Published online Jan 7, 2020. doi: 10.3748/wjg.v26.i1.21
Peer-review started: November 26, 2019
First decision: December 12, 2019
Revised: December 15, 2019
Accepted: December 22, 2019
Article in press: December 22, 2019
Published online: January 7, 2020
Phosphatidylinositol-3,4,5-trisphosphate dependent Rac exchange factor 1 (PREX1) was reported to be overexpressed in some cancers and involved in cancer development, but its expression and significance in gastric cancer remain unclear.
To evaluate the expression of PREX1 in gastric cancer and its significance in the development of gastric cancer, especially to evaluate the potential mechanism of PREX1 in gastric cancer.
Bioinformatic analysis was performed in order to examine the expression of PREX1 in gastric cancer. The relationship between the survival rate of gastric cancer patients and PREX1 expression was assessed by Kaplan Meier portal. The Gene Set Enrichment Analysis and the correlation between PREX1 and transforming growth factor (TGF) β1 pathway-related mediators were evaluated by cBioPortal for Cancer Genomics. Western blotting and reverse transcriptase polymerase chain reaction assay were used to test the role of TGFβ1 on the expression of PREX1. Western blotting and dual-luciferase reporter system was used to evaluate the effect of PREX1 on the activation of TGFβ1 pathway. Wound healing and Transwell assay were used to assess the effect of PREX1 on the metastasis activity of gastric cancer cells.
PREX1 was overexpressed in the gastric tumors, and the expression levels were positively associated with the development of gastric cancer. Also, the high expression of PREX1 revealed poor prognosis, especially for those advanced and specific intestinal gastric cancer patients. PREX1 was closely involved in the positive regulation of cell adhesion and positively correlated with TGFβ1-related mediators. Furthermore, TGFβ1 could induce the expression of PREX1 at both the protein and mRNA level. Also, PREX1 could activate the TGFβ1 pathway. The induced PREX1 could increase the migration and invasion activity of gastric cancer cells.
PREX1 is overexpressed in gastric cancer, and the high level of PREX1 predicts poor prognosis. PREX1 is closely associated with TGFβ signaling and promotes the metastasis of gastric cancer cells.
Core tip: In this study, we fully identified the overexpression of phosphatidylinositol-3,4,5-trisphosphate dependent Rac exchange factor 1 (PREX1) in gastric cancer and its positive correlation with the gastric cancer progression. High PREX1 expression predicts poor prognosis in the specific intestinal-type gastric cancer patients. PREX1 is closely involved with cell adhesion, and PREX1 has a positive feedback loop regulation with transforming growth factor β1 pathway to promote the metastasis of gastric cancer cells. PREX1 may be a novel target for the drug development of gastric cancer, and PREX1 acts as a predictor for prognosis of intestinal-specific gastric cancer.
- Citation: Shao Q, Chen ZM. Feedback regulation between phosphatidylinositol-3,4,5-trisphosphate dependent Rac exchange factor 1 and transforming growth factor β1 and prognostic value in gastric cancer. World J Gastroenterol 2020; 26(1): 21-34
- URL: https://www.wjgnet.com/1007-9327/full/v26/i1/21.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i1.21
Gastric cancer is one of the most common digestive tract tumors. It has the characteristics of high malignancy and high metastasis[1]. In recent years, the incidence of gastric cancer continues to increase, which is closely related to food safety, poison exposure, and bad living habits[2], such as environmental toxins, malnutrition, etc. At present clinical practice, the curative treatment of gastric cancer is relatively rare. Furthermore, due to the poor early diagnosis of gastric cancer, most patients with gastric cancer are diagnosed at the advanced stage, so the efficiency of treatment is not satisfactory[3-5]. The clinical summary represents that the 5-year survival rate is relatively low, and due to the tumor type, the patient's quality of life is significantly reduced[6]. Therefore, exploring novel mediators of gastric cancer is of great significance in the therapy of gastric cancer.
Phosphatidylinositol-3,4,5-trisphosphate dependent Rac exchange factor 1 (PREX1) was identified as an activator of Rac[7,8]. A previous publication showed that PREX1 interacts with a G protein-coupled receptor and activates a downstream signaling pathway[9,10]. PREX1 is an indispensable factor in the regulation of neutrophil function[11,12]. Welch et al[13] reported that PREX1 was an important regulator in neutrophil functions such as the induction of reactive oxygen species and the inflammation response. Moreover, PREX1 has the ability to regulate neutrophil chemotaxis. A detailed molecular mechanism study showed that PREX1 can activate Rac1/2, thus regulating neutrophil migration[14,15]. In addition, PREX1 mediates the signaling regulation between the T-cell antigen receptor and CXCR4[16,17]. Therefore, PREX1 may be a prospective target for immune disease.
Many reports of PREX1 also focus on the study of cancer. PREX1 was shown to be overexpressed in breast tumor tissues and to be positively correlated with the clinical-pathological feature, which could predict the prognosis for breast cancer patients[18-20]. Other studies also reported the overexpression of PREX1 in some cancers, including prostate cancer, kidney cancer, ovarian cancer, glioblastoma and thyroid cancer, and that this overexpression was involved in the proliferation and migration of cancer cells[21-24]. Notably, the latest study found abnormal methylation of PREX1 in gastric cancer. Liu et al[25] reported that hypermethylation of PREX1 represented a higher survival rate. However, the expression of PREX1 and its significance in gastric cancer remain unclear. In this study, we identified the expression of PREX1 in different types of gastric cancer. This study also revealed the association between PREX1 expression with the development of gastric cancer. Furthermore, the clinical significance of PREX1 was assessed using survival analysis. We firstly report that PREX1 expression is a specific prognosis biomarker for intestinal-type gastric cancer. Notably, we also evaluated the potential mechanism regulation in gastric cancer. Interestingly, we report for the first time that PREX1 has a positive feedback loop with the transforming growth factor (TGF) β1 signaling pathway and participates in the process of gastric cancer metastasis. This study further demonstrates the expression of PREX1 in gastric cancer and evaluates its significance in the clinical finding. Furthermore, the feedback loop regulation between TGFβ1 and PREX1 has been firstly addressed, and PREX1 might be a novel target for the treatment of gastric cancer.
SGC-7901, BGC-823 cells, and HEK293T cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, United States). The recombinant human TGFβ1 protein (240-B-002) was purchased from R&D Systems (Minneapolis, MN, United States). Anti-TGF-β 1 antibody (ab92486) and anti-PREX1 antibody (ab124231) were purchased from Abcam (Cambridge, MA, United States). Anti-β actin was provided from Santa Cruz Biotechnology (Dallas, TX, United States). Lipofectamine 2000 transfection reagent, Dulbecco's modified eagle's medium, fetal bovine serum (FBS), antibiotic reagent, and RMPI 1640 medium were purchased from Thermo Fisher (Waltham, MA, United States). Dual-luciferase reporter kit and crystal violet staining were obtained from Beyotime (Wuhan, China). Protease inhibitor cocktail was purchased from APExBio (Houston, TX, United States). Lentivirus concentration solution, radioimmunoprecipitation assay lysis buffer, bicinchoninic acid protein concentration kit, total RNA isolation reagent, first-strand cDNA synthesis kit, enhanced chemiluminescence system, and SYBR quantitative polymerase chain reaction (PCR) kit were obtained from Yeasen Biotechnology (Shanghai, China).
Oncomine analysis
The Cancer Genome Atlas (TCGA) analysis of gastric cancer was achieved by Oncomine portal. The relative gene expression of PREX1 was expressed as copy number units. The different histology of gastric cancer included diffuse gastric adenocarcinoma, gastric tubular adenocarcinoma, gastric intestinal-type adenocarcinoma, mucinous gastric adenocarcinoma, and gastric mixed adenocarcinoma. The 236 cases of blood sample from gastric cancer patients were also included in this study. The detailed analysis condition was as follows: P value < 1E-4, fold change > 2, the gene ranks was top 10%. The patient’s information was obtained from sample filters with clinical outcome, and the expression of PREX1 in gastric cancer patients with different stage and tumor grade and with lymph node metastasis or not were assessed in this study. Datasets included in the survival analysis are shown in Table 1[26-31].
The overall survival (OS) and post-progression survival (PPS) rate were conducted in Kaplan Meier plotter. The classification of high expression patients and low expression patients was split by median expression value. The biased arrays were excluded in the survival analysis. In total, 631 patients were included to compare OS between PREX1 high expression and low expression patients, and 384 patients were included in the PPS analysis. The restrict analysis to the subtype was the Lauren classification method. In the OS analysis, 269 samples with intestinal gastric cancer and 240 samples with diffuse gastric cancer were included. The statistical analysis was performed using the logrank test and Cox regression analysis.
The co-expression analysis of PREX1 in gastric cancer was performed in the cBioPortal for Cancer Genomics. P value less than 0.01 was set as a filter for significant co-expression network analysis. The co-expression gene list was subjected to the Gene Set Enrichment Analysis (GSEA) with a biological process. The enrichment score and ranked list metric were included in the GSEA analysis. The correlation analysis between PREX1 and TGFβ1, TGFβ2, intercellular adhesion molecule-1, integrin alpha (ITGA) 4, and ITGA5 of TCGA gastric cancer were conducted with cBioPortal for Cancer Genomics. The correlation level analysis was assessed by the Pearson method.
Human embryonic kidney HEK293T cells were cultured in the Dulbecco's modified eagle's medium with 10% FBS. SGC-7901 and BGC-823 cells were cultured in RMPI1640 medium and supplemented with 10% FBS, 200 mg/mL streptomycin, and 200 U/mL penicillin. Lipofectamine 2000 was applied in the cell transfection. HEK293T cells were plated in the 10 cm cell dish, and when the cell density was about 80%, the cells were subjected to the transfection. The full length of human PREX1 or control vector plasmid was transfected combined with pCMV-dR8.91 and pCMV-VSVG plasmids into HEK293T cells. The detailed procedure was as follows: the overexpression or vector plasmid, pCMV-dR8.91 plasmid, and pCMV-VSVG plasmid were diluted in the Opti-MEM medium, the ratio between the three plasmids was 5:3:2. Lipofectamine 2000 reagent was diluted in the Opti-MEM medium, and the concentration was confirmed according to the manufacturer’s protocol. After further culture for 48 h, the supernatant was collected for lentivirus concentration with solution kit. The viral particle of PREX1 overexpression and vector control were supplemented in the culture medium with 5 μg/mL polybrene. With the incorporation of polybrene, the effect of overexpression transfection could be enhanced.
The human full length of PREX1 is 4980 bp. The full length of PREX1 was cloned into pCDH-CMV-MCS-EF1-Puro lentivirus system. The primer for PREX1 transcript was as follows: forward primer, 5’-GCTCTAGAATGGAGGCGCCCAGCGGCAG-3’; reverse primer, 5’-CGGAATTCTCAGAGGTCCCCATCCAC-3’. The PREX1 transcript amplification was constructed with PrimeStar DNA polymerase. Due to the high content of GC in the forward primer, the PCR reaction solution contained 10% dimethylsulfoxide. Furthermore, considering the big Tm value difference between forward and reverse primer, the two-step PCR method was applied. The restriction enzymes XbaI and EcoRI were used to insert PREX1 full length into a pCDH-CMV-MCS-EF1-Puro vector.
The cells were washed with cold phosphate-buffered saline (PBS) buffer twice, and the cells were lysed with radioimmunoprecipitation assay solution on ice for 30 min. The lysis buffer was supplemented with protease inhibitor cocktail at the work concentration. The cell lysis buffer was collected and centrifuged at 12000 g/min for 15 min, and the supernatant was collected and subjected to the bicinchoninic acid protein concentration assay. The equivalent protein was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane. The membrane was blocked with non-fatty milk at room temperature for 1 h, and then the membrane was washed with Tris buffered saline with tween buffer. The membranes were incubated with indicated primary antibody (1:1000) at 4 °C overnight. The membranes were washed with Tris buffered saline with tween three times and incubated with the indicated second antibody at room temperature for 1 h. Enhanced chemiluminescence system was used to detect the protein expression.
The cells were collected and washed with cold PBS buffer twice, and the cells were lysed with total RNA isolation reagent at room temperature for 10 min. Trichloromethane was added, and the sample was vortexed for 1 min and centrifuged at 12000 g for 10 min. The supernatant was collected in a new tube and supplemented with cold isopropanol. The solution was mixed and centrifuged at 12000 g for 10 min. The white sediment was the total RNA and washed with 75% ethanol twice for RNA purification. The first-strand cDNA synthesis was conducted according to the manufacturer’s protocol. Relative mRNA levels of the indicated target gene was evaluated with SYBR green assay, and the 2-△△CT method was used to display the mRNA levels. The primer for real-time quantitative polymerase chain reaction as followed: PREX1 (forward primer: 5’-GGCATTCCTGCATCGCATC-3’, reverse primer: 5’-CGGGTGTAAACAATACTCCAAGG-3’, amplicon size: 151 bp), β-actin (forward primer: 5’-CATGTACGTTGCTATCCAGGC-3’, reverse primer: 5’-CTCCTT AATGTCACGCACGAT-3’, amplicon size: 250 bp).
HEK293T cells were seeded in a 24-well plate, and the cell density was approximately 80%. The cells were transfected with Smad3 reporter plasmid pGL3.PT3 and housekeeping Rellina plasmid. Simultaneously, the culture medium was supplemented with lentivirus particle of PREX1 overexpression and vector control. Twenty-four h later, the cells were treated with recombinant human TGFβ1 protein at the density of 20 ng/mL. The cells were washed with cold PBS buffer and lysed with reporter lysis on ice for 15 min, and the lysis was collected and centrifuged at 12000 g at 4 °C. The supernatant was collected into a white 96-well plate, and the firefly luciferin substrate was added into the supernatant and immediately mixed for 10 s, and the fluorescence value was measured using Rellina luciferase substrate.
The cells were seeded into 6-well plates and cultured for 24 h. When the cell confluence was about 100%, a yellow tip was used to develop a scratch, and the cells were washed with PBS three times. The lentivirus for PREX1 overexpression and vector control were supplemented, and the cells were cultured for another 48 h. Cell images were acquired by Zeiss microscope.
The Transwell unit was pretreated with matrigel and stored at 37 °C for 6 h, and the cells were seeded into the Transwell unit for culture. After 48 h culture, the matrigel was removed from the Transwell and stained with crystal violet. The invasive cells were numbered by Zeiss microscope.
The gene levels are expressed as log2 (copy number units) in Oncomine analysis, and the difference between two groups was analyzed by Student’s t-test. The survival rate analysis was conducted with the Kaplan Meier method, and logrank P value was conducted to evaluate the statistical significance. The correlation analysis was achieved by the Pearson method.
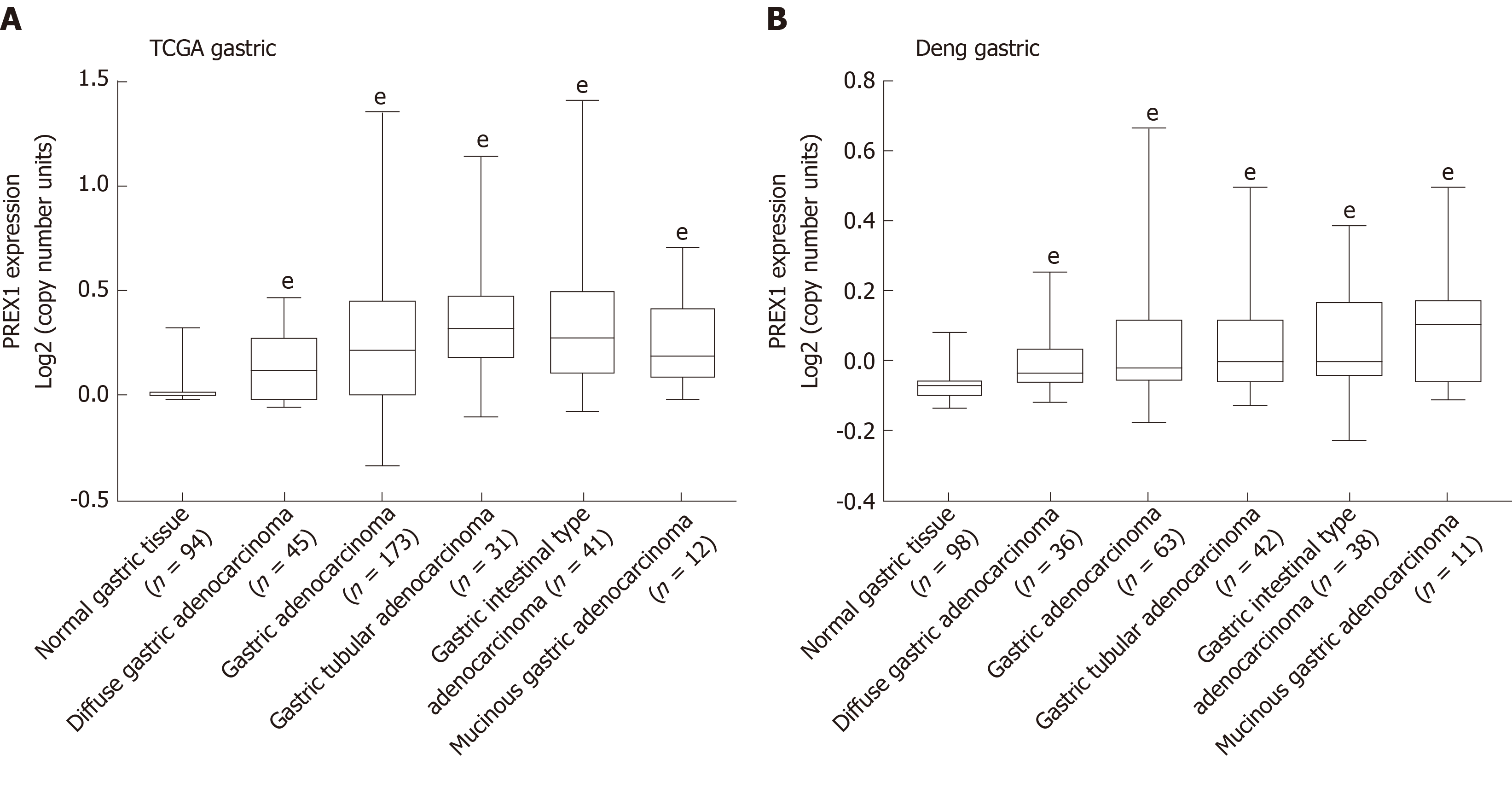
Previous studies demonstrated that PREX1 was overexpressed in some cancers[15,18,23], but its expression in gastric cancer remains unclear. To understand fully the value of PREX1 in gastric cancer, we firstly examined the expression of PREX1 in gastric cancer. As shown in Figure 1A, the analysis of TCGA gastric cancer revealed that PREX1 was highly expressed in different histology types of gastric tumor tissues. To confirm the overexpression of PREX1 in gastric tumor tissues, Deng gastric RNA-sequencing analysis was conducted and demonstrated that PREX1 was highly expressed in different histology of gastric cancer (Figure 1B), suggesting that PREX1 was a general mediator in gastric cancer[32].
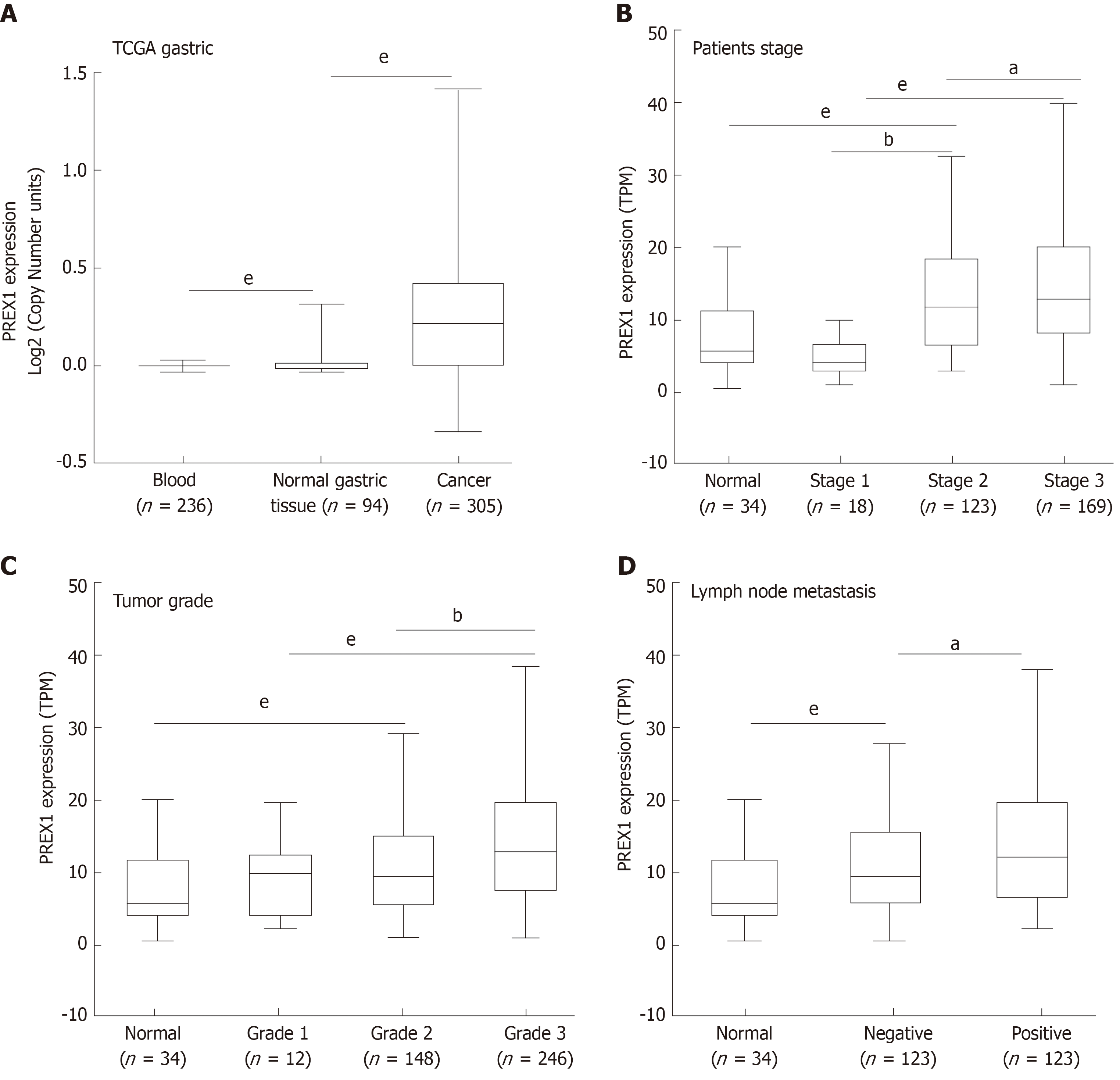
In this study, we identified that PREX1 was overexpressed in the gastric tumor tissues. Considering the significance of PREX1 in the function regulation of neutrophils and macrophage[10,12], we examined the expression of PREX1 in the blood of 236 gastric cancer patients. The results showed that the level of PREX1 in gastric cancer patient’s blood was not significantly that than in normal gastric cancer. To understand the expression of PREX1 in the development of gastric cancer, the level of PREX1 in the different stages of gastric cancer patients was assessed. As shown in Figure 2B, the level of PREX1 in patients with stage I was not remarkably different than in normal gastric tissues. However, the expression of PREX1 was significantly enhanced in the advanced stage. A similar result was also observed in the PREX1 expression analysis of different tumor grades. There was no significant difference between patients with tumor grade I and normal gastric tissues, and PREX1 level was remarkably elevated in the higher tumor grade (Figure 2C). Furthermore, the expression of PREX1 in gastric cancer patients with lymph node metastasis or not was evaluated, and the result showed that the level of PREX1 was significantly increased in patients with gastric cancer tumor with lymph node metastasis compared to those without lymph node metastasis (Figure 2D). Based on these findings, we hypothesized that PREX1 expression might be a marker for advanced-stage gastric cancer.
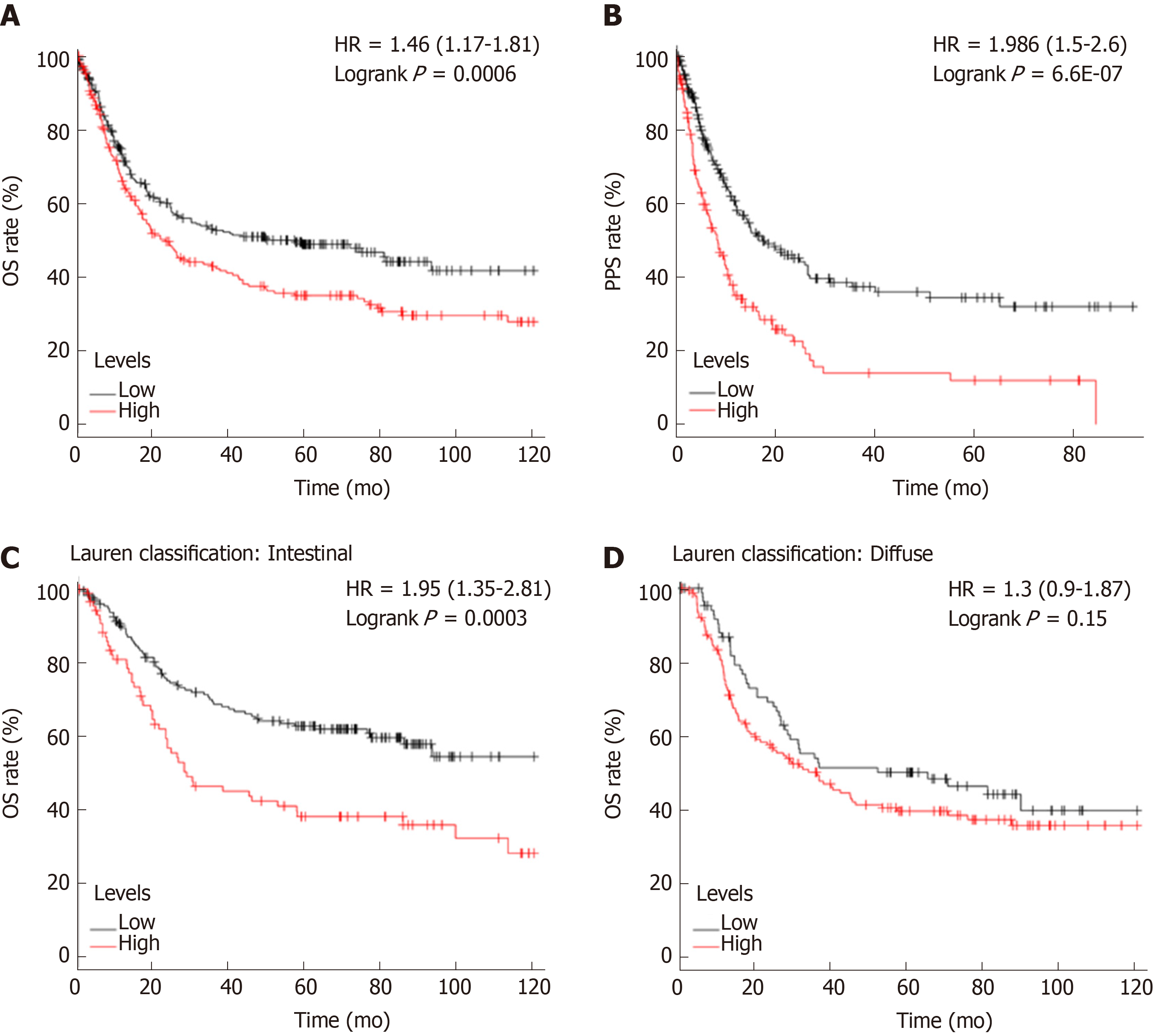
As mentioned previously, PREX1 expression was varied in gastric cancers with different histology, PREX1 expression was elevated in the advanced stage of gastric cancer patients, and PREX1 might serve as a marker for the diagnosis of advanced gastric cancer patients. Thus, we hypothesized that PREX1 might be a prognosis marker for gastric cancer patients. To confirm the hypothesis, we evaluated the relationship between PREX1 expression and the OS and PPS rates of gastric cancer patients. As shown in Figure 3A, the gastric cancer patients with high expression of PREX1 represented a lower OS rate [logrank P = 0.006, hazard ratio = 1.46 (1.17-1.81)]. In detail, the median survival of the high expression cohort was 35.9 mo, but the median survival of the low expression cohort was 78.6 mo. PREX1 expression was elevated in the advanced gastric cancer patients compared with the early-stage patients. Regarding PPS, patients with high PREX1 expression displayed a lower survival rate [Figure 3B, logrank P = 6.6E-07, hazard ratio = 1.986 (1.5-2.6)]. Combined with higher expression in the advanced gastric cancer patients, PREX1 might be a significant biomarker as a prognosis predictor of gastric cancer patients, especially for those patients with advanced stage. In clinical practice, gastric cancer has two main histology types, diffuse gastric cancer and intestinal gastric cancer[33]. In further evaluation, we examined if PREX1 expression affected the survival rate in different histology types of gastric cancer patients. Interestingly, as shown in Figure 3C and D, high PREX1 expression patients display a lower survival rate in specific intestinal gastric cancer patients. However, the survival rate of high PREX1 expression and low expression patients was not significantly different in diffuse gastric cancer patients. So, PREX1 expression was a novel prognosis biomarker for gastric cancer patients, especially for advanced patients and intestinal gastric cancer patients.
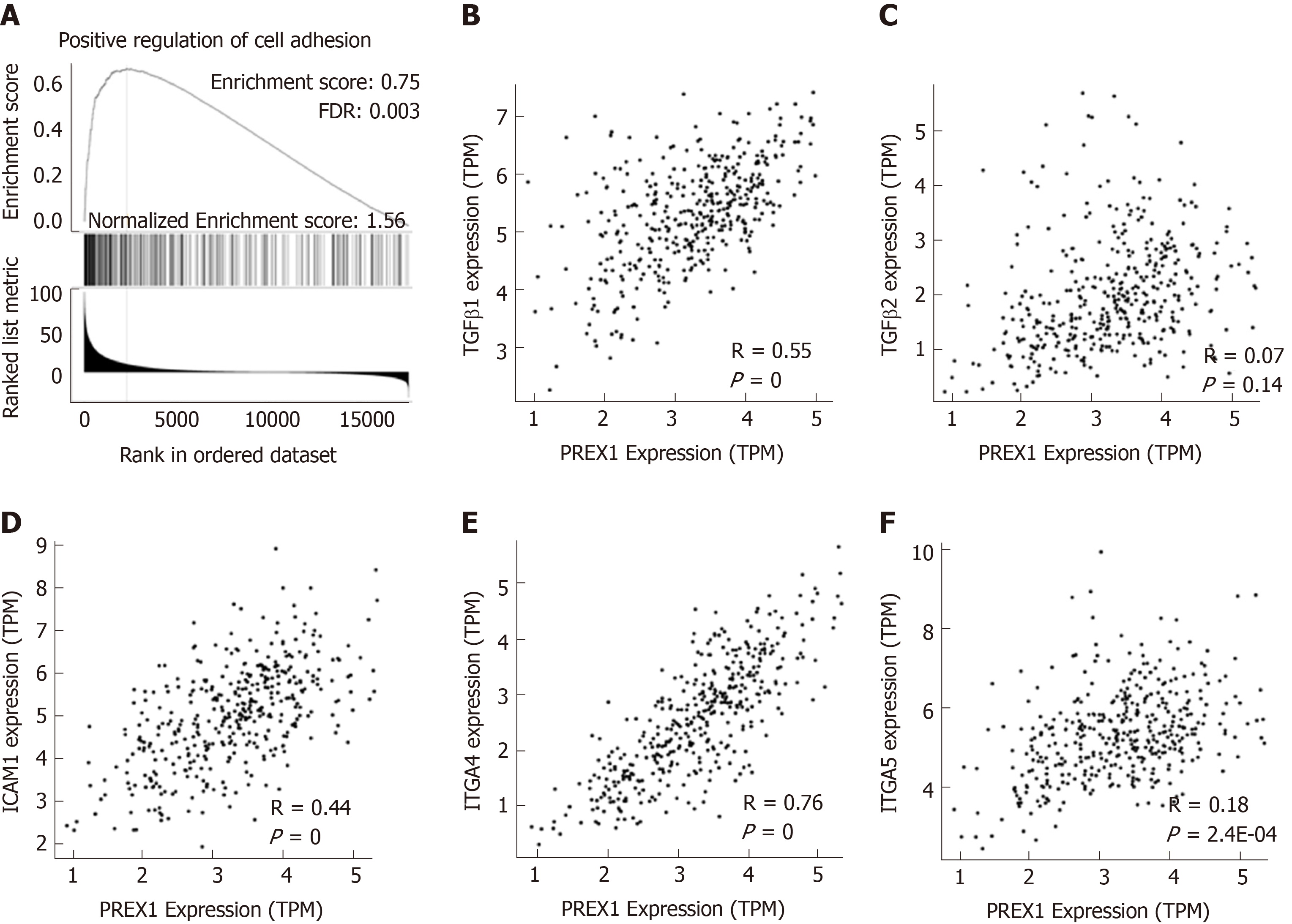
PREX1 was overexpressed in the gastric tumor tissues, and the expression of PREX1 could serve as a prognosis predictor for gastric cancer patients, especially for those patients with advanced-stage and intestinal histology. These results demonstrated that PREX1 might be an important mediator in the development of gastric cancer. Thus, we evaluated the potential mechanism regulation involved in the function of PREX1 in gastric cancer. Firstly, the co-expression network of PREX1 was conducted, and the GSEA was applied to study the potential effect of PREX1 on the biological process. As shown in Figure 4A, PREX1 expression was closely associated with the positive regulation of cell adhesion, with the higher enrichment score (normalized enrichment score = 1.56, false discovery rate = 0.003). To confirm further this result, the correlation between PREX1 and other important mediators were conducted. TGFβ signaling pathway is an important mediator in cancer development and is was closely involved in the migration and invasion of cancer cells[34]. As PREX1 expression was positively associated with cell adhesion, we evaluated the correlation between PREX1 and TGFβ signaling pathway. PREX1 was positively correlated with TGFβ1 (Figure 4B), and there was no significant correlation between PREX1 and TGFβ2 (Figure 4C). In further analysis downstream of TGFβ pathway, a similar phenomenon was observed in the correlation analysis of intercellular adhesion molecule-1 (intercellular adhesion molecule-1, R = 0.44, Figure 4D), ITGA4 (ITGA4, R = 0.76, Figure 4E), and ITGA5 (ITGA5, R = 0.18, Figure 4F). These data revealed that PREX1 was positively associated with the cell adhesion and migration process, which are important stimulus factors in the development of some cancers.
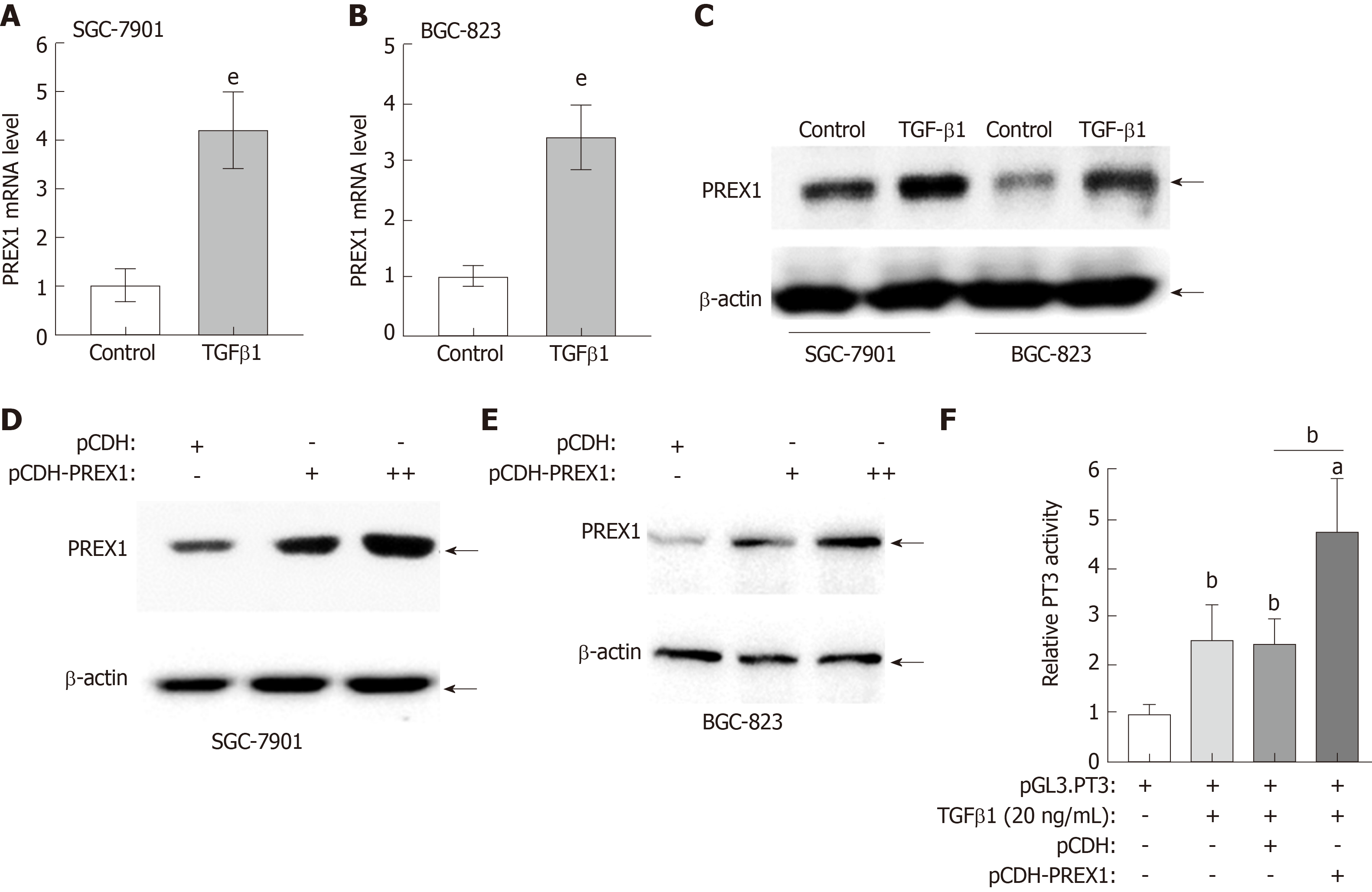
PREX1 was closely involved in the TGFβ1 signaling pathway, as described above. In this study, we further confirm the interaction between PREX1 and TGFβ1. As shown in Figure 5A and B, recombinant human TGFβ1 protein treatment could induce PREX1 mRNA levels in SGC-7901 and BGC-823 cell lines. The protein expression was evaluated by western blotting, and the result showed recombinant human TGFβ1 protein could remarkably increase PREX1 protein expression (Figure 5C). Feedback regulation is an essential pattern in the signaling transduction. As TGFβ1 can induce PREX1 expression, we hypothesized that PREX1 might affect the TGFβ1 pathway. To confirm the hypothesis, SGC-7901 and BGC-823 cells were overexpressed with PREX1, and we found that overexpression of PREX1 could activate the TGFβ1 pathway (Figure 5D and E). Furthermore, a Smad3-luciferase reporter (Luc-PT3) was utilized to study the effect of PREX1 on the TGFβ downstream signaling pathway. As shown in Figure 5F, overexpression of PREX1 could enhance TGFβ1-induced Smad3 luciferase activity. Hence, PREX1 has a feedback loop with TGFβ1 pathway.
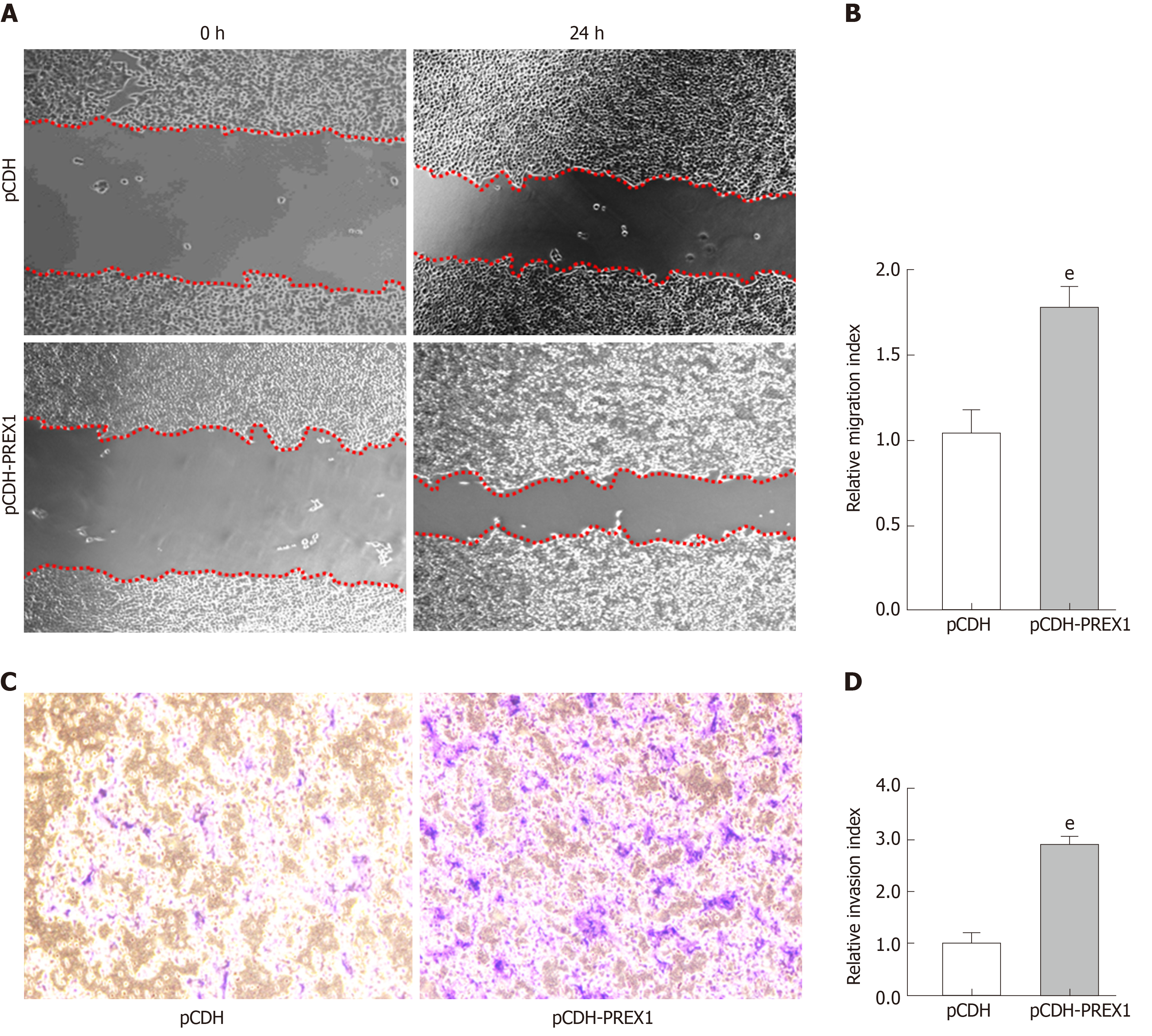
PREX1 was overexpressed in gastric cancer, PREX1 was closely involved in the biological process of cell adhesion, and a mechanism study revealed that PREX1 has a feedback loop with the TGFβ1 pathway, which has been widely reported as a promoter in cancer metastasis[34]. Therefore, we examined the effect of PREX1 in the migration and invasion of gastric cancer cells. As Figure 6A and 6B show, when PREX1 is overexpressed with lentivirus particle in SGC-7901 cells, the migration rate was increased compared with lentivirus vector control. Furthermore, the Transwell assay assessed that overexpression of PREX1 enhances invasion in SGC-7901 cells. Taken together, these results revealed that PREX1 was closely associated with TGFβ1 and regulated the metastasis process of gastric cancer cells.
The incidence and mortality of gastric cancer continue to rise in recent years, and the current clinical treatment of gastric cancer mainly depends on surgery, chemotherapy, and targeted therapy[35]. As targeted therapy can be directly applied to tumors, compared with surgery, radiotherapy, and chemotherapy, its side effects are relatively small[35,36]. Targeted therapeutic therapies for gastric cancer are mainly targeted to epidermal growth factor receptor and vascular endothelial growth factor receptor (VEGFR)[37]. However, more and more studies have confirmed the presence of epidermal growth factor receptor and vascular endothelial growth factor receptor mutations in gastric cancer patients, resulting in the failure of targeted therapy[38]. Therefore, the development of new targets is of great significance for the treatment of gastric cancer.
PREX1 has been reported to be overexpressed in some cancers, including breast, prostate, ovarian, and thyroid cancer, and PREX1 expression is closely associated with cancer development[18,39]. Furthermore, some studies about the mechanism regulation of PREX1 revealed that PREX1 could activate phosphatidylinositol 3-kinase signaling pathway, a cancer facilitating factor[18]. However, the study of PREX1 in gastric cancer remains unclear and needs further elaboration. In this study, we firstly identified that PREX1 is highly expressed in different types of gastric cancer (Figure 1) and that PREX1 expression is positively correlated with progression of gastric cancer (Figure 2), suggesting that PREX1 is a potential target for gastric cancer. To understand fully the significance of PREX1 in the development of gastric cancer, the effect of PREX1 on the survival analysis was conducted, and high PREX1 expression in patients predicted poor prognosis (Figure 3A and B). Interestingly, the OS analysis in intestinal-type gastric cancer revealed PREX1 is a significant biomarker for the prediction of poor prognosis, but it is not applicable to diffuse-type gastric cancer (Figure 3C and D). As PREX1 was overexpressed in both diffuse-type and intestinal-type gastric cancer (Figure 1), PREX1 might be a specific molecule in the heterogeneity of gastric cancer. Based on these data, we believe that PREX1 may be a novel target for gastric cancer therapy, especially in the intestinal-type gastric cancer.
PREX1 was reportedly involved in the regulation of some signaling pathways[39]. For example, PREX1 could activate phosphatidylinositol 3-kinase/AKT and MEK/ERK pathways to promote the development of breast cancer[18], and PREX1 could also interact with protein kinase A and cooperate with platelet-derived growth factor receptor β in the regulation of cell migration[14,40]. As PREX1 is an important regulator in the development of gastric cancer, the mechanism study of PREX1 in gastric cancer showed that PREX1 is closely involved with regulation of cell adhesion (Figure 4). Further evaluation represents that PREX1 has a positive feedback loop with TGFβ pathway (Figure 5), which is an essential factor in the development of some cancers, especially in the promotion of metastasis. Indeed, the metastasis promoting activity of PREX1 was also confirmed in the wound healing and Transwell assay (Figure 6). In summary, this study fully evaluated the overexpression of PREX1 in gastric cancer and demonstrated that PREX1 expression could act as a poor prognosis predictor for the specific intestinal-type gastric cancer. PREX1 is a prompting factor in the metastasis of gastric cancer cells, and PREX1 might be a novel drug target and a valuable prognostic biomarker for gastric cancer.
Phosphatidylinositol-3,4,5-trisphosphate dependent Rac exchange factor 1 (PREX1) is considered a pro-oncogene is some cancer types and is involved in some common cancer-promoting pathways, such as phosphatidylinositol 3-kinase/AKT and MEK/ERK. However, its expression and clinical value in gastric cancer has not been reported. Gastric cancer is a common high mortality disease, and identifying a novel therapeutic target could be valuable.
The goal was to confirm the expression of PREX1 in gastric cancer tissues and to correlate PREX1 level and disease development. The potential mechanism of PREX1 in the regulation of transforming growth factor (TGF) β pathway was also examined.
To evaluate the level and clinical value of PREX1 in the gastric cancer and to determine the potential mechanism in the regulation of gastric cancer metastasis.
The Cancer Genome Atlas and Oncomine portal were used to test the level of PREX1 in gastric cancer tissues. The Kaplan Meier was utilized to evaluate the survival rate in high and low PREX1 expressing patient groups, including the examination of overall survival (OS) and post-progression survival (PPS). The Gene Set Enrichment Analysis was applied to screen the potential PREX1-mediated pathways. The correlation analysis was achieved in the cBioPortal for Cancer Genomics. The mRNA level of PREX1 was examined by reverse transcription quantitative polymerase chain reaction, and the protein level was evaluated by western blotting assay. The dual luciferase reporter assay was applied to test the activation of the TGFβ pathway. The effect of PREX1 in the regulation of metastasis in gastric cancer was assessed by wound healing and Transwell assays.
PREX1 was highly expressed in gastric cancer tissues, and the expression level of PREX1 was positively correlated with patient stage, tumor grade, and lymph node metastasis. Furthermore, the high PREX1 level patients showed lower survival rate in OS and PPS, and the difference in OS was only discovered in the intestinal-specific gastric cancer patients. PREX1 expression was also closely linked with the cell adhesion and TGF-β related regulators. TGF-β1 could induce PREX1 expression, and PREX1 could activate TGF-β pathway. Overexpression of PREX1 could enhance the migration and invasion activity in vitro.
PREX1 is overexpressed in gastric cancer tissues and is involved in the development of gastric cancer. PREX1 could serve as a value prognostic biomarker in the prediction of OS and PPS. The mechanism study showed PREX1 is closely involved with the regulation of the cell adhesion process and TGF-β pathway in gastric cancer. PREX1 has a feedback regulation relationship with TGF-β and acts an enhancer in the regulation of metastasis in gastric cancer.
In this study, we firstly identified that PREX1 was overexpressed in gastric cancer and involved in the development of disease. PREX1 could act as a valuable prognostic marker, and the feedback regulation between PREX1 and TGF-β signaling pathway might contribute to the metastasis of gastric cancer cells. In future work, the detailed regulation between TGF-β and PREX1 should be studied as well as whether PREX1 could directly interact with TGF-β.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aykan NF, Tanabe S S-Editor: Tang JZ L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Hunt RH, Camilleri M, Crowe SE, El-Omar EM, Fox JG, Kuipers EJ, Malfertheiner P, McColl KE, Pritchard DM, Rugge M, Sonnenberg A, Sugano K, Tack J. The stomach in health and disease. Gut. 2015;64:1650-1668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 2. | Banks M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, Uedo N, Bhandari P, Pritchard DM, Kuipers EJ, Rodriguez-Justo M, Novelli MR, Ragunath K, Shepherd N, Dinis-Ribeiro M. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68:1545-1575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 295] [Article Influence: 59.0] [Reference Citation Analysis (1)] |
| 3. | Kinami S, Nakamura N, Tomita Y, Miyata T, Fujita H, Ueda N, Kosaka T. Precision surgical approach with lymph-node dissection in early gastric cancer. World J Gastroenterol. 2019;25:1640-1652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 27] [Cited by in F6Publishing: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Deng JY, Liang H. Clinical significance of lymph node metastasis in gastric cancer. World J Gastroenterol. 2014;20:3967-3975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 111] [Cited by in F6Publishing: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 5. | Machlowska J, Maciejewski R, Sitarz R. The Pattern of Signatures in Gastric Cancer Prognosis. Int J Mol Sci. 2018;19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean Practice Guideline for Gastric Cancer 2018: an Evidence-based, Multi-disciplinary Approach. J Gastric Cancer. 2019;19:1-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 273] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 7. | Marei H, Malliri A. GEFs: Dual regulation of Rac1 signaling. Small GTPases. 2017;8:90-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Lucato CM, Halls ML, Ooms LM, Liu HJ, Mitchell CA, Whisstock JC, Ellisdon AM. The Phosphatidylinositol (3,4,5)-Trisphosphate-dependent Rac Exchanger 1·Ras-related C3 Botulinum Toxin Substrate 1 (P-Rex1·Rac1) Complex Reveals the Basis of Rac1 Activation in Breast Cancer Cells. J Biol Chem. 2015;290:20827-20840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Campbell AP, Smrcka AV. Targeting G protein-coupled receptor signalling by blocking G proteins. Nat Rev Drug Discov. 2018;17:789-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 10. | Nie B, Cheng N, Dinauer MC, Ye RD. Characterization of P-Rex1 for its role in fMet-Leu-Phe-induced superoxide production in reconstituted COS(phox) cells. Cell Signal. 2010;22:770-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Pan D, Barber MA, Hornigold K, Baker MJ, Toth JM, Oxley D, Welch HC. Norbin Stimulates the Catalytic Activity and Plasma Membrane Localization of the Guanine-Nucleotide Exchange Factor P-Rex1. J Biol Chem. 2016;291:6359-6375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Liang Q, Cheng N, Zhang G, Liang Y, Qian F, Wu D, Ye RD. Identification of P-Rex1 as an anti-inflammatory and anti-fibrogenic target for pulmonary fibrosis. Sci Rep. 2016;6:25785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Welch HC, Condliffe AM, Milne LJ, Ferguson GJ, Hill K, Webb LM, Okkenhaug K, Coadwell WJ, Andrews SR, Thelen M, Jones GE, Hawkins PT, Stephens LR. P-Rex1 regulates neutrophil function. Curr Biol. 2005;15:1867-1873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Chávez-Vargas L, Adame-García SR, Cervantes-Villagrana RD, Castillo-Kauil A, Bruystens JG, Fukuhara S, Taylor SS, Mochizuki N, Reyes-Cruz G, Vázquez-Prado J. Protein Kinase A (PKA) Type I Interacts with P-Rex1, a Rac Guanine Nucleotide Exchange Factor: Effect On Pka Localization And P-Rex1 Signaling. J Biol Chem. 2016;291:6182-6199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Xu X, Jin T. The Novel Functions of the PLC/PKC/PKD Signaling Axis in G Protein-Coupled Receptor-Mediated Chemotaxis of Neutrophils. J Immunol Res. 2015;2015:817604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Kremer KN, Dinkel BA, Sterner RM, Osborne DG, Jevremovic D, Hedin KE. TCR-CXCR4 signaling stabilizes cytokine mRNA transcripts via a PREX1-Rac1 pathway: implications for CTCL. Blood. 2017;130:982-994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Dinkel BA, Kremer KN, Rollins MR, Medlyn MJ, Hedin KE. GRK2 mediates TCR-induced transactivation of CXCR4 and TCR-CXCR4 complex formation that drives PI3Kγ/PREX1 signaling and T cell cytokine secretion. J Biol Chem. 2018;293:14022-14039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Dillon LM, Bean JR, Yang W, Shee K, Symonds LK, Balko JM, McDonald WH, Liu S, Gonzalez-Angulo AM, Mills GB, Arteaga CL, Miller TW. P-REX1 creates a positive feedback loop to activate growth factor receptor, PI3K/AKT and MEK/ERK signaling in breast cancer. Oncogene. 2015;34:3968-3976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Lopez-Haber C, Barrio-Real L, Casado-Medrano V, Kazanietz MG. Heregulin/ErbB3 Signaling Enhances CXCR4-Driven Rac1 Activation and Breast Cancer Cell Motility via Hypoxia-Inducible Factor 1α. Mol Cell Biol. 2016;36:2011-2026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Marotti JD, Muller KE, Tafe LJ, Demidenko E, Miller TW. P-Rex1 Expression in Invasive Breast Cancer in relation to Receptor Status and Distant Metastatic Site. Int J Breast Cancer. 2017;2017:4537532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Goel HL, Pursell B, Shultz LD, Greiner DL, Brekken RA, Vander Kooi CW, Mercurio AM. P-Rex1 Promotes Resistance to VEGF/VEGFR-Targeted Therapy in Prostate Cancer. Cell Rep. 2016;14:2193-2208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Zervantonakis IK, Iavarone C, Chen HY, Selfors LM, Palakurthi S, Liu JF, Drapkin R, Matulonis U, Leverson JD, Sampath D, Mills GB, Brugge JS. Systems analysis of apoptotic priming in ovarian cancer identifies vulnerabilities and predictors of drug response. Nat Commun. 2017;8:365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Gont A, Daneshmand M, Woulfe J, Lavictoire SJ, Lorimer IA. PREX1 integrates G protein-coupled receptor and phosphoinositide 3-kinase signaling to promote glioblastoma invasion. Oncotarget. 2017;8:8559-8573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Bisarro Dos Reis M, Barros-Filho MC, Marchi FA, Beltrami CM, Kuasne H, Pinto CAL, Ambatipudi S, Herceg Z, Kowalski LP, Rogatto SR. Prognostic Classifier Based on Genome-Wide DNA Methylation Profiling in Well-Differentiated Thyroid Tumors. J Clin Endocrinol Metab. 2017;102:4089-4099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Liu D, Ma X, Yang F, Xiao D, Jia Y, Wang Y. Discovery and validation of methylated-differentially expressed genes in Helicobacter pylori-induced gastric cancer. Cancer Gene Ther. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Kim HK, Choi IJ, Kim CG, Kim HS, Oshima A, Yamada Y, Arao T, Nishio K, Michalowski A, Green JE. Three-gene predictor of clinical outcome for gastric cancer patients treated with chemotherapy. Pharmacogenomics J. 2012;12:119-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Ooi CH, Ivanova T, Wu J, Lee M, Tan IB, Tao J, Ward L, Koo JH, Gopalakrishnan V, Zhu Y, Cheng LL, Lee J, Rha SY, Chung HC, Ganesan K, So J, Soo KC, Lim D, Chan WH, Wong WK, Bowtell D, Yeoh KG, Grabsch H, Boussioutas A, Tan P. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 2009;5:e1000676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 303] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 28. | Förster S, Gretschel S, Jöns T, Yashiro M, Kemmner W. THBS4, a novel stromal molecule of diffuse-type gastric adenocarcinomas, identified by transcriptome-wide expression profiling. Mod Pathol. 2011;24:1390-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Li WQ, Hu N, Burton VH, Yang HH, Su H, Conway CM, Wang L, Wang C, Ding T, Xu Y, Giffen C, Abnet CC, Goldstein AM, Hewitt SM, Taylor PR. PLCE1 mRNA and protein expression and survival of patients with esophageal squamous cell carcinoma and gastric adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2014;23:1579-1588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Busuttil RA, George J, Tothill RW, Ioculano K, Kowalczyk A, Mitchell C, Lade S, Tan P, Haviv I, Boussioutas A. A signature predicting poor prognosis in gastric and ovarian cancer represents a coordinated macrophage and stromal response. Clin Cancer Res. 2014;20:2761-2772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1071] [Cited by in F6Publishing: 1330] [Article Influence: 147.8] [Reference Citation Analysis (0)] |
| 32. | Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB, Zhang S, Lee M, Wu J, Lim KH, Lei Z, Goh G, Lim QY, Tan AL, Sin Poh DY, Riahi S, Bell S, Shi MM, Linnartz R, Zhu F, Yeoh KG, Toh HC, Yong WP, Cheong HC, Rha SY, Boussioutas A, Grabsch H, Rozen S, Tan P. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61:673-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 478] [Cited by in F6Publishing: 498] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 33. | Lee JH, Chang KK, Yoon C, Tang LH, Strong VE, Yoon SS. Lauren Histologic Type Is the Most Important Factor Associated With Pattern of Recurrence Following Resection of Gastric Adenocarcinoma. Ann Surg. 2018;267:105-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 34. | Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1114] [Cited by in F6Publishing: 1124] [Article Influence: 93.7] [Reference Citation Analysis (0)] |
| 35. | Lazăr DC, Tăban S, Cornianu M, Faur A, Goldiş A. New advances in targeted gastric cancer treatment. World J Gastroenterol. 2016;22:6776-6799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 58] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 36. | Lazăr DC, Avram MF, Romoșan I, Cornianu M, Tăban S, Goldiș A. Prognostic significance of tumor immune microenvironment and immunotherapy: Novel insights and future perspectives in gastric cancer. World J Gastroenterol. 2018;24:3583-3616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 94] [Cited by in F6Publishing: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 37. | Deng W, Gu L, Li X, Zheng J, Zhang Y, Duan B, Cui J, Dong J, Du J. CD24 associates with EGFR and supports EGF/EGFR signaling via RhoA in gastric cancer cells. J Transl Med. 2016;14:32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Riquelme I, Saavedra K, Espinoza JA, Weber H, García P, Nervi B, Garrido M, Corvalán AH, Roa JC, Bizama C. Molecular classification of gastric cancer: Towards a pathway-driven targeted therapy. Oncotarget. 2015;6:24750-24779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 39. | Welch HC. Regulation and function of P-Rex family Rac-GEFs. Small GTPases. 2015;6:49-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Campbell AD, Lawn S, McGarry LC, Welch HC, Ozanne BW, Norman JC. P-Rex1 cooperates with PDGFRβ to drive cellular migration in 3D microenvironments. PLoS One. 2013;8:e53982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |