Published online May 26, 2015. doi: 10.4252/wjsc.v7.i4.691
Peer-review started: September 10, 2014
First decision: December 17, 2014
Revised: January 26, 2015
Accepted: February 4, 2015
Article in press: February 9, 2015
Published online: May 26, 2015
Rotator cuff tears are frequent shoulder problems that are usually dealt with surgical repair. Despite improved surgical techniques, the tendon-to-bone healing rate is unsatisfactory due to difficulties in restoring the delicate transitional tissue between bone and tendon. It is essential to understand the molecular mechanisms that determine this failure. The study of the molecular environment during embryogenesis and during normal healing after injury is key in devising strategies to get a successful repair. Mesenchymal stem cells (MSC) can differentiate into different mesodermal tissues and have a strong paracrine, anti-inflammatory, immunoregulatory and angiogenic potential. Stem cell therapy is thus a potentially effective therapy to enhance rotator cuff healing. Promising results have been reported with the use of autologous MSC of different origins in animal studies: they have shown to have better healing properties, increasing the amount of fibrocartilage formation and improving the orientation of fibrocartilage fibers with less immunologic response and reduced lymphocyte infiltration. All these changes lead to an increase in biomechanical strength. However, animal research is still inconclusive and more experimental studies are needed before human application. Future directions include expanded stem cell therapy in combination with growth factors or different scaffolds as well as new stem cell types and gene therapy.
Core tip: Current surgical techniques in rotator cuff repair do not achieve good tendon-to-bone healing. The use of stem cells to improve healing is a promising alternative. Different in vivo animal studies have shown good results in achieving restoration of the native enthesis. However, human studies are scarce so the use of stem cell therapy in rotator cuff repair should still be considered and experimental technique. Further basic and clinical research is needed.
- Citation: Mora MV, Ibán MAR, Heredia JD, Laakso RB, Cuéllar R, Arranz MG. Stem cell therapy in the management of shoulder rotator cuff disorders. World J Stem Cells 2015; 7(4): 691-699
- URL: https://www.wjgnet.com/1948-0210/full/v7/i4/691.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i4.691
The rotator cuff is a structure formed by the tendinous insertions of a group of muscles that dynamically stabilize the glenohumeral joint. Rotator cuff disease is the most common condition of the shoulder for which patients seek treatment and can be found in 30% to 50% of the population aged older than 50 years[1,2]. However, it also affects athletes and active individuals regardless of age and activity level.
Rotator cuff tears often require surgical treatment in order to increase function and decrease pain[3,4]. The objective of the treatment is the repair of the damaged tendons. Whether or not healing of the tear is a prognostic factor on function and pain after rotator cuff repair has been controversial. However, most of the authors have found that tear recurrence determines lower functional scores and a decrease in patient satisfaction[5-7]. In an attempt to improve the strength of the surgical repair, new materials and surgical techniques that aim to reproduce the anatomical footprint of the rotator cuff have been proposed[8,9]. Despite these significant technical advances, several studies have shown a persistently high failure rate of tendon to bone rotator cuff repair that ranges from 30% to 94%[6,10,11].
The main problem with failure in rotator cuff repair is probably biologic, as it is well known that the delicate and highly specialized fibro-cartilaginous transition zone between the rotator cuff and the bone does not regenerate after repair[12,13]. Standard tendon to bone repair techniques attain only a fibro-vascular scar tissue that has relatively poor mechanical properties[14]. Thus, the focus in research has changed from mechanical improvement of the repair techniques to finding ways to improve the biological environment around that repair[15-22]. This would include growth factors (GF), bone morphogenetic proteins (BMPs) as well as stem cells. The hypothesis is that biological therapies might facilitate the regeneration of the normal tendon-to-bone insertion microarchitecture and limit the amount of scar tissue. In this direction, isolated GF or platelet rich plasma has been recently used with variable results but stems cell are a more promising alternative[23-25].
Stem cells have demonstrated great potential in enhancing the biologic healing process based on their influence in angiogenesis and the inflammatory pattern[26]. However, several questions still remain before they can be used clinically for augmenting tendon to bone healing. The purpose of this paper is to outline the current knowledge on the role stem cell therapy might have in dealing with rotator cuff tears and the future implications of the ongoing research’s results.
Tissue regeneration in the tendon-to-bone interphase is a complex process. The stiffness difference between tendon and bone is responsible for significant mechanical stress in the regeneration zone[27]. The enthesis represents a transitional tissue that allows for efficient energy transmission due to the gradual changes that occur in its microstructure, its histological characteristics and its biomechanical behaviour.
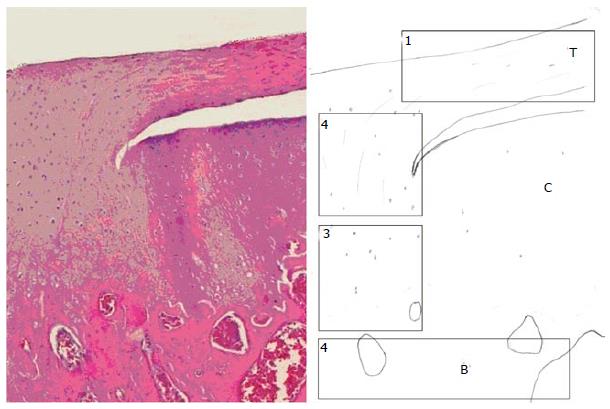
The enthesis has been divided into four zones: tendon, non-mineralized fibrocartilage, mineralized fibrocartilage and bone[28] (Figure 1 and Table 1). In the tendon area (zone 1) there is a predominance of type I collagen fibres together with a small amount of decorin which is a small cellular or pericellular matrix proteoglycan; In the non-mineralized fibrocartilage area (zone 2), type II and III collagen fibres are predominant and small amounts of type I, IX and X collagen fibres have also been detected. Aggregans and decorine are also present. Zone 3 is constituted by the mineralized fibrocartilage, with a highly specialized mineralized content and type I collagen fibres. Lastly, zone 4 is characterized for a bone-alike composition, as it corresponds to the bony insertional area. As previously mentioned, it has been demonstrated that this specialized tissue does not regenerate after injury and repair. The fibro vascular tissue that substitutes the native enthesis is characterized by a predominance of type III collagen due to the excessive formation of scar tissue and the absence of fibrocartilage.
| Zone | Histological characteristics | Collagen type | Extracellular matrix composition |
| Zone 1 | Tendon | I | Decorin |
| Zone 2 | Non-mineralized fibrocartilage | II and III ( small amounts of I, IX and X) | Aggrecan and decorin |
| Zone 3 | Mineralized fibrocartilage | II (small amounts of X) | Aggrecan and mineral component |
| Zone 4 | Bone | I | Mineral component |
The reparative process can be divided into 3 phases (inflammatory, reparative and remodelling) and numerous cells and cytokines have been implicated[13,29,30]. Diaz-Heredia et al[30] have studied the gradual variation of vascular endothelial growth factor (VEGF), interleukin-1 (IL-1) and transforming growth factor-β1 (TGF-β1) in an animal model of rotator cuff tears in rats. Some authors have pointed out that the inability to regenerate the native enthesis could be caused by the incomplete expression of the genes implicated in its formation[28]. During embryogenic development, healing occurs without expression of TGF-β1 but with expression of TGF-β3, which determines an absence of scar tissue. On the contrary, during postnatal life, TGF-β1 is active during the three phases of the healing process[13,30,31]. Another important group of factors widely studied are BMP-12, 13 and 14 as well as fibroblast growth factor-β (FGF-β and insulin like growth factor-1 (IGF-1). Matrix metalloproteinases are multi-domain proteinases regulated by tissue inhibitors of metalloproteinases (TIMPs) and play a determinant role in the remodelling phase.
The enthesis structure is developed successfully during embryogenic period so knowledge of the biological mechanism of its development could help in pinpointing which factors are relevant in trying to regenerate the native transitional tissue[28]. Galatz et al[32] found that the mature fibrocartilage does not appear until 21 d after birth. Supraspinatus fibroblasts expressed type I collagen during all the process. Type II collagen was expressed firstly in the non-mineralized fibrocartilage and at 7 d in the mineralized fibrocartilage, where it persisted until 56 d. Type X collagen was initially seen in mineralized collagen at 14 d and it persisted until 56 d. There was a change in the presence from TGF-β3 to TGF-β1 at 15 d. The gradual expression of different factors present in the development of the physeal plate as (sex determining region Y)-box 9, Scleraxis, Patched 1, Parathyroid hormone-related protein (PTHrP) and Indian Heddegog (Ihh) has also been studied[32-34]. It has been proposed that the stratification in the structure and composition along the different zones of the enthesis could be a consequence of the gradual expression of these and other factors. For example, the amount of mineral deposit in the mature enthesis could be determined by the presence of osteogenic factors such as runt-related transcription factor 2 (Runx2) and bone morphogenetic protein-2 (BMP2). On the other hand, the formation of fibrocartilage could be related to a greater expression of PTHrP, Ihh and Sox9. Lastly, tendon development would be conditioned by the expression of BMP-12, tenomoduline and scleraxis[32]. Scleraxis is a protein member of the basic helix-loop-helix superfamily of transcription factors.
In the past decades, as mentioned before, numerous biology-based strategies have been developed in order to improve the rate and quality of healing in rotator cuff models. The main areas of research, apart from stem cells, are matrix metalloproteinase (MMP) inhibitors and GF.
MMP expression is increased in degenerative rotator cuff tissue and it is known to cause progressive weakness in extracellular matrix. They are involved in tumoral growth, aneurysmatic disease and post-surgical tissue remodelling in the rotator cuff[35-37]. Tissular metalloproteinase inhibitors are thus, potential biological tools. In particular, inhibition of MMP-13, a MMP that is increased in degenerative rotator cuff tears, allows for higher amount of fibrocartilage formation, better collagen fiber organization and higher load to ultimate failure in the enthesis[36,38].
GF factors are key in the development of the different enthesis zones. The regeneration of the most specialized zone, the mineralized fibrocartilage, can be stimulated by osteoinductive factors[39]. The GF are usually delivered with a vehicle, such as augmented sutures, fibrin gels or collagen sponges[16,40,41]. Rodeo et al[16] developed an animal model of supraspinatus repair in sheep in which they used BMP-2 to 7, TGFβ1, TGFβ2, TGF-β3 and FGF. They detected better histologic and biomechanical properties[16]. Other investigators have obtained similar results with BMP-12[42], BMP-13[17], BMP-14[43], FGF[40,44], IGF-1[45] and PDGF-b[15]. Some of these factors seem to play different roles depending in which zone of the enthesis they act or the timing of their effects.
The most widespread treatment, however, is platelet rich plasma (PRP) obtained from autologous blood[46]. It has been proposed that PRP facilitates coagulation and homeostasis, stimulates wound closure, restores intraarticular hyaluronic acid, equilibrates angiogenesis, promotes glucosamine synthesis and serves as a cellular support for migration and differentiation[23]. Despite the variable results obtained, it has been used for muscular, ligamentous, tendinous or cartilaginous injuries[47-50]. With regards to its application in rotator cuff tears, the results have also been controversial. Neither Sánchez Márquez et al[24] or Ruiz-Moneo et al[51] found any relevant clinical improvement with the use of PRP to augment suture in massive tears. However, other investigators support the use of PRP in selected cases[52]. For example, Randelli et al[25] in a prospective randomized clinical trial, found less postoperative pain and accelerated healing rate in patients with non-massive rotator cuff tears but there were no differences in functional scores and re-rupture rate. Due to the chronic nature of these injuries, it has been suggested that PRP application should be serial in order to enhance its benefits[53]. Another explanation for this fact is that the expression of growth factors is ephemeral. In this context, stem cell and gene therapies could be a more definitive and long-lasting treatment.
The use of stem cell therapy in the regeneration of musculoskeletal tissue is a very dynamic field. MSCs of different origins, with their innate ability to differentiate into several mesenchymal tissues including bone, fat, muscle and tendon have been used extensively in tissue repair. Applications in which its usefulness has been confirmed are: treatment of bone defects, cartilage regeneration, meniscal regeneration and healing, management of tendinopathies and management of muscle lesions[54-56]. Investigators usually prefer adult MSCs over embryonic of fetal stem cells as the former are usually locally available and easier to obtain for the treatment of these non-life-threatening problems. Furthermore, the low immunogenicity of MSCs allows for the use of allogenic strains[57].
Some authors have also performed extensive research in animal bone-to-tendon healing models. Until recently the most widespread model reproduced the integration of anterior cruciate ligament tendinous grafts in a bone tunnel. In this animal model, the hamstring grafts are introduced into bony tunnels in both femoral and tibiae bones and pull out strength is tested. Lim et al[58] have used MSCs in this model in rabbits and found a significant increase in maximum load to failure.
It was not until 2009 that MSC therapy was applied to a rotator cuff model, since then the available literature has grown consistently. MSCs of different origins have been used for rotator cuff repair. Different tissue sources have been identified: bone marrow, adipose tissue, muscle, synovia, periosteum, tendon, dermis and umbilical cord or peripheral blood, have all been evaluated as sources of multipotent and pluripotent cell[26]. Although generally speaking MSCs of different origins have similar biological potential, there is increasing knowledge that certain MSC populations are better than others for specific tissue regeneration[59-61]. Table 2 shows the main animal investigations performed on rotator cuff repair.
| Ref. | Animal | Type of cells | Tendon repair model | Method of delivery | Results |
| Gulotta et al[20] | Rat | Allogenic BM-MSC | Supraspinatus tendon Acute repair | Fibrin glue carrier | No differences in structure, composition or strength at the repair site |
| Gulotta et al[63] | Rat | Allogenic BM-MSCs transduced with MT1- MMP | Supraspinatus tendon Acute repair | Fibrin glue carrier | Improved fibrocartilage Improved biomechanical strength |
| Gulotta et al[64] | Rat | Allogenic BM-MSCs transduced with human BMP-13 | Supraspinatus tendon Acute repair | Fibrin glue carrier | No differences in structure, composition or strength at the repair site |
| Gulotta et al[65] | Rat | Allogenic BM-MSCs transduced with sleraxis | Supraspinatus tendon Acute repair | Fibrin glue carrier | Improved fibrocartilage Improved mechanical resistance and stiffness |
| Shen et al[74] | Rabbit | Allogenic T-MSCs | Supraspinatus tendon Acute repair | Seeded scaffold (silk-collagen) | T-MSCs differentiated into tenocytes Improved collagen content Improved biological environment Less inflammation |
| Kida et al[62] | Rat | Autologous BM-MSC | Supraspinatus tendon Acute repair | Transosseous drilling | BM-MSCs infiltrated the repaired tendon Improved mechanical resistance |
| Oh et al[71] | Rabbit | Allogenic A-MSCs | Subscapularis tendon Chronic repair | Injection | Improved muscle function Improved tendon healing Decreased fatty infiltration |
The principal source for stem cell-enhanced healing of the rotator cuff has been autologous bone marrow (BM-MSCs). Gulotta et al[20] performed an experimental unilateral detachment of supraspinatus tendon and a transosseous repair in rats. BM-MSCs were harvested by performing lavage of intramedullary canals of long bones with Hank’s Balanced Salt Solution (Gibco, Gaithersburg, MD). They showed that MSCs were present at the repair site and that they were metabolically active. Although they did not find significant differences in between the treated and untreated groups, at 4 wk, there was a higher amount of fibrocartilage formation and better orientation of fibrocartilage fibers.
In order to reproduce rotator cuff surgery, Kida et al[62] designed a study in which they performed additional drilling to the greater tuberosity to release bone marrow and allow bone marrow cells to migrate into the suture zone. They tested chimeric rats that expressed green fluorescent protein in the bone marrow cells and looked for the expression of this protein after a period of 2, 4 and 8 wk. It seems that drilling and the subsequent migration of stem cells might improve maximum load to failure at 4 and 8 wk.
More recently, Gulotta et al[63-65] have used genetically modified MSCs in order to express scleraxis and produce MIT1 and BMP-13 with promising results. MSCs genetically modified to over-express MT1-MMP might be useful for augmenting suture as it has demonstrated improved biomechanical strength at 4 wk based on a higher presence of fibrocartilage[63]. Results of studies with application of MSCs genetically modified to overexpress BMP-13[64] were not that successful. On the contrary, MSCs genetically modified with Scx demonstrated to promote better biomechanical characteristics at 2 wk[65].
Adipose Tissue derived stem cells (AMSC) have also shown multipotentiality in vitro[66]. Due to its mesodermal origin, they can differentiate into adipose lineage cells[67], osteogenic cells[68], chondrogenic cells[59] and myogenic cells[69]. In vivo, they have also demonstrated their capacity to differentiate into adipose tissue using different scaffolds as polyglicolic acid, collagen sponges or fibrin gel[54,70,71].
Recently, Oh et al[71] have published the first study in a rotator cuff model using AMSCs. Four groups were compared for a suture of the subscapularis tendon in rabbit using saline, saline and AMSCs, only AMSCs and only suture. They found better healing properties and a capacity of regeneration after fatty infiltration of the muscle.
Muscle-derived stem cells (M-MSCs) have been isolated using a modification of a method known as the preplate technique[72]. Pelinkovic et al[73] have shown that the injection of M-MSCs into the supraspinatus tendon of athymic rats resulted in the engraftment of transplanted cells in a pattern with a morphology comparable to resident tendon fibers. The authors suggest that more studies are necessary before assuming that M-MSCs can improve rotator cuff healing.
Lastly, Shen et al[74] performed a study using tenocyte-derived stem cells (T-MSCs) proliferated in vitro and obtained from human fetal Achilles tendon samples. Implantation of this type of cells in the rabbit rotator cuff defect did not elicit an immunologic response but increased fibroblastic cell ingrowth and reduced infiltration of lymphocytes.
Cell adhesion to the scaffold depends on the interaction that is established in between the scaffold microstructure and the cell surface receptors denominated integrins. Transmembrane contacts are key factor for MSC survival, proliferation and differentiation[75]. Numerous studies have investigated the behaviour of stem cells in different scaffolds and have demonstrated that the scaffold can determine the differentiation capacity into one or other lineages[75]. Two different types of interactions have been described: physical and biochemical. Vehicles that maintain the rounded shape of the cells and avoid contact in between them, promote the chondrogenic differentiation and avoid expression of type I collagen. Porous gelatine vehicles or those that use fibrin favour a fibro cartilaginous phenotype due to the expression of collagen types I and II[76].
Although there is a lack of consensus on whether the application of stem cells to enhance the rotator cuff healing is effective or not, some authors have started developing different strategies for the clinical application of the experimental findings.
Beitzel et al[77] studied the quantity and characteristics of BM-MSCs obtained from proximal humerus and distal femur bone marrow aspiration and found them comparable, supporting the previous experimental research by Kida et al[62]. Rotator cuff derived MSCs have been isolated and compared to BM-derived stem cells. It seems that the myogenic potential of MSCs derived from rotator cuff cells is higher than for BM-MSCs[78]. Randelli et al[79] could isolate tenocyte-derived stem cells from supraspinatus tendon and long head of biceps tendon. Utsunomiya et al[80] also studied the subacromial bursa as a potential source for MSCs and found that the synovial cells found in the bursa were a good cell source.
Ellera Gomes et al[81] published their work in 14 patients with a complete tear of the rotator cuff that was repaired in a trans osseous fashion through a mini-incision augmenting the suture with mononuclear stem cells from iliac crest bone marrow aspirate. At 12 mo, 12 of the 14 tears had healed according to clinical and magnetic resonance imaging results[81]. This is the only published investigation on clinical application of stem cells in rotator cuff tears.
Lastly, Beitzel et al[75] have also focused their attention in how different scaffolds behave in humans in order to extrapolate results obtained from experimental research. MSCs adhesion, proliferation, and scaffold morphology were evaluated by histologic analysis and electron microscopy. According to their findings, significant differences existed: non cross-linked porcine collagen scaffolds showed superior results for cell adhesion and proliferation, as well as on histologic evaluation.
Advanced stem cell therapy and gene therapy represent the most feasible option in order to improve rotator cuff healing[21]. A better knowledge of the molecular phases of embryogenesis of the enthesis as well as the injury and healing patterns have allowed to identify the growth factors and proteins to target[13,28].
A combination of stem cells, modified before implantation, using exposure to different growth factors or modifications to the culture conditions to generate a desired phenotype is one of the most investigated pathways[26]. Moreover, the newly recognized anti-inflammatory and antiapoptotic impact of MSCs on tissue healing may provide a great potential for functional restoration[76,82].
On the other hand, specific growth factor supplementation, in the form of transgenic therapy may allow longer-term tendon repair and potential return to function. Fetal-derived embryonic stem cell-like cells have recently been evaluated for tendon and ligament repair. More recently, induced pluripotent stem cells, developed by genetically reprogramming adult-sourced cells, may be particularly beneficial in the challenging environment of rotator cuff injury. Generation of iPS cells can use viral or, more recently, nonviral vector delivery of reprogramming genes. However, these transgenic therapies lack safety clearance when it comes to oncologic and teratogenic risks[26].
Lastly, stem cells associated to bio or nanotechnology can control the proliferation and differentiation into complex, viable 3D tissues. So we might be able to use biodegradable polymer scaffolds to promote cell growth and differentiation and formation of 3D structures. This could be useful in order to avoid scarring during the healing process.
Current literature regarding the clinical use of stem cells in rotator cuff tears is limited. Although in vivo animal studies have shown promising results to enhance tendon-to-bone healing, the use of stem cell therapy in rotator cuff should still be considered an experimental technique. Further basic and clinical research is needed.
P- Reviewer: Hanypsiak BT, Li SC, Liu L, Pixley J S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Gomoll AH, Katz JN, Warner JJ, Millett PJ. Rotator cuff disorders: recognition and management among patients with shoulder pain. Arthritis Rheum. 2004;50:3751-3761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford). 2004;43:131-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 315] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 3. | Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006;88:1699-1704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Harryman DT, Hettrich CM, Smith KL, Campbell B, Sidles JA, Matsen FA. A prospective multipractice investigation of patients with full-thickness rotator cuff tears: the importance of comorbidities, practice, and other covariables on self-assessed shoulder function and health status. J Bone Joint Surg Am. 2003;85-A:690-696. [PubMed] [Cited in This Article: ] |
| 5. | Yoo JH, Cho NS, Rhee YG. Effect of postoperative repair integrity on health-related quality of life after rotator cuff repair: healed versus retear group. Am J Sports Med. 2013;41:2637-2644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Kim HM, Caldwell JM, Buza JA, Fink LA, Ahmad CS, Bigliani LU, Levine WN. Factors affecting satisfaction and shoulder function in patients with a recurrent rotator cuff tear. J Bone Joint Surg Am. 2014;96:106-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96:99-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: Footprint contact characteristics for a transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16:461-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 287] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 9. | Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 10. | Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15:290-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 413] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 11. | Tashjian RZ, Hollins AM, Kim HM, Teefey SA, Middleton WD, Steger-May K, Galatz LM, Yamaguchi K. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010;38:2435-2442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 12. | Kobayashi M, Itoi E, Minagawa H, Miyakoshi N, Takahashi S, Tuoheti Y, Okada K, Shimada Y. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J Shoulder Elbow Surg. 2006;15:371-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Galatz LM, Sandell LJ, Rothermich SY, Das R, Mastny A, Havlioglu N, Silva MJ, Thomopoulos S. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006;24:541-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res. 2008;466:622-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Dines JS, Grande DA, Dines DM. Tissue engineering and rotator cuff tendon healing. J Shoulder Elbow Surg. 2007;16:S204-S207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Rodeo SA. Biologic augmentation of rotator cuff tendon repair. J Shoulder Elbow Surg. 2007;16:S191-S197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Murray DH, Kubiak EN, Jazrawi LM, Araghi A, Kummer F, Loebenberg MI, Zuckerman JD. The effect of cartilage-derived morphogenetic protein 2 on initial healing of a rotator cuff defect in a rat model. J Shoulder Elbow Surg. 2007;16:251-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Maniscalco P, Gambera D, Lunati A, Vox G, Fossombroni V, Beretta R, Crainz E. The “Cascade” membrane: a new PRP device for tendon ruptures. Description and case report on rotator cuff tendon. Acta Biomed. 2008;79:223-226. [PubMed] [Cited in This Article: ] |
| 19. | Derwin KA, Codsi MJ, Milks RA, Baker AR, McCarron JA, Iannotti JP. Rotator cuff repair augmentation in a canine model with use of a woven poly-L-lactide device. J Bone Joint Surg Am. 2009;91:1159-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37:2126-2133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 21. | Isaac C, Gharaibeh B, Witt M, Wright VJ, Huard J. Biologic approaches to enhance rotator cuff healing after injury. J Shoulder Elbow Surg. 2012;21:181-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Milano G, Saccomanno MF, Careri S, Taccardo G, De Vitis R, Fabbriciani C. Efficacy of marrow-stimulating technique in arthroscopic rotator cuff repair: a prospective randomized study. Arthroscopy. 2013;29:802-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26:269-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 24. | Sánchez Márquez JM, Martínez Díez JM, Barco R, Antuña S. Functional results after arthroscopic repair of massive rotator cuff tears; influence of the application platelet-rich plasma combined with fibrin. Revista Española De Cirugía Ortopédica Y Traumatología (English Edition). 2011;55:282-287. [Cited in This Article: ] |
| 25. | Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20:518-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 327] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 26. | Nixon AJ, Watts AE, Schnabel LV. Cell- and gene-based approaches to tendon regeneration. J Shoulder Elbow Surg. 2012;21:278-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Lui P, Zhang P, Chan K, Qin L. Biology and augmentation of tendon-bone insertion repair. J Orthop Surg Res. 2010;5:59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Thomopoulos S, Genin GM, Galatz LM. The development and morphogenesis of the tendon-to-bone insertion - what development can teach us about healing. J Musculoskelet Neuronal Interact. 2010;10:35-45. [PubMed] [Cited in This Article: ] |
| 29. | Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg. 1998;7:599-605. [PubMed] [Cited in This Article: ] |
| 30. | Diaz Heredia J, Ruiz Ibán M. A, Martinez Botas M, Aranda Romero F, Moros Marco S, Gonzalez Lizan F, Martin Gamero FJP. Respuesta a la lesión del manguito de los rotadores:Variación en la expresión de factores de crecimiento durante las primeras 8 semanas poslesión. Estudio experimental en ratas. Cuadernos De Artroscopia. 2012;19:31-38. [Cited in This Article: ] |
| 31. | Cowin AJ, Holmes TM, Brosnan P, Ferguson MW. Expression of TGF-beta and its receptors in murine fetal and adult dermal wounds. Eur J Dermatol. 2001;11:424-431. [PubMed] [Cited in This Article: ] |
| 32. | Galatz L, Rothermich S, VanderPloeg K, Petersen B, Sandell L, Thomopoulos S. Development of the supraspinatus tendon-to-bone insertion: localized expression of extracellular matrix and growth factor genes. J Orthop Res. 2007;25:1621-1628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N, Deng JM, Zhang Z, Martin JF, Behringer RR, Nakamura T. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci USA. 2005;102:14665-14670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 414] [Cited by in F6Publishing: 423] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 34. | Chen X, Yin Z, Chen JL, Shen WL, Liu HH, Tang QM, Fang Z, Lu LR, Ji J, Ouyang HW. Force and scleraxis synergistically promote the commitment of human ES cells derived MSCs to tenocytes. Sci Rep. 2012;2:977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 35. | Garofalo R, Cesari E, Vinci E, Castagna A. Role of metalloproteinases in rotator cuff tear. Sports Med Arthrosc. 2011;19:207-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Del Buono A, Oliva F, Longo UG, Rodeo SA, Orchard J, Denaro V, Maffulli N. Metalloproteases and rotator cuff disease. J Shoulder Elbow Surg. 2012;21:200-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 37. | Castagna A, Cesari E, Garofalo R, Gigante A, Conti M, Markopoulos N, Maffulli N. Matrix metalloproteases and their inhibitors are altered in torn rotator cuff tendons, but also in the macroscopically and histologically intact portion of those tendons. Muscles Ligaments Tendons J. 2013;3:132-138. [PubMed] [Cited in This Article: ] |
| 38. | Bedi A, Fox AJ, Kovacevic D, Deng XH, Warren RF, Rodeo SA. Doxycycline-mediated inhibition of matrix metalloproteinases improves healing after rotator cuff repair. Am J Sports Med. 2010;38:308-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 39. | Dean BJ, Franklin SL, Carr AJ. A systematic review of the histological and molecular changes in rotator cuff disease. Bone Joint Res. 2012;1:158-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Kovacevic D, Fox AJ, Bedi A, Ying L, Deng XH, Warren RF, Rodeo SA. Calcium-phosphate matrix with or without TGF-β3 improves tendon-bone healing after rotator cuff repair. Am J Sports Med. 2011;39:811-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 41. | Chung SW, Song BW, Kim YH, Park KU, Oh JH. Effect of platelet-rich plasma and porcine dermal collagen graft augmentation for rotator cuff healing in a rabbit model. Am J Sports Med. 2013;41:2909-2918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Seeherman HJ, Archambault JM, Rodeo SA, Turner AS, Zekas L, D’Augusta D, Li XJ, Smith E, Wozney JM. rhBMP-12 accelerates healing of rotator cuff repairs in a sheep model. J Bone Joint Surg Am. 2008;90:2206-2219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 43. | Chhabra A, Tsou D, Clark RT, Gaschen V, Hunziker EB, Mikic B. GDF-5 deficiency in mice delays Achilles tendon healing. J Orthop Res. 2003;21:826-835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Thomopoulos S, Harwood FL, Silva MJ, Amiel D, Gelberman RH. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg Am. 2005;30:441-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 45. | Chaudhury S, Carr AJ. Lessons we can learn from gene expression patterns in rotator cuff tears and tendinopathies. J Shoulder Elbow Surg. 2012;21:191-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Chahal J, Van Thiel GS, Mall N, Heard W, Bach BR, Cole BJ, Nicholson GP, Verma NN, Whelan DB, Romeo AA. The role of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy. 2012;28:1718-1727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 47. | Sánchez M, Anitua E, Orive G, Padilla S. A biological approach to orthopaedic surgery: are they lost in translation? Arthroscopy. 2013;29:969-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2013;12:CD010071. [Cited in This Article: ] |
| 49. | Ahmad Z, Brooks R, Kang SN, Weaver H, Nunney I, Tytherleigh-Strong G, Rushton N. The effect of platelet-rich plasma on clinical outcomes in lateral epicondylitis. Arthroscopy. 2013;29:1851-1862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Raeissadat SA, Sedighipour L, Rayegani SM, Bahrami MH, Bayat M, Rahimi R. Effect of Platelet-Rich Plasma (PRP) versus Autologous Whole Blood on Pain and Function Improvement in Tennis Elbow: A Randomized Clinical Trial. Pain Res Treat. 2014;2014:191525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Ruiz-Moneo P, Molano-Muñoz J, Prieto E, Algorta J. Plasma rich in growth factors in arthroscopic rotator cuff repair: a randomized, double-blind, controlled clinical trial. Arthroscopy. 2013;29:2-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 52. | Gumina S, Campagna V, Ferrazza G, Giannicola G, Fratalocchi F, Milani A, Postacchini F. Use of platelet-leukocyte membrane in arthroscopic repair of large rotator cuff tears: a prospective randomized study. J Bone Joint Surg Am. 2012;94:1345-1352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 53. | Anitua E, Sánchez M, Orive G. The importance of understanding what is platelet-rich growth factor (PRGF) and what is not. J Shoulder Elbow Surg. 2011;20:e23-e24; author reply e24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Lendeckel S, Jödicke A, Christophis P, Heidinger K, Wolff J, Fraser JK, Hedrick MH, Berthold L, Howaldt HP. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32:370-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 455] [Cited by in F6Publishing: 432] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 55. | Ruiz-Ibán MÁ, Díaz-Heredia J, García-Gómez I, Gonzalez-Lizán F, Elías-Martín E, Abraira V. The effect of the addition of adipose-derived mesenchymal stem cells to a meniscal repair in the avascular zone: an experimental study in rabbits. Arthroscopy. 2011;27:1688-1696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 56. | Mazzocca AD, McCarthy MB, Chowaniec D, Cote MP, Judson CH, Apostolakos J, Solovyova O, Beitzel K, Arciero RA. Bone marrow-derived mesenchymal stem cells obtained during arthroscopic rotator cuff repair surgery show potential for tendon cell differentiation after treatment with insulin. Arthroscopy. 2011;27:1459-1471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 57. | García-Gómez I, Elvira G, Zapata AG, Lamana ML, Ramírez M, Castro JG, Arranz MG, Vicente A, Bueren J, García-Olmo D. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2010;10:1453-1468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 58. | Lim JK, Hui J, Li L, Thambyah A, Goh J, Lee EH. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20:899-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 59. | Ogawa R, Mizuno H, Hyakusoku H, Watanabe A, Migita M, Shimada T. Chondrogenic and osteogenic differentiation of adipose-derived stem cells isolated from GFP transgenic mice. J Nippon Med Sch. 2004;71:240-241. [PubMed] [Cited in This Article: ] |
| 60. | Seo MJ, Suh SY, Bae YC, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 401] [Cited by in F6Publishing: 388] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 61. | Brzoska M, Geiger H, Gauer S, Baer P. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem Biophys Res Commun. 2005;330:142-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 62. | Kida Y, Morihara T, Matsuda K, Kajikawa Y, Tachiiri H, Iwata Y, Sawamura K, Yoshida A, Oshima Y, Ikeda T. Bone marrow-derived cells from the footprint infiltrate into the repaired rotator cuff. J Shoulder Elbow Surg. 2013;22:197-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 63. | Gulotta LV, Kovacevic D, Montgomery S, Ehteshami JR, Packer JD, Rodeo SA. Stem cells genetically modified with the developmental gene MT1-MMP improve regeneration of the supraspinatus tendon-to-bone insertion site. Am J Sports Med. 2010;38:1429-1437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 64. | Gulotta LV, Kovacevic D, Packer JD, Ehteshami JR, Rodeo SA. Adenoviral-mediated gene transfer of human bone morphogenetic protein-13 does not improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39:180-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 65. | Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39:1282-1289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 66. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 67. | Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5703] [Cited by in F6Publishing: 5564] [Article Influence: 241.9] [Reference Citation Analysis (0)] |
| 68. | Gimble JM, Grayson W, Guilak F, Lopez MJ, Vunjak-Novakovic G. Adipose tissue as a stem cell source for musculoskeletal regeneration. Front Biosci (Schol Ed). 2011;3:69-81. [PubMed] [Cited in This Article: ] |
| 69. | Bacou F, el Andalousi RB, Daussin PA, Micallef JP, Levin JM, Chammas M, Casteilla L, Reyne Y, Nouguès J. Transplantation of adipose tissue-derived stromal cells increases mass and functional capacity of damaged skeletal muscle. Cell Transplant. 2004;13:103-111. [PubMed] [Cited in This Article: ] |
| 70. | Mizuno H. Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. J Nippon Med Sch. 2009;76:56-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 71. | Oh JH, Chung SW, Kim SH, Chung JY, Kim JY. 2013 Neer Award: Effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elbow Surg. 2014;23:445-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 72. | Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M, Péault B, Cummins J, Huard J. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc. 2008;3:1501-1509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 73. | Pelinkovic D, Lee JY, Engelhardt M, Rodosky M, Cummins J, Fu FH, Huard J. Muscle cell-mediated gene delivery to the rotator cuff. Tissue Eng. 2003;9:143-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Shen W, Chen J, Yin Z, Chen X, Liu H, Heng BC, Chen W, Ouyang HW. Allogenous tendon stem/progenitor cells in silk scaffold for functional shoulder repair. Cell Transplant. 2012;21:943-958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 75. | Beitzel K, McCarthy MB, Cote MP, Russell RP, Apostolakos J, Ramos DM, Kumbar SG, Imhoff AB, Arciero RA, Mazzocca AD. Properties of biologic scaffolds and their response to mesenchymal stem cells. Arthroscopy. 2014;30:289-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 76. | Gimble JM, Bunnell BA, Guilak F. Human adipose-derived cells: an update on the transition to clinical translation. Regen Med. 2012;7:225-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 77. | Beitzel K, McCarthy MB, Cote MP, Durant TJ, Chowaniec DM, Solovyova O, Russell RP, Arciero RA, Mazzocca AD. Comparison of mesenchymal stem cells (osteoprogenitors) harvested from proximal humerus and distal femur during arthroscopic surgery. Arthroscopy. 2013;29:301-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 78. | Tsai CC, Huang TF, Ma HL, Chiang ER, Hung SC. Isolation of mesenchymal stem cells from shoulder rotator cuff: a potential source for muscle and tendon repair. Cell Transplant. 2013;22:413-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 79. | Randelli P, Conforti E, Piccoli M, Ragone V, Creo P, Cirillo F, Masuzzo P, Tringali C, Cabitza P, Tettamanti G. Isolation and characterization of 2 new human rotator cuff and long head of biceps tendon cells possessing stem cell-like self-renewal and multipotential differentiation capacity. Am J Sports Med. 2013;41:1653-1664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 80. | Utsunomiya H, Uchida S, Sekiya I, Sakai A, Moridera K, Nakamura T. Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. Am J Sports Med. 2013;41:657-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 81. | Ellera Gomes JL, da Silva RC, Silla LM, Abreu MR, Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc. 2012;20:373-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 82. | Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 810] [Cited by in F6Publishing: 987] [Article Influence: 49.4] [Reference Citation Analysis (0)] |