Published online Aug 26, 2010. doi: 10.4252/wjsc.v2.i4.67
Revised: June 26, 2010
Accepted: July 3, 2010
Published online: August 26, 2010
Mesenchymal stem cells (MSCs) are non-hematopoietic stem cells with the capacity to differentiate into tissues of both mesenchymal and non-mesenchymal origin. MSCs can differentiate into osteoblastic, chondrogenic, and adipogenic lineages, although recent studies have demonstrated that MSCs are also able to differentiate into other lineages, including neuronal and cardiomyogenic lineages. Since their original isolation from the bone marrow, MSCs have been successfully harvested from many other tissues. Their ease of isolation and ex vivo expansion combined with their immunoprivileged nature has made these cells popular candidates for stem cell therapies. These cells have the potential to alter disease pathophysiology through many modalities including cytokine secretion, capacity to differentiate along various lineages, immune modulation and direct cell-cell interaction with diseased tissue. Here we first review basic features of MSC biology including MSC characteristics in culture, homing mechanisms, differentiation capabilities and immune modulation. We then highlight some in vivo and clinical evidence supporting the therapeutic roles of MSCs and their uses in orthopedic, autoimmune, and ischemic disorders.
- Citation: Rastegar F, Shenaq D, Huang J, Zhang W, Zhang BQ, He BC, Chen L, Zuo GW, Luo Q, Shi Q, Wagner ER, Huang E, Gao Y, Gao JL, Kim SH, Zhou JZ, Bi Y, Su Y, Zhu G, Luo J, Luo X, Qin J, Reid RR, Luu HH, Haydon RC, Deng ZL, He TC. Mesenchymal stem cells: Molecular characteristics and clinical applications. World J Stem Cells 2010; 2(4): 67-80
- URL: https://www.wjgnet.com/1948-0210/full/v2/i4/67.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v2.i4.67
Mesenchymal stem cells (MSCs) are a heterogeneous population of cells with the potential to differentiate into diverse somatic lineages. They were originally described by Friedentstein and colleagues 40 years ago as adherent cells with a fibroblast-like appearance capable of differentiating into osteocytes, chondrocytes, adipocytes, tenocytes and myocytes[1-3]. In recent years MSCs have attracted significant attention for their potential role in elucidating differentiation pathways, promoting tissue engineering and function as gene vectors and immunomodulators in autoimmune diseases.
Stem cells are defined by their ability to remain undifferentiated for a prolonged period while retaining the potential to differentiate along one lineage (unipotent), multiple lineages (multipotent) or into all three germ layers (pluripotent)[4]. While MSCs were initially defined by their ability to differentiate into cells of mesodermal origin, recent studies have provided support for their capacity to differentiate into cells from all three germ layers[5]. In addition, MSCs have other characteristics making them attractive modalities in treating human disease. These cells are described as MHC II negative cells, lacking co-stimulatory molecules such as CD40, CD80 and CD86, which permits allogenic transplantation without immunosuppression[6]. Furthermore, they can be easily isolated from an autologous source, enabling easy accessibility for therapeutic intent. MSCs can provide therapeutic benefit through the secretion of specific cytokines, ex vivo genetic modification and direct cell-cell contact. As such, these cells have been studied for their use in diverse diseases ranging from genetic disorders to tissue ischemia. In this review we summarize the current understanding of MSC biology and conclude by revealing some relevant clinical applications.
After their initial isolation from humans, MSCs have since been successfully harvested from many other species including: mouse, rat, dog and horse[6-14]. They have also been isolated from almost every type of tissue, including: periosteum, brain, liver, bone marrow, adipose, skeletal muscle, amniotic fluid and hair follicle[15-24]. When harvested from the bone marrow, MSCs make up a minute fraction of nucleated cells and account for approximately 0.001%-0.01% of all cells in each aspirate, depending on the technique[25]. However, the therapeutic application of MSCs often requires a large number of cells, which requires ex vivo expansion post-harvest. Of note, MSCs have also been isolated from pathologic sites such as joints damaged by rheumatoid arthritis and in such cases they demonstrate characteristic up-regulation of BMP receptors. While cells isolated from various tissues share many similar characteristics, they exhibit minor differences in their expression profile and differentiation potential[26].
In order to utilize MSCs in a translational fashion, it is important to expand these cells ex vivo in order to obtain sufficient quantities for therapeutic uses. While stem cells are capable of continuous regeneration and expansion throughout an individual’s life, they demonstrate limited proliferation and differentiation capacity in an ex-vivo setting[27]. Mesenchymal stem cell capacity to expand is highly variable, even amongst two samples from the same patient[27]. While still a source of debate, some studies have also suggested that MSCs have the capacity to undergo malignant transformation in vitro[28]. This variability has posed a challenge in comparing data from different groups. Numerous factors including culture parameters such as nutritional level, cell confluence, oxygen level, number of passages and plastic surface quality influence MSC behavior[29]. Vacanti et al[30] examined passage number and its effect on MSC characteristics. They compared early (< 5 passages) to late MSCs (> 15 passages) and demonstrated that late MSCs had characteristics associated with cell aging as depicted by actin accumulation and reduced substrate adherence[30]. Furthermore, early MSCs remain pluripotent, while late MSCs had limited differentiation capacity[30]. In addressing this concern, investigators have attempted to study the stem cell niche in hopes of mimicking this environment in an ex-vivo setting to allow for more predictable cellular behavior.
One key difference between the culture environment and the in vivo setting is the amount of oxygen to which the stem cells are exposed. Even though oxygen levels amongst various tissues differ greatly, its concentration is always significantly less than atmospheric oxygen levels[31]. Bone marrow has a characteristically hypoxic environment with oxygen concentrations very similar to ischemic tissue[32]. MSCs that are cultured in hypoxic environments demonstrate greater expansion and differentiation potential. Some studies have suggested that these characteristics may in part relate to the up-regulation of telomerase activity in cells cultured in hypoxic conditions[33]. These observations suggest that MSCs utilize low oxygen environments in vivo to proliferate and renew, differentiating only when they approach blood vessels that expose them to higher oxygen tension.
Telomere length is another important factor that regulates differentiation and proliferation capacity. The in vitro culture environment causes significant telomere shortening, accounting for the loss of 100 bps per passage as compared to the 17 bps lost per year in vivo[34]. Studies have also shown that loss of telomerase activity prevents MSC differentiation, while over-expression of telomerase enables cells to maintain self-renewal and multipotential characteristics over a 3-year culture period[35,36]. Genetically engineered cells designed to over-express telomerase have also shown greater resistance to oxidative stress through the up-regulation of stress response genes[37]. Asymmetric cell division can also cause loss of multipotentiality by diluting stem cells[38]. To address this concern, Lee et al[39] successfully utilized the purine nucleoside xanthosine to suppress asymmetric cell division in hepatically-derived stem cells, which led to the retention of their differentiation capacity and inhibition of senescence.
Kinetic studies support 3 phases in MSC growth: (1) an initial lag phase lasting 3-5 d followed by (2) rapid expansion and finally, and (3) a stationary phase[40]. Transition from the lag phase to rapid expansion is initiated by an increase in secretion of dickkopf-1 (Dkk-1), an inhibitor of the Wnt signaling cascade[40,41]. This results in decreased β-catenin levels, causing decreased cell proliferation[40]. In addition, the interaction between epidermal growth factor receptor-1 (HER-1) and heparin-binding epidermal growth factor (HB-EGF) promotes MSC proliferation and inhibits differentiation in various selective media[42].
To date, no cellular markers or receptors have been found to be unique to MSCs. In order to facilitate a more unified approach to studying MSC biology, the International Society of Cryotherapy has devised three criteria needed to identify MSCs: (1) plastic adherence of the isolated cells in culture; (2) expression of cluster of differentiation (CD) markers such as CD105, CD73, and CD90 in > 95% of the culture with absent expression of markers including CD34, CD45, CD14 or CD11B, CD79A or CD19 and human leukocyte antigen-DR (HLA-DR) in > 95% of the culture; and 3) capacity to differentiate into osteocytes, adipocytes and chondrocytes[43].
In addition, with minor differences in expression patterns from one tissue source to another, all MSCs express embryonic cell markers such as Oct4, Nanog, and stage-specific embryonic antigen-4 (SSEA-4)[44]. Further variation in MSC characteristics has been associated with the age of the donor, with a direct correlation exiting between advanced age and decreased osteogenic potential. This fact may in part contribute to disorders, such as osteoporosis, seen primarily in the aging population[45]. A similar decrease in cell number as a function of age has also been documented in satellite cells[46]. In addition, MSCs isolated from older donors demonstrate lower proliferation potential which may provide an explanation for the reduced healing capacity observed in older patients[47].
MSCs possess a unique capacity to home toward injured tissue[48]. While the exact mechanism underlying MSC homing is not yet fully understood, some studies have shed light on factors that may govern MSC trafficking. Rochefort et al[49] demonstrated a 15-fold increase in the pool of circulating MSCs when rats were placed in hypoxic chamber over a 3-wk period. This increase in circulating cell number was specific to MSCs, while the number of other hematopoietic precursors remained unchanged. Studies suggest that such hypoxic induced cell trafficking may, in part, be a function of a Matrix metalloproteinase (MMP)-dependent pathway[50]. Stromal cell-derive factor-1(SDF-1) also plays a critical role in MSC recruitment and tissue regeneration through its selective expression at sites of injury[51,52]. Ceradini and colleagues demonstrated that recruitment of CXC chemokine receptor-4 (CXCR-4) positive progenitor cells to injured tissue is mediated by hypoxia-inducible factor-1 (HIF-1) which promotes over-expression of SDF-1 in a gradient proportional to tissue hypoxia[32]. Age dependent decline in HIF-1 expression has also been documented and this may contribute to the impaired ability of MSC homing and tissue repair observed in the elderly[53]. A CXCR4-SDF-1 dependent homing mechanism has also been seen with MSC migration toward sites of malignant growth[54,55], selectively targeting the hypoxic environment of cancer stroma. Frount-mediated clustering of chemokine receptor 2 (CCR2) has also been shown to be critical for the reorganization of cytoskeleton during cellular migration[56].
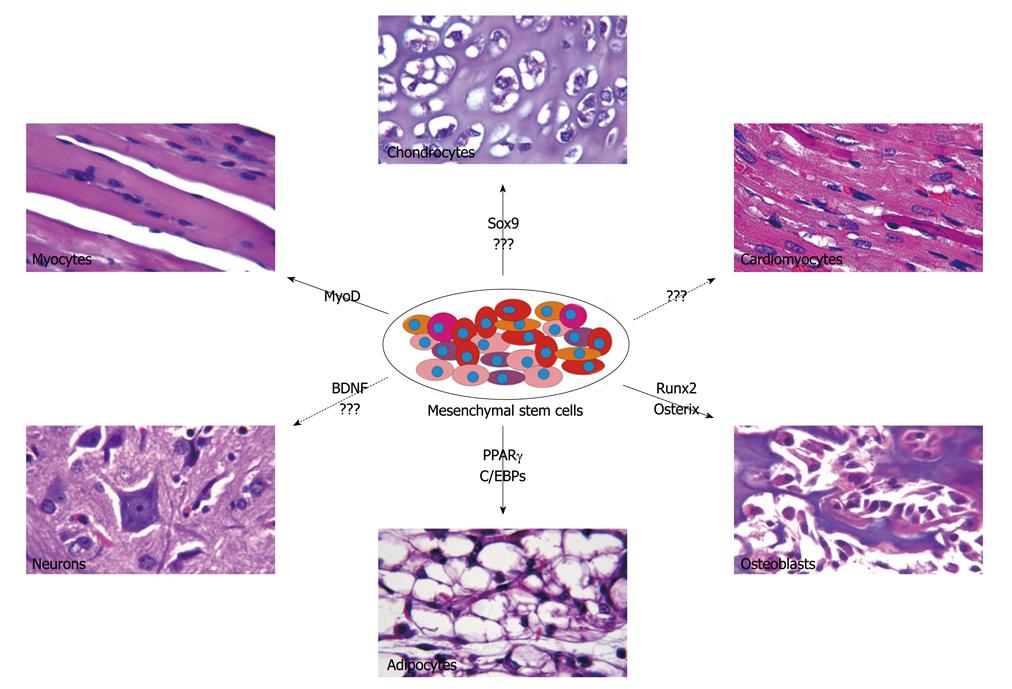
MSC potency extends beyond the conventional mesodermal lineages and includes differentiation into liver, kidney, muscle, skin, neural and cardiac cells[57]. The multipotency of MSCs has allowed for significant progress in our understanding of differentiation pathways of various lineages for tissue engineering and therapeutic purposes (Figure 1). Runt-related transcription factor 2 (Runx2) has been considered as a master regulatory gene responsible for early osteogenic differentiation[58]. Runx2 acts synergistically with transforming growth factor-β (TGFβ) to up-regulate expression of interleukin 11 (IL-11), which reduces adipogenesis while promoting chondrocytic and osteoblastic differentiation[59]. The Runx2 inhibition of adipogenesis is an effect modulated by direct interaction with peroxisome proliferator-activated receptor γ (PPARγ), an adipogenic inducing factor[60]. While Runx2 acts early to promote osteoblastic differentiation, osterix, another important osteogenic inducer, suppresses chondrogenesis and promotes osteoblastic differentiation at a later stage. Low levels of osterix are sufficient to inhibit chondrogenesis while a high expression level is necessary for osteogenic differentiation[61]. Furthermore, ex vivo MSCs have successfully differentiated into osteoblasts under exposure to various stimuli including growth factors such as bone morphogenetic proteins (BMPs) and Wnts[1,3,41,62-68], magnetic field stimulation[69] and culture in osteogenic media supplemented by dexamethasone and ascorbate[70,71]. The selective capability to promote osteogenic differentiation has vast clinical implications.
Chondrogenesis is a multi-step cellular event which requires commitment of MSCs, followed by their aggregation and differentiation into chondrocytes[72]. In-vitro differentiation of MSCs into a chondrogenic lineage has been studied through exposure to growth factors, co-culture with cartilage or nucleus pulposus and overexpression of specific genes such as SRY-box 9 (Sox9) to promote chondrocytic differentiation[73,74]. Sox9 cooperates with its downstream proteins Sox 5 and Sox6 to promote chondroycte proliferation, maturation and matrix formation[72]. Overexpression of Sox5, Sox 6 and Sox 9 in cultured cells[75] and ectopic expression of Sox9 in mice induce the expression of type II collagen[76,77]. Furthermore, TGFβ has been shown to play an important role in chondrogenic differentiation, an effect that is synergistically enhanced when co-administered with BMP-2[73,78]. In addition, previous studies had suggested a lower chondrogenic potential in adipose derived MSCs, a characteristic that was overcome by the up-regulation of TGFβ receptor I expression[79]. The ability to promote chondrogenic differentiation in MSCs has significant clinical implication for treating conditions such as intervertebral disc degeneration[80].
MSC capacity to differentiate into an adipogenic lineage has been extensively studied. PPAR-γ plays a critical role in this process by regulating the function of many adipocyte specific genes[81]. In addition, PPARγ interacts with members of the CCAAT/enhancer binding protein (C/EBP) family to regulate adipogenesis[82]. Cells can also be induced to undergo adipogenesis through exposure to exogenous factors or by culturing them in adipogenic media containing insulin and dexamethasone[62,83,84]. Factors critical in adipogenesis have been viewed as candidates for treatment of obesity and related disorders.
MSCs also possess the ability to differentiate across various lineages when exposed to certain chemical environments. Unlike the mechanism responsible for growth factor induced differentiation, the pathways governing chemical mediated differentiation is poorly understood but is routinely used in the in-vitro setting. Adipogenic media consists of dexamethasone, insulin, isobutylmethylxanthine, and indomethacin, which in combination lead to increased expression of PPAR-γ and other adipose specific factors such as lipoprotein lipase[25]. Treatment of MSCs with 5-azacytidine promotes cardiomyocyte differentiation and leads to improved cardiac function in the swine myocardial infarction model[11]. When incubated with nicotinamide and beta-mercaptoethanol, MSCs undergo islet cell differentiation and modulate glucose levels in diabetic rat models[85].
The ability of MSCs to modulate immune responses was first described by Bartholomew and colleagues, who demonstrated that injection of allogeneic MSCs prolonged skin graft survival in baboons[86]. Both in vivo and in vitro studies have provided support for the immunomodulatory role of MSCs. However, the mechanism underlying this immunomodulation is still not fully understood. Interestingly, MSCs have both immune enhancing and suppressing capability. They can promote immune function by serving as antigen presenting cells (APCs) through an autocrine interferon-γ (IFN-γ) dependent pathway. However, when the level of IFN-γ increases above a threshold, it directly inhibits antigen presentation and promotes immune suppression[87]. This narrow window of immune activity suggests that MSCs may provide protection against foreign antigens while limiting damage caused by an exacerbated inflammatory response. IFN-γ promotes MSC induced immune suppression by up-regulating B7-H1, an inhibitory surface molecule on stem cells, suggesting that cell-cell contact is important for immune function of MSCs[88]. While cell-to-cell contact plays a significant role in immune modulation, secretion of soluble factors is also critical to MSCs’ immune-regulatory role[89,90].
MSCs are capable of interacting with various cell lines responsible for mounting an immune reaction. They secrete soluble factors that arrest B-cells in the G0/G1 phase, inhibit B-cell differentiation, and impair B-cell chemotaxis[89]. They modulate monocyte function in a contact independent process through IL1-β secretion, which promotes MSC TGFβ1 expression, leading to inhibition of alloreactive T-cells and the down-regulation of activation markers such as CD25, CD38 and CD 69[91]. Furthermore, they modulate function of dendritic cells, NK cells and T-cells[92-95].
MSC–mediated T-cell suppression is a complex process with many inconsistent reports found in the literature. Nitric oxide (NO) has been shown to play an important role in this process. Sato et al[96] showed that MSCs produce NO in the presence of CD4+ and CD8+ T cells, resulting in the suppression of Stat5 phosphorylation and of T-cell proliferation. This suppression was reversed when either prostaglandin or NO synthase (NOS) was inhibited, leading to T-cell proliferation[96]. In addition, Ren and colleagues successfully showed that the concomitant presence of IFN-γ and either tumor necrosis factor-α (TNF-α), IL1-α or IL1-β was sufficient to promote MSC immunosuppression[97]. Their effect was mediated by the increased expression of chemokines and inducible nitric oxide synthase (iNOS) by MSCs, suggesting that pro-inflammatory cytokines are essential in promoting this immune function[97]. MSCs’ ability to inhibit allogenic T-cell has also been shown to be partly dependent on indoleamine 2,3-dioxygenase (IDO) mediated tryptophan catabolism[98]. A better understanding of the complex pathways responsible for the immunomodulatory role of MSCs will enable more effective therapeutic applications for immune diseases refractory to today’s treatment protocols.
Effectiveness of mesenchymal stem cell administration has been studied in various in vivo disease models (Table 1), some of which will be mentioned in this review. In addition, we will describe some of the currently reported uses of MSC therapy in the clinical setting (Table 2) and highlight their effectiveness.
| Animal | Disease model | Outcome |
| Baboon | Skin graft transplantation | Prolonged skin graft survival[169] |
| Mouse | GvHD | Prevention of GvHD[124,170] |
| EAE | Prevention of EAE development and improved functional recovery[129,171] | |
| CIA | Improved arthritic symptoms[172] | |
| NOD | Prevent T-cell mediated beta-cell destruction[173] | |
| SLE | Improved symptoms, serological markers and renal function[137] | |
| Lung injury | Decreased severity of endotoxin induced lung injury and improved survival[174] | |
| Rat | Glomerulonephritis | Accelerate glomerular healing[175] |
| Critical size defect | Improved healing and function[176] | |
| Experimental colitis | Improved survival and healing[177] | |
| Dilated rat cardiomyopathy | Improved cardiac function[178] | |
| Heart transplantation | Participate in tissue repair by giving rise to myofibroblasts and cardiomyocytes[179] | |
| Myocardial infarction | Improved cardiac function and survival[143] | |
| Cerebral ischemia | Reduced gross lesion volume and improved functional recovery[151] |
| Indications | Source | Rout of administration | Outcome |
| Myocardial infarction | Allogenic BM | IV | Increased GSS and EF[148] |
| Autologous BM | Intracoronary | Improved LVF[149] | |
| Cartilage defect | Autologous BM | Direct site transplantation | Improved clinical symptom and coverage of defect[110] |
| Osteogenesis imperfecta | Allogenic BM | IV | Growth acceleration[117] |
| Fetal MSC | Intrauterine transplantation | Osteoblastic differentiation and reduced fracture[119] | |
| Critical size defect | Autologous BM | Scaffold loaded | Faster full recovery of limb function than bone graft[103] |
| MLD and hurler syndrome | Allogenic BM | IV | Improved nerve conduction velocity in MLD patient and increased bone mineral density[167] |
| Severe idiopathic aplastic anemia | Allogenic BM | IV | Improved stroma[168] |
| Crohn's disease | Adipose derived stem cell | Intralesional | Improved fistula and quality of life[136] |
The ability of MSCs to differentiate into osteoblasts, tenocytes and chondrocytes has attracted interest for their use in the orthopedic setting. One such application is the treatment of non-unions or critical size bone defects. Incomplete post-fracture healing of bone longer than 6 mo is referred to as non-union and varies in incidence from 5%-20% depending on fracture type[98-100]. Currently, the mainstay of therapy for critical defects is autologous bone-grafting, which has disadvantages including limited availability and significant donor site morbidity. Stem cell therapy provides an alternative approach enabling bone formation locally at defect sites. MSCs loaded onto a porous ceramic cylinder provide significant healing potential in critical size defects in the canine model[101]. Cells modified to express osteoinductive factors also demonstrate enhanced bone healing at defect sites[102]. Our group has recently demonstrated that BMP-9 is one of the most osteogenic factors in promoting ectopic bone formation from MSCs[68]. Clinical trials also support the therapeutic benefit of MSC therapy in enhancing bone healing.
Quarto et al[103] have utilized autologous MSCs loaded onto macroporous hydroxyapatite scaffolds to demonstrate full limb functional recovery in a significantly shorter period than for traditional bone grafting. MSC-engineered bone demonstrated significant durability when used for treatment of critical size defects in long bones[104]. The therapeutic efficacy of MSC promotion of bone healing is directly correlated with the number of progenitor cells available in the graft[105]. The unique capability of MSCs to promote bone healing combined with their ease of isolation provides a more effective therapy with less morbidity for patients suffering from critical size bone defects.
Traditionally, joint cartilage defects were managed through local injection of autologous chondrocyte suspensions expanded in vitro[106]. Currently, research is underway to delineate the role of MSCs in cartilage repair. Wakitani et al[107]
utilized MSCs to treat full-thickness articular cartilage defects in a rabbit model. The MSC treated group demonstrated total repair of subchondral bone 2 wk after implantation. MSC repair of full-thickness cartilage defects was found to be superior to tissue repair by chondrocytes, fibroblasts or human umbilical cord blood (hUCB) stem cells in relation to cell arrangement, subchondral bone remodeling, and integration with surrounding cartilage[108].
In comparison to animal studies, there are limited reports outlining the role of MSCs in promoting cartilage repair in the human clinical setting. Two patients with full thickness articular patellar defects experienced significant improvement in symptoms post autologous MSC transplantation with fibrocartilage repair[109]. The same group reported a similar therapeutic benefit in three different patients with nine defects in five knees[110]. In an another case report, autologous bone marrow transplantation in an athlete with a full-thickness articular cartilage defect of the femoral condyle led to significant improvement in symptoms and the resumption of previous activities[111]. Improvements in osteoarthritic knees treated by MSCs were also noted via histology and arthroscopy[112]. While these results are promising, our ability to utilize MSC therapy routinely for common clinical applications is limited by our incomplete understanding of their specific role and function.
Allogenic MSC transplantation has also shown to be beneficial in treating genetic bone disorders such as osteogenesis imperfecta and hypophosphatasia. Osteogenesis imperfecta (OI) is characterized by skeletal fragility and connective tissue alterations. The defect usually results from alteration in the production of type I collagen by osteoblasts, which manifests clinically as short stature, skeletal deformities and low density bones that are prone to fracture. Traditionally, this disorder had been treated through pharmacological means to enhance bone mass and decrease bone resorption[113,114]. However, the immunoprivileged nature of MSCs combined with their ability to undergo osteoblastic differentiation has made these cells highly favorable candidates for treatment of genetic disorders such as OI.
In vivo murine models of OI have demonstrated selective incorporation of MSCs in bony tissue with subsequently reduced fracture rates and increased bone strength[115]. In addition, these engrafted cells demonstrate higher efficiency in bone matrix formation[116]. Clinically, MSC therapy has led to enhanced growth and increased mineral content post-allogenic MSC transplantation[117,118]. Successful in utero-transplantation of MSCs has also been reported in a fetus with severe OI who after birth demonstrated regular growth and psychomotor development[119].
Hypophosphatasia, another genetic disorder of mesenchymal origin, is caused by a mutation in tissue non-specific alkaline phosphatase (TNALP). This syndrome has a highly variable clinical presentation, ranging from still birth without bone mineralization to early loss of teeth in the absence of skeletal symptoms. Limited clinical studies have demonstrated that cellular therapy utilizing allogenic MSCs will allow donor cells to engraft in the skeletal microenvironment, express sufficient TNALP and improve bone mineralization[120]. An 8-mo old suffering from severe hypophosphatasia with poor prognosis experienced significant clinical improvement 3 mo after receiving an allogenic bone marrow cell transplant from a haplo-identical sibling. At last follow up, the patient at age six years was ambulatory without significant clinical symptoms[121]. Another 8-mo old female diagnosed with severe hypophosphatasia also experienced significant symptomatic and clinical improvement after allogenic MSC transplantation. At eight years, the patient now suffers from only a mild form of hypophosphatasia[120]. These promising outcomes open the door to new therapeutic modalities for diseases that have shown minimal response to current management protocols.
Autoimmune diseases have great clinical implications and are associated with significant patient morbidity. MSCs have been shown to inhibit immune response against minor histocompatibility antigens[122]. Animal models of Graft-versus-host disease (GVHD) have suggested that bone marrow or adipose-derived MSCs have the same immunosuppressive effect and lead to significant symptomatic improvement[123,124]. This immunomodulatory role was utilized to manage a patient with treatment-resistant grade IV GVHD who subsequently demonstrated significant clinical improvement[125]. In a phase II clinical trial on patients with steroid-resistant, severe, acute GVHD, MSC treatment led to lower transplantation related mortality and higher 2-year survival post-MSC transplantation[126].
Multiple sclerosis (MS) is a devastating disease with limited effective treatment protocols at present[127]. Given the minimal success in treatment of this disease, research has focused on identifying the therapeutic potential of MSC therapy in enhancing patients’ quality of life. Experimental Autoimmune Encephalomyelitis (EAE) is a murine model of MS, which enables study of the pathogenesis of this disease[128]. In vivo MSC treatment leads to T-cell anergy in the secondary lymphoid tissue in the absence of T-cell apoptosis[129]. Over expression of human ciliary neurotrophic factor (CNTF) enhances the therapeutic benefit of MSCs in EAE by inhibiting inflammation and stimulating oligodendrogenesis[129]. MSC transplantation also inhibits Th1 inflammatory response and promotes expression of anti-inflammatory cytokines in EAE mice[130]. There are limited clinical reports on the therapeutic role of MSC therapy in patients with MS; however, one pilot study demonstrated improvement in sensory, pyramidal and cerebral function in 6/10 patients without any side effects[131].
Joint destruction in rheumatoid arthritis (RA) is a T-cell mediated disease that results in significant patient morbidity. Collagen induced arthritis (CIA) is an animal model of RA that is widely used to delineate the pathogenesis of this disease[132]. MSC therapy leads to significant cytokine dependent improvement in arthritic symptoms in transplanted animals[133]. MSCs are able to prevent immune destruction of joint architecture by inhibiting collagen-reactive T-cells even after differentiation into chondrocytes[134]. To date no clinical trials have reported the role of MSC transplantation in patients suffering from RA.
In patients undergoing renal transplantation, administration of donor MSCs lead to a dose dependent immune suppression by inhibiting proliferation in alloreactive T-cells[135]. Furthermore, the therapeutic role of MSC has also been investigated in patients with Crohn’s disease. In a phase II clinical trial of patients with Crohn’s disease and complex perianal fistulas, MSC therapy lead to enhanced fistula healing and increased the quality of life in treated patients[136]. Therapeutic role of MSCs has also been documented in patients with systemic lupus erythematosus (SLE)[137]. While the above mentioned applications of MSC therapy are promising, significant strides in our understanding is needed prior to utilization of such therapy as a routine method of managing autoimmune diseases.
MSCs’ propensity to migrate toward ischemic tissue through a CXCR4-SDF-1 dependent pathway and their ability to proliferate and differentiate into different cell types has made them attractive modalities for treatment of ischemic injuries such as myocardial infarction and cerebral ischemia. Heart failure constitutes a significant medical problem that is associated with reduced quality of life, lower life expectancy and increased medical costs[138]. Current management is focused on symptomatic improvement but a means of reversing the changes associated with heart disease is still lacking. However, stem cell biology and regenerative medicine have introduced a unique method of replacing damaged cardiac tissue with healthy cells, as in vitro studies have successfully demonstrated MSC differentiation into cardiomyocytes[139,140].
MSC therapy post myocardial infarction (MI) can improve left ventricular function[141,142]. Miyahara et al[143] demonstrated that, in addition to improving cardiac function, MSC transplantation significantly enhanced survival rate in post-MI rats. Even though MSCs demonstrate the capacity to differentiate into cardiomyocytes, this ability is limited. Their beneficial effect has been attributed at least in part to their propensity for cytokine secretion. These angiogenic promoting factors protect the surrounding cells in the ischemic tissue[144] in a paracrine manner and can be synergistically enhanced when cells are genetically altered to express angiogenic and pro-survival factors[145-147]. In a randomized double-blinded study, patients receiving IV infusion of MSCs post-MI demonstrated significant enhancement in cardiovascular function[148]. Similar therapeutic benefit was reported in patients receiving intracoronary MSC administration compared to placebo[149]. The ability of MSCs to differentiate into cardiomyocytes, combined with their capacity to secrete cytokines that enhance tissue repair, will enable increased survival and quality of life by improving cardiac repair and function after myocardial infarction.
MSCs have the capacity to differentiate into neuron-like cells in the presence of epidermal growth factor (EGF) or bone-derived neurotrophic factor (BDNF)[150]. In addition, they have the ability to promote angiogenesis and tissue repair, which are key functions that play a beneficial role post-ischemic injury. In a rat model of middle cerebral occlusion, MSC administration led to significant reduction in gross lesion volume and improved functional recovery. These symptomatic improvements were more pronounced when MSCs where administered in a large dose immediately post ischemia as compared to temporally dispersed dosing[151]. MSC efficacy in this setting may rely upon their secretion of time-appropriate cytokines, as suggested by Omori et al[151]. MSCs also have the capacity to enhance proliferation of endogenous neural stem cells and protect newborn cells from the deleterious environment found at sites of ischemia.
In mice with embolic middle cerebral artery occlusion, MSCs successfully migrate, survive and differentiate into neuron-like cells after transplantation into the striatum, thus enhancing functional recovery[152]. In vivo studies have suggested that MSC therapy leads to early restoration of cerebral blood flow and blood brain barrier integrity post ischemic injury, which may play a part in their beneficial therapeutic role[153]. MSCs also facilitate axonal sprouting and remyelination after stroke[154,155]. While there are many modalities of MSC administration, intravenous transplantation is superior to striatal delivery when measured by long-term functional recovery in animal stroke models[156].
In a clinical trial, autologous transplantation of MSCs in 30 patients with middle cerbral artery (MCA) infarcts and severe neurological deficits resulted in significant improvement in the treated group without any adverse reactions after serial follow-up evaluations[157]. The safety associated with studies such as these set the stage for a more comprehensive exploration of the potential role of MSCs in humans. Hopefully, in the near future MSC therapy will lead to enhanced quality of life for patients whose everyday activities have been compromised by cerebral ischemia.
As previously mentioned, MSCs have the propensity to migrate to hypoxic environments such as ischemic tissue and tumor stroma[158]. Furthermore, the high local concentration of paracrine growth factors, including IL-8, transforming growth factor-ss1 (TGF-ss1) and vascular endothelial growth factor (VEGF) found at sites of tumor formation, selectively recruits MSCs to sites of tumor growth[159]. Such selective migration, combined with the capacity to act as vectors for gene therapy, has suggested that MSCs could be used to provide localized anti-neoplastic treatments while minimizing systemic side effects. Studeny et al[160]
utilized adenoviral transfected MSCs expressing INF-β to successfully suppress tumor growth and increase survival in a mouse xenograft model. Furthermore, inducible TRAIL-expressing MSCs have been shown to successfully suppress metastatic disease in the murine model[161]. Moreover, Khakoo et al[158] illustrated that MSCs can also exert their anti-neoplastic effect through direct cell-to-cell contact, which inhibits Akt activation in Kaposi sarcoma cells. This effect was abrogated using neutralizing antibody against E-cadherin[158]. MSCs help to slow tumor proliferation by promoting apoptosis and cell cycle arrest in G0/G1phase[162]. In addition, MSCs can directly enhance the immune response against cancer cells by taking up and presenting tumor associated antigens to enhance the CD8 mediated anti-tumor response[163].
While many studies have described MSCs’ anti-neoplastic characteristics, others have raised concern regarding ability of MSCs to promote tumor formation. MSCs may serve as important contributors to the cancer stroma, promoting cell survival and metastasis[164]. The active recruitment of MSCs to the site of tumor formation, combined with their pro-angiogenic characteristics, has suggested that these cells are capable of enhancing tumor growth[165]. Furthermore, MSCs have been found to act as precursors of cancers such as gastric carcinoma through their selective recruitment to sites of chronic inflammation followed by their ability to undergo metaplasia and subsequently dysplasia to become cancerous cells[166]. It’s hoped that improvement in our understanding of MSCs and their role in oncogenesis will enable more insight into cancer formation and also allow more innovative therapeutic approaches.
The characteristics of MSCs have attracted the attention of many researchers hoping to utilize the unique properties of these cells for diverse therapeutic applications. With their ability to differentiate into multiple lineages, migrate toward injured tissue, and propensity to secrete factors identified to be important in tissue repair, it is easy to imagine how a reduction in MSC number or functional capacity can result in impaired healing, as seen in the aging population. As our understanding of mesenchymal stem cell biology and other fields such as molecular genetics improves, it may be possible to genetically alter these “rescue cells” and enhance their capacity to heal common clinical diseases. In addition, their ability to modulate immune responses and specific homing capacity towards sites of malignant growth makes MSCs attractive cellular vehicles for the treatment of autoimmune disease and solid cancers. Their ease of accessibility combined with their immune-privileged nature makes these cells great therapeutic tools. While MSC therapy is very promising, the lack of unique phenotypic markers and inefficient extraction are factors currently limiting their use. With further advancement in our understanding of their biology and behavior during ex vivo expansion these cells will play an important role in managing disorders that lack effective treatment.
Peer reviewers: Guangwen Ren, PhD, Department of Molecular Genetics, Microbiology and Immunology, Robert Wood Johnson Medical School-UMDNJ, 675 Hoes Lane West, Piscataway, NJ 08854, United States; Chie-Pein Chen, MD, PhD, Professor, Division of High Risk Pregnancy, Mackay Memorial Hospital, 92 Sec. 2, Zhong-Shan North Road, Taipei 104, Taiwan, China
S- Editor Wang JL L- Editor Hughes D E- Editor Yang C
| 1. | Deng ZL, Sharff KA, Tang N, Song WX, Luo J, Luo X, Chen J, Bennett E, Reid R, Manning D. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001-2021. [Cited in This Article: ] |
| 2. | Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230-247. [Cited in This Article: ] |
| 3. | Luu HH, Song WX, Luo X, Manning D, Luo J, Deng ZL, Sharff KA, Montag AG, Haydon RC, He TC. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25:665-677. [Cited in This Article: ] |
| 4. | Young HE. Existence of reserve quiescent stem cells in adults, from amphibians to humans. Curr Top Microbiol Immunol. 2004;280:71-109. [Cited in This Article: ] |
| 5. | D'Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513-522. [Cited in This Article: ] |
| 6. | Igura K, Zhang X, Takahashi K, Mitsuru A, Yamaguchi S, Takashi TA. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy. 2004;6:543-553. [Cited in This Article: ] |
| 7. | Airey JA, Almeida-Porada G, Colletti EJ, Porada CD, Chamberlain J, Movsesian M, Sutko JL, Zanjani ED. Human mesenchymal stem cells form Purkinje fibers in fetal sheep heart. Circulation. 2004;109:1401-1407. [Cited in This Article: ] |
| 8. | Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003;89:1235-1249. [Cited in This Article: ] |
| 9. | Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Isolation and multilineage differentiation of bovine bone marrow mesenchymal stem cells. Cell Tissue Res. 2005;319:243-253. [Cited in This Article: ] |
| 10. | Devine SM, Bartholomew AM, Mahmud N, Nelson M, Patil S, Hardy W, Sturgeon C, Hewett T, Chung T, Stock W. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001;29:244-255. [Cited in This Article: ] |
| 11. | Moscoso I, Centeno A, López E, Rodriguez-Barbosa JI, Santamarina I, Filgueira P, Sánchez MJ, Domínguez-Perles R, Peñuelas-Rivas G, Domenech N. Differentiation "in vitro" of primary and immortalized porcine mesenchymal stem cells into cardiomyocytes for cell transplantation. Transplant Proc. 2005;37:481-482. [Cited in This Article: ] |
| 12. | Ringe J, Häupl T, Sittinger M. [Mesenchymal stem cells for tissue engineering of bone and cartilage]. Med Klin (Munich). 2003;98 Suppl 2:35-40. [Cited in This Article: ] |
| 13. | Santa María L, Rojas CV, Minguell JJ. Signals from damaged but not undamaged skeletal muscle induce myogenic differentiation of rat bone-marrow-derived mesenchymal stem cells. Exp Cell Res. 2004;300:418-426. [Cited in This Article: ] |
| 14. | Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150-156. [Cited in This Article: ] |
| 15. | Amoh Y, Li L, Campillo R, Kawahara K, Katsuoka K, Penman S, Hoffman RM. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci USA. 2005;102:17734-17738. [Cited in This Article: ] |
| 16. | Bjerknes M, Cheng H. Intestinal epithelial stem cells and progenitors. Methods Enzymol. 2006;419:337-383. [Cited in This Article: ] |
| 17. | Coles BL, Angénieux B, Inoue T, Del Rio-Tsonis K, Spence JR, McInnes RR, Arsenijevic Y, van der Kooy D. Facile isolation and the characterization of human retinal stem cells. Proc Natl Acad Sci USA. 2004;101:15772-15777. [Cited in This Article: ] |
| 18. | da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204-2213. [Cited in This Article: ] |
| 19. | Davis AA, Temple S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature. 1994;372:263-266. [Cited in This Article: ] |
| 20. | In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338-1345. [Cited in This Article: ] |
| 21. | Liu Z, Martin LJ. Pluripotent fates and tissue regenerative potential of adult olfactory bulb neural stem and progenitor cells. J Neurotrauma. 2004;21:1479-1499. [Cited in This Article: ] |
| 22. | Ringe J, Leinhase I, Stich S, Loch A, Neumann K, Haisch A, Häupl T, Manz R, Kaps C, Sittinger M. Human mastoid periosteum-derived stem cells: promising candidates for skeletal tissue engineering. J Tissue Eng Regen Med. 2008;2:136-146. [Cited in This Article: ] |
| 23. | Sinanan AC, Hunt NP, Lewis MP. Human adult craniofacial muscle-derived cells: neural-cell adhesion-molecule (NCAM; CD56)-expressing cells appear to contain multipotential stem cells. Biotechnol Appl Biochem. 2004;40:25-34. [Cited in This Article: ] |
| 24. | Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228. [Cited in This Article: ] |
| 25. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [Cited in This Article: ] |
| 26. | Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402-1416. [Cited in This Article: ] |
| 27. | Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275-281. [Cited in This Article: ] |
| 28. | Røsland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lønning PE. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331-5339. [Cited in This Article: ] |
| 29. | Barrilleaux B, Phinney DG, Prockop DJ, O'Connor KC. Review: ex vivo engineering of living tissues with adult stem cells. Tissue Eng. 2006;12:3007-3019. [Cited in This Article: ] |
| 30. | Vacanti V, Kong E, Suzuki G, Sato K, Canty JM, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol. 2005;205:194-201. [Cited in This Article: ] |
| 31. | Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220:562-568. [Cited in This Article: ] |
| 32. | Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858-864. [Cited in This Article: ] |
| 33. | D'Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971-2981. [Cited in This Article: ] |
| 34. | Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675-682. [Cited in This Article: ] |
| 35. | Abdallah BM, Haack-Sørensen M, Burns JS, Elsnab B, Jakob F, Hokland P, Kassem M. Maintenance of differentiation potential of human bone marrow mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene despite [corrected] extensive proliferation. Biochem Biophys Res Commun. 2005;326:527-538. [Cited in This Article: ] |
| 36. | Liu L, DiGirolamo CM, Navarro PA, Blasco MA, Keefe DL. Telomerase deficiency impairs differentiation of mesenchymal stem cells. Exp Cell Res. 2004;294:1-8. [Cited in This Article: ] |
| 37. | Armstrong L, Saretzki G, Peters H, Wappler I, Evans J, Hole N, von Zglinicki T, Lako M. Overexpression of telomerase confers growth advantage, stress resistance, and enhanced differentiation of ESCs toward the hematopoietic lineage. Stem Cells. 2005;23:516-29. [Cited in This Article: ] |
| 38. | Rambhatla L, Bohn SA, Stadler PB, Boyd JT, Coss RA, Sherley JL. Cellular Senescence: Ex Vivo p53-Dependent Asymmetric Cell Kinetics. J Biomed Biotechnol. 2001;1:28-37. [Cited in This Article: ] |
| 39. | Lee HS, Crane GG, Merok JR, Tunstead JR, Hatch NL, Panchalingam K, Powers MJ, Griffith LG, Sherley JL. Clonal expansion of adult rat hepatic stem cell lines by suppression of asymmetric cell kinetics (SACK). Biotechnol Bioeng. 2003;83:760-771. [Cited in This Article: ] |
| 40. | Gregory CA, Singh H, Perry AS, Prockop DJ. The Wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem. 2003;278:28067-28078. [Cited in This Article: ] |
| 41. | Luo J, Chen J, Deng ZL, Luo X, Song WX, Sharff KA, Tang N, Haydon RC, Luu HH, He TC. Wnt signaling and human diseases: what are the therapeutic implications? Lab Invest. 2007;87:97-103. [Cited in This Article: ] |
| 42. | Krampera M, Pasini A, Rigo A, Scupoli MT, Tecchio C, Malpeli G, Scarpa A, Dazzi F, Pizzolo G, Vinante F. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood. 2005;106:59-66. [Cited in This Article: ] |
| 43. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [Cited in This Article: ] |
| 44. | Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743-1751. [Cited in This Article: ] |
| 45. | D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115-1122. [Cited in This Article: ] |
| 46. | Gibson MC, Schultz E. Age-related differences in absolute numbers of skeletal muscle satellite cells. Muscle Nerve. 1983;6:574-580. [Cited in This Article: ] |
| 47. | Schäfer R, Knauf U, Zweyer M, Högemeier O, de Guarrini F, Liu X, Eichhorn HJ, Koch FW, Mundegar RR, Erzen I. Age dependence of the human skeletal muscle stem cell in forming muscle tissue. Artif Organs. 2006;30:130-140. [Cited in This Article: ] |
| 48. | Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160-169. [Cited in This Article: ] |
| 49. | Rochefort GY, Delorme B, Lopez A, Hérault O, Bonnet P, Charbord P, Eder V, Domenech J. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24:2202-2208. [Cited in This Article: ] |
| 50. | Annabi B, Lee YT, Turcotte S, Naud E, Desrosiers RR, Champagne M, Eliopoulos N, Galipeau J, Béliveau R. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells. 2003;21:337-347. [Cited in This Article: ] |
| 51. | Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697-703. [Cited in This Article: ] |
| 52. | Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322-1328. [Cited in This Article: ] |
| 53. | Rivard A, Berthou-Soulie L, Principe N, Kearney M, Curry C, Branellec D, Semenza GL, Isner JM. Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J Biol Chem. 2000;275:29643-29647. [Cited in This Article: ] |
| 54. | Zagzag D, Krishnamachary B, Yee H, Okuyama H, Chiriboga L, Ali MA, Melamed J, Semenza GL. Stromal cell-derived factor-1alpha and CXCR4 expression in hemangioblastoma and clear cell-renal cell carcinoma: von Hippel-Lindau loss-of-function induces expression of a ligand and its receptor. Cancer Res. 2005;65:6178-6188. [Cited in This Article: ] |
| 55. | Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50-56. [Cited in This Article: ] |
| 56. | Belema-Bedada F, Uchida S, Martire A, Kostin S, Braun T. Efficient homing of multipotent adult mesenchymal stem cells depends on FROUNT-mediated clustering of CCR2. Cell Stem Cell. 2008;2:566-575. [Cited in This Article: ] |
| 57. | Arthur A, Zannettino A, Gronthos S. The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. J Cell Physiol. 2009;218:237-245. [Cited in This Article: ] |
| 58. | Fujita T, Azuma Y, Fukuyama R, Hattori Y, Yoshida C, Koida M, Ogita K, Komori T. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J Cell Biol. 2004;166:85-95. [Cited in This Article: ] |
| 59. | Enomoto H, Furuichi T, Zanma A, Yamana K, Yoshida C, Sumitani S, Yamamoto H, Enomoto-Iwamoto M, Iwamoto M, Komori T. Runx2 deficiency in chondrocytes causes adipogenic changes in vitro. J Cell Sci. 2004;117:417-425. [Cited in This Article: ] |
| 60. | Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846-855. [Cited in This Article: ] |
| 61. | Tominaga H, Maeda S, Miyoshi H, Miyazono K, Komiya S, Imamura T. Expression of osterix inhibits bone morphogenetic protein-induced chondrogenic differentiation of mesenchymal progenitor cells. J Bone Miner Metab. 2009;27:36-45. [Cited in This Article: ] |
| 62. | Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, Jiang W, Luu HH, Luo J, Szatkowski JP. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11:1312-1320. [Cited in This Article: ] |
| 63. | Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, Montag AG. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:55958-55968. [Cited in This Article: ] |
| 64. | Peng Y, Kang Q, Cheng H, Li X, Sun MH, Jiang W, Luu HH, Park JY, Haydon RC, He TC. Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. J Cell Biochem. 2003;90:1149-1165. [Cited in This Article: ] |
| 65. | Peng Y, Kang Q, Luo Q, Jiang W, Si W, Liu BA, Luu HH, Park JK, Li X, Luo J. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:32941-32949. [Cited in This Article: ] |
| 66. | Shea CM, Edgar CM, Einhorn TA, Gerstenfeld LC. BMP treatment of C3H10T1/2 mesenchymal stem cells induces both chondrogenesis and osteogenesis. J Cell Biochem. 2003;90:1112-1127. [Cited in This Article: ] |
| 67. | Si W, Kang Q, Luu HH, Park JK, Luo Q, Song WX, Jiang W, Luo X, Li X, Yin H. CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Mol Cell Biol. 2006;26:2955-2964. [Cited in This Article: ] |
| 68. | Tang N, Song WX, Luo J, Luo X, Chen J, Sharff KA, Bi Y, He BC, Huang JY, Zhu GH. BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. J Cell Mol Med. 2009;13:2448-2464. [Cited in This Article: ] |
| 69. | Singh P, YashRoy RC, Hoque M. Augmented bone-matrix formation and osteogenesis under magnetic field stimulation in vivo XRD, TEM and SEM investigations. Indian J Biochem Biophys. 2006;43:167-172. [Cited in This Article: ] |
| 70. | Choi YH, Burdick MD, Strieter RM. Human circulating fibrocytes have the capacity to differentiate osteoblasts and chondrocytes. Int J Biochem Cell Biol. 2010;42:662-671. [Cited in This Article: ] |
| 71. | Oreffo RO, Kusec V, Romberg S, Triffitt JT. Human bone marrow osteoprogenitors express estrogen receptor-alpha and bone morphogenetic proteins 2 and 4 mRNA during osteoblastic differentiation. J Cell Biochem. 1999;75:382-392. [Cited in This Article: ] |
| 72. | Akiyama H. Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol. 2008;18:213-219. [Cited in This Article: ] |
| 73. | Shen B, Wei A, Tao H, Diwan AD, Ma DD. BMP-2 enhances TGF-beta3-mediated chondrogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in alginate bead culture. Tissue Eng Part A. 2009;15:1311-1320. [Cited in This Article: ] |
| 74. | Tsuchiya H, Kitoh H, Sugiura F, Ishiguro N. Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2003;301:338-343. [Cited in This Article: ] |
| 75. | Ikeda T, Kamekura S, Mabuchi A, Kou I, Seki S, Takato T, Nakamura K, Kawaguchi H, Ikegawa S, Chung UI. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 2004;50:3561-3573. [Cited in This Article: ] |
| 76. | Paul R, Haydon RC, Cheng H, Ishikawa A, Nenadovich N, Jiang W, Zhou L, Breyer B, Feng T, Gupta P. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine (Phila Pa 1976). 2003;28:755-763. [Cited in This Article: ] |
| 77. | Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, Ling KW, Sham MH, Koopman P, Tam PP, Cheah KS. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997;16:174-178. [Cited in This Article: ] |
| 78. | Mehlhorn AT, Schmal H, Kaiser S, Lepski G, Finkenzeller G, Stark GB, Südkamp NP. Mesenchymal stem cells maintain TGF-beta-mediated chondrogenic phenotype in alginate bead culture. Tissue Eng. 2006;12:1393-1403. [Cited in This Article: ] |
| 79. | Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F, Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682-691. [Cited in This Article: ] |
| 80. | Richardson SM, Walker RV, Parker S, Rhodes NP, Hunt JA, Freemont AJ, Hoyland JA. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells. 2006;24:707-716. [Cited in This Article: ] |
| 81. | Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145-171. [Cited in This Article: ] |
| 82. | Farmer SR. Regulation of PPARgamma activity during adipogenesis. Int J Obes (Lond). 2005;29 Suppl 1:S13-S16. [Cited in This Article: ] |
| 83. | Yin J, Jin X, Beck S, Kang DH, Hong Z, Li Z, Jin Y, Zhang Q, Choi YJ, Kim SC. In vitro myogenic and adipogenic differentiation model of genetically engineered bovine embryonic fibroblast cell lines. Biotechnol Lett. 2010;32:195-202. [Cited in This Article: ] |
| 84. | Oishi K, Noguchi H, Yukawa H, Hayashi S. Differential ability of somatic stem cells. Cell Transplant. 2009;18:581-589. [Cited in This Article: ] |
| 85. | Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol. 2004;10:3016-3020. [Cited in This Article: ] |
| 86. | Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42-48. [Cited in This Article: ] |
| 87. | Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, Rameshwar P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood. 2006;107:4817-4824. [Cited in This Article: ] |
| 88. | Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, Shen B, Yin S, Liu W, Cui L. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18:846-857. [Cited in This Article: ] |
| 89. | Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367-372. [Cited in This Article: ] |
| 90. | Xu G, Zhang L, Ren G, Yuan Z, Zhang Y, Zhao RC, Shi Y. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 2007;17:240-248. [Cited in This Article: ] |
| 91. | Groh ME, Maitra B, Szekely E, Koç ON. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 2005;33:928-934. [Cited in This Article: ] |
| 92. | Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576-6583. [Cited in This Article: ] |
| 93. | Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noël D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025-2032. [Cited in This Article: ] |
| 94. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [Cited in This Article: ] |
| 95. | Le Blanc K, Rasmusson I, Götherström C, Seidel C, Sundberg B, Sundin M, Rosendahl K, Tammik C, Ringdén O. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004;60:307-315. [Cited in This Article: ] |
| 96. | Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228-234. [Cited in This Article: ] |
| 97. | Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141-150. [Cited in This Article: ] |
| 98. | Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619-4621. [Cited in This Article: ] |
| 99. | Marsh D. Concepts of fracture union, delayed union, and nonunion. Clin Orthop Relat Res. 1998;S22-S30. [Cited in This Article: ] |
| 100. | Einhorn TA. Enhancement of fracture healing. Instr Course Lect. 1996;45:401-416. [Cited in This Article: ] |
| 101. | Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998;80:985-996. [Cited in This Article: ] |
| 102. | Peterson B, Zhang J, Iglesias R, Kabo M, Hedrick M, Benhaim P, Lieberman JR. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11:120-129. [Cited in This Article: ] |
| 103. | Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385-386. [Cited in This Article: ] |
| 104. | Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, Mastrogiacomo M, Cancedda R. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13:947-955. [Cited in This Article: ] |
| 105. | Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87:1430-1437. [Cited in This Article: ] |
| 106. | Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-895. [Cited in This Article: ] |
| 107. | Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579-592. [Cited in This Article: ] |
| 108. | Yan H, Yu C. Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy. 2007;23:178-187. [Cited in This Article: ] |
| 109. | Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13:595-600. [Cited in This Article: ] |
| 110. | Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1:74-79. [Cited in This Article: ] |
| 111. | Kuroda R, Ishida K, Matsumoto T, Akisue T, Fujioka H, Mizuno K, Ohgushi H, Wakitani S, Kurosaka M. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226-231. [Cited in This Article: ] |
| 112. | Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199-206. [Cited in This Article: ] |
| 113. | King D, Chase J, Havey RM, Voronov L, Sartori M, McEwen HA, Beamer WG, Patwardhan AG. Effects of growth hormone transgene expression on vertebrae in a mouse model of osteogenesis imperfecta. Spine (Phila Pa 1976). 2005;30:1491-1495. [Cited in This Article: ] |
| 114. | Rauch F, Glorieux FH. Osteogenesis imperfecta, current and future medical treatment. Am J Med Genet C Semin Med Genet. 2005;139C:31-37. [Cited in This Article: ] |
| 115. | Guillot PV, Abass O, Bassett JH, Shefelbine SJ, Bou-Gharios G, Chan J, Kurata H, Williams GR, Polak J, Fisk NM. Intrauterine transplantation of human fetal mesenchymal stem cells from first-trimester blood repairs bone and reduces fractures in osteogenesis imperfecta mice. Blood. 2008;111:1717-25. [Cited in This Article: ] |
| 116. | Panaroni C, Gioia R, Lupi A, Besio R, Goldstein SA, Kreider J, Leikin S, Vera JC, Mertz EL, Perilli E. In utero transplantation of adult bone marrow decreases perinatal lethality and rescues the bone phenotype in the knockin murine model for classical, dominant osteogenesis imperfecta. Blood. 2009;114:459-468. [Cited in This Article: ] |
| 117. | Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932-8937. [Cited in This Article: ] |
| 118. | Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309-313. [Cited in This Article: ] |
| 119. | Le Blanc K, Götherström C, Ringdén O, Hassan M, McMahon R, Horwitz E, Anneren G, Axelsson O, Nunn J, Ewald U. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005;79:1607-1614. [Cited in This Article: ] |
| 120. | Cahill RA, Wenkert D, Perlman SA, Steele A, Coburn SP, McAlister WH, Mumm S, Whyte MP. Infantile hypophosphatasia: transplantation therapy trial using bone fragments and cultured osteoblasts. J Clin Endocrinol Metab. 2007;92:2923-2930. [Cited in This Article: ] |
| 121. | Whyte MP, Kurtzberg J, McAlister WH, Mumm S, Podgornik MN, Coburn SP, Ryan LM, Miller CR, Gottesman GS, Smith AK. Marrow cell transplantation for infantile hypophosphatasia. J Bone Miner Res. 2003;18:624-636. [Cited in This Article: ] |
| 122. | Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722-3729. [Cited in This Article: ] |
| 123. | Tian Y, Deng YB, Huang YJ, Wang Y. Bone marrow-derived mesenchymal stem cells decrease acute graft-versus-host disease after allogeneic hematopoietic stem cells transplantation. Immunol Invest. 2008;37:29-42. [Cited in This Article: ] |
| 124. | Yañez R, Lamana ML, García-Castro J, Colmenero I, Ramírez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582-2591. [Cited in This Article: ] |
| 125. | Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439-1441. [Cited in This Article: ] |
| 126. | Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579-1586. [Cited in This Article: ] |
| 127. | Karussis D, Kassis I, Kurkalli BG, Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci. 2008;265:131-135. [Cited in This Article: ] |
| 128. | Fuller KG, Olson JK, Howard LM, Croxford JL, Miller SD. Mouse models of multiple sclerosis: experimental autoimmune encephalomyelitis and Theiler's virus-induced demyelinating disease. Methods Mol Med. 2004;102:339-361. [Cited in This Article: ] |
| 129. | Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755-1761. [Cited in This Article: ] |
| 130. | Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD, Miller RH. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192-1203. [Cited in This Article: ] |
| 131. | Mohyeddin Bonab M, Yazdanbakhsh S, Lotfi J, Alimoghaddom K, Talebian F, Hooshmand F, Ghavamzadeh A, Nikbin B. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J Immunol. 2007;4:50-57. [Cited in This Article: ] |
| 132. | Williams RO. Collagen-induced arthritis as a model for rheumatoid arthritis. Methods Mol Med. 2004;98:207-216. [Cited in This Article: ] |
| 133. | Mao F, Xu WR, Qian H, Zhu W, Yan YM, Shao QX, Xu HX. Immunosuppressive effects of mesenchymal stem cells in collagen-induced mouse arthritis. Inflamm Res. 2010;59:219-225. [Cited in This Article: ] |
| 134. | Zheng ZH, Li XY, Ding J, Jia JF, Zhu P. Allogeneic mesenchymal stem cell and mesenchymal stem cell-differentiated chondrocyte suppress the responses of type II collagen-reactive T cells in rheumatoid arthritis. Rheumatology (Oxford). 2008;47:22-30. [Cited in This Article: ] |
| 135. | Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Alwayn IP, Weimar W, Hoogduijn MJ. Donor-derived mesenchymal stem cells suppress alloreactivity of kidney transplant patients. Transplantation. 2009;87:896-906. [Cited in This Article: ] |
| 136. | Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79-86. [Cited in This Article: ] |
| 137. | Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421-1432. [Cited in This Article: ] |
| 139. | Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697-705. [Cited in This Article: ] |
| 140. | Shiota M, Heike T, Haruyama M, Baba S, Tsuchiya A, Fujino H, Kobayashi H, Kato T, Umeda K, Yoshimoto M. Isolation and characterization of bone marrow-derived mesenchymal progenitor cells with myogenic and neuronal properties. Exp Cell Res. 2007;313:1008-1023. [Cited in This Article: ] |
| 141. | Grauss RW, van Tuyn J, Steendijk P, Winter EM, Pijnappels DA, Hogers B, Gittenberger-De Groot AC, van der Geest R, van der Laarse A, de Vries AA. Forced myocardin expression enhances the therapeutic effect of human mesenchymal stem cells after transplantation in ischemic mouse hearts. Stem Cells. 2008;26:1083-1093. [Cited in This Article: ] |
| 142. | Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102:11474-11479. [Cited in This Article: ] |
| 143. | Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459-465. [Cited in This Article: ] |
| 144. | Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076-1084. [Cited in This Article: ] |
| 145. | Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840-850. [Cited in This Article: ] |
| 146. | Tang J, Wang J, Zheng F, Kong X, Guo L, Yang J, Zhang L, Huang Y. Combination of chemokine and angiogenic factor genes and mesenchymal stem cells could enhance angiogenesis and improve cardiac function after acute myocardial infarction in rats. Mol Cell Biochem. 2010;339:107-118. [Cited in This Article: ] |
| 147. | Zhang Z, Deb A, Zhang Z, Pachori A, He W, Guo J, Pratt R, Dzau VJ. Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. J Mol Cell Cardiol. 2009;46:370-377. [Cited in This Article: ] |
| 148. | Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277-2286. [Cited in This Article: ] |
| 149. | Dill T, Schächinger V, Rolf A, Möllmann S, Thiele H, Tillmanns H, Assmus B, Dimmeler S, Zeiher AM, Hamm C. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. Am Heart J. 2009;157:541-547. [Cited in This Article: ] |
| 150. | Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247-256. [Cited in This Article: ] |
| 151. | Omori Y, Honmou O, Harada K, Suzuki J, Houkin K, Kocsis JD. Optimization of a therapeutic protocol for intravenous injection of human mesenchymal stem cells after cerebral ischemia in adult rats. Brain Res. 2008;1236:30-38. [Cited in This Article: ] |
| 152. | Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, Zhang Z. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311-1319. [Cited in This Article: ] |
| 153. | Borlongan CV, Lind JG, Dillon-Carter O, Yu G, Hadman M, Cheng C, Carroll J, Hess DC. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res. 2004;1010:108-116. [Cited in This Article: ] |
| 154. | Liu Z, Li Y, Zhang X, Savant-Bhonsale S, Chopp M. Contralesional axonal remodeling of the corticospinal system in adult rats after stroke and bone marrow stromal cell treatment. Stroke. 2008;39:2571-2577. [Cited in This Article: ] |
| 155. | Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, Chopp M. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137:393-399. [Cited in This Article: ] |
| 156. | Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S, Hart C, Sanchez-Ramos J, Sanberg PR. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res. 2003;73:296-307. [Cited in This Article: ] |
| 157. | Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874-882. [Cited in This Article: ] |
| 158. | Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006;203:1235-1247. [Cited in This Article: ] |
| 159. | Birnbaum T, Roider J, Schankin CJ, Padovan CS, Schichor C, Goldbrunner R, Straube A. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J Neurooncol. 2007;83:241-247. [Cited in This Article: ] |
| 160. | Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593-1603. [Cited in This Article: ] |
| 161. | Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69:4134-4142. [Cited in This Article: ] |
| 162. | Lu YR, Yuan Y, Wang XJ, Wei LL, Chen YN, Cong C, Li SF, Long D, Tan WD, Mao YQ. The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer Biol Ther. 2008;7:245-251. [Cited in This Article: ] |
| 163. | Ma B, Ren J, Jiang HF, Jia J. [Antitumor activities against hepatocellular carcinoma induced by bone marrow mesenchymal stem cells pulsed with tumor-derived exosomes]. Beijing Da Xue Xue Bao. 2008;40:494-499. [Cited in This Article: ] |
| 164. | Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557-563. [Cited in This Article: ] |
| 165. | Tang YL, Zhao Q, Qin X, Shen L, Cheng L, Ge J, Phillips MI. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80:229-236; discussion 236-237. [Cited in This Article: ] |
| 166. | Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568-1571. [Cited in This Article: ] |
| 167. | Koç ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant. 2002;30:215-222. [Cited in This Article: ] |
| 168. | Fouillard L, Bensidhoum M, Bories D, Bonte H, Lopez M, Moseley AM, Smith A, Lesage S, Beaujean F, Thierry D. Engraftment of allogeneic mesenchymal stem cells in the bone marrow of a patient with severe idiopathic aplastic anemia improves stroma. Leukemia. 2003;17:474-476. [Cited in This Article: ] |
| 169. | Pozzi S, Lisini D, Podestà M, Bernardo ME, Sessarego N, Piaggio G, Cometa A, Giorgiani G, Mina T, Buldini B. Donor multipotent mesenchymal stromal cells may engraft in pediatric patients given either cord blood or bone marrow transplantation. Exp Hematol. 2006;34:934-942. [Cited in This Article: ] |
| 170. | Tisato V, Naresh K, Girdlestone J, Navarrete C, Dazzi F. Mesenchymal stem cells of cord blood origin are effective at preventing but not treating graft-versus-host disease. Leukemia. 2007;21:1992-9. [Cited in This Article: ] |
| 171. | Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, Mitchell JB, Hammill L, Vanguri P, Chopp M. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005;195:16-26. [Cited in This Article: ] |
| 172. | Mao F, Xu WR, Qian H, Zhu W, Yan YM, Shao QX, Xu HX. Immunosuppressive effects of mesenchymal stem cells in collagen-induced mouse arthritis. Inflamm Res. 2010;59:219-225. [Cited in This Article: ] |
| 173. | Madec AM, Mallone R, Afonso G, Abou Mrad E, Mesnier A, Eljaafari A, Thivolet C. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia. 2009;52:1391-1399. [Cited in This Article: ] |
| 174. | Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855-1863. [Cited in This Article: ] |
| 175. | Kunter U, Rong S, Djuric Z, Boor P, Müller-Newen G, Yu D, Floege J. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol. 2006;17:2202-2212. [Cited in This Article: ] |
| 176. | Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998;16:155-162. [Cited in This Article: ] |
| 177. | González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978-989. [Cited in This Article: ] |
| 178. | Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128-1135. [Cited in This Article: ] |
| 179. | Wu GD, Bowdish ME, Jin YS, Zhu H, Mitsuhashi N, Barsky LW, Barr ML. Contribution of mesenchymal progenitor cells to tissue repair in rat cardiac allografts undergoing chronic rejection. J Heart Lung Transplant. 2005;24:2160-2169. [Cited in This Article: ] |