Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1144
Revised: January 4, 2003
Accepted: January 13, 2003
Published online: June 15, 2003
Tumor angiogenesis is the proliferation of a network of blood vessels that penetrates into cancerous growths, supplying nutrients and oxygen and removing waste products. The process of angiogenesis plays an important role in many physiological and pathological conditions. Solid tumors depend on angiogenesis for growth and metastasis in a hostile environment. In the prevascular phase, the tumor is rarely larger than 2 to 3 mm3 and may contain a million or more cells. Up to this size, tumor cells can obtain the necessary oxygen and nutrient supplies required for growth and survival by simple passive diffusion. The properties of tumors to release and induce several angiogenic and anti-angiogenic factors which play crucial roles in regulating endothelial cell (EC) proliferation, migration, apoptosis or survival, cell-cell and cell-matrix adhesion through different intracellular signaling are thought to be the essential mechanisms during tumor-induced angiogenesis. Tumor angiogenesis actually starts with tumor cells releasing molecules that send signals to surrounding normal host tissue. This signaling activates certain genes in the host tissue that, in turn, make proteins to encourage growth of new blood vessels. In this review, we focus the mechanisms of tumor-induced angiogenesis, with an emphasis on the regulatory role of several angiogenic and anti-angiogenic agents during the angiogenic process in tumors. Advances in understanding the mechanisms of tumor angiogenesis have led to the development of several most effective anti-angiogenic and anti-metastatic therapeutic agents and also have provided several techniques for the regulation of cancer's angiogenic switch. The suggestion is made that standard cytotoxic chemotherapy and angiogenesis inhibitors used in combination may produce complementary therapeutic benefits in the treatment of cancer.
- Citation: Gupta MK, Qin RY. Mechanism and its regulation of tumor-induced angiogenesis. World J Gastroenterol 2003; 9(6): 1144-1155
- URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1144.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1144
Angiogenesis is a complex multi-step process involving extensive interplay between cells, soluble factors, and extracellular matrix (ECM) components. Four distinct sequential steps in angiogenesis include: (1) degradation of basement membrane by proteases; (2) migration of endothelial cells (ECs) into the interstitial space and sprouting; (3) ECs proliferation at the migrating tip; (4) lumen formation, generation of new basement membrane with the recruitment of pericyte, formation of anastomoses and finally blood flow[1]. The angiogenic response in the microvasculature is associated with changes in cellular adhesive interactions between adjacent ECs, pericytes and surrounding ECM. In the process of active neovascularization, activated ECs reorganize their cytoskeleton, express cell surface adhesion molecules such as integrins and selectins, secrete proteolytic enzymes, and remodel their adjacent ECM. These events are followed by the formation of capillary buds. Autocrine and/or paracrine angiogenic factors must be present to induce EC migration, proliferation, elongation, orientation and differentiation leading to the re-establishment of the basement membrane, lumen formation and anastomosis with other new or pre-existing vessels, eventually leading to the formation of intact microvessels.
Angiogenic phenotype serves the development of malignant tumor at multiple stages. Tumor cells may overexpress one or more of the positive regulators of angiogenesis, may mobilize an angiogenic protein from the ECM, may recruit host cells such as macrophages (which produce their own angiogenic proteins), or may engage in a combination of these processes. Tumor angiogenesis is mediated by tumor-secreted angiogenic growth factors that interact with their surface receptors expressed on ECs. The most commonly found angiogenic growth factors such as VEGF and bFGF, when encounter ECs, they bind to the tyrosine kinase receptors on ECs membrane. Binding leads to dimerization of the receptors and activation of autophosphorylation of tyrosines on the receptor surface and thereby initiates the several signaling proteins (including PI3-kinase, Src, Grb2/m-SOS-1 (a nucleotide exchange factor for Ras) and signal transducers and activators of transcriptions (STATs) each of which contains src-homology-2 (SH-2) domains[2]. Binding of the SH-2 regions of these proteins to the phosphotyrosines on the receptor tyrosine kinases (RTKs) activates several pathways that are crucial for triggering the cell cycle machinery. The most well studied pathway passes through the GTP-binding protein Ras and activates the mitogen activated protein kinase (MAPK) cascade and subsequently transcription factors in the nucleus[2]. Up-regulation of an angiogenic factor is not sufficient in itself for a tumor cell to become angiogenic, however, certain negative regulators or inhibitors of vessel growth may need to be down-regulated[3]. If there is a preponderance of angiogenic factors in the local milieu, the neovasculature may persist as capillaries, or differentiate into mature venules or arterioles. If instead, the local milieu changes such that there is a preponderance of angiostatic factors, the neovessels can regress. The angiostatic factors that mediate regression can do so either by inducing apoptosis or cell cycle arrest of ECs. Thus, the switch to the angiogenic phenotype is regulated by a change in the local equilibrium between positive and negative regulators of the growth of microvessels[1,3].
Vascular endothelial growth factor (VEGF), also known as vascular permeability factor (VPF), is a heparin-binding angiogenic growth factor, and is highly expressed in various types of tumors. It may increase ECs permeability by enhancing the activity of vesicular-vacuolar organelles, clustered vesicles in ECs lining small vessels that facilitate transport of metabolites between luminal and abluminal plasma membranes[4]. Alternatively, VEGF may enhance permeability through mitogen-activated protein (MAP) kinase signal transduction cascade by loosening adhering junctions between ECs in a monolayer via rearrangement of cadherin/catenin complexes[5,6]. In addition, recent studies have shown that VEGF enhances ECs permeability by activating PKB/Akt, endothelial nitric-oxide synthase (eNOS), and MAP kinase dependent pathways using human umbilical vein endothelial cell[7] (Figure 1). Increased vascular permeability may allow the extravasation of plasma proteins and formation of ECM favorable to endothelial and stromal cell migration.
VEGF is an EC specific mitogen. VEGF, after binding to its high affinity receptors (Flt-1/VEGFR-1, Flk-1/KDR/VEGFR-2), promotes the formation of the second messenger via hydrolysis of inositol, thus induces the autophosphorylation of the receptors in the presence of heparin-like molecules, and open phosphatidylinositol metabolic signal transduction pathways, activates MAP kinases in EC and thereby VEGF exerts its mitogenic effect by promoting EC proliferation[8,9].
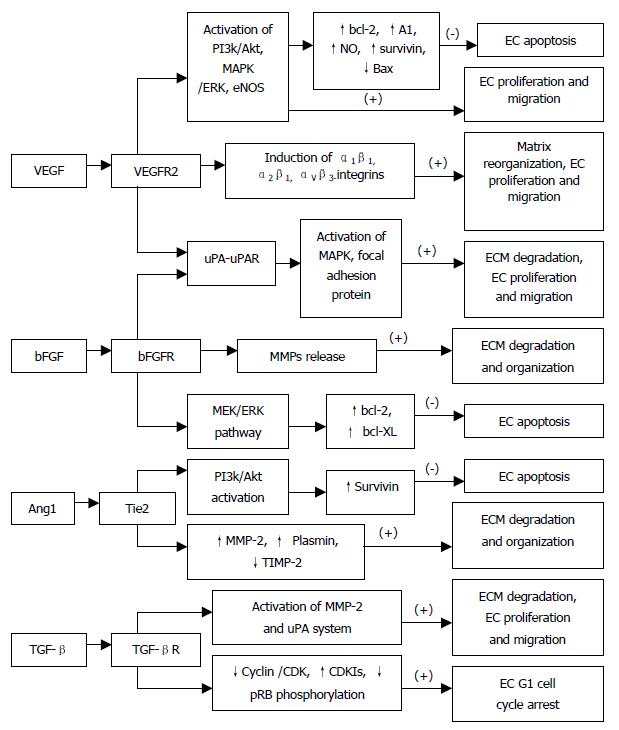
VEGF induces a balanced system of proteolysis that can remodel ECM components necessary for angiogenesis. VEGF stimulates EC production of urokinase-like plasminogen activator (uPA), tissue type plasminogen activator (tPA) and plasminogen activator inhibitor-1 (PAI-1)[10,11], proteolytic enzymes, tissue factors, and interstitial collagenase[12]. Plasminogen activators activate plasminogen to plasmin, which can break down ECM components. In addition to remodeling the basement membrane, uPA bound to uPAR also mediates intracellular signal transduction in ECs. Tang et al[13] have demonstrated that uPAR occupancy on ECs results in the phosphorylation of focal adhesion proteins and the activation of MAP kinase through which uPA influences EC migration and proliferation (Figure 2).
Moreover, VEGF has been shown to exhibit its angiogenic effect by inducing expression of the α1β1, α2β1 and αvβ3-integrins, which promote cell migration, proliferation and matrix reorganization (Figure 2), and α1β1, α2β1 and αvβ3 antagonists may prove effective on inhibiting VEGF-driven angiogenesis associated with cancers and other pathologies through apoptosis[14,15]. VEGF, in addition to a very specific mitogen for vascular EC, is a potent pro-survival factor for ECs in newly formed immature vessels. Several endothelial survival factors (VEGF, angiopoietin-1 and αvβ3) suppress p53, p21, p16 and p27, and proapoptotic protein Bax, whereas they variably activate the survival PI3k/Akt, p42/44 MAP kinases, bcl-2, A1 and survivin pathways[16-20](Figure 2). It was reported that p42/p44 MAP kinases promoted VEGF expression by activating its transcription via recruitment of the AP-2/Sp1 (activator protein-2) complex on the proximal region (-88/-66) of the VEGF promoter and by direct phosphorylation of hypoxia-inducible factor 1 alpha (HIF-1 alpha)[21]. Pharmacological inhibition of PI3K or transfection with a dominant-negative Akt mutant abolished the antiapoptotic effect of VEGF on ECs. In addition to the PI3K/Akt pathway, ras-dependent signaling pathways might also play an important role at least for VEGF signaling. Thus, H-rasV12G down-regulation leads to profound tumor regression, which is initially characterized by massive apoptosis of tumor- and host-derived ECs[22]. Therefore, apoptosis induction is resistant to enforced VEGF expression, suggesting that VEGF requires an intact Ras-dependent signaling pathway to mediate its apoptosis inhibitory effect[22]. And also, VEGF via the KDR/Flt-1 receptor induces enhanced expression of the serine-threonine protein kinase Akt[19], a downstream target of PI3-kinase, which potently blocks apoptosis by interfering with various apoptosis signaling pathways[23,24], promotes EC migration[25], and enhances the expression of the hypoxia-inducible factor (HIF), which is known to stimulate VEGF expression[26], suggesting a potent proangiogenic effect[27,28]. These findings have identified the VEGFR2 and the PI3K/Akt signal transduction pathway as crucial elements in promoting EC survival induced by VEGF. The downstream effector pathways mediating the antiapoptotic VEGF effect include Akt-dependent activation of the endothelial nitric oxide synthase (NOS)[29,30], resulting in an enhanced endothelial NO synthesis, which, in turn promotes EC survival (Figure 2). Gupta et al[31] demonstrated that the VEGF-induced activation of the MAPK/extracellular signal-regulated kinase (ERK) pathway and inhibition of the stress-activated protein kinase/c-Jun amino-terminal kinase pathway is also implicated in the antiapoptotic effect mediated by VEGF (Figure 2). Interestingly, the activation of the PI3K/Akt pathway mediates not only the antiapoptotic effect but also the migratory effect of VEGF on ECs via Akt-dependent phosphorylation and activation of eNOS[32] (Figure 2).
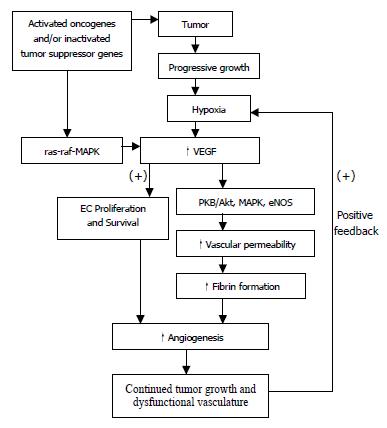
The expression of VEGF mRNA is highest in hypoxic tumor cells adjacent to necrotic areas. Hypoxia-induced transcription of VEGF mRNA is apparently mediated, at least in part, by the binding of hypoxia-inducible factor 1 (HIF-1) to an HIF-1 binding site located in the VEGF promoter, and by the activation of a stress inducible PI3K/Akt pathway[26,33]. In fact, progressive growth of tumor creates ongoing hypoxia, which up-regulates several pro-angiogenic compounds including VEGF, bFGF, IL-8, TNF-α, TGF-βetc. These compounds, via several mechanisms such as increase of vessel hyperpermeability, release of plasma proteins, induction of proteases, fibrin formation, EC proliferation, migration etc, promote angiogenesis and fibrinolysis resulting in continued tumor growth and dysfunctional vasculature, which further positively feedback to create continuing hypoxia inside tumors (Figure 1).
Fibroblast growth factors (FGFs) and their receptors are overexpressed in various types of cancers, and are important tumor angiogenic and ECs survival factors. Pardo et al[34]. reported that bFGF induced expression of the antiapoptotic proteins bcl-XL and bcl-2 via the MEK/ERK signaling pathway (Figure 2). Expression of VEGF mRNA in the tumor is increased by bFGF overexpression, and the bFGF-induced tumor development is significantly inhibited by treatment with KDR/Flk-1 neutralizing monoclonal antibody (mAb), which suggests that bFGF synergistically augments VEGF-mediated hepatocellular carcinoma development and angiogenesis, at least in part, by induction of VEGF through KDR/Flk-1[35]. In addition, bFGF induces an increase of VEGF mRNA in vascular smooth muscle cells[36] and an increase in VEGF receptors in microvascular ECs[37]. aFGF and bFGF are mitogenic for ECs and stimulate ECs migration as well as ECs production of plasminogen activator (PA) and collagenase that are capable of degrading basement membrane[38] (Figure 2). FGFs are responsible for production of ECM and release of matrix metalloproteinases (MMPs) for selective degradation and organization of ECM[39] (Figure 2).
Binding of FGFs to their high affinity receptors causes the activation of the intrinsic tyrosine kinase and a cascade of events, leading eventually to the induction of immediate early gene transcription, and to cell proliferation. FGFs receptors dimerize upon ligand binding, and transphosphorylate at tyrosine residue. Angiogenic growth factors, like bFGF and VEGF165, require interaction with heparin sulfate (HS) in order to induce a proliferative signal through tyrosine kinase receptors. Binding of bFGF to high affinity cell surface receptor sites can be modulated by heparin-mimicking compounds (i.e. RG-13577) that can modulate abnormal bFGF signaling by disrupting bFGF mediated autocrine loop, compete with heparin sulfate (HS) on binding to bFGF, bind the growth factor, and prevent receptor binding and/or dimerization[40], and by proteolytic enzymes (e.g. MMP-2) that cleave the ectodomain of the receptor. These effects are associated with profound inhibition of bFGF mediated signal transduction (tyrosine phosphorrylation) and proliferation of vascular ECs[40]. Spontaneous migration of ECs is inhibited by neutralizing antibodies to bFGF, suggesting an autocrine of bFGF synthesized and released by the ECs themselves[38]. A dominant-negative receptor, which, when co-expressed with FGF receptors (FGFRs), can block the activation and signal transduction. In addition, the ligand-specific targeting of toxin to tumor cells expressing FGFRs and the compounds that bind and inactivate FGF ligands, can block ECs proliferation.
It has been proposed that angiopoietin-1 (Ang1) and angiopoietin-2 (Ang2) are pro-angiogenic and anti-angiogenic owing to their respective agonist and antagonist signaling action through the Tie2 receptor[41]. Lobov et al[41] have demonstrated that in vivo, in the presence of endogenous VEGF-A, Ang2 promotes a rapid increase in capillary diameter, remodeling of the basal lamina, proliferation and migration of ECs, and stimulates sprouting of new blood vessels. By contrast, Ang2 promotes ECs death and vessel regression if the activity of endogenous VEGF is inhibited[41]. It was reported that Ang1 induced phosphorylation of Tie2 and the p85 subunit of PI 3'-kinase and increased PI 3'-kinase activity in a dose-dependent manner, suggesting that the Tie2 receptor, PI 3'-kinase, and Akt are crucial elements in signal transduction pathway leading to EC survival induced by the paracrine activity of Ang1[42] (Figure 2). Alternatively, Ang1 prevents EC apoptosis via Akt/survivin pathway by activating a critical survival messenger, Akt, and by up-regulating a broad spectrum apoptosis inhibitor, survivin[43,44] (Figure 2), but has no effect on the expression of bcl-2 and XIAP[44]. Moreover, Ang1-induced migratory effect might be mediated through PI 3'-kinase activity dependent tyrosine phosphorylation of p125FAK, which plays a key role in regulating dynamic changes in actin cytoskeleton organization during EC migration[45]. Increased plasmin and MMP-2 secretion, and suppressed TIMP-2 secretion by Ang1 from ECs are also important determinants for inducing ECs sprouting[45] (Figure 2). In contrast, the PI 3'-kinase inhibitors have been found to inhibit Ang1-stimulated tyrosine phosphorylation of p125FAK, and secretion of MMP-2 and plasmin from ECs and migration[45]. Ang2 blocks Ang1-mediated Tie2 autophosphorylation in ECs and acts as a check point on Ang1/Tie2-mediated angiogenesis to prevent excessive branching and sprouting of blood vessels by promoting destabilization of blood vessels.
Transforming growth factor-betas (TGF-βs) are multifunctional polypeptides that regulate cell growth and differentiation, ECM deposition, cellular adhesion properties, angiogenesis and immune functions. TGF-β1 acts through the TGF-β type I and type II receptors to activate intracellular mediators, such as Smad proteins, the p38 MAPK, and the ERK pathway[46]. TGF-β1 mRNA levels by activin receptor-like kinase 5 (ALK5) independent of p38 MAPK activation[46]. In contrast, TGF-β1 induction of fibronectin (FN) mRNA requires p38 MAPK activity[46]. TGF-β1 induction of PAI-1 and TSP-1 mRNA uses at least ALK5 and possibly the p38 MAPK pathway[46]. TGF-β secreted by most cultured cells is in biologically inactive form, and cannot bind TGF-β receptors; the latent TGF-β is activated by proteases such as plasmin and cathepsin D, low pH, chaotropic agents such as urea, and heat[47,48]. Several studies suggested that VEGF increases plasminogen activator (PA) activity in vascular ECs[11] and that plasmin is able to activate latent TGF- β1[49,50] which decreases Flk-1 expression and thereby negatively regulates the VEGF/Flk-1 signal transduction pathway in EC[51], raise the possibility that a complex self-regulating mechanism of VEGF signal transduction may exist during angiogenesis[50]. However, immunohistochemical study has shown that TGF-β1 might be associated with tumor progression by indirectly stimulating angiogenesis through the up-regulation of VEGF expression in gastric carcinoma [52]. In addition, TGF-β1 inhibits the generation of the anti-angiogenic molecule angiostatin by human pancreatic cancer cells in a time- and dose-dependent manner, and this effect is mediated through modulation of the plasminogen/plasmin system[53].
TGF-β not only inhibits the activity and expression of cyclins and CDKs but also induces the cyclin-dependent kinase inhibitors (CDKIs) p15, p27 and p15, which bind to the cyclin/CDKs, preventing phosphorylation of pRB and thereby arresting most epithelial cells (including ECs) at late G1 phase[54] (Figure 2). The effects of TGF-β1 on endothelial tube formation may be mediated through a net antiproteolytic activity by modulating uPA and PAI levels[55]. Ellenrieder et al[56] reported that TGF-β treatment of PANC-1 and IMIM-PC1 cells resulted in strong up-regulation of expression and activity of both matrix metalloproteinase-2 (MMP-2) and the uPA system, and treatment with MMP inhibitors or inhibitors of the uPA system caused significant reduction of TGF-β-induced invasiveness in both cell lines suggesting that TGF-β acts in an autocrine manner to induce tumor cell invasion, which is mediated by MMP-2 and the uPA system (Figure 2). Furthermore, TGF-β indirectly stimulates angiogenesis by the recruitment of inflammatory mediators that secrete angiogenic factors. Thus, TGF-β regulates vascular remodeling through its pleiotropic effects on different cell types.
Up-regulation of MMP activity, favoring proteolytic degradation of the basement membrane and ECM, has been linked to tumor growth and metastasis, as well as tumor-associated angiogenesis. IL-8 mRNA is up-regulated in neoplastic tissues, such as non-small cell lung cancer[57] and that its expression correlates with the extent of neovascularization, tumor progression and survival. And also, MMP-2 mRNA level is increased in tumor cells transfected with IL-8, but VEGF and bFGF mRNA levels are unchanged[58,59] suggesting that IL-8-induced MMP-2 production is a major mechanism by which tumor cells induce angiogenesis. IL-8 can also be up-regulated by hypoxia, suggesting that the environment plays a major role in regulating IL-8 expression and metastasis[58]. MMPs induce tumor angiogenesis by degrading ECM and thereby release angiogenic mitogens that have been shown to be stored within the matrix. In addition, MMP-2 and MMP-3 are able to release soluble FGF receptor 1 (FGFR1)[60] and soluble 12-kDa immunoreactive and mitogenic heparin-binding epidermal growth factor (HB-EGF)[61], respectively. MMP-2 has been shown to directly modulate melanoma cell adhesion, spreading on ECM and invasion[62], and an inhibitor of MMP-2 significantly inhibits growth and neovascularization of tumors implanted into chick chorioallantoic membrane (CAM) by preventing MMP-2 binding to αvβ3 and blocking cell surface collagenolytic activity[63]. Furthermore, MMP-9, as well as MMP-2 proteolytically cleave and activate latent TGF-β, and promote tumor invasion and angiogenesis[64].
Oncogenes are found to be activated and tumor suppressor genes are found to be inactivated in tumor, and hence promote tumor growth and angiogenesis through different mechanisms (Figure 1). It has been shown that VEGF is introduced by K- or H-ras mutant gene, v-src and v-raf in transformed fibroblast and ECs. Other angiogenic factors such as VEGF, TNF-α, TGF-β have been shown to be up-regulated by mutant ras[65]. These effect may be mediated through a ras-raf-MAP kinase signal transduction pathway (Figure 1), which results in activation of promoter regions of genes of angiogenic growth factors[66]. Moreover, expression of ras, either constitutive or transient, potentiated the induction of VEGF by hypoxia[67].
p53 is an important suppressor gene, which inhibits the angiogenic process by inducing thrombospondin-1, down-regulating VEGF and NOS and, in addition, down-regulating hypoxia-induced angiogenesis, either inducing apoptosis or enhancing anti-angiogenic factors[68]. A transient transfection of mutated p53 results in up-regulation of VEGF mRNA in NIH3T3 cells[69]. In contrast, adenovirus-mediated wild-type p53 overexpression down-regulats CD40-induced VEGF expression and transmigration in human multiple myeloma cells expressing mutant p53[70]. And, we have previously demonstrated that the expression of Flt-1 receptor is significantly correlated with p53 mutation gene, not obviously with ras mutation gene in pancreatic carcinoma cells, which suggest that wild type p53, after mutation, might lose the suppressive function to the expression of Flt-1 receptor, thus results in neovascularization of pancreatic neoplasm and promotes the growth of tumor cells, whereas ras mutation may take part in neovascularization through other approaches. Recombinant wild type p53 represses bFGF mRNA translation in rabbit reticulocyte lysate, in a dose- dependent manner via blocking translation initiation by preventing 80S ribosome formation on an mRNA bearing the bFGF mRNA leader sequence[71]. Moreover, adenoviral vector-mediated wild type p53 transduction results in tumor regression, at least in part, via anti-angiogenesis mediated by the down-modulation of FGF binding protein, a secreted protein required for the activation of angiogenic factor bFGF[72]. In addition, wild type p53 gene transfer significantly reduces cell invasiveness in vitro via a decrease in the secreted levels of MMP-2 in mutated p53 human melanoma cell lines[73]. Biologically, p53 acts at a G1/S check point, postponding DNA replication after certain cell stress, such as DNA damage[74], and also induces the apoptotic pathway of cell death[75].
Leading anti-angiogenic targets that have been identified are[76,77]: (1) inhibition of the growth factors that promote endothelial proliferation; (2) inhibition of the proteases required for ECs to penetrate basement membrane and form new blood vessels; (3) disruption of specific intracellular signal transduction pathway; (4) induction of EC apoptosis or inhibition of EC survival; (5) inhibition of endothelial bone marrow precursor cells; and (6) inhibition of αvβ3-integrin-vitronect interaction that is pivotal in mediating ECs adhesion to ECM during neovascularizatioin[77].
One broad class of angiogenesis inhibitors is made up of drugs that target growth factors such as bFGF and VEGF. The factors tend to bind to heparin, a property that may trap them within the ECM and may thereby govern their bioavailability. Hence, the early generation of drugs is heparin-like (e.g. Pentosan polysulfate), especially with regard to carrying multiple negative charges that promote growth factor binding. However, receptor targeting agents can impede tumor growth and metastasis by interfering, at specific growth-factor receptors, such as those for FGFs and VEGF, with the transduction of angiogenic stimuli into intracellular responses. In these pathways, the receptors are transmembrane tyrosine kinases, in which ligand binding to an extracellular domain induces autophosphorylation of an intracellular kinase domain. Each kinase then functions as an activator of downstream signals. To disrupt such a sequence, a drug may compete for receptor binding and prevent tyrosine kinase autophosphorylation. Inhibitors of VEGF family include: (1) anti-VEGF mAb[78]: directly neutralizes VEGF proteins, and inhibits biological activities of VEGF; (2) soluble VEGF receptors: specifically bind to VEGF, indirectly block the function of VEGF with receptors; (3) inhibitors of VEGF receptors[79]: bind to VEGF receptors and block their functions with VEGF; (4) inhibitors of VEGF signal transduction: interfere a series of signal transduction pathways by blocking autophosphorylation of VEGF receptors; (5) VEGF antisense[80]: is a specific nucleotide sequence, which binds to VEGF mRNA and thereby interferes VEGF mRNA translation and VEGF protein formation. A recent study has shown that the VEGFR2 DNAzyme can cleave its substrate efficiently in a concentration- and time-dependent manner, inhibit the proliferation of EC with a concomitant reduction of VEGFR2 mRNA, and inhibit tumor growth in vivo[81].
More than 40 endogenous angiogenesis inhibitors have been characterized, and they are divided into 4 major groups: interferons (IFNs), proteolytic fragments, interleukins (ILs), and tissue inhibitors of metalloproteinases (TIMPs)[82].
Interferons The interferons (INF-α, -β, and -γ) are members of a family of secreted glycoproteins, which have direct or indirect inhibitory effect on tumor angiogenesis and growth. IFN-α/β have been reported to down-regulate the expression of pro-angiogenic factor MMP-9 mRNA and protein in different cancers[83-86]. Also, IFN-α/β down-regulate IL-8 expression in bladder cancer[83-84]. Several studies demonstrated that the administration of optimal biological dose of IFN-α/b decreased the expression of bFGF mRNA and protein and microvessel density in the tumors and, in addition, induced EC apoptosis[83-85,87]. Sasamura et al[88]. demonstrated that IFN-α/β had mild inhibitory effects on VEGF mRNA and bFGF mRNA expression, whereas IFN-α did not significantly decrease the level of either VEGF mRNA or bFGF mRNA in renal cell carcinoma. However, some studies demonstrated that IFN-α/β treatment did not cause the reduction of bFGF and VEGF levels in serum from patients with carcinoid tumours[89] and leukemia[86]. Thus, anti-angiogenic effect of IFNs treatment might be mediated by the regulation of different angiogenic factors in different tumors in dose- and time-dependent manner. Moreover, IFN-γ is presumed to induce its anti-angiogenic effects through the secretion of IFN-γ inducible protein 10 (IP-10) and monokine induced by IFN-γ[90]. Finally, IFNs have antitumor properties, which may be mediated through a direct cytotoxic effect on tumor cells, augmentation of immunogenicity of tumor by up-regulation of major histocompatibility (MHC) classes I and II and tumor associated antigens, and/or activation of macrophages, T lymphocytes and natural killer cells[89].
Interleukins It was reported that interleukins (ILs) having a Glu-Leu-Arg (ELR) motif at the NH2 terminus, such as IL-8, enhance angiogenesis, and those that lack this sequence, such as IL-4, inhibit it[91]. IL-4 inhibits in vivo neovascularization induced by bFGF in the rat cornea and blocks the migration of microvascular ECs toward bFGF in vitro[92]. However, it has been shown that IL-1α, a representative cytokine of activated macrophages, induces angiogenesis through the enhanced expression of various angiogenic factors such as VEGF, IL-8, and bFGF[93]. And also, IL-6 was found to counteract the apoptotic effect mediated by wild type p53[75]. Several studies have reported that IL-12 suppresses the expression of VEGF mRNA[94,95], bFGF[94] and MMP-9 mRNA[94]. Additionally, IL-12 was found to stimulate mRNA expression of IFN-γ and its inducible anti-angiogenic chemokine IFNγ-inducible protein (IP-10) in ECs cultured with IL-12[95]. IL-12 significantly promotes apoptosis and inhibits proliferation rate of human tumors and extensive necrosis in the murine, and thereby reducing tumor vessel density[95]. Furthermore, the in vivo inhibition of neovascularization in IL-10-secreting tumors might be mediated by the ability of IL-10 to down-regulate the synthesis of VEGF, IL-1β, TNF-α, IL-6, and MMP-9 in tumor-associated macrophages[96]. And also, IL-10 inhibits tumor metastasis through a natural killer (NK) cell-dependent mechanism[96].
Tissue inhibitors of metalloproteinases Remodeled ECM components comprise a scaffold upon which ECs can adhere, migrate, and form tubes, and deposition of these components forms the basal lamina that ensheaths endothelial and mural cells. In vitro migration of ECs through gelatin is significantly inhibited by overexpressed TIMP-1[97]. Murphy et al[98] reported that TIMP-2, but not TIMP-1, inhibited bFGF-induced EC proliferation. TIMP-2 is able to inhibit soluble FGFR1 released by MMP-2[60]. Transfection of the highly metastatic B6F10 murine melanoma cell line with TIMP-2 cDNA showed the reduced levels of blood vessel formation and diminished induction of EC migration and invasion[99]. Studies have shown that the overexpression of TIMP-3 induces the apoptotic cell death of a number of cancer cell lines and rat vascular smooth muscle cells through the stabilization of TNF-alpha receptors on the cell surface, perhaps by inhibiting a receptor shedding metalloproteinase[100,101]. Furthermore, anti-angiogenic and antitumor effects of TIMP-3 appear to be mediated, in part, by decreased expression of vascular endothelial (VE)-cadherin by ECs in the presence of TIMP-3 in an in vitro assay and in TIMP-3-overexpressing tumors[102]. Finally, TIMP-1, TIMP-2, TIMP-3 and TIMP-4 inhibit neovascularization by inhibiting MMP-1, MMP-2, and MMP-9 induced breakdown of surrounding matrix[103]. Thus, the multiple effects of TIMPs on both endothelial and tumor cells migration render MMPs attractive targets for tumor therapy.
Proteolytic fragments Most of these fragments are derived from ECM components, such as collagen or fibronectin, or from enzymes such as plasminogen and MMP-2 that remodel ECM. Perhaps the most characterized inhibitors in this class are angiostatin and endostatin.
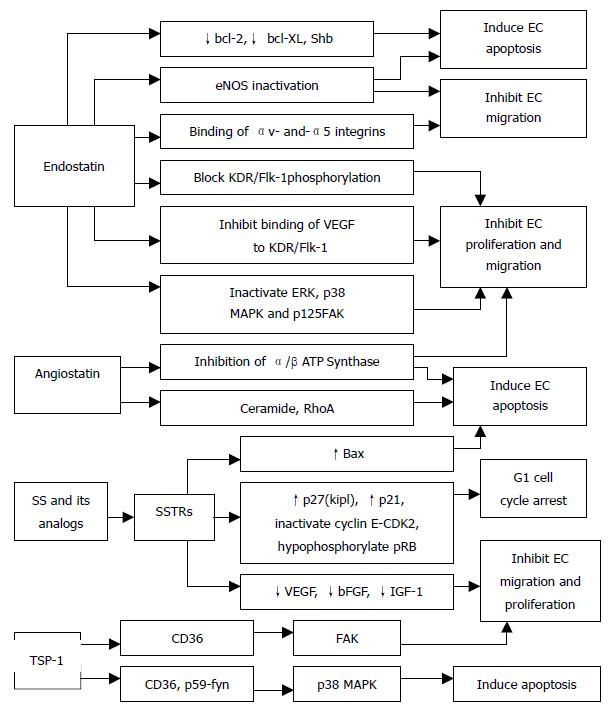
Angiostatin The anti-angiogenic effect of angiostatin, a 38-kDa internal fragment of plasminogen, may be mediated, at least in part, by their ability to down-regulate VEGF expression within the tumor[104]. Angiostatin inhibits hepatocyte growth factor (HGF)-induced phosphorylation of c-met, Akt, and ERK1/2, and thereby exerts its anti-angiogenic effect via disruption of HGF/c-met signaling[105]. Intraperitoneal administration of angiostatin potently inhibits the neovascularization and metastasis formation in mice observed after a primary tumor has been removed[106]. It has been shown that binding of angiostatin to the α/β-subunits of plasma membrane-localized ATP synthase may suppress endothelial-surface ATP metabolism and thereby mediates its anti-angiogenic effects and the down-regulation of EC proliferation and migration[107,108] (Figure 3). Further, adenoviral mediated angiostatin gene transfer selectively inhibits EC proliferation and disrupts the G2/M transition induced by M-phase-promoting factors, and that ECs show a significant mitosis arrest that is correlated with the down-regulation of the M-phase phosphoproteins[109]. Other studies have shown that angiostatin treatment significantly increases the apoptosis of EC and tumor cells, and decreases density of tumor blood vessels[109-111]. Angiostatin was found to produce a transient increase in ceramide that correlates with actin stress fiber reorganization, detachment and death[112] and, in addition, treatment with angiostatin or ceramide resulted in the activation of RhoA, an important effector of cytoskeletal structure[112] (Figure 3). Angiostatin can selectively regulate the expression of E-selectin and thereby inhibits the proliferation of ECs.
Endostatin It is a 20-kDa fragment of type XVIII collagen that has been identified as a factor produced by hemangioendothelioma cells that inhibits ECs proliferation, angiogenesis and tumor growth. The mechanisms by which endostatin inhibits VEGF-induced proliferation and migration of ECs are (Figure 3): First, endostatin blocks the VEGF-induced tyrosine phosphorylation of KDR/Flk-1 in ECs[113]. Second, endostatin suppresses the VEGF-induced activation of ERK, p38 MAPK, and p125FAK, which are downstream events of the KDR/Flk-1 signaling and are involved in the mitogenic and motogenic activities of VEGF in ECs[113]. Third, endostatin inhibits the binding of VEGF to ECs and to its cell surface receptor, KDR/Flk-1[113]. Finally, endostatin directly binds to KDR/Flk-1 but not to VEGF[113]. Endostatin was found to exhibit its anti-migratory effect by reducing VEGF-induced phosphorylation of endothelial NOS (eNOS)[114] (Figure 3). Rehn et al[115] demonstrated that soluble endostatin was capable of binding to αv- and α5-integrins, thereby inhibiting the integrin functions, such as EC migration (Figure 3). In addition, endostatin may exert its antiproliferative and anti-angiogenic effects by competing with bFGF for binding to cell surface heparan sulphate proteoglycans, which could disrupt the mitogenic growth factor signaling[116]. Endostatin induces a significant decrease in EC proliferation in the basal state and after stimulation by neuropeptide Y and bombesin[117]. Endostatin potently inhibits both the extracellular activation of proMMP-2 by inhibition of membrane-type 1 MMP (MT1-MMP) and the catalytic activity of MMP-2 and thereby can block the invasiveness of ECs and tumor cells[118]. The proapoptotic activity of endostatin appears to be mediated via tyrosine kinase signaling[119] and reduction of antiapoptotic proteins bcl-2 and bcl-XL without affecting the level of the proapoptotic Bax protein[120] (Figure 3). Furthermore, the Shb adaptor protein has been suggested to be involved in the mediation of the apoptotic signaling of endostatin[119] (Figure 3).
Somatostatin (SS) and its analogs inhibit the proliferation of somatostatin receptors (SSTRs) positive endocrine neoplasm. The antiproliferative action of SS is signaled via five specific G-protein coupled receptors (SSTR1-SSTR5), which initiate pertussis toxin sensitive-G protein dependent, and tyrosine phosphatase mediated cell growth arrest or apoptosis according to receptor subtypes and target cells. It has been shown that activation of SSTR1, 2, 4, and 5 induce G1 cell cycle arrest through the ability of SS to maintain high levels of CDKIs p27(Kip1) and p21, and inactivate cyclin E-CDK2 complexes, thus leading to hypophosphorylation of pRb[121,122](Figure 3). Moreover, somatostatin-mediated growth inhibition of normal and cancer pancreatic acinar cells is triggered via an inhibition of PI3-kinase signaling pathway[123]. SS may directly stimulate tumor apoptosis via sstr3-dependent G protein signaling, causing the induction of suppressor gene p53 and proapoptotic protein Bax[124](Figure 3). Our recent investigation reported that the low expression or loss of SSTR2 gene was more negatively correlated with the over-expression of p53 and ras mutation genes, which might take part in the angiogenesis of pancreatic neoplasm, whereas there was no significant relationship between SSTR2 and DPC4 (deleted in pancreatic cancer, locus 4), which suggested that there was different regulatory pathway in neovascularization of pancreatic neoplasm. Albini et al[125] provided evidence that SS inhibits Kaposi sarcoma associated angiogenesis by inhibiting both EC proliferation and invasion, and also by inhibiting migration of monocytes, which are important mediators of the angiogenic cascade, and are able to produce survival factors that, in turn, activate ECs. In addition, SS induces a significant decrease in basal and stimulates EC proliferation in HUVEC, and also decreases number of capillaries[117]. CAM model study showed that unlabeled SS analogs inhibited angiogenesis, which was proportional to the ability of the analogs to inhibit growth hormone (GH) production[126].
It is defined that SSTR subtypes are responsible for the specific post-receptor signal transduction mechanisms involved in octreotide's inhibition of angiogenesis[126]. The intracellular signal transduction mechanisms involved in this angiogenic inhibition include the G^sub i^-binding protein, cAMP, and calcium[127]. Further, SS and its analogs induce their biologic effects by interacting with specific receptors that are coupled to a variety of signal transduction pathways involving adenylate cyclase, guanylate cyclase, ionic conductance channels, phospholipase C-β, phospholipase A2, and tyrosine phosphatase and protein dephosphorylation and thereby regulate cell growth[128,129]. The best characterized pathway involves the inhibition of adenylate cyclase, leading to a reduction in intracellular cAMP levels. Antiproliferative effects that are mediated through SSTR1 and SSTR2, involve the stimulation of tyrosin phosphatases, however SSTR5 appears to be coupled to inositol phospholipid/calcium pathway[130]. Mentlein et al[131] reported that cultivated cells from solid human gliomas of different stages and glioma cell lines secreted variable amounts of VEGF, which was reduced between 25% and 80% of control levels depending on the glioma by co-incubation with SS or SSTR2-selective agonists (octreotide and L-054522) in dose-dependent manner. Growth factor-induced (EGF, bFGF) VEGF synthesis could also be suppressed to < 50% by co-incubation with SS or SSTR2-selective agonists, which was less pronounced in hypoxia-induced VEGF synthesis[131]. And also, SS and octreotide diminished the proliferative activity of cultured murine ECs HECa10 vs controls; however, SS and octreotide did not change the release of VEGF into supernatants of 24-h or 72-h EC cultures[132]. A recent study has demonstrated that SS 14 can reduce bFGF-induced corneal angiogenesis[133].
In summary, the mechanisms of action of tumor growth inhibition by SS and its analogs are[134]: (1) inhibition of the secretion of hormones, such as GH, insulin and/or gastrointestinal hormones; (2) direct or indirect (via GH) inhibition of IGF-1 and/or other growth factors that exert a stimulatory effect on tumor growth. On the other hand, SS analogs can selectively stimulate the formation of IGF-binding protein 1, and thereby interfering with IGF-1 action at the receptor level; (3) inhibition of angiogenesis through different mechanisms; (4) direct antimitotic effects of growth factors, which act on tyrosine kinase receptors such as EGF and FGF, via SSTRs on the tumor cells; (5) modulation of immunological activity.
Thrombospondin-1 (TSP-1) is a naturally occurring inhibitor of angiogenesis that limits vessel density in normal tissues and curtails tumor growth. TSP-1 exerts its anti-angiogenic activity via binding to the CD36 receptor by triggering an apoptotic signaling pathway[135]. Binding of TSP-1 to CD36 receptor leads to the recruitment of the Src-related kinase, p59-fyn, and to activation of p38 MAPK. The activation of the p38 MAPK has been shown to be p59-fyn-dependent and to require a caspase-3-like proteolytic activity[135]. Furthermore, activated p38 MAPK leads to the activation of caspase-3 and to apoptosis[135] (Figure 3). Interestingly, the apoptotic effect of TSP-1 is restricted to ECs activated to take part in the angiogenic process and not in quiescent vessels[135]. TSP-1 acts through CD36 to modulate the activity of focal adhesion kinase (FAK) and thus inhibits EC migration and proliferation[136] (Figure 3). TSP-1 can effectively inhibit chemotaxis in vitro and neovascularization in vivo, induced by several angiogenic stimuli. These include protein that acts via tyrosine kinase receptors (VEGF, bFGF, aFGF, PDGF), via G proteins (IL-8), via serine/threonine kinase receptors (TGF-β), and also lipids (PGE-1)[137,138].
Angiogenesis is a complex process that depends on the coordination of many different activities in several cell types. The angiogenic response in the microvasculature is associated with changes in cellular adhesive interactions between adjacent ECs, pericytes, fibroblasts, and immune mediators express many different cytokines and growth factors that react with other cells or ECM components to affect ECs migration, proliferation, tube formation, and vessel stabilization. As one or more of the positive regulators of angiogenesis are up-regulated, and simultaneously, certain negative regulators of angiogenesis are down-regulated, tumors become angiogenic. Interestingly, different angiogenic regulators, sometimes, function through the same mechanism and a single angiogenic regulator, sometimes, functions through different mechanisms. Hence, the anti-angiogenic therapy can be realized through the regulation of 'angiogenic switch' by interfering with different mechanisms.
Anti-angiogenic agents, if administered before a tumor develops or becomes vascular supply dependent, would therefore theoretically act similarly to a vaccine in preventing tumor development, not just tumor growth. However, it is notable that anti-angiogenic therapy represents a treatment, not a cure, for cancer. A cure for cancer can be realized only by targeting the agents and mechanisms that cause normal cells to become tumorigenic. The anti-angiogenic therapy of cancer, nonetheless, represents a highly effective strategy for destroying tumors because fundamental requirement of tumor growth is dependent on a blood supply. Unlike standard chemotherapy that targets tumor cells and other proliferating cells, angiogenesis inhibitors target dividing ECs that have been recruited into the tumor bed. For example, certain tubulin-binding agents such as combretastin A-4, exhibit a selective toxicity for proliferating ECs in vitro and causing a vascular collapse in tumor models in vivo via apoptosis and the subsequent death of much larger numbers of tumor cells[139]. Thus, specific anti-angiogenic therapy has little or no toxicities such as gastrointestinal symptoms and myelosuppression that are characteristic of standard chemotherapeutic regimens, does not require that the therapeutic agent enter any tumor cells nor cross the blood brain barrier, controls tumor growth independently of growth fraction or tumor cell heterogeneity or even tumor cell type, and does not induce acquired drug resistance[140]. Further, since normal vasculature in the adult is quiescent, the appropriate use of selective angiogenic inhibitors may be expected to confer a degree of specificity that is not obtainable with the nonspecific modalities of chemotherapy and radiation therapy and to allow for relatively nontoxic, long-term treatment of tumors.
Because anti-angiogenic agents are expected to be cytostatic rather than cytotoxic, they may be particularly effective in combination with cytotoxic agents, even used in advanced cases of pancreatic, colon, and hormone-refractory prostate cancer, thereby targeting not only DNA synthesis and cell division but also the biologic behavior of tumor cells. The following guidelines are suggested to improve the therapeutic efficacy of endogenous angiogenesis inhibitors in clinical trials: (1) after surgery or radiotherapy to prevent recurrence of distant metastases; (2) combinatorial therapies, for example, in combination with conventional chemotherapy, radiotherapy and vaccine therapy or immunotherapy, and also, in combination with several angiogenesis inhibitors rather than a single inhibitor; (3) targeting therapy. Angiogenesis inhibitors may be specifically targeted to the disease locus at high concentrations rather than be widely distributed in the entire body; (4) gene therapy, several advantages including prolonged therapy, low doses of DNA molecules, and less frequent injections may be achieved by anti-angiogenic gene therapy with endogenous angiogenesis inhibitors; (5) more potent angiogenesis inhibitors should be discovered; (6) prolonged half-lives. Slow-release of angiogenesis inhibitors in the body reaches a steady-state level in the circulation.
Remarkably diverse groups of anti-angiogenic drugs are currently undergoing evaluation in phase I, II or III clinical trials. However, there are still some difficulties associated with the clinical evaluation of these drugs efficacy. In the experimental animal model, tumors can be removed and examined for therapeutic efficacy such as changes in the extent of vascularization, vascular structure, EC viability or apoptosis, as well as for markers of angiogenic activity, e.g. VEGF expression. But in the clinical situation, taking serial biopsies of metastatic tumors may not be a particularly practical or desirable approach. For this, reliable surrogate markers of tumor angiogenesis in serum or urine, and non-invasive strategy may be necessary. Several studies have successfully used various non-invasive medical imaging strategies (e.g. MRI, Doppler ultrasound) to monitor changes in tumor blood flow, vascular structure and permeability[141-143]. Indeed, there are considerable research efforts underway in this field. In addition, there are obvious concerns about delayed toxicity associated with long-term anti-angiogenic therapy, and physiological angiogenesis affected by anti-angiogenic drugs such as wound healing in a cancer patient, reproductive angiogenesis (e.g. corpus luteum development in adult females, development of the vasculature in developing embryos), in neonates and children. In this concern, a potentially significant development in the near future could be the use of genomics based technologies to uncover a large number of highly (or even totally) specific molecular markers for the activated ECs of newly formed blood vessels.
In the near future, the outcome of ongoing clinical trials will give us more insights into the potential of anti-angiogenic approaches to treat cancer.
Edited by Wu XN
| 1. | Bussolino F, Mantovani A, Persico G. Molecular mechanisms of blood vessel formation. Trends Biochem Sci. 1997;22:251-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 330] [Cited by in F6Publishing: 335] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 2. | Sebti SM, Hamilton AD. Design of growth factor antagonists with antiangiogenic and antitumor properties. Oncogene. 2000;19:6566-6573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994;265:1582-1584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1008] [Cited by in F6Publishing: 1073] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 4. | Kohn S, Nagy JA, Dvorak HF, Dvorak AM. Pathways of macromolecular tracer transport across venules and small veins. Structural basis for the hyperpermeability of tumor blood vessels. Lab Invest. 1992;67:596-607. [PubMed] [Cited in This Article: ] |
| 5. | Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci. 1998;111:1853-1865. [PubMed] [Cited in This Article: ] |
| 6. | Kevil CG, Payne DK, Mire E, Alexander JS. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J Biol Chem. 1998;273:15099-15103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 233] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Lal BK, Varma S, Pappas PJ, Hobson RW, Durán WN. VEGF increases permeability of the endothelial cell monolayer by activation of PKB/akt, endothelial nitric-oxide synthase, and MAP kinase pathways. Microvasc Res. 2001;62:252-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 2001;280:C1358-C1366. [PubMed] [Cited in This Article: ] |
| 9. | Doanes AM, Hegland DD, Sethi R, Kovesdi I, Bruder JT, Finkel T. VEGF stimulates MAPK through a pathway that is unique for receptor tyrosine kinases. Biochem Biophys Res Commun. 1999;255:545-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Mandriota SJ, Seghezzi G, Vassalli JD, Ferrara N, Wasi S, Mazzieri R, Mignatti P, Pepper MS. Vascular endothelial growth factor increases urokinase receptor expression in vascular endothelial cells. J Biol Chem. 1995;270:9709-9716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 209] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Pepper MS, Ferrara N, Orci L, Montesano R. Vascular endothelial growth factor (VEGF) induces plasminogen activators and plasminogen activator inhibitor-1 in microvascular endothelial cells. Biochem Biophys Res Commun. 1991;181:902-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 412] [Cited by in F6Publishing: 439] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Unemori EN, Ferrara N, Bauer EA, Amento EP. Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J Cell Physiol. 1992;153:557-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 346] [Cited by in F6Publishing: 375] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 13. | Tang H, Kerins DM, Hao Q, Inagami T, Vaughan DE. The urokinase-type plasminogen activator receptor mediates tyrosine phosphorylation of focal adhesion proteins and activation of mitogen-activated protein kinase in cultured endothelial cells. J Biol Chem. 1998;273:18268-18272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 119] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M. Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc Natl Acad Sci U S A. 1997;94:13612-13617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 352] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 15. | Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol. 1996;149:293-305. [PubMed] [Cited in This Article: ] |
| 16. | Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2935] [Cited by in F6Publishing: 2813] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 17. | Watanabe Y, Lee SW, Detmar M, Ajioka I, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) delays and induces escape from senescence in human dermal microvascular endothelial cells. Oncogene. 1997;14:2025-2032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 98] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273:13313-13316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 687] [Cited by in F6Publishing: 723] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 19. | Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336-30343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1473] [Cited by in F6Publishing: 1552] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 20. | Wheeler-Jones C, Abu-Ghazaleh R, Cospedal R, Houliston RA, Martin J, Zachary I. Vascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase A2 in endothelial cells via p42/p44 mitogen-activated protein kinase. FEBS Lett. 1997;420:28-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 202] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Berra E, Milanini J, Richard DE, Le Gall M, Viñals F, Gothié E, Roux D, Pagès G, Pouysségur J. Signaling angiogenesis via p42/p44 MAP kinase and hypoxia. Biochem Pharmacol. 2000;60:1171-1178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N, Shen Q, O'Hagan R, Pantginis J, Zhou H. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400:468-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 681] [Cited by in F6Publishing: 715] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 23. | Khwaja A. Akt is more than just a Bad kinase. Nature. 1999;401:33-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 221] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349-16354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 460] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 25. | Morales-Ruiz M, Fulton D, Sowa G, Languino LR, Fujio Y, Walsh K, Sessa WC. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ Res. 2000;86:892-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 307] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 26. | Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997;90:3322-3331. [PubMed] [Cited in This Article: ] |
| 27. | Dimmeler S, Zeiher AM. Akt takes center stage in angiogenesis signaling. Circ Res. 2000;86:4-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 194] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci U S A. 2000;97:1749-1753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 415] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 29. | Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2723] [Cited by in F6Publishing: 2678] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 30. | Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2015] [Cited by in F6Publishing: 2023] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 31. | Gupta K, Kshirsagar S, Li W, Gui L, Ramakrishnan S, Gupta P, Law PY, Hebbel RP. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res. 1999;247:495-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 292] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 32. | Dimmeler S, Dernbach E, Zeiher AM. Phosphorylation of the endothelial nitric oxide synthase at ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett. 2000;477:258-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 293] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 33. | Büchler P, Reber HA, Büchler M, Shrinkante S, Büchler MW, Friess H, Semenza GL, Hines OJ. Hypoxia-inducible factor 1 regulates vascular endothelial growth factor expression in human pancreatic cancer. Pancreas. 2003;26:56-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 34. | Pardo OE, Arcaro A, Salerno G, Raguz S, Downward J, Seckl MJ. Fibroblast growth factor-2 induces translational regulation of Bcl-XL and Bcl-2 via a MEK-dependent pathway: correlation with resistance to etoposide-induced apoptosis. J Biol Chem. 2002;277:12040-12046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, Huber J, Nakatani T, Tsujinoue H, Yanase K. Synergistic effect of basic fibroblast growth factor and vascular endothelial growth factor in murine hepatocellular carcinoma. Hepatology. 2002;35:834-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Stavri GT, Zachary IC, Baskerville PA, Martin JF, Erusalimsky JD. Basic fibroblast growth factor upregulates the expression of vascular endothelial growth factor in vascular smooth muscle cells. Synergistic interaction with hypoxia. Circulation. 1995;92:11-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 255] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 37. | Mandriota SJ, Pepper MS. Vascular endothelial growth factor-induced in vitro angiogenesis and plasminogen activator expression are dependent on endogenous basic fibroblast growth factor. J Cell Sci. 1997;110:2293-2302. [PubMed] [Cited in This Article: ] |
| 38. | Sato Y, Rifkin DB. Autocrine activities of basic fibroblast growth factor: regulation of endothelial cell movement, plasminogen activator synthesis, and DNA synthesis. J Cell Biol. 1988;107:1199-1205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 536] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 39. | Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977;14:53-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 914] [Cited by in F6Publishing: 858] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 40. | Miao HQ, Ornitz DM, Aingorn E, Ben-Sasson SA, Vlodavsky I. Modulation of fibroblast growth factor-2 receptor binding, dimerization, signaling, and angiogenic activity by a synthetic heparin-mimicking polyanionic compound. J Clin Invest. 1997;99:1565-1575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A. 2002;99:11205-11210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 478] [Cited by in F6Publishing: 492] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 42. | Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3'-Kinase/Akt signal transduction pathway. Circ Res. 1998;86:24-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 441] [Cited by in F6Publishing: 453] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 43. | Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O'Connor DS, Li F, Altieri DC, Sessa WC. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102-9105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 468] [Cited by in F6Publishing: 475] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 44. | Harfouche R, Hasséssian HM, Guo Y, Faivre V, Srikant CB, Yancopoulos GD, Hussain SN. Mechanisms which mediate the antiapoptotic effects of angiopoietin-1 on endothelial cells. Microvasc Res. 2002;64:135-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Kim I, Kim HG, Moon SO, Chae SW, So JN, Koh KN, Ahn BC, Koh GY. Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res. 2000;86:952-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 46. | Laping NJ, Grygielko E, Mathur A, Butter S, Bomberger J, Tweed C, Martin W, Fornwald J, Lehr R, Harling J. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002;62:58-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 497] [Cited by in F6Publishing: 490] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 47. | Lawrence DA, Pircher R, Jullien P. Conversion of a high molecular weight latent beta-TGF from chicken embryo fibroblasts into a low molecular weight active beta-TGF under acidic conditions. Biochem Biophys Res Commun. 1985;133:1026-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 289] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 48. | Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988;106:1659-1665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 659] [Cited by in F6Publishing: 706] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 49. | Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol. 1990;110:1361-1367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 543] [Cited by in F6Publishing: 574] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 50. | Sato Y, Tsuboi R, Lyons R, Moses H, Rifkin DB. Characterization of the activation of latent TGF-beta by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J Cell Biol. 1990;111:757-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 341] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 51. | Mandriota SJ, Menoud PA, Pepper MS. Transforming growth factor beta 1 down-regulates vascular endothelial growth factor receptor 2/flk-1 expression in vascular endothelial cells. J Biol Chem. 1996;271:11500-11505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 126] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Saito H, Tsujitani S, Oka S, Kondo A, Ikeguchi M, Maeta M, Kaibara N. The expression of transforming growth factor-beta1 is significantly correlated with the expression of vascular endothelial growth factor and poor prognosis of patients with advanced gastric carcinoma. Cancer. 1999;86:1455-1462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 53. | O'Mahony CA, Albo D, Tuszynski GP, Berger DH. Transforming growth factor-beta 1 inhibits generation of angiostatin by human pancreatic cancer cells. Surgery. 1998;124:388-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Sherr CJ. Cancer cell cycles. Science. 1996;274:1672-1677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3965] [Cited by in F6Publishing: 3946] [Article Influence: 140.9] [Reference Citation Analysis (0)] |
| 55. | Pepper MS, Belin D, Montesano R, Orci L, Vassalli JD. Transforming growth factor-beta 1 modulates basic fibroblast growth factor-induced proteolytic and angiogenic properties of endothelial cells in vitro. J Cell Biol. 1990;111:743-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 368] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 56. | Ellenrieder V, Hendler SF, Ruhland C, Boeck W, Adler G, Gress TM. TGF-beta-induced invasiveness of pancreatic cancer cells is mediated by matrix metalloproteinase-2 and the urokinase plasminogen activator system. Int J Cancer. 2001;93:204-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 57. | Yuan A, Yang PC, Yu CJ, Chen WJ, Lin FY, Kuo SH, Luh KT. Interleukin-8 messenger ribonucleic acid expression correlates with tumor progression, tumor angiogenesis, patient survival, and timing of relapse in non-small-cell lung cancer. Am J Respir Crit Care Med. 2000;162:1957-1963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 132] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 58. | Bar-Eli M. Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology. 1999;67:12-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 187] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 59. | Kitadai Y, Takahashi Y, Haruma K, Naka K, Sumii K, Yokozaki H, Yasui W, Mukaida N, Ohmoto Y, Kajiyama G. Transfection of interleukin-8 increases angiogenesis and tumorigenesis of human gastric carcinoma cells in nude mice. Br J Cancer. 1999;81:647-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 123] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Levi E, Fridman R, Miao HQ, Ma YS, Yayon A, Vlodavsky I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci U S A. 1996;93:7069-7074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 260] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 61. | Suzuki M, Raab G, Moses MA, Fernandez CA, Klagsbrun M. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J Biol Chem. 1997;272:31730-31737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 223] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 62. | Ray JM, Stetler-Stevenson WG. Gelatinase A activity directly modulates melanoma cell adhesion and spreading. EMBO J. 1995;14:908-917. [PubMed] [Cited in This Article: ] |
| 63. | Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell. 1998;92:391-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 465] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 64. | Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163-176. [PubMed] [Cited in This Article: ] |
| 65. | Rak J, Filmus J, Finkenzeller G, Grugel S, Marmé D, Kerbel RS. Oncogenes as inducers of tumor angiogenesis. Cancer Metastasis Rev. 1995;14:263-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 170] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 66. | Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, Moghaddam A, Whitehouse R, Bicknell R, Harris AL. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963-969. [PubMed] [Cited in This Article: ] |
| 67. | Mazure NM, Chen EY, Yeh P, Laderoute KR, Giaccia AJ. Oncogenic transformation and hypoxia synergistically act to modulate vascular endothelial growth factor expression. Cancer Res. 1996;56:3436-3440. [PubMed] [Cited in This Article: ] |
| 68. | Chiarugi V, Magnelli L, Gallo O. Cox-2, iNOS and p53 as play-makers of tumor angiogenesis (review). Int J Mol Med. 1998;2:715-719. [PubMed] [Cited in This Article: ] |
| 69. | Kieser A, Weich HA, Brandner G, Marmé D, Kolch W. Mutant p53 potentiates protein kinase C induction of vascular endothelial growth factor expression. Oncogene. 1994;9:963-969. [PubMed] [Cited in This Article: ] |
| 70. | Tai YT, Podar K, Gupta D, Lin B, Young G, Akiyama M, Anderson KC. CD40 activation induces p53-dependent vascular endothelial growth factor secretion in human multiple myeloma cells. Blood. 2002;99:1419-1427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | Galy B, Créancier L, Prado-Lourenço L, Prats AC, Prats H. p53 directs conformational change and translation initiation blockade of human fibroblast growth factor 2 mRNA. Oncogene. 2001;20:4613-4620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Sherif ZA, Nakai S, Pirollo KF, Rait A, Chang EH. Downmodulation of bFGF-binding protein expression following restoration of p53 function. Cancer Gene Ther. 2001;8:771-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Toschi E, Rota R, Antonini A, Melillo G, Capogrossi MC. Wild-type p53 gene transfer inhibits invasion and reduces matrix metalloproteinase-2 levels in p53-mutated human melanoma cells. J Invest Dermatol. 2000;114:1188-1194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304-6311. [PubMed] [Cited in This Article: ] |
| 75. | Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991;352:345-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1402] [Cited by in F6Publishing: 1556] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 76. | Eckhardt SG. Angiogenesis inhibitors as cancer therapy. Hosp Pract (1995). 1999;34:63-68, 77-79, 83-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 77. | Oehler MK, Bicknell R. The promise of anti-angiogenic cancer therapy. Br J Cancer. 2000;82:749-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Zhang W, Ran S, Sambade M, Huang X, Thorpe PE. A monoclonal antibody that blocks VEGF binding to VEGFR2 (KDR/Flk-1) inhibits vascular expression of Flk-1 and tumor growth in an orthotopic human breast cancer model. Angiogenesis. 2002;5:35-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Witte L, Hicklin DJ, Zhu Z, Pytowski B, Kotanides H, Rockwell P, Böhlen P. Monoclonal antibodies targeting the VEGF receptor-2 (Flk1/KDR) as an anti-angiogenic therapeutic strategy. Cancer Metastasis Rev. 1998;17:155-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 256] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 80. | Im SA, Gomez-Manzano C, Fueyo J, Liu TJ, Ke LD, Kim JS, Lee HY, Steck PA, Kyritsis AP, Yung WK. Antiangiogenesis treatment for gliomas: transfer of antisense-vascular endothelial growth factor inhibits tumor growth in vivo. Cancer Res. 1999;59:895-900. [PubMed] [Cited in This Article: ] |
| 81. | Zhang L, Gasper WJ, Stass SA, Ioffe OB, Davis MA, Mixson AJ. Angiogenic inhibition mediated by a DNAzyme that targets vascular endothelial growth factor receptor 2. Cancer Res. 2002;62:5463-5469. [PubMed] [Cited in This Article: ] |
| 82. | Feldman AL, Libutti SK. Progress in antiangiogenic gene therapy of cancer. Cancer. 2000;89:1181-1194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 83. | Slaton JW, Perrotte P, Inoue K, Dinney CP, Fidler IJ. Interferon-alpha-mediated down-regulation of angiogenesis-related genes and therapy of bladder cancer are dependent on optimization of biological dose and schedule. Clin Cancer Res. 1999;5:2726-2734. [PubMed] [Cited in This Article: ] |
| 84. | Izawa JI, Sweeney P, Perrotte P, Kedar D, Dong Z, Slaton JW, Karashima T, Inoue K, Benedict WF, Dinney CP. Inhibition of tumorigenicity and metastasis of human bladder cancer growing in athymic mice by interferon-beta gene therapy results partially from various antiangiogenic effects including endothelial cell apoptosis. Clin Cancer Res. 2002;8:1258-1270. [PubMed] [Cited in This Article: ] |
| 85. | Ozawa S, Shinohara H, Kanayama HO, Bruns CJ, Bucana CD, Ellis LM, Davis DW, Fidler IJ. Suppression of angiogenesis and therapy of human colon cancer liver metastasis by systemic administration of interferon-alpha. Neoplasia. 2001;3:154-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 86. | Bauvois B, Dumont J, Mathiot C, Kolb JP. Production of matrix metalloproteinase-9 in early stage B-CLL: suppression by interferons. Leukemia. 2002;16:791-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 87. | Marler JJ, Rubin JB, Trede NS, Connors S, Grier H, Upton J, Mulliken JB, Folkman J. Successful antiangiogenic therapy of giant cell angioblastoma with interferon alfa 2b: report of 2 cases. Pediatrics. 2002;109:E37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 88. | Sasamura H, Takahashi A, Miyao N, Yanase M, Masumori N, Kitamura H, Itoh N, Tsukamoto T. Inhibitory effect on expression of angiogenic factors by antiangiogenic agents in renal cell carcinoma. Br J Cancer. 2002;86:768-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 89. | Nilsson A, Janson ET, Eriksson B, Larsson A. Levels of angiogenic peptides in sera from patients with carcinoid tumours during alpha-interferon treatment. Anticancer Res. 2001;21:4087-4090. [PubMed] [Cited in This Article: ] |
| 90. | Jonasch E, Haluska FG. Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist. 2001;6:34-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 382] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 91. | Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348-27357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 897] [Cited by in F6Publishing: 862] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 92. | Volpert OV, Fong T, Koch AE, Peterson JD, Waltenbaugh C, Tepper RI, Bouck NP. Inhibition of angiogenesis by interleukin 4. J Exp Med. 1998;188:1039-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 184] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 93. | Torisu H, Ono M, Kiryu H, Furue M, Ohmoto Y, Nakayama J, Nishioka Y, Sone S, Kuwano M. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFalpha and IL-1alpha. Int J Cancer. 2000;85:182-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 94. | Oshikawa K, Rakhmilevich AL, Shi F, Sondel PM, Yang N, Mahvi DM. Interleukin 12 gene transfer into skin distant from the tumor site elicits antimetastatic effects equivalent to local gene transfer. Hum Gene Ther. 2001;12:149-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Duda DG, Sunamura M, Lozonschi L, Kodama T, Egawa S, Matsumoto G, Shimamura H, Shibuya K, Takeda K, Matsuno S. Direct in vitro evidence and in vivo analysis of the antiangiogenesis effects of interleukin 12. Cancer Res. 2000;60:1111-1116. [PubMed] [Cited in This Article: ] |
| 96. | Huang S, Ullrich SE, Bar-Eli M. Regulation of tumor growth and metastasis by interleukin-10: the melanoma experience. J Interferon Cytokine Res. 1999;19:697-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 97. | Fernandez HA, Kallenbach K, Seghezzi G, Grossi E, Colvin S, Schneider R, Mignatti P, Galloway A. Inhibition of endothelial cell migration by gene transfer of tissue inhibitor of metalloproteinases-1. J Surg Res. 1999;82:156-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 98. | Murphy AN, Unsworth EJ, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinases-2 inhibits bFGF-induced human microvascular endothelial cell proliferation. J Cell Physiol. 1993;157:351-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 248] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 99. | Valente P, Fassina G, Melchiori A, Masiello L, Cilli M, Vacca A, Onisto M, Santi L, Stetler-Stevenson WG, Albini A. TIMP-2 over-expression reduces invasion and angiogenesis and protects B16F10 melanoma cells from apoptosis. Int J Cancer. 1998;75:246-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 100. | Smith MR, Kung H, Durum SK, Colburn NH, Sun Y. TIMP-3 induces cell death by stabilizing TNF-alpha receptors on the surface of human colon carcinoma cells. Cytokine. 1997;9:770-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 155] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 101. | Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest. 1998;101:1478-1487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 330] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 102. | Spurbeck WW, Ng CY, Strom TS, Vanin EF, Davidoff AM. Enforced expression of tissue inhibitor of matrix metalloproteinase-3 affects functional capillary morphogenesis and inhibits tumor growth in a murine tumor model. Blood. 2002;100:3361-3368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 103. | Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111-122. [PubMed] [Cited in This Article: ] |
| 104. | Hajitou A, Grignet C, Devy L, Berndt S, Blacher S, Deroanne CF, Bajou K, Fong T, Chiang Y, Foidart JM. The antitumoral effect of endostatin and angiostatin is associated with a down-regulation of vascular endothelial growth factor expression in tumor cells. FASEB J. 2002;16:1802-1804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 105. | Wajih N, Sane DC. Angiostatin selectively inhibits signaling by hepatocyte growth factor in endothelial and smooth muscle cells. Blood. 2003;101:1857-1863. [PubMed] [Cited in This Article: ] |
| 106. | Cao Y, Ji RW, Davidson D, Schaller J, Marti D, Söhndel S, McCance SG, O'Reilly MS, Llinás M, Folkman J. Kringle domains of human angiostatin. Characterization of the anti-proliferative activity on endothelial cells. J Biol Chem. 1996;271:29461-29467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 277] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 107. | Moser TL, Stack MS, Asplin I, Enghild JJ, Højrup P, Everitt L, Hubchak S, Schnaper HW, Pizzo SV. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci U S A. 1999;96:2811-2816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 373] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 108. | Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK, Cheek DJ, Pizzo SV. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci U S A. 2001;98:6656-6661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 109. | Griscelli F, Li H, Bennaceur-Griscelli A, Soria J, Opolon P, Soria C, Perricaudet M, Yeh P, Lu H. Angiostatin gene transfer: inhibition of tumor growth in vivo by blockage of endothelial cell proliferation associated with a mitosis arrest. Proc Natl Acad Sci U S A. 1998;95:6367-6372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 195] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 110. | Sun X, Kanwar JR, Leung E, Lehnert K, Wang D, Krissansen GW. Angiostatin enhances B7.1-mediated cancer immunotherapy independently of effects on vascular endothelial growth factor expression. Cancer Gene Ther. 2001;8:719-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Lucas R, Holmgren L, Garcia I, Jimenez B, Mandriota SJ, Borlat F, Sim BK, Wu Z, Grau GE, Shing Y. Multiple forms of angiostatin induce apoptosis in endothelial cells. Blood. 1998;92:4730-4741. [PubMed] [Cited in This Article: ] |
| 112. | Gupta N, Nodzenski E, Khodarev NN, Yu J, Khorasani L, Beckett MA, Kufe DW, Weichselbaum RR. Angiostatin effects on endothelial cells mediated by ceramide and RhoA. EMBO Rep. 2001;2:536-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 113. | Kim YM, Hwang S, Kim YM, Pyun BJ, Kim TY, Lee ST, Gho YS, Kwon YG. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J Biol Chem. 2002;277:27872-27879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 309] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 114. | Urbich C, Reissner A, Chavakis E, Dernbach E, Haendeler J, Fleming I, Zeiher AM, Kaszkin M, Dimmeler S. Dephosphorylation of endothelial nitric oxide synthase contributes to the anti-angiogenic effects of endostatin. FASEB J. 2002;16:706-708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 115. | Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo C, Pihlajaniemi T, Alitalo K, Vuori K. Interaction of endostatin with integrins implicated in angiogenesis. Proc Natl Acad Sci U S A. 2001;98:1024-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 269] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 116. | Hohenester E, Sasaki T, Olsen BR, Timpl R. Crystal structure of the angiogenesis inhibitor endostatin at 1.5 A resolution. EMBO J. 1998;17:1656-1664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 166] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 117. | Marion-Audibert AM, Nejjari M, Pourreyron C, Anderson W, Gouysse G, Jacquier MF, Dumortier J, Scoazec JY. [Effects of endocrine peptides on proliferation, migration and differentiation of human endothelial cells]. Gastroenterol Clin Biol. 2000;24:644-648. [PubMed] [Cited in This Article: ] |
| 118. | Kim YM, Jang JW, Lee OH, Yeon J, Choi EY, Kim KW, Lee ST, Kwon YG. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase. Cancer Res. 2000;60:5410-5413. [PubMed] [Cited in This Article: ] |
| 119. | Dixelius J, Larsson H, Sasaki T, Holmqvist K, Lu L, Engström A, Timpl R, Welsh M, Claesson-Welsh L. Endostatin-induced tyrosine kinase signaling through the Shb adaptor protein regulates endothelial cell apoptosis. Blood. 2000;95:3403-3411. [PubMed] [Cited in This Article: ] |
| 120. | Dhanabal M, Ramchandran R, Waterman MJ, Lu H, Knebelmann B, Segal M, Sukhatme VP. Endostatin induces endothelial cell apoptosis. J Biol Chem. 1999;274:11721-11726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 415] [Cited by in F6Publishing: 422] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 121. | Pagès P, Benali N, Saint-Laurent N, Estève JP, Schally AV, Tkaczuk J, Vaysse N, Susini C, Buscail L. sst2 somatostatin receptor mediates cell cycle arrest and induction of p27(Kip1). Evidence for the role of SHP-1. J Biol Chem. 1999;274:15186-15193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 122. | Sharma K, Patel YC, Srikant CB. C-terminal region of human somatostatin receptor 5 is required for induction of Rb and G1 cell cycle arrest. Mol Endocrinol. 1999;13:82-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 123. | Charland S, Boucher MJ, Houde M, Rivard N. Somatostatin inhibits Akt phosphorylation and cell cycle entry, but not p42/p44 mitogen-activated protein (MAP) kinase activation in normal and tumoral pancreatic acinar cells. Endocrinology. 2001;142:121-128. [PubMed] [DOI] [Cited in This Article: ] |
| 124. | Sharma K, Patel YC, Srikant CB. Subtype-selective induction of wild-type p53 and apoptosis, but not cell cycle arrest, by human somatostatin receptor 3. Mol Endocrinol. 1996;10:1688-1696. [PubMed] [Cited in This Article: ] |
| 125. | Albini A, Florio T, Giunciuglio D, Masiello L, Carlone S, Corsaro A, Thellung S, Cai T, Noonan DM, Schettini G. Somatostatin controls Kaposi's sarcoma tumor growth through inhibition of angiogenesis. FASEB J. 1999;13:647-655. [PubMed] [Cited in This Article: ] |
| 126. | Woltering EA, Watson JC, Alperin-Lea RC, Sharma C, Keenan E, Kurozawa D, Barrie R. Somatostatin analogs: angiogenesis inhibitors with novel mechanisms of action. Invest New Drugs. 1997;15:77-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 127. | Gulec SA, Gaffga CM, Anthony CT, Su LJ, O'Leary JP, Woltering EA. Antiangiogenic therapy with somatostatin receptor-mediated in situ radiation. Am Surg. 2001;67:1068-1071. [PubMed] [Cited in This Article: ] |
| 128. | Patel YC. Molecular pharmacology of somatostatin receptor subtypes. J Endocrinol Invest. 1997;20:348-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 185] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 129. | Lewin MJ. The somatostatin receptor in the GI tract. Annu Rev Physiol. 1992;54:455-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 130. | Buscail L, Estève JP, Saint-Laurent N, Bertrand V, Reisine T, O'Carroll AM, Bell GI, Schally AV, Vaysse N, Susini C. Inhibition of cell proliferation by the somatostatin analogue RC-160 is mediated by somatostatin receptor subtypes SSTR2 and SSTR5 through different mechanisms. Proc Natl Acad Sci U S A. 1995;92:1580-1584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 202] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 131. | Mentlein R, Eichler O, Forstreuter F, Held-Feindt J. Somatostatin inhibits the production of vascular endothelial growth factor in human glioma cells. Int J Cancer. 2001;92:545-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 132. | Lawnicka H, Stepień H, Wyczółkowska J, Kolago B, Kunert-Radek J, Komorowski J. Effect of somatostatin and octreotide on proliferation and vascular endothelial growth factor secretion from murine endothelial cell line (HECa10) culture. Biochem Biophys Res Commun. 2000;268:567-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 133. | Wu PC, Liu CC, Chen CH, Kou HK, Shen SC, Lu CY, Chou WY, Sung MT, Yang LC. Inhibition of experimental angiogenesis of cornea by somatostatin. Graefes Arch Clin Exp Ophthalmol. 2003;241:63-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 134. | Lamberts SW, Krenning EP, Reubi JC. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr Rev. 1991;12:450-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 559] [Cited by in F6Publishing: 511] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 135. | Jiménez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 742] [Cited by in F6Publishing: 738] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 136. | Gilmore AP, Romer LH. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996;7:1209-1224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 404] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 137. | Volpert OV, Lawler J, Bouck NP. A human fibrosarcoma inhibits systemic angiogenesis and the growth of experimental metastases via thrombospondin-1. Proc Natl Acad Sci U S A. 1998;95:6343-6348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 132] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 138. | Panetti TS, Chen H, Misenheimer TM, Getzler SB, Mosher DF. Endothelial cell mitogenesis induced by LPA: inhibition by thrombospondin-1 and thrombospondin-2. J Lab Clin Med. 1997;129:208-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 139. | Iyer S, Chaplin DJ, Rosenthal DS, Boulares AH, Li LY, Smulson ME. Induction of apoptosis in proliferating human endothelial cells by the tumor-specific antiangiogenesis agent combretastatin A-4. Cancer Res. 1998;58:4510-4514. [PubMed] [Cited in This Article: ] |
| 140. | Boehm T, Folkman J, Browder T, O'Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1201] [Cited by in F6Publishing: 1123] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 141. | Jayson GC, Zweit J, Jackson A, Mulatero C, Julyan P, Ranson M, Broughton L, Wagstaff J, Hakannson L, Groenewegen G. Molecular imaging and biological evaluation of HuMV833 anti-VEGF antibody: implications for trial design of antiangiogenic antibodies. J Natl Cancer Inst. 2002;94:1484-1493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 213] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 142. | Gossmann A, Helbich TH, Kuriyama N, Ostrowitzki S, Roberts TP, Shames DM, van Bruggen N, Wendland MF, Israel MA, Brasch RC. Dynamic contrast-enhanced magnetic resonance imaging as a surrogate marker of tumor response to anti-angiogenic therapy in a xenograft model of glioblastoma multiforme. J Magn Reson Imaging. 2002;15:233-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 143. | Kim SW, Park SS, Ahn SJ, Chung KW, Moon WK, Im JG, Yeo JS, Chung JK, Noh DY. Identification of angiogenesis in primary breast carcinoma according to the image analysis. Breast Cancer Res Treat. 2002;74:121-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |