Published online Apr 15, 2003. doi: 10.3748/wjg.v9.i4.800
Revised: November 21, 2002
Accepted: November 28, 2002
Published online: April 15, 2003
AIM: To study the detail mechanism of interaction between PKC and GRK2 and the effect of GRK2 on activity of PKC.
METHODS: The cDNA of pleckstrin homology (PH) domain located in GRK2 residue 548 to 660 was amplified by PCR with the mRNA of human GRK2 (β1-adrenergic receptor kinase) as template isolated from human fresh placenta, the expression vector pGEX-PH inserted with the aboved cDNA sequence for GRK2 PH domain protein and the expression vectors for GST (glutathion-s-transferase) -GRK2 PH domain fusion protein, BTK (Bruton’s tyrosine kinase) PH domain and GST protein were constructed. The expression of GRK2 in culture mammalian cells (6 cell lines: PC-3, MDCK, SGC7901, Jurkat cell etc.) was determined by SDS-PAGE and Co-immunoprecipitation. The binding of GRK2 PH domain, GST-GRK2 PH domain fusion protein and BTK PH domain to PKC in vitro were detected by SDS-PAGE and Western blot, upon prolonged stimulation of epinephrine, the binding of GRK2 to PKC was also detected by western blot and Co-immunoprecipitation.
RESULTS: The binding of GRK2 PH domain to PKC in vitro was confirmed by western blot, as were the binding upon prolonged stimulation of epinephrine and the binding of BTK PH domain to PKC. In the present study, GRK2 PH domain was associated with PKC and down-regulated PKC activity, but Btk PH domain up-regulated PKC activity as compared with GRK2 PH domain.
CONCLUSION: GRK2 can bind with PKC and down-regulated PKC activity.
- Citation: Yang XL, Zhang YL, Lai ZS, Xing FY, Liu YH. Pleckstrin homology domain of G protein-coupled receptor kinase-2 binds to PKC and affects the activity of PKC kinase. World J Gastroenterol 2003; 9(4): 800-803
- URL: https://www.wjgnet.com/1007-9327/full/v9/i4/800.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i4.800
G protein-coupled receptor kinase-2 (GRK), also known as β1-adrenergic receptor kinase (β-ARK1), plays an important role in agonist-induced desensitization of the β-adrenergic receptors. Activation of protein kinase C (PKC) is able to stimulate phosphorylation and activation of GRKs and induce desensitization of G protein-coupled receptor. However, the detail mechanism of interaction between PKC and GRK2 and the effect of GRK2 on activity of PKC remain unknown. Pleckstrin homology (PH) domain is a kind of functional domain containing 120 amino acids, which exist on many protein molecules that involved in cellular signal transduction. A PH domain located in GRK2 residue 548 to 660 may play a significant role in mediating interaction between PKC and GRK2. In the present study, we showed that PKC could associate with PH domain of GRK2 in pull-down assay in vitro. Co-immunoprecipitation displayed binding of PKC to GRK2 in intact Jurkat cells after prolonged stimulation of epinephrine. Assay of PKCβ1 kinase activity indicated that the binding of the PH domain of GRK2 to PKCβ1 could down-regulate the activity of PKCβ1 kinase. Thus, GRK2 may play a negative feedback regulatory role on PKCβ1 activity in interaction between GRK2 and PKCβ1.
G protein-coupled receptor kinase-2 is a member of the GRK family existing of at least 6 GRKs[1]. GRKs are implicated in agonist-induced phosphorylation and homologous desensitization of G protein-coupled receptor (GPR). Phosphorylation sites of the GPR by GRK are located in the carboxyl tail of the receptor and involved amino acid residues at positions serine 404 408, and 410[2]. These phosphorylation sites are thought to initiate desensitization of GPR.
It has been recently reported that protein kinase C (PKC) could phosphorylate and activate GRK which sequentially mediated desensitization of GPR[3]. In addition, PKC could directly phosphorylate GPR and initiate desensitization of the receptors[4]. Thus, PKC likely participates in phosphorylation and homologous desensitization of adrenergic receptor at multiple levels. Furthermore, both activated GRK and PKC are recruited to the specific membrane and GRK may localize on the membran ce via its PH do main bin ding to phosphatidylinositol phosphates. Both recruitment to membrane will facilitate the association of PKC with GRK. Deletion or mutation of the PH domain will abolish GRK faculty of phosphorylating and activating GPR[5]. PH domain of GRK plays a pivotal role in mediating the phosphorylation and desensitization of the receptors. However, as to detail mechanism of PKC, how to interact with and activate GRK remains unclear.
PH domain has been distinguished from more than 100 molecules that are implicated in signaling and other biological function. The main function of PH domains is to mediate protein-phospholipid and protein-protein interaction. Up to now, several molecules such as phosphatidylinositol phosphates, Gβγ, RACK1, PKC, Gα subunit-12 and F-actin have been identified as ligands of PH domains. It was reported by Yao et al[6] (1994) that the PH domain of Btk and Itk interacted with protein kinase C. Afterwards, the interactions between PKC and other PH domains of the molecules were also confirmed by different groups. Those PH domains that bind to PKC share a high homology in 1st-4 thβ strands[7] which formats a relatively reserved face binding to PKC. But other PH domains that can interact with PKC remain to be identified. PKC is a family of serine/threonine protein kinase, which plays significant roles in numerous cellular responses (including cell proliferation, differentiation, growth control, tumor progression, apoptosis etc.)[8]. Besides the event of PKC phosphorylating GRK2 discussed above, PKC can phosphorylate GRK5 and modulate the activity of GRK5[9]; PKC can also lead to olfactory signal termination and desensitization[17] via other GRK2; Agonist stimulation results in oxytocin receptor interaction with GRK and PKC[10]. Moreover, PKC presents a manner of phosphorylating directly to muscarinic receptor and leading to the desensitization receptor[11].
Like most interaction between signaling molecules, PKC is likely associated with GRK2 before phosphorylating and activating GRK2. To elucidate detail mechanism of interaction between GRK2 and PKC, we reported the study on interaction between PKC and GRK2. It is expected that present study will provide new insights into understanding of the modulation mechanism of PKC in GRK2 mediating signaling pathway. It is also expected that the present investigation can illustrate the importance of the GRK2 PH domain in interaction with PKC.
Anti-GRK2, anti-PKCβ1 and secondary antibodies conjugated with horseradish peroxidase were purchased from Santa Cruz Biotechnology. PKC assay kit (Sigma TECTTM PKC assay system) and reverse transcription kit were from Promega. Glutathione-agarose beads was from Sigma. Expression vector pGEX-4T-1 and ECL detection kit (for western blotting) were from Amersham Pharmacia Biotech. PVDF membrane was from Millipore. Rabbit IgG was from Sino-American company (China). Protein A-agarose was from Pierce Inc.
Construction of expression vector pGEX-PH The mRNA of human GRK2 (β1-adrenergic receptor kinase) was isolated from fresh human placenta[12], and reverse transcription was performed according to the kit protocol. cDNA of PH domain (residue 548 to 660)[13] was amplified by PCR with oligonucleotides containing BamHI sites (forward, CGGGATCCGGCCACGAGGAAGACTAC) and EcoRI sites (reverse, GGAATTCTCACCGCTGCACCAGCTGCTG). The products were purified from agarose gel and the resulting fragments were digested with BamHI and EcoRI, respectively. The resulting fragments were ligated in frame between BamHI and EcoRI sites of plasmid pUC-19. The resulting plasmid (termed pUC-PH) was resolved by sequencing. The fragments were excised from pUC-PH and ligated in frame into expression vector pGEX-4T-1, named pGEX-PH.
Expression and purification of GST fusion protein GST-GRK2 PH domain, Btk PH domian and GST were expressed in Escherichia coli strain DH5α. Here was Btk PH domian positive control[6]. The expression was induced with 0.2 mM of IPTG at 26 °C for 1 hour in LB medium containing 1% glucose. Briefly, the bacteria were resuspended in ice-cold STE buffer (10 mM Tris-HCl, 150 mM NaCl,1.0 mM EDTA,1.0 mM phenylmethylsulfonyl fluoride and 0.1 mg/mL lysozyme, pH8.0) on ice for 20 min. The treated bacteria were added dithiothreitol to 5.0 mM and sarkosyl to 1.5% on ice until the solution became viscous, and then lysed by sonicate (30-40 output,1 min × 2, at 4 °C). Cell lysate was centrifuged at 10000 × g for 20 min at 4 °C. Component of both supernatant and the pellet was separated by SDS-PAGE to determine the distribution of the fusion protein. The supernatant was added Triton X-100 to 2%, and then filtered with 0.45 mm membrane. The solution was saved as the fraction containing interested proteins, and then the treated glutathione-agarose beads were added to solution at 4 °C for 2 hours with occasional gentle mixing. The beads were washed with PBS and centrifuged at 800 × g, 4 °C for 3 min × 5 times. The pellet was subjected to SDS-PAGE in 12% gels. In order to utilize 1 equivalent amount of GST and fusion proteins for the following assay, the protein concentration was modulated according to band sizes in SDS-GAGE gel.
Determination of GRK2 expression in mammalian cell lines MDCK, SGC7901, DU145, PC-3, U937 and Jurkat cells were maintained in Dulbecco’s modified eagle’s medium or 1640 medium with 10% (v/v) new born bovine serum and a 5% CO2 humidified atmosphere at 37 °C. In brief, the 107 cells were harvested by centrifuging or scraping off, respectively, washed by PBS at 4 °C and then incubated with 1 ml of lysis buffer (20 mM Tris-HCl, pH7.5, 10 mM KCl, 1 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 10 µg/mL leupeptin and 1 µg/mL aprotinin) on ice for 20 min with casually and vigorously vortexing. The mixtures were centrifuged at 12000 × g for 10 min at 4 °C. The supernatants were saved as GRK2-contained or PKB-contained preparations for western blot.
Cell culture and lysis Jurkat T lymphocytes were maintained as above with RPMI 1640 medium. The 2 × 107 cells were divided into 2 parts and were serum-freed for 8 h. followed by 0.5 h treatment without (Control) or with epinephrine. The stimulation was stopped by removal of the medium and adding 3 ml of ice-cold phosphate-buffered saline, which was also used for two similar washing steps with centrifugation at 500 × g for 5 min.
Protein-protein interaction assay Approximately 20 µg of the GST and GST fusion proteins immobilized on glutathione-agarose beads was incubated with the 0.3 ml of Jurkat T lymphocyte supernatant at room temperature for 2 hours with gentle mixing. The beads were then washed five times with1 ml of ice-cold PBST (PBS with 0.05% tween-20). The eventual bead pellet was resolved by SDS-PAGE. Equivalent amount of the fusion protein or GST alone was used for the following assay.
Western blot-the resolved proteins were electrotransferred onto PVDF membrane. The membrane was blocked by incubating with 5% gelatin in PBST at 37 °C for 1 h with gentle vibration, and then incubated with 1 µg of anti-PKCβ1 antibody at 4 °C overnight. The membrane was then washed 5 times with PBST and incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody at room temperature for 1 h with gentle vibration. The membrane was finally washed 6 times with PBST to remove unbound secondary antibody and visualized by using ECL (Amersham Pharmacia Biotech).
Co-immunoprecipitation assays 107 Jurkat cells were lysed in 1 ml of lysis buffer (20 mM sodium phosphate, pH7.5, 500 mM NaCl, 0.1% SDS, 1% NP-40, 0.5% sodium deoxycholate, 0.02% sodium azide, 0.5 mM phenylmethylsulfonyl fluoride, µg/mL leupeptin and 2 µg/mL aprotinin). Briefly, 10 µg of anti-PKCβ1, or anti-GRK2 antibodies and rabbit IgG were added to 200 µl of the lysate, respectively, then the samples were incubated overnight at 4 °C. The products were added 40 ml of immobilized protein A (Pierce Inc) and incubated at room temperature for 2 h with gentle mixing. The products were added 0.5 ml lysis buffer (without protease inhibitor) and centrifuged at at 500 × g, 4 °C for 3 min. The supernatant was discarded and this procedure was repeated for 6 times. In brief, the pellets were subjected to SDS-PAGE. The proteins were electrotransferred onto PVDF membranes. The membranes were blocked with gelatin and incubated with anti-PKCβ1 or anti-GRK2 antibodies, respectively. The membranes were probed and visualized as described above.
PKC kinase assay The fusion proteins and GST were purified as described above, and washed extensively with PBST. After washing, the GST-fusion proteins and GST were eluted with 20 mM of reduced glutathione in 50 mM Tris·HCl (pH8.0). The eluted proteins were concentrated using a Centricon (Millipore). Protein concentrations were determined with Bradford reagent (Bio-Rad). Equivalent amounts of the protein were added to the 100 µl lysate of Jurkat cells. According to manufacturer’s instruction (Sigma TECTTM PKC assay system), the activity of PKC in the lysate was determined by subtracting the radioactivity of the reaction with control buffer and 100 µM PKC peptide inhibitor from that of the reaction without activated buffer nor the PKC inhibitor. The experiment was performed in triplicate samples for each groups. The data were presented as percentage of PKC activity and the control was used as 100%.
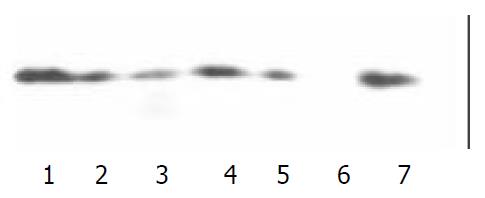
To search for potential binding between the expressed proteins (GST-GRK2 PH domain, GST-Btk PH domain and GST) and PKCβ1, the proteins immobilized on glutathione-agarose beads were added to lysate of Jurkat cells, and then the products were subjected to SDS-PAGE and Western blot. The potential PKCβ1 was probed with anti- PKCβ1 antibody. As shown in Figure 1, the GST fusion proteins encompassing GRK2 PH domain and GST-Btk PH domain associated specifically with PKCβ1 because the GST did not pull out PKCβ1 under the same experimental conditions. Binding of Btk PH domain to PKCβ1 was formerly approved. We confirmed the binding of GRK2 PH domain to PKCβ1 which was specific in vitro.
In order to detect the association of GRK2 with PKC in vivo, GRK2 expression of 6 cell lines were confirmed by western blot in advance. The same amount of the protein was utilized in SDS-PAGE. The results indicated GRK2 were expressed in PC-3, MDCK,7901 and Jurkat cell lines. The GRK2-antibody could react with GRK1 in MDCK and 7901 cell lines,and the antibody could specifically recognize GRK2 in Jurkat cells. Thus, the following Co-immunoprecipitation assays was performed in Jurkat cells (Figure 2).
Phosphorylation and activation of GRK by PKC enhance desensitization of G protein-coupled receptor. In the pull-down assay, our result indicated that the PH domain of GRK2 was associated specifically with PKC in vitro. To determine the association of PKC with GRK2in vivo after stimulation of the agonist of β-ARK, 100 µm epinephrine was added to the cells in 1640 medium for 30 min. Co-immunoprecipitation was performed with specific anti-GRK2 and anti-PKC antibodies in co-precipitation and detection of PKC and GRK. After stimulation of 100 µm epinephrine for 30 min, PKC was detected out in the precipitate of GRK antibody and GRK was detected out in precipitate of PKC antibody, respectively. In contrast, the PKC did not bind to GRK2 in the intact cells without stimulation of epinephrine. Thus, the results indicated that the association of PKC with GRK2 was involved in GRK2 activation and desensitization of G protein-coupled receptor (Figure 3).
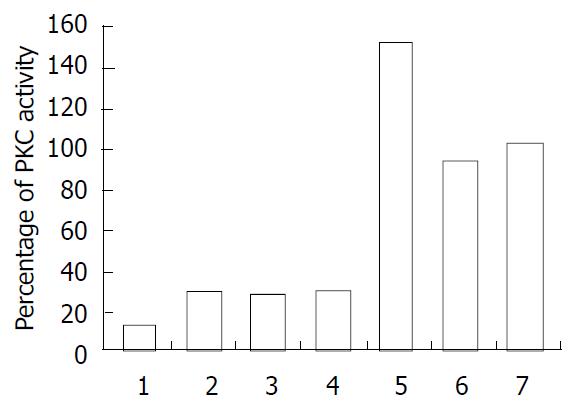
Effects of proteins on PKC activity were determined as described under the experimental Procedures. After purification as described above, the expressed proteins were eluted and dialysed in PBS. Equivalent amounts (20 µg) of GST-GRK2 PH domain, GST-Btk PH domain and GST were added to the lysate of Jurkat cell lysate (100 µl) in determining PKC activity. When Btk associated with PKC, Btk activity was down-regulated by PKC and PKC activity was up-regulated by Btk PH domain. In the present study, GRK2 PH domain was associated with PKC and down-regulated PKC activity but Btk PH domain up-regulated PKC activity as compared with GST and untreated cells (Figure 4).
The GRK family is composed of six members, namly GRK 1-6. Of these, GRK2 phosphorylates G protein-coupled receptors (GPCRs) and plays an important role in mediating homologous desensitization of GPCRs. Chuang’s work showned the activity of GRK2 was increased[14] in a protein kinase C (PKC)-dependent manner. Further study demonstrated that PKC could directly phosphorylate GRK. This study suggested that GRK could be activated via phosphorylation of PKC. Recent work had led to the identification of phosphorylation site at GRK2, the study of Krasel and co-worker showed that PKC phosphorylated GRK2 at serine 29[15], but the detail mechanism of PKCβ1 recognizing and binding to GRK2 remains unknown. In present study, we reported that GRK2 could bind to PKC via its carboxyl-terminal PH domain in vitro, which was a fundamental event in mediating interaction between PKC and GRK2. The present result suggested the GRK ph domain had an analogous fashion to PH domain of Btk and Itk, which could interact with protein kinase C[6] in our previous study. The PH domain of GRK2 is probably involved in directly binding of GRK2 to PKC and facilitates phosphorylation and activation of GRK2 by PKC.
Stimulation of agonist (epinephrine) on adrenergic receptor results in association of PKCβ1 with GRK2 in Jurkat cells. GRK2 could through its PH domain can directly bind to PKC and then be phosphorylated by PKC. But in intact cells (without stimulation of epinephrine), PKC and GRK2 are in dissociated state. The epinephrine stimulation on adrenergic receptor may activate phospholipase C (PLC), and initiates a cascade of events. The stimulation causes PLC-mediated hydrolysis of inositol phospholipids, and results in calcium mobilization and production of second messenger diacylglycerol (DAG). The DAG and Ca2+ are activators of PKC, and cause activation of PKC[16]. In the present study, activated PKC is likely to bind to and phosphorylate GRK2 directly, which phosphorylates G-protein coupled receptor and results in receptor desensitization. In this signaling pathway, activation of PKC appears to be prior to receptor desensitization and is of fundamental importance in regulating the receptor desensitization.
Those PH domains from Btk, β-spectrin, dynamin and amino-terminal of AFAP-110 known directly binding to PKC share a high homology at first three β-sheets[7]. These β-sheets form conserved structure elements and may specifically bind to PKC. Table 1 showed the residue comparison of PH domains from GRK2 and GRK3 with these PH domains. The PH domain of GRK2 and GRK3 matches that consensus well. This may form a structured base of PH domains binding to PKC.
| PH domain (source) | β1 | β2 | β3 |
| Btk | ESIFLKT--SQQKKKTSPLS--NFKKRLFLLT-VH-KLSYYEYDF | ||
| β-spectrin | MEGFLNRKH-EWEAHNKKASSR-SWHNVYCVIN-NQ--EMGFYKDAK | ||
| Dynamin | RKGWLTI---NNIGIMKGGS----KEYWFVLT--AE--NLSWYKDDE | ||
| AFAP, Nt PH domain | ICAFLLRKK-RFGQ----------WTKLLCVIK-EN--KLLCYKSSK | ||
| Consensus | 1* +* | ++***** | +L 1p |
| GRK2 | MHGYMSKM--GNPFLTQ-----WQRRYFYLF---PN--RLEWRGEGEA | ||
| GRK3 | MHGYMLKL--GNPFLTQ-----WQRRYFYLF---PN--RLEWRGEGES | ||
The previous study revealed PKC inhibited the activity of Btk kinase via binding to Btk PH domain[6], but the binding effect on PKC activity remains unclear. In the present study, the effects on PKC activity of Btk and GRK2 PH domains were determined with GST. Compared with GST or control, Btk PH domain could increase PKC kinase activity, but GRK2 PH domain inhibited PKC kinase activity. The mechanism of activation or inhibition of PKC activity might lie in the PH domains binding to PKC regulatory domain and regulating activity of PKC kinase. Biological function of Btk PH domain inhibiting PKC activity will be laid in future investigation. The present study indicated that PKC through its binding to the PH domain of GRK and facilitates phosphorylation and activation of GRK by PKC when stimulated by epinephrine. On the other hand, GRK2 PH domain appeared to play a negative feedback regulatory role on PKC activity. GRK2 immediately turns off PKC kinase activity on GRK after PKC completed phosphorylation and activation of GRK.
Edited by Wu XN
| 1. | Sallese M, Iacovelli L, Cumashi A, Capobianco L, Cuomo L, De Blasi A. Regulation of G protein-coupled receptor kinase subtypes by calcium sensor proteins. Biochim Biophys Acta. 2000;1498:112-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Diviani D, Lattion AL, Cotecchia S. Characterization of the phosphorylation sites involved in G protein-coupled receptor kinase- and protein kinase C-mediated desensitization of the alpha1B-adrenergic receptor. J Biol Chem. 1997;272:28712-28719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 102] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Winstel R, Freund S, Krasel C, Hoppe E, Lohse MJ. Protein kinase cross-talk: membrane targeting of the beta-adrenergic receptor kinase by protein kinase C. Proc Natl Acad Sci USA. 1996;93:2105-2109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 129] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | García-Sáinz JA, Vázquez-Prado J, del Carmen Medina L. Alpha 1-adrenoceptors: function and phosphorylation. Eur J Pharmacol. 2000;389:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Touhara K. Effects of mutations in pleckstrin homology domain on beta-adrenergic receptor kinase activity in intact cells. Biochem Biophys Res Commun. 1998;252:669-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Yao L, Kawakami Y, Kawakami T. The pleckstrin homology domain of Bruton tyrosine kinase interacts with protein kinase C. Proc Natl Acad Sci USA. 1994;91:9175-9179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 263] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Baisden JM, Qian Y, Zot HM, Flynn DC. The actin filament-associated protein AFAP-110 is an adaptor protein that modulates changes in actin filament integrity. Oncogene. 2001;20:6435-6447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Nishizuka Y. The protein kinase C family and lipid mediators for transmembrane signaling and cell regulation. Alcohol Clin Exp Res. 2001;25:3S-7S. [PubMed] [Cited in This Article: ] |
| 9. | Pronin AN, Benovic JL. Regulation of the G protein-coupled receptor kinase GRK5 by protein kinase C. J Biol Chem. 1997;272:3806-3812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Berrada K, Plesnicher CL, Luo X, Thibonnier M. Dynamic inter-action of human vasopressin/oxytocin receptor subtypes with G protein-coupled receptor kinases and protein kinase C after agonist stimulation. J Biol Chem. 2000;275:27229-27237. [Cited in This Article: ] |
| 11. | Hosey MM, Benovic JL, DebBurman SK, Richardson RM. Multiple mechanisms involving protein phosphorylation are linked to desensitization of muscarinic receptors. Life Sci. 1995;56:951-955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40518] [Cited by in F6Publishing: 38830] [Article Influence: 1049.5] [Reference Citation Analysis (0)] |
| 13. | Chuang TT, LeVine H, De Blasi A. Phosphorylation and activation of beta-adrenergic receptor kinase by protein kinase C. J Biol Chem. 1995;270:18660-18665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 136] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Chuang TT, Sallese M, Ambrosini G, Parruti G, De Blasi A. High expression of beta-adrenergic receptor kinase in human periph-eral blood leukocytes. Isoproterenol and platelet activating factor can induce kinase translocation. J Biol Chem. 1992;267:6886-6892. [Cited in This Article: ] |
| 15. | Krasel C, Dammeier S, Winstel R, Brockmann J, Mischak H, Lohse MJ. Phosphorylation of GRK2 by protein kinase C abolishes its inhibition by calmodulin. J Biol Chem. 2001;276:1911-1915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Rasmussen H, Isales CM, Calle R, Throckmorton D, Anderson M, Gasalla-Herraiz J, McCarthy R. Diacylglycerol production, Ca2 influx, and protein kinase C activation in sustained cellu-lar responses. Endocr Rev. 1995;16:649-681. [Cited in This Article: ] |
| 17. | Bruch RC, Kang J, Moore ML, Medler KF. Protein kinase C and receptor kinase gene expression in olfactory receptor neurons. J Neurobiol. 1997;33:387-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 18. | Zheng J, Cahill SM, Lemmon MA, Fushman D, Schlessinger J, Cowburn D. Identification of the binding site for acidic phospholipids on the pH domain of dynamin: implications for stimulation of GTPase activity. J Mol Biol. 1996;255:14-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 216] [Article Influence: 7.7] [Reference Citation Analysis (0)] |