Published online Aug 15, 2000. doi: 10.3748/wjg.v6.i4.540
Revised: February 22, 2000
Accepted: March 4, 2000
Published online: August 15, 2000
AIM: To investigate effect o f losartan, an AT1 receptor antagonist, on hepatic fibrosis induced by CCl4; and to determine whether or not AT1 receptors are expressed on hepatic stellate cells.
METHODS AND RESULTS: Fifty male Sprague-Dawley rats, weighing (180 ± 20) g, were randomized into five groups (control group, model group, and three los artan treated groups), in which all rats were given the subcutaneous injection o f 40% CCl4 (every 3 days for 6 weeks) except for rats of control group. Rats of losartan-treated groups were treated with losartan (20 mg/kg, 10 mg/kg, 5 mg/kg, daily gavage). After 6 weeks liver tissue and serum samples of all rats were examined. Serum hyaluronic acid (HA), procollagen type III (PC III) were detected by radioimmunoassays. van Giesion collagen staining was used to evaluate the extracellular matrix of rats with liver fibrosis. The expression of AT1 receptors, transforming growth factor-beta (TGF-β), and alpha-smooth muscle actin (α-SMA) in liver tissue were determined by immunohistochemical techniques. Compared with model group, serum ALT and AST of losartan-treated groups were significantly reduced (t = 4. 20, P < 0.01 and t = 4.57, P < 0.01). Serum HA and PC III also had significant differences (t = 3.53, P < 0.01 and t = 2.20, P < 0.05). The degree of fibrosis was improved by losartan and correlated with the expressions of AT1 receptors, TGF-β, and α-SMA in liver tissue.
CONCLUSION: AT1 receptor antagonist, losartan, could limit the progression of the hepatic fibrosis induced by CCl4. The mechanism may be relat ed to the decrease in the expression of AT1 receptors and TGF-β, a meliorating the injury of hepatocytes; activation of local renin-angiotensin system might relate to hepatic fibrosis; and during progression of fibrosis, activated hepatic stellate cells might express AT1 receptors.
- Citation: Wei HS, Li DG, Lu HM, Zhan YT, Wang ZR, Huang X, Zhang J, Cheng JL, Xu QF. Effects of AT1 receptor antagonist, losartan, on rat hepatic fibrosis induced by CCl4. World J Gastroenterol 2000; 6(4): 540-545
- URL: https://www.wjgnet.com/1007-9327/full/v6/i4/540.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i4.540
Hepatic fibrosis, which may ultimately lead to cirrhosis, is associated with most chronic liver diseases, and is characterized by the net accumulation of extracellular matrix (ECM), including collagen, glycoproteins, and proteoglycans [1,2]. Many reports have suggested that hepatic stellate cells (HSCs) are the major producers of ECM in liver injury, and play a prominent role in liver fibrosis[3-7]. Tissue repair after acute liver damage involves “activation” of “quiescent” HSCs to myofibroblast-like cells[8-12]. Transform ing growth factor-beta (TGF-β) is a pleiotropic cytokine that has been assigned a key role in epithelial repair, and HSCs were shown to its main source[13-16]. In cultured HSCs, TGF-β-mediated up-regul ation of collagen and other ECM components mRNA was time and dose-dependent[17,18]. In the past years, significant progress has been made in our understanding of this pathologic mechanism, however, few effective drugs can slow the progression of the fibrosis[19,20].
Over the past decade, preventing the formation of angiotensin II by angiotensin-converting enzyme (ACE) inhibitors has revolutionized the therapy of hypertension and especially of congestive heart failure[21]. Recen tly, a number of studies demonstr ated that ACE inhibitors also effectively limited the progression of cardiac, renal and pulmonary interstitial fibrosis[22-26]. Recent work has shown that angiotensin II type 1 (AT1) recep tor antagonist, losartan, can also ameliorate the renal and cardiac fibrosis[27,28]. The prevailing hypothesis for the main mechanism was suppressing the expression of TGF-β in kidney and heart, rather than its dynamic effects[29-32]. Based on this and other information, we hypothesized that the AT1 receptor antagonist, losartan, could also limit the progression of hepatic fibrosis. To explore our speculation, the present study was designed to investigate the effect of losartan on rat’s hepatic fibrosis induced by CCl4, and determine whether or not there was expression of AT1 receptor on hepatic s tellate cells.
Fifty male Sprague-Dawley rats, weighing 180 ± 20 g, were purchased from Animal Center of Shanghai Medical University (Shanghai, China). Losartan was obtained f rom MSD Co. (England). Polyclonal rabbit antibody to rat TGF-β was purchased from Boster Biotech Co. (Wuhan, China). Monoclonal antibody of alpha-sm ooth muscle actin (α-SMA) was purchased from Maixin Biotech Co. (Fuzhou, China). Hyaluronic acid (HA) and procollagen type III (PC III) radioimmunoassays kits were purchased from Navy Shanghai Medical In stitute (Shanghai, China).
Fifty rats were randomized into five groups (control group, model group and three losartan-treated groups) in which all rats were given subcutaneous injection of 40% CCl4 (0.3 mL/100 g, every 3 days for 6 weeks) except for rats o f co ntrol group (only given injection of same dose of olive oil). In an initial experiment, rats of losartan-treated groups were treated with losartan (20 mg/kg, 10 mg/kg, 5 mg/kg by daily gavage). After six weeks, all rats were sacrificed. Serum was collected and stored at -20 °C for analysis of aspartate transaminase (AST) and alanine transaminase (ALT) activity by standard enzymatic methods. The serum levels of PC III and HA was determined by radioimmunoassays.
The liver sections were fixed in a 10% solution of formaldehyde in 0.1 mol/L pho sphate-buffered saline (pH 7.4), and embedded in paraffin. Five-micrometer sli des were prepared. van Giesion collagen staining was used to evaluate the ECM of rats. According to van Giesion collagen staining, the degree of fibrosis was divided into five grades (0-5). Specimens were scored blindly by the histologist and were also ranked blindly for severity of fibrosis. The expression of AT1 receptor (anti-rat rabbit polyclonal antibody was the product of Santa Cruz Biote ch Co), TGF-β and α-SMA were detected by immunohisto chemical techniques.
Data are presented as -x±sx. Comparision between two groups was made using Student’s t test. Difference of fibrosis between model and losartan-treated groups was compared using Ridit analysis. The test was con sidered significant at P < 0.05.
Compared with control group, ALT and AST increased significantly in fibrotic rats in the model group, but only marginally in losartan treated rats. ALT and AST activities were significantly lower in 20 mg losartan-treated group than in model group of CCl4 rats. The effects were associated with doses of losartan (Table 1).
As expected, serum levels of HA and PCIII increased in rats of model group. Serum HA levels were approximately three times higher in rats of model g roup than rats of control group. There was a tendency towards a decrease in HA and PC III levels in losartan treated group (Table 2).
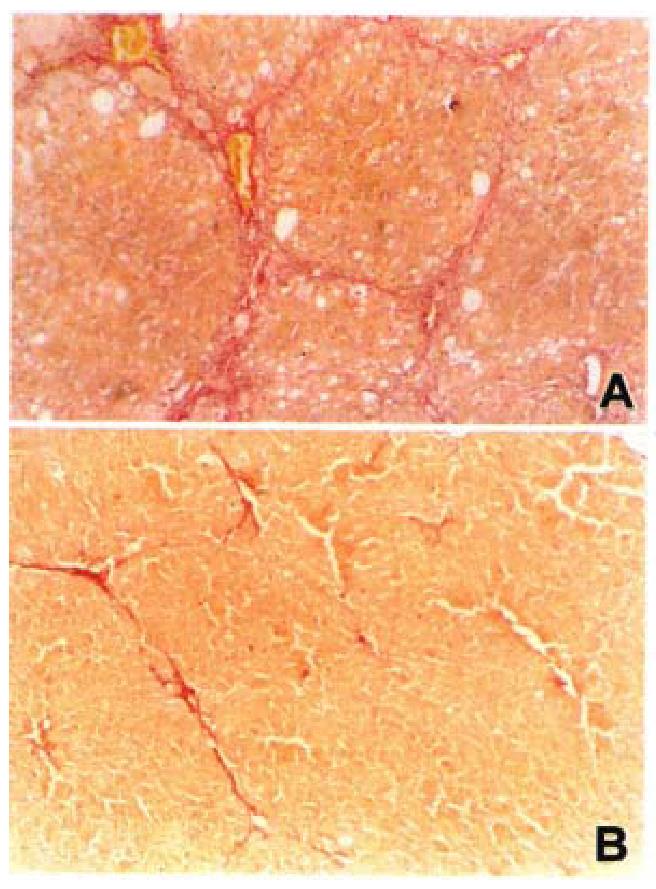
Piecemeal and lobular necrosis was obvious in the CCl4 model compared to rats in control group. The lobular necrosis was significantly decreased by losartan in three treated groups. There was an increase in the area of fibrosis in model rats compared with rats in control group. There was a significant decrease in the losartan treated rats (Table 3, Figure 1).
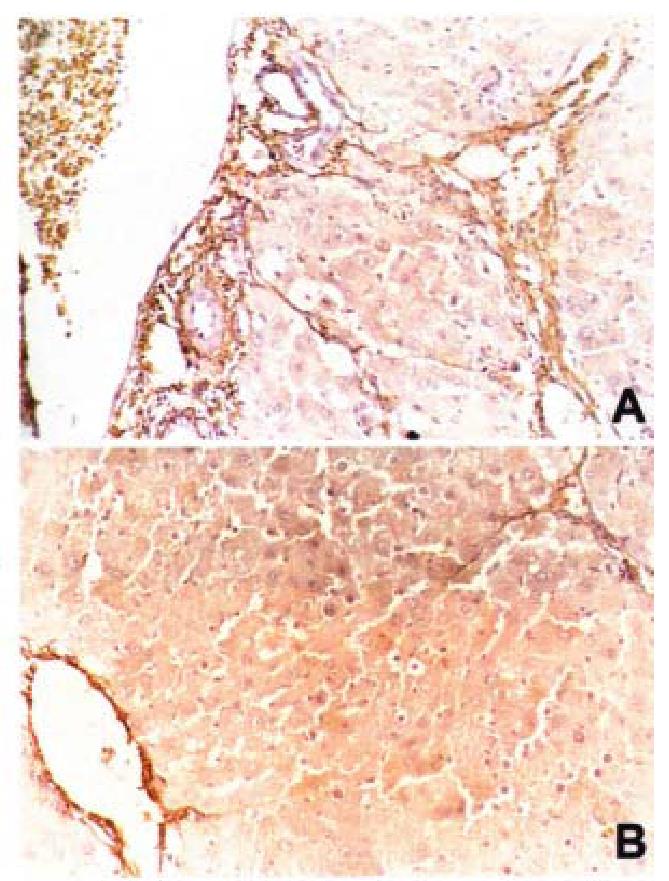
Compared with normal liver tissue, in which AT1 receptors mainly locate in vasculature, in the fibrotic liver tissue, the expression of AT1 receptors significan tly enhanced, and scattered in fibrotic areas. The expression of AT1 receptors was markedly reduced by losartan (Figure 2). The expression of α-SMA was a marker of HSC activation. Immnohistochemical dete ction demonstrated that vascular smooth muscle cells and pericytes were positive for α-SMA in control livers, whereas HSCs strongly positive of α-SMA were observed in rats of model group, and they were scattered along the sinusoidal walls. Many α-SMA-positive HSCs were detected in the area of centrilobular and periportal fibrotic bands in rats of model group. Compared with model group, liver of rats treated with losartan, showed mark edly reduced numbers of α-SMApositive HSC. At same time, its serum levels of PC III and LN were also significantly decreased (P < 0.05).
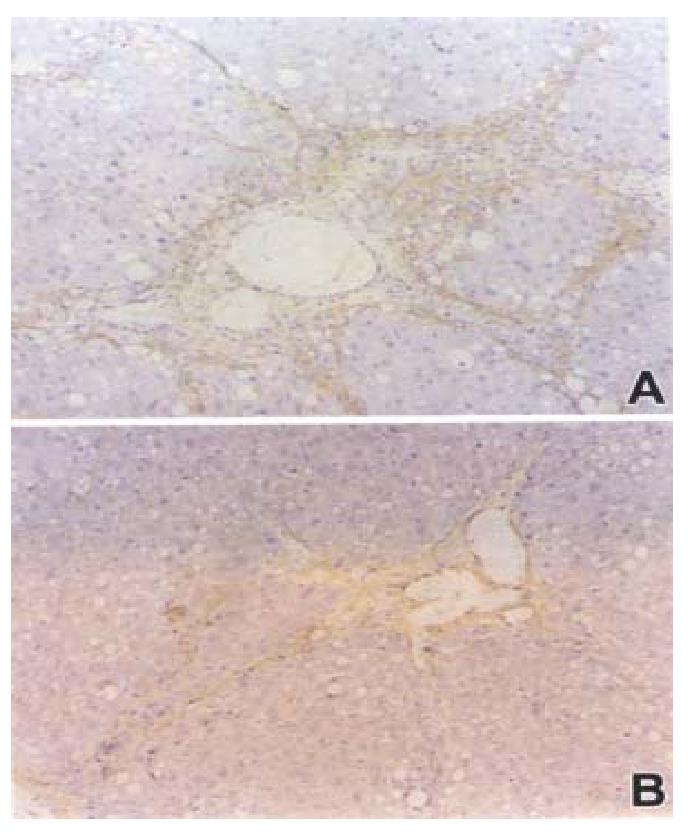
Similar to the α-SMA, TGF-β was also strongly expre ssed in areas of periportal fibrotic bands in rats of model group. In contrast, livers of rats treated with losartan showed significantly reduced numbers of TGF-β-positive cells (Figure 3).
Our study firstly demonstrated that AT1 receptor antagonist, losartan, could slow the progression of hepatic fibrosis induced by CCl4. Activated HSC might express AT1 receptors in fibrotic liver tissue.
Over the last decade, many lines of evidence have demonstrated that local RAS ac tivation was the major mechanism of cardiac and renal interstitial fibrosis[33-37]. In vivo studies have shown that ACE inhibitors and AT1 receptor s antagonists can limit the progression of cardiac, renal, and pulmonary fibrosis[23,38-41], and the mechanism is independent of their dynamic effects. This is based on several in vitro and in vivo findings. First, all cardiac and renal fibroblasts express AT1 receptors; secondly, Ang II induces mitogenic response, increases protein synthesis, production of collagen, and TGF-β in fibroblasts in a dose-dependent manner[29,42,43]. These results support the notion that Ang II can both directly act on fibroblasts and/or enhance the expression of TGF-β.
In the present study, the three different doses (20 mg/kg, 10 mg/kg, 5 mg/kg) of losartan were given to the rats. As the results shown, losartan could limit the progression of the hepatic fibrosis in a dose-dependent manner. At the dose not influencing systolic blood pressure (10 mg/kg) in the normotensive rats[44], losartan could attenuate the fibrosis, but even at a very low dose (5 mg/kg), had a weak but significant effect. In agreement with this study, Boffa et al[45] also reported that losartan completely prevented collagen I gene activation and attenuated the degree of fibrosis without influencing the systolic pressure in the kidneys of transgenic mice. This suggested that Ang II might act as an important regulator in the progression of hepatic fibrosis induced by CCl4.
Using the immunohistochemical methods, we observed the expression of AT1 receptors in liver tissue. As the results shown, this is the first report that confirms that the liver tissue expresses AT1 receptors. Compared with normal rats, where AT1 receptors are mainly located in the vasculature, the expression of AT1 receptors were significantly enhanced in rats of model group, which were mainly located in fibrotic area, and correlated with the degree of fibrosis. The results suggested that the expression of AT1 receptors might relate to hepatic fibrogenesis. Furthermore, immunostaining indicated that the distribution of AT1 receptor correlated with the expression of TGF-β and α-SMA. As has b een recently reported, in a model of chronic cyclosporine (CsA), TGF-β played a role in CsA-induced tubulointerstitial fibrosis and arteriolopath y by stimulating ECM protein synthesis and inhibiting ECM degradation. Losartan resulted in decreasing expression of TGF-β and synthesis of ECM[46]. Zhang et al[28] have also reported that losartan is effective in reducing the increasing expression of AT1 receptors and ECM protein in infarcted heart tissue. Based on the above data, we postulated that expression of AT1 receptor might be related to the activation and ECM synthesis of HSCs.
Another interesting finding in our study was that losartan could ameliorate the hepatocyte injury, as reflected by the release of liver enzymes. The explanation for this observation is not known, but it might relate to protection of the hepatocyte from free radical-mediated damage. Recently, Anthuber et al[47] demonstrated that non-thiol-containing ACE inhibitor, enalapril, could attenuate the hepatocyte injury induced by ischemia/reperfusion. The prevailing mechanism of action was considered to relate with modulation of the angiotens in, bradykinin, and prostacyclin metabolism. Whether losartan has some as-yet-unknown, specific, protective property, remains to be determined in future studies.
That transforming growth factor β(TGF-β) is a key molecule responsible for tissue fibrosis, provides a basis for targeting TGF-β as an antifibrotic agent[48-50]. Recently, Sun et al[31] found that the early induction of TGF-β 1 via the angiot ensin II type 1 receptor played a major role in the development of cardiac fibrosis in infarcted heart. Shihab et al[46] described that losartan reduced TGF-β overproduction in a dose-dependent manner, slowing the rate of renal interstitial fibrosis[22].
Overproduction of TGF-β and activation of HSC are key processes in the progression of hepatic fibrosis. Our results demonstrated that losartan could reduce the expression of TGF-β and α-SMA in liver tissue and suppress fibrosis procession. It is suggested that RAS also parti cipates in the progression of hepatic fibrosis induced by CCl4 in rats.
In conclusion, our results demonstrated that (1) AT1 receptor antagonist, losar tan, could limit the progression of the hepatic fibrosis induced by CCl4. Th e mechanism may be related to the decrease in the expression of AT1 receptors an d TGF-β, ameliorating the injury of hepatocyte; (2) activation of local renin-angiotensin system might relate to hepatic fibrogenesis; (3) in the progression of fibrosis, activated hepatic stellate cells might express AT1 receptor.
Edited by Zhu QR proofread by Mittra S
| 1. | Cheng ML, Wu YY, Huang KF, Luo TY, Ding YS, Lu YY, Liu RC, Wu J. Clinical study on the treatment of liver fibrosis due to hepatitis B by IFN-alpha(1) and traditional medicine preparation. World J Gastroenterol. 1999;5:267-269. [PubMed] [Cited in This Article: ] |
| 2. | Liu P, Liu C, Xu LM, Hu YY, Xue HM, Liu CH, Zhang ZQ. Effects of Fuzheng Huayu 319 recipe on liver fibrosis in chronic hepatitis B. World J Gastroenterol. 1998;4:348-353. [PubMed] [Cited in This Article: ] |
| 3. | Wang YJ, Sun ZQ, Quan QZ, Yu JJ. Fat-storing cells and liver fibrosis. China Natl J New Gastroenterol. 1996;2:58-60. [Cited in This Article: ] |
| 4. | Huang ZG, Zhai WR, Zhang YE, Zhang XR. Study of heteroserum-induced rat liver fibrosis model and its mechanism. World J Gastroenterol. 1998;4:206-209. [PubMed] [Cited in This Article: ] |
| 5. | Yang XB, Huang ZM, Cao WB, Zheng M, Chen HY, Zhang JZ, Tao JH, Lu LJ, Sui X, Liu J. Study on liver injury models induced by CCl4 D-Gal and ANIT in mice:. World J Gastroenterol. 1998;4:63. [Cited in This Article: ] |
| 6. | Feng ZJ, Niu RM, Ren XL, Yao XX. Cellular immune function and liver damage in post hepatitic cirrhosis. China Natl J New Gastroenterol. 1997;3:22. [Cited in This Article: ] |
| 7. | Lu LG, Zeng MD, Li JQ, Hua J, Fan JG, Qiu DK. Study on the role of free fatty acids in proliferation of rat hepatic stellate cells (II). World J Gastroenterol. 1998;4:500-502. [PubMed] [Cited in This Article: ] |
| 8. | Lu LG, Zeng MD, Li JQ, Hua J, Fan JG, Fan ZP, Qiu DK. Effect of lipid on proliferation and activation of rat hepatic stellate cells (I). World J Gastroenterol. 1998;4:497-499. [PubMed] [Cited in This Article: ] |
| 9. | Li DG, Lu HM, Chen YW. Progress in studies of tetrandrine against hepatofibrosis. World J Gastroenterol. 1998;4:377-379. [PubMed] [Cited in This Article: ] |
| 10. | Xu LM, Liu C, Liu P. Effect of amygdalin on proliferation of rat hepatic fat-storing cells and collagen production in vitro. China Natl J New Gastroenterol. 1997;3:103. [Cited in This Article: ] |
| 11. | Du WD, Zhang YE, Zhai WR, Zhou XM. Dynamic changes of type I,III and IV collagen synthesis and distribution of collagen-producing cells in carbon tetrachloride-induced rat liver fibrosis. World J Gastroenterol. 1999;5:397-403. [PubMed] [Cited in This Article: ] |
| 12. | Gao ZL, Li DG, Lu HM, Gu XH. The effect of retinoic acid on Ito cell proliferation and content of DNA and RNA. World J Gastroenterol. 1999;5:443-444. [PubMed] [Cited in This Article: ] |
| 13. | Roulot D, Sevcsik AM, Coste T, Strosberg AD, Marullo S. Role of transforming growth factor beta type II receptor in hepatic fibrosis: studies of human chronic hepatitis C and experimental fibrosis in rats. Hepatology. 1999;29:1730-1738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Wrana JL. Transforming growth factor-beta signaling and cirrhosis. Hepatology. 1999;29:1909-1910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Wu CH. Fibrodynamics-elucidation of the mechanisms and sites of liver fibrogenesis. World J Gastroenterol. 1999;5:388-390. [PubMed] [Cited in This Article: ] |
| 16. | Cai DY, Zhao G, Chen JC, Ye GM, Bing FH, Fan BW. Therapeutic effect of Zijin capsule in liver fibrosis in rats. World J Gastroenterol. 1998;4:260-263. [PubMed] [Cited in This Article: ] |
| 17. | Saile B, Matthes N, Knittel T, Ramadori G. Transforming growth factor beta and tumor necrosis factor alpha inhibit both apoptosis and proliferation of activated rat hepatic stellate cells. Hepatology. 1999;30:196-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 130] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Huang ZS, Wang ZW, Liu MP, Zhong SQ, Li QM, Rong XL. Protective effects of polydatin against CCl(4)-induced injury to primarily cultured rat hepatocytes. World J Gastroenterol. 1999;5:41-44. [PubMed] [Cited in This Article: ] |
| 19. | Yasuda H, Imai E, Shiota A, Fujise N, Morinaga T, Higashio K. Antifibrogenic effect of a deletion variant of hepatocyte growth factor on liver fibrosis in rats. Hepatology. 1996;24:636-642. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Wang YJ, Li MD, Wang YM, Nie QH, Chen GZ. Experimental study of bioartificial liver with cultured human liver cells. World J Gastroenterol. 1999;5:135-137. [PubMed] [Cited in This Article: ] |
| 21. | Lombardi WL, Litwin SE. Angiotensin-converting enzyme inhibitors: congestive heart failure and beyond. Coron Artery Dis. 1999;10:361-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Matoba S, Tatsumi T, Keira N, Kawahara A, Akashi K, Kobara M, Asayama J, Nakagawa M. Cardioprotective effect of angiotensin-converting enzyme inhibition against hypoxia/reoxygenation injury in cultured rat cardiac myocytes. Circulation. 1999;99:817-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Uhal BD, Gidea C, Bargout R, Bifero A, Ibarra-Sunga O, Papp M, Flynn K, Filippatos G. Captopril inhibits apoptosis in human lung epithelial cells: a potential antifibrotic mechanism. Am J Physiol. 1998;275:L1013-L1017. [PubMed] [Cited in This Article: ] |
| 24. | Valentin JP, Sechi LA, Griffin CA, Humphreys MH, Schambelan M. The renin-angiotensin system and compensatory renal hypertrophy in the rat. Am J Hypertens. 1997;10:397-402. [PubMed] [Cited in This Article: ] |
| 25. | Borghi C, Bacchelli S, Esposti DD, Bignamini A, Magnani B, Ambrosioni E. Effects of the administration of an angiotensin-converting enzyme inhibitor during the acute phase of myocardial infarction in patients with arterial hypertension. SMILE Study Investigators. Survival of Myocardial Infarction Long-term Evaluation. Am J Hypertens. 1999;12:665-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Levy EM. Angiotensin converting enzyme inhibitors: first line therapy in patients with diabetic hypertension. Curr Opin Nephrol Hypertens. 1999;8:333-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Silvestre JS, Heymes C, Oubénaïssa A, Robert V, Aupetit-Faisant B, Carayon A, Swynghedauw B, Delcayre C. Activation of cardiac aldosterone production in rat myocardial infarction: effect of angiotensin II receptor blockade and role in cardiac fibrosis. Circulation. 1999;99:2694-2701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 246] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Zhang GX, Pu SY, Yang YZ, Shen XD, Peng TQ, Chen HZ. Effect of losartan and captopril on expression of cardiac angiotensin II AT1 receptor mRNA in rats following myocardial infarction. Zhongguo Yaoli Xuebao. 1997;18:431-434. [PubMed] [Cited in This Article: ] |
| 29. | Fern RJ, Yesko CM, Thornhill BA, Kim HS, Smithies O, Chevalier RL. Reduced angiotensinogen expression attenuates renal interstitial fibrosis in obstructive nephropathy in mice. J Clin Invest. 1999;103:39-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. 1997;29:1947-1958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 268] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 31. | Sun Y, Zhang JQ, Zhang J, Ramires FJ. Angiotensin II, transforming growth factor-beta1 and repair in the infarcted heart. J Mol Cell Cardiol. 1998;30:1559-1569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 186] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Ruiz-Ortega M, Egido J. Angiotensin II modulates cell growth-related events and synthesis of matrix proteins in renal interstitial fibroblasts. Kidney Int. 1997;52:1497-1510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 144] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Morrissey JJ, Klahr S. Effect of AT2 receptor blockade on the pathogenesis of renal fibrosis. Am J Physiol. 1999;276:F39-F45. [PubMed] [Cited in This Article: ] |
| 34. | Schuijt MP, van Kats JP, de Zeeuw S, Duncker DJ, Verdouw PD, Schalekamp MA, Danser AH. Cardiac interstitial fluid levels of angiotensin I and II in the pig. J Hypertens. 1999;17:1885-1891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Mento PF, Pica ME, Hilepo J, Chang J, Hirsch L, Wilkes BM. Increased expression of glomerular AT1 receptors in rats with myocardial infarction. Am J Physiol. 1998;275:H1247-H1253. [PubMed] [Cited in This Article: ] |
| 36. | Heymes C, Silvestre JS, Llorens-Cortes C, Chevalier B, Marotte F, Levy BI, Swynghedauw B, Samuel JL. Cardiac senescence is associated with enhanced expression of angiotensin II receptor subtypes. Endocrinology. 1998;139:2579-2587. [PubMed] [Cited in This Article: ] |
| 37. | Yamazaki T, Komuro I, Yazaki Y. Role of the renin-angiotensin system in cardiac hypertrophy. Am J Cardiol. 1999;83:53H-57H. [PubMed] [Cited in This Article: ] |
| 38. | Makino N, Hata T, Sugano M, Dixon IM, Yanaga T. Regression of hypertrophy after myocardial infarction is produced by the chronic blockade of angiotensin type 1 receptor in rats. J Mol Cell Cardiol. 1996;28:507-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Shen J, Xu Y. Inhibitory effects of captopril on hypoxia-induced proliferation and collagen synthesis in pulmonary vascular smooth muscle cells. Zhongguo Yaoli Xuebao. 1999;20:349-352. [PubMed] [Cited in This Article: ] |
| 40. | Kontogiannis J, Burns KD. Role of AT1 angiotensin II receptors in renal ischemic injury. Am J Physiol. 1998;274:F79-F90. [PubMed] [Cited in This Article: ] |
| 41. | Yang BC, Phillips MI, Zhang YC, Kimura B, Shen LP, Mehta P, Mehta JL. Critical role of AT1 receptor expression after ischemia/reperfusion in isolated rat hearts: beneficial effect of antisense oligodeoxynucleotides directed at AT1 receptor mRNA. Circ Res. 1998;83:552-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Thai H, Raya T. Angiotensin II receptor blockers. Coron Artery Dis. 1999;10:377-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 43. | Zhang X, O'Malley Y, Robbins ME. Angiotensin II-induced modulation of rat mesangial cell phenotype. Radiat Res. 1999;151:725-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Takemoto M, Egashira K, Tomita H, Usui M, Okamoto H, Kitabatake A, Shimokawa H, Sueishi K, Takeshita A. Chronic angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade: effects on cardiovascular remodeling in rats induced by the long-term blockade of nitric oxide synthesis. Hypertension. 1997;30:1621-1627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 113] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Boffa JJ, Tharaux PL, Placier S, Ardaillou R, Dussaule JC, Chatziantoniou C. Angiotensin II activates collagen type I gene in the renal vasculature of transgenic mice during inhibition of nitric oxide synthesis: evidence for an endothelin-mediated mechanism. Circulation. 1999;100:1901-1908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Shihab FS, Bennett WM, Tanner AM, Andoh TF. Angiotensin II blockade decreases TGF-beta1 and matrix proteins in cyclosporine nephropathy. Kidney Int. 1997;52:660-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 174] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 47. | Anthuber M, Farkas S, Rihl M, Menger MD, Schildberg FW, Jauch KW, Messmer K. Angiotensin-converting enzyme inhibition by enalapril: a novel approach to reduce ischemia/reperfusion damage after experimental liver transplantation. Hepatology. 1997;25:648-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Basile DP. The transforming growth factor beta system in kidney disease and repair: recent progress and future directions. Curr Opin Nephrol Hypertens. 1999;8:21-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res. 1998;40:352-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 295] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 50. | Isaka Y, Akagi Y, Ando Y, Tsujie M, Sudo T, Ohno N, Border WA, Noble NA, Kaneda Y, Hori M. Gene therapy by transforming growth factor-beta receptor-IgG Fc chimera suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int. 1999;55:465-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |