17989] [Cited by in F6Publishing: 17055] [Article Influence: 947.5] [Reference Citation Analysis (0)] |
| 13. | Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Aït-Hamou N, Leschik J, Pellestor F, Ramirez JM, De Vos J. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011;25:2248-2253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 347] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 14. | Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1110] [Cited by in F6Publishing: 1234] [Article Influence: 94.9] [Reference Citation Analysis (0)] |
| 15. | Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1084] [Cited by in F6Publishing: 1083] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 16. | Ruckh JM, Zhao JW, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJ. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 426] [Cited by in F6Publishing: 479] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 17. | Salpeter SJ, Khalaileh A, Weinberg-Corem N, Ziv O, Glaser B, Dor Y. Systemic regulation of the age-related decline of pancreatic β-cell replication. Diabetes. 2013;62:2843-2848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 636] [Cited by in F6Publishing: 693] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 19. | Carlson ME, Silva HS, Conboy IM. Aging of signal transduction pathways, and pathology. Exp Cell Res. 2008;314:1951-1961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Kellner J, Liu L, Zhou D. Inhibition of p38 mitogen-activated protein kinase promotes ex vivo hematopoietic stem cell expansion. Stem Cells Dev. 2011;20:1143-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Price FD, von Maltzahn J, Bentzinger CF, Dumont NA, Yin H, Chang NC, Wilson DH, Frenette J, Rudnicki MA. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat Med. 2014;20:1174-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 272] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 22. | Zhai XY, Yan P, Zhang J, Song HF, Yin WJ, Gong H, Li H, Wu J, Xie J, Li RK. Knockdown of SIRT6 Enables Human Bone Marrow Mesenchymal Stem Cell Senescence. Rejuvenation Res. 2016; Mar 14; Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 23. | Carlson ME, Suetta C, Conboy MJ, Aagaard P, Mackey A, Kjaer M, Conboy I. Molecular aging and rejuvenation of human muscle stem cells. EMBO Mol Med. 2009;1:381-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 24. | Elabd C, Basillais A, Beaupied H, Breuil V, Wagner N, Scheideler M, Zaragosi LE, Massiéra F, Lemichez E, Trajanoski Z. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells. 2008;26:2399-2407. [PubMed] [Cited in This Article: ] |
| 25. | Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, Kung S, Jiang KP, Conboy IM. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun. 2014;5:4082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 257] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 26. | Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454:528-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 352] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 27. | Petersson M. Opposite effects of oxytocin on proliferation of osteosarcoma cell lines. Regul Pept. 2008;150:50-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 694] [Cited by in F6Publishing: 743] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 29. | Yamashita AS, Geraldo MV, Fuziwara CS, Kulcsar MA, Friguglietti CU, da Costa RB, Baia GS, Kimura ET. Notch pathway is activated by MAPK signaling and influences papillary thyroid cancer proliferation. Transl Oncol. 2013;6:197-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Hasson P, Paroush Z. Crosstalk between the EGFR and other signalling pathways at the level of the global transcriptional corepressor Groucho/TLE. Br J Cancer. 2006;94:771-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Zhang Y, Derynck R. Regulation of Smad signalling by protein associations and signalling crosstalk. Trends Cell Biol. 1999;9:274-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 204] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 32. | Tian Q, He XC, Hood L, Li L. Bridging the BMP and Wnt pathways by PI3 kinase/Akt and 14-3-3zeta. Cell Cycle. 2005;4:215-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY). 2012;4:535-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 209] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 34. | Pacheco LM, Gomez LA, Dias J, Ziebarth NM, Howard GA, Schiller PC. Progerin expression disrupts critical adult stem cell functions involved in tissue repair. Aging (Albany NY). 2014;6:1049-1063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Infante A, Gago A, de Eguino GR, Calvo-Fernández T, Gómez-Vallejo V, Llop J, Schlangen K, Fullaondo A, Aransay AM, Martín A. Prelamin A accumulation and stress conditions induce impaired Oct-1 activity and autophagy in prematurely aged human mesenchymal stem cell. Aging (Albany NY). 2014;6:264-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 575] [Cited by in F6Publishing: 605] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 37. | Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metab. 2015;22:164-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 396] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 38. | Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 650] [Cited by in F6Publishing: 711] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 39. | Baht GS, Silkstone D, Vi L, Nadesan P, Amani Y, Whetstone H, Wei Q, Alman BA. Exposure to a youthful circulaton rejuvenates bone repair through modulation of β-catenin. Nat Commun. 2015;6:7131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 40. | Anisimov VN. Metformin for aging and cancer prevention. Aging (Albany NY). 2010;2:760-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 41. | Jeong J, Conboy MJ, Conboy IM. Pharmacological inhibition of myostatin/TGF-β receptor/pSmad3 signaling rescues muscle regenerative responses in mouse model of type 1 diabetes. Acta Pharmacol Sin. 2013;34:1052-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Yousef H, Conboy MJ, Morgenthaler A, Schlesinger C, Bugaj L, Paliwal P, Greer C, Conboy IM, Schaffer D. Systemic attenuation of the TGF-β pathway by a single drug simultaneously rejuvenates hippocampal neurogenesis and myogenesis in the same old mammal. Oncotarget. 2015;6:11959-11978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 43. | Blagosklonny MV. Aging, stem cells, and mammalian target of rapamycin: a prospect of pharmacologic rejuvenation of aging stem cells. Rejuvenation Res. 2008;11:801-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY). 2012;4:159-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 45. | Jagtap S, Meganathan K, Wagh V, Winkler J, Hescheler J, Sachinidis A. Chemoprotective mechanism of the natural compounds, epigallocatechin-3-O-gallate, quercetin and curcumin against cancer and cardiovascular diseases. Curr Med Chem. 2009;16:1451-1462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 46. | Johnson SM, Gulhati P, Arrieta I, Wang X, Uchida T, Gao T, Evers BM. Curcumin inhibits proliferation of colorectal carcinoma by modulating Akt/mTOR signaling. Anticancer Res. 2009;29:3185-3190. [PubMed] [Cited in This Article: ] |
| 47. | Vergara D, Simeone P, Toraldo D, Del Boccio P, Vergaro V, Leporatti S, Pieragostino D, Tinelli A, De Domenico S, Alberti S. Resveratrol downregulates Akt/GSK and ERK signalling pathways in OVCAR-3 ovarian cancer cells. Mol Biosyst. 2012;8:1078-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 48. | Honoki K, Tsujiuchi T. Senescence bypass in mesenchymal stem cells: a potential pathogenesis and implications of pro-senescence therapy in sarcomas. Expert Rev Anticancer Ther. 2013;13:983-996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |