INTRODUCTION
Liver cell adenoma (LCA) is a benign neoplasm composed of cells that closely resemble normal hepatocytes. The lesion arises in normal liver. LCA typically develops in the setting of a hormonal or metabolic abnormality which stimulates hepatocyte proliferation. Exogenous steroid hormone ingestion is undoubtedly the most common such stimulus. Hence, oral contraceptive steroids are the cause of most LCAs[1] although some cases are related to glycogen storage diseases. LCA is still uncommon in countries where oral contraceptives are not used[2-4]. Although the incidence is very low, LCA has been reported in men, children, and women not taking oral contraceptives.
We report here a case of LCA with transformation to hepatocellular carcinoma (HCC) in a woman who had received oral contraceptives for only one month 30 years before.
CASE REPORT
A 57-year-old woman attended hospital because of slight abdominal fullness. She was referred to our hospital because of a liver mass detected by abdominal CT in June 2001. Computed tomography revealed a single mass at the left hepatic lobe measuring 10 × 10 × 8 cm. Her past history was unremarkable and she had no history of drug or alcohol abuse. At the age of thirty she had taken oral contraceptives for one month. Physical examination revealed no hepatomegaly or other abnormalities. The results of urinalysis and peripheral blood analysis were normal. Biochemical findings for blood, i.e. AST, ALT, LDH, γ-GTP, cholinesterase, total bilirubin, and total protein, were also normal. Hepatitis B surface (HBs) antigen, anti-HBs antibody, and anti-hepatitis C virus antibody were negative. Although serum protein induced by the absence of vitamin K, or by increased antagonist-II (PIVKA-II) levels, was elevated, serum alpha fetoprotein levels were normal (18 ng/mL). On June 11, PIVKA-II was 234 μAU/mL and increased rapidly to 3503 μAU/mL on July 19. Levels of other tumor markers, i.e., CEA and CA 19-9 were all within normal limits.
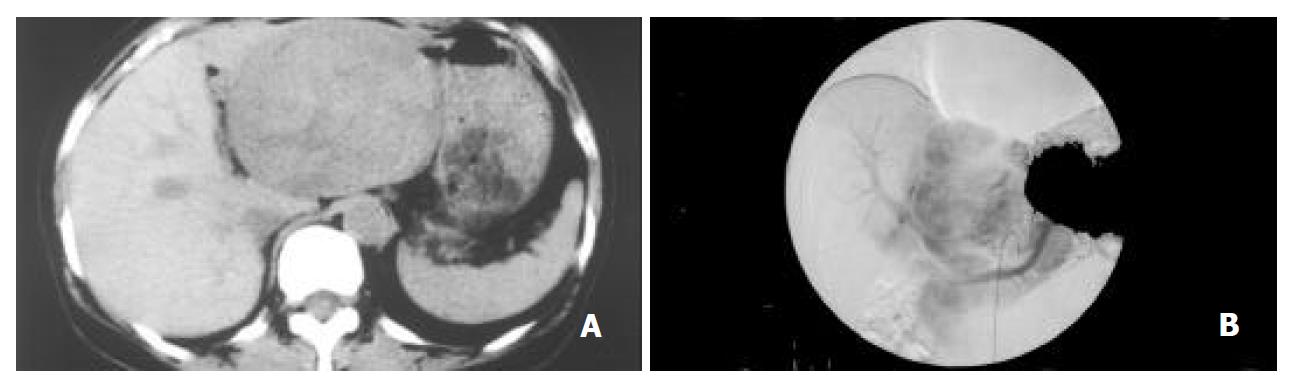
Abdominal ultrasonography revealed a highly echoic lesion, measuring 10 × 9 cm in the left posterior segment of the liver. Computed tomography of the abdomen confirmed a large low-density mass, measuring 10.5 × 8.5 cm in size. Celiac angiography demonstrated a large hypervascular mass (Figure 1). On CT angiography, the mass lesion was enhanced heterogeneously during the early phase, and a sharply demarcated tumor stain was noted during the late phase. This abnormal lesion of the liver was also detected as iso-intensity on T1-weighted and hyper-and hypo-intensity on T2-weighted magnetic resonance imaging (MRI). Therefore, a left lobectomy of the liver was performed under the clinical diagnosis of HCC, in August 2001. After resection of the tumor, serum PIVKA-II fell to the normal level.
Figure 1 A: Computed tomography revealing a large tumor in the left lobe.
B: Celiac angiography during the arterial phase, showing a large hypervascular lesion.
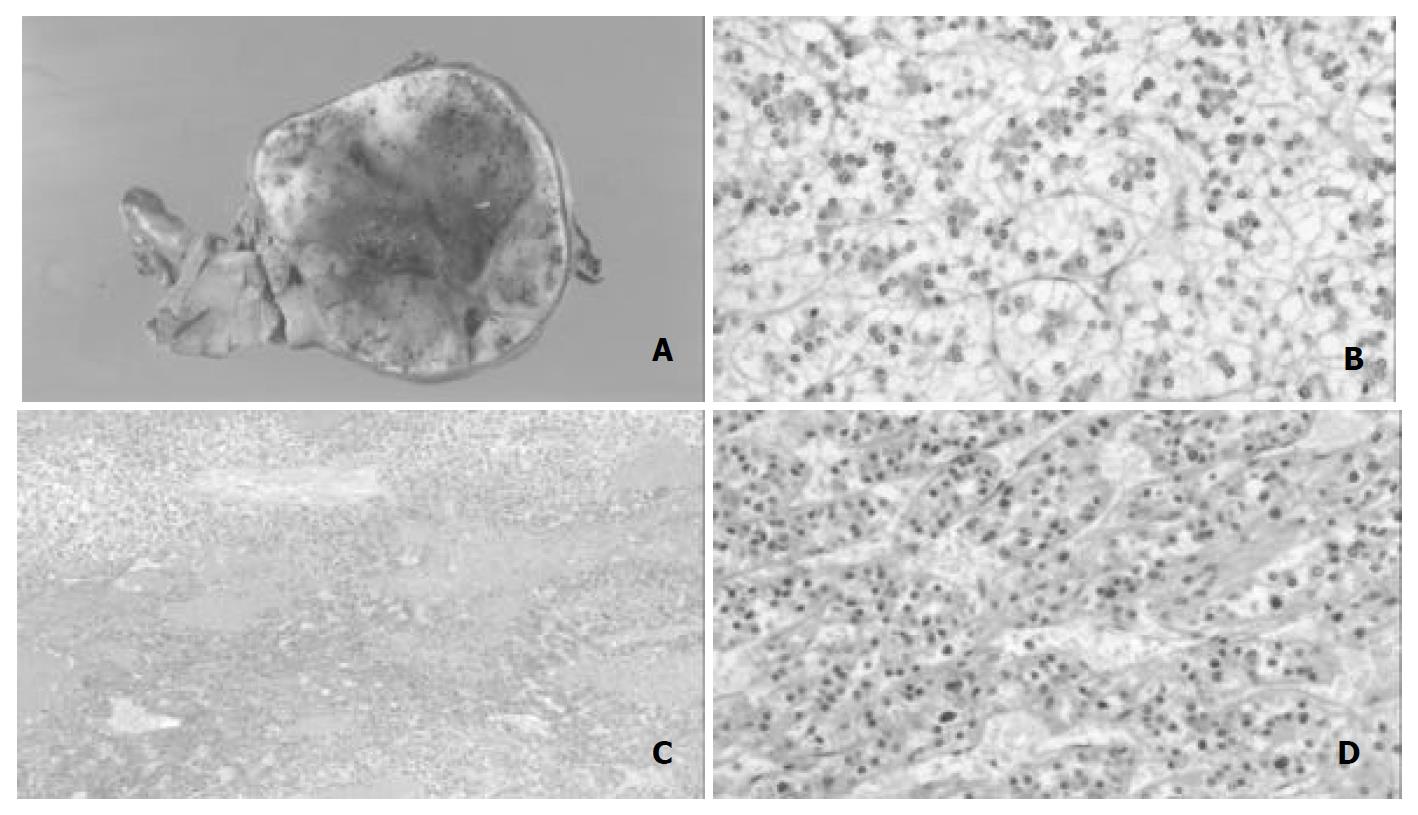
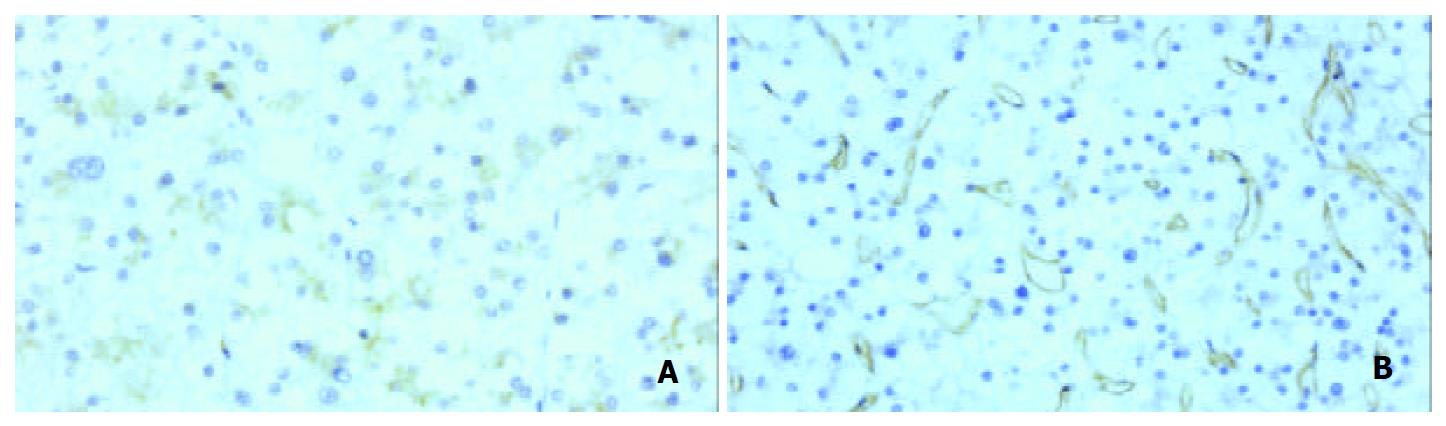
The surrounding liver tissue revealed no fibrosis or cirrhosis. The tumor was solitary and spherical, and measured 10 × 10 × 8 cm (Figure 2A). On the cut surface, the tumor was firm and well encapsulated. The color varied from yellow-white to reddish brown. There were irregular scars and a variegated appearance with hemorrhage. Microscopically, tumor cells were of uniform size but larger and paler than non-tumor hepatocytes in the surrounding tissue, and arranged in small sheets and cords with an occasional acinar pattern (Figure 2B). Background hepatic tissue looked to be normal. Tumor cell cytoplasm was clear and hydropic with eosinophilic granules around the nucleus in each cell. PAS staining revealed abundant accumulation of glycogen in the cytoplasm. The nuclei of tumor cells were uniform and regular, and mitosis was not seen. Thin-walled vascular channels were scattered throughout the tumor. Peliosis hepatic-like sinusoidal dilatation and hemorrhage were present especially in the central part of the tumor (Figure 2C). Portal tracts were absent throughout the tumor. These histological findings indicated LCA, but not simple LCA because of the following features. Distinct areas with a mid-trabecular pattern were evident (Figure 2D). Reticulin fibers were well represented in each sheet, and most cell plates were of two-cell thickness. However, three or more cell plates were present in some hypercellular areas. Pseudoglandular structures were not encountered . Immunohistochemistry revealed that PIVKA-II (18B7, Eisai Co. Ltd.; Tokyo) was positive both in the adenoma cells and in mid-trabecular areas (Figure 3A). CD34 (Nichirei, Tokyo, Japan) was positive throughout the endothelial cells of the tumor (Figure 3B). Ki-67 (Dako) was positive sporadically in mid-trabecular area and tumor cells with enlarged nuclei. AFP (Dako, Demark), P53 (DO-7, Dako), estrogen receptor (Novocastra, Newcastle, UK), and progesterone receptor (Novocastra) were all negative throughout.
Figure 2 A: Macroscopic appearance of resected tumor.
The tumor was incompletely encapsulated by thin fibrous tissues, and its cut surface was tan-yellowish. Hemorrhage was observed inside part of the tumor. B: Microscopic appearance of tumor. Tumor cells were relatively uniform and had clear eosinophilic cytoplasm with small round nuclei. C: Trabecular structures were spo-radically encountered in association with hemorrhage. D: The tumor cells were arranged predominantly in a thin trabecular pattern with moderate nuclear atypia.
Figure 3 Immunohistochemistry.
A: PIVKA-II. B: CD34. PIVKA-II was positive in the tumor cells with or without nuclear atypism and trabecular structure (A). CD34 was expressed diffusely in the sinusoidal endothelial cells (B).
DISCUSSION
Oral contraceptive steroids are the cause of most LCAs, as demonstrated by epidemiological case-control studies conducted in the 1970’s[5]. The tumor is nearly always found in women aged 15 to 45, with an incidence estimated to be 3 to 4 per 100000 long-term contraceptive steroid users per year. An incidence of only 1 per million occurs in non-users or women with less than 2 years’ exposure to contraceptive steroids. The risk increases with the duration of contraceptive steroid use and with the potency of the preparation. The present case was not related to oral contraceptives although she had a very short period of oral contraceptive use about 30 years before.
Malignant transformation of LCAs is rare, because most are resected on discovery. However, there were a few well documented cases of hepatocellular carcinoma arising in unresected solitary and multiple adenomas[6-8]. This might represent an adenoma-carcinoma progression sequence in hepatocellular neoplasia, similar to that seen in colon cancer[9]. Intratumoral hemorrhage and intraperitoneal tumoral rupture occasionally occurred in LCA, so that surgical excision has been usually advised for LCA to prevent the risk of rupture and hemorrhage, and malignant transformation[10].
Distinguishing LCA from well-differentiated HCC by histopathology is a difficult task in small biopsies and occasionally even in resected tumor specimens. LCA can be differentiated clinically from HCC on the basis of tumor marker abnormalities, such as AFP and PIVKA-II. Gene analysis is one of the useful methods distinguishing these tumors, although it is not practical in ordinary diagnostic procedure. Multiple chromosomal aberrations detected by CGH, including gains or losses in one or more of six chromosomes (1q, 4q, 8p, 8q, 16p, and 17p) have been reported in hepatocellular carcinomas, but not in LCAs[11]. The detection of frequent aberrations supported a diagnosis of carcinoma and made LCA unlikely, as consistently reported by several investigators[12,13].
LCA has a small but not negligible risk of malignant transformation into HCC. LCAs are rare tumors which may be difficult to differentiate from well differentiated HCCs. In the present case the key finding of malignant transformation into HCC was the presence of an irregular mid-trabecular growth pattern as determined histologically. p53 and Ki-67 are useful markers for differentiation of LCA from HCC. p53 protein was detected in the nuclei of tumor cells of 8.5% to 44% of HCC. In contrast, immunostaining of p53 was seen in none of the focal nodular hyperplasias examined, and also in none of the LCAs. In addition, mutant p53 expression in HCC was positively correlated with tumor grade[14,15]. Ki-67, assessed using the monoclonal antibody MIB-1, is expressed in the nuclei of cells of HCC, but not in adenoma cells. The nuclei of LCAs are typically uniform and regular, the nuclear/cytoplasmic ratio is normal, and mitosis is not seen. In the present case, p53 was negative throughout the tumor, even in the areas with a trabecular growth pattern that was the key finding of malignant transformation. Ki-67 was sporadically positive in mid-trabecular area and relatively large nuclei, but not many as conventional HCC.
In contrast to the results of Ki-67 and p53 staining, those of CD34 and PIVKA-II supported malignant transformation. CD34 was reported to show diffuse staining of a large number of sinusoids in HCC in contrast to LCA where the staining was focal or identified only marginal sinusoids[16]. However, consideration should be given to the possibility of LCA and focal nodular hyperplasia, which could also exhibit significantly diffuse CD34 reactivity[17]. PIVKA-II levels were elevated in most patients with HCC. Although LCAs with elevated serum levels of PIVKA-II have been sporadically reported, these were relatively low. Diffuse positive reactions of CD34 on sinusoid endothelial cells in the area of suspicious HCC and marked increase of PIVKA-II (3503 mAU/mL), as seen in the present case, might indicate malignant transformation. The evaluation of a hepatic nodule is a very common clinical problem. Identification of malignant features remains, at times, inconsistent and controversial, and the distinction of HCC from dysplastic nodule can be difficult. Establishment of diagnostic standards for malignant features should be expected. Finally, this case was reviewed by four Western experts, and was diagnosed as LCA with malignant transformation to HCC (personal communication).